Abstract
Sacituzumab govitecan (SG) is an antibody-drug conjugate (ADC) targeting TROP2, which has recently been approved for treatment-refractory metastatic urothelial cancer (UC). However, the variability of TROP2 expression across different bladder cancer (BC) subtypes, as well as after enfortumab vedotin (EV) exposure, remains unknown. Using gene expression data from four clinical cohorts with >1400 patient samples of muscle-invasive BC and a BC tissue microarray, we found that TROP2 mRNA and protein are highly expressed across basal, luminal, and stroma-rich subtypes, but depleted in the neuroendocrine subtype. In addition, TROP2 mRNA levels are correlated with NECTIN4 mRNA but are more highly expressed than NECTIN4 mRNA in patient cohorts and BC cell lines. Moreover, CRISPR/Cas9-mediated knockdown of TROP2 demonstrates that its expression is one factor governing SG sensitivity. After prolonged EV exposure, cells can downregulate NECTIN4, leading to EV resistance, but retain TROP2 expression and remain sensitive to SG, suggesting nonoverlapping resistance mechanisms to these ADCs. While our findings warrant further validation, they have significant implications for biomarker development, patient selection, and treatment sequencing in the clinic as well as clinical trial design and stratification for metastatic BC patients.
Keywords: Urothelial cancer, Bladder cancer, Sacituzumab govitecan, Enfortumab vedotin, Molecular subtypes, Antibody-drug conjugate
Patient summary:
In this report, we investigated the expression levels of the drug target TROP2 across different molecular subtypes of bladder cancer in multiple patient cohorts and cell lines. We found high levels of TROP2 in most subtypes except in the neuroendocrine subtype. Overall, TROP2 gene expression is higher than NECTIN4 gene expression, and cells resistant to enfortumab vedotin (EV), a NECTIN4-targeting antibody-drug conjugate, remain sensitive to sacituzumab govitecan (SG). Our findings suggest that SG may be effective across most bladder cancer subtypes, including the bladder cancers previously treated with EV.
The surface protein TROP2, encoded by the gene TACSTD2/ TROP2, is a calcium signaling transmembrane protein that is highly expressed in multiple cancers including bladder urothelial carcinoma (UC; Supplementary Fig. 1) and is associated with poor survival [1]. Sacituzumab govitecan (SG; prior name IMMU-132) is an antibody-drug conjugate (ADC) that delivers SN-38, a topoisomerase inhibitor, to tumor cells expressing TROP2. A recent phase 2 trial of SG (TROPHY-U-01, NCT03547973) in patients with heavily pretreated, locally advanced, or metastatic UC demonstrated a 27% overall response rate, leading to expedited approval by the Food and Drug Administration [2]. Additional trials are underway to confirm SG efficacy (TROPiCS-04, NCT04527991) and to evaluate drug combinations, including with the NECTIN4-targeting ADC enfortumab vedotin (EV) (NCT04724018). As SG moves into earlier disease states and is incorporated into combinations with other ADCs, understanding the mechanisms of sensitivity and resistance is critical to maximize clinical efficacy.
The effectiveness of an ADC is dependent on both high tumor expression of the target surface protein and tumor sensitivity to the toxic payload. Molecular subtyping of UC has highlighted differences in oncogenic mechanisms and clinicopathologic features [3]. Whether TROP2 mRNA is ubiquitously and uniformly expressed across the six consensus molecular subtypes of UC has not been reported. Further, whether exposure or resistance to one ADC (eg, EV) confers resistance to another ADC (eg, SG) remains unknown. Here, we interrogated TROP2 gene expression in multiple clinical datasets, evaluated cell line sensitivity to EV and SG, and assessed gene expression variability across bladder cancer (BC) subtypes to better understand the potential for combination therapy. Further methods are available in the Supplementary material.
To assess TROP2 mRNA expression across the six molecular subtypes of BC, we analyzed TROP2 mRNA expression in four patient cohorts (n = 1483 samples) with localized muscle-invasive bladder tumors (in studies by Seiler et al [4], Sjödahl et al [5], Robertson et al [6], and NCT02609269). Clinical characteristics were described previously [7]. Using the consensus classifier subtypes [3], we found comparable median expression between luminal (luminal papillary, luminal nonspecified, and luminal unstable), basal, and stroma-rich subtypes, with greater variability in expression among basal and stroma-rich subtypes in all four cohorts (Fig. 1A–D and Supplementary Table 1). Expression variability within basal subtypes was mirrored in basal BC cell lines (Supplementary Fig. 2). Surprisingly, there was lower TROP2 expression in neuroendocrine (NE)-like subtypes, which we validated by immunohistochemistry using tissue microarrays and a collection of NE BC specimens (Fig. 1E, and Supplementary Fig. 3 and 4); TROP2 was previously shown to be enriched in and a driver of NE prostate cancer [8]. We also found that TROP2 mRNA levels were well correlated with TROP2 protein levels (Spearman’s rank correlation r = 0.62, p < 0.0001; Fig. 1F and Supplementary Fig. 4), suggesting that gene expression may serve as a proxy for protein expression, in accordance with prior studies [9,10]. Finally, in patients with advanced disease treated on the IMvigor210 clinical trial [11], TROP2 expression was similar across different metastatic sites and was similar in patients with locally advanced and metastatic UC (Supplementary Fig. 5). Together, these results may predict comparable SG effectiveness across most non-NE BC subtypes.
Fig. 1 –
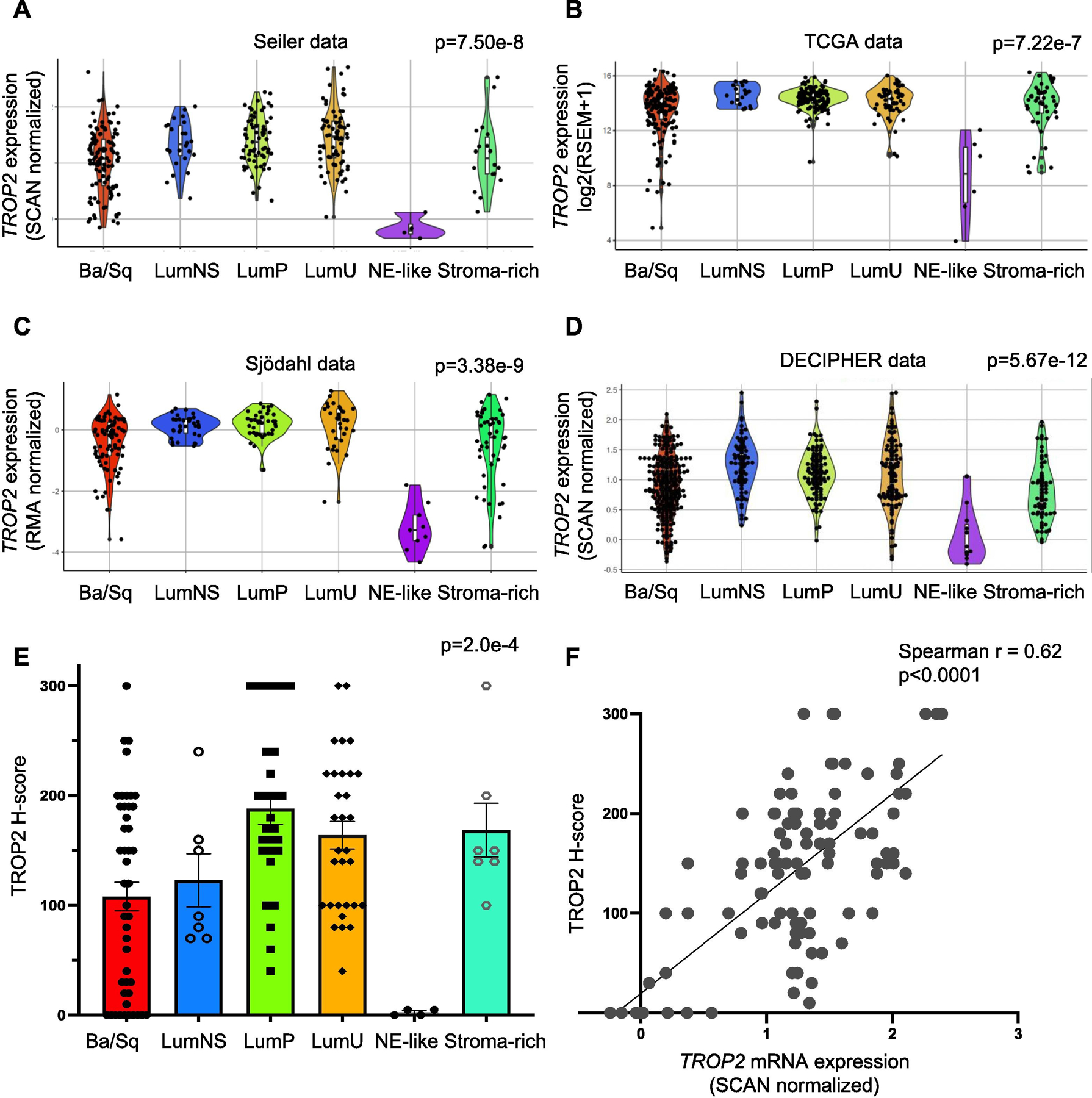
TROP2 mRNA and TROP2 protein expression across the molecular subtypes of muscle-invasive bladder cancer (MIBC). Violin plots showing TROP2 mRNA expression levels by consensus molecular subtypes in the (A) Seiler, (B) TCGA, (C) Sjödahl, and (D) Decipher cohorts. (E) Immunohistochemistry for TROP2 was performed using a bladder cancer TMA (n = 80 samples, in duplicate). H scores for TROP2 were assigned in a blinded manner, and subtypes were determined previously. The average TROP2 H score ± SEM is shown for each subtype. The p value from Kruskal-Wallis testing is shown for each cohort in panels A–E. (F) Scatter plot showing the correlation between TROP2 protein (H score) and TROP2 mRNA expression levels. The Spearman’s rho coefficient is shown (p < 0.0001). Ba/Sq = basal/Squamous; LumNS = luminal nonspecified; LumP = luminal papillary; LumU = luminal unstable; NE = neuroendocrine; SEM = standard error of the mean; TCGA = The Cancer Genome Atlas; TMA = tissue microarray.
To assess the potential effectiveness of TROP2- and NECTIN4-targeting ADCs, we compared TROP2 and NECTIN4 mRNA expression in the patient cohorts and 35 BC cell lines. We found that TROP2 and NECTIN4 mRNA expression is positively correlated (Spearman’s rank correlation r > 0.4, p < 0.0001; Supplementary Fig. 6), but that TROP2 mRNA is overall more highly expressed than NECTIN4 mRNA in patient samples (p < 1e-56; Fig. 2A and B) and cell lines (p < 0.0001; Fig. 2C). We also found either no correlation or a negative correlation between TROP2, PDCD1 (encoding PD1), and CD274 (encoding PD-L1) in patient cohorts (Supplementary Fig. 7).
Fig. 2 –
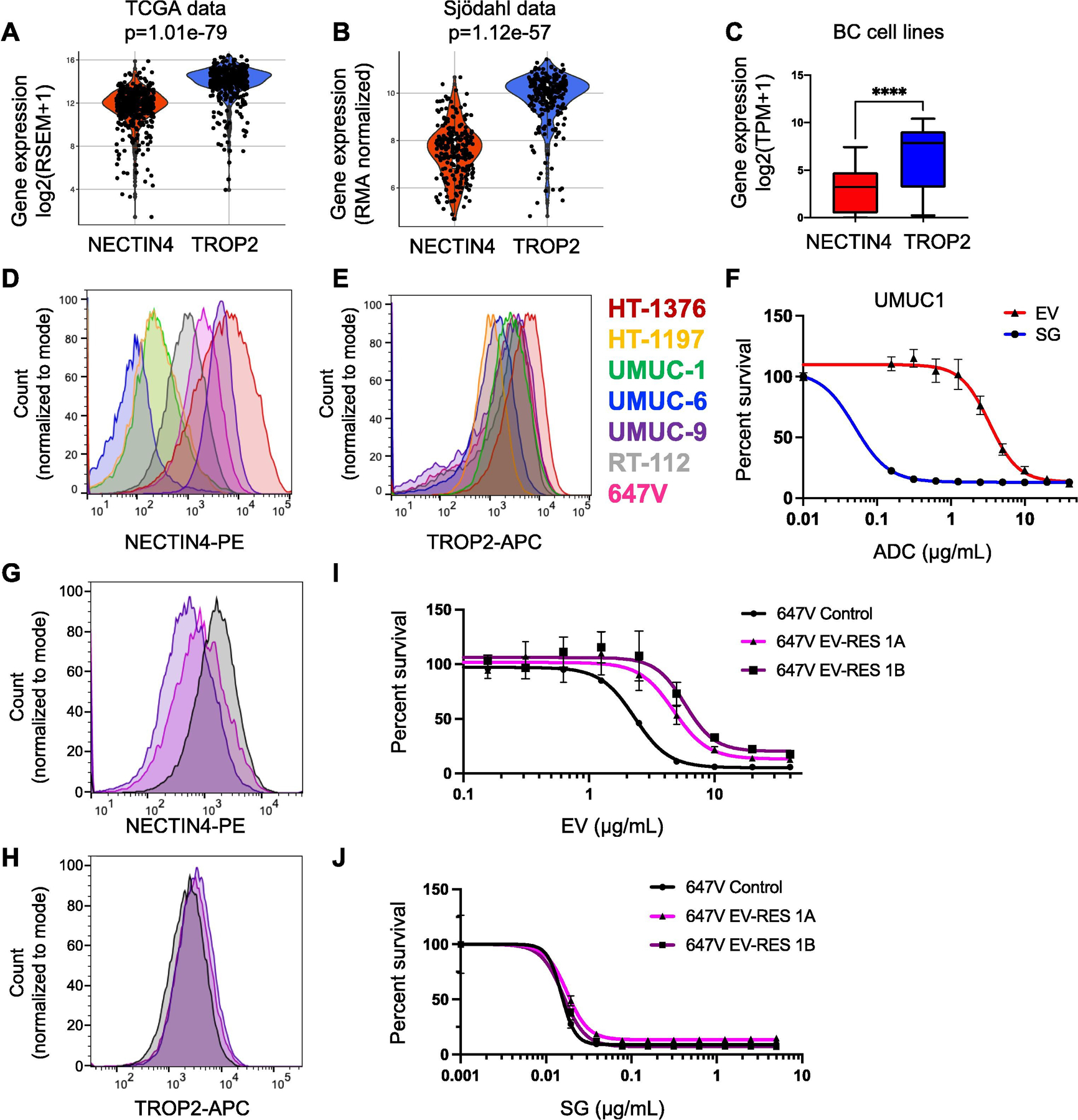
TROP2 and NECTIN4 mRNA expression in MIBC and correlation to antibody-drug conjugate response. Violin plots of NECTIN4 and TROP2 mRNA levels in the (A) TCGA and (B) Sjödahlcohorts. The p value from Wilcoxon rank-sum testing is shown for each cohort. (C) Box and whisker plot of NECTIN4 and TROP2 mRNA expression in 35 urothelial carcinoma cell lines. (D) NECTIN4 and (E) TROP2 surface protein expression in seven bladder cancer cell lines. (F) Dose-response curves to the antibody drug conjugates (ADCs) enfortumab vedotin (EV) and sacituzumab govitecan (SG) in the UMUC-1 cell line. (G) NECTIN4 and (H) TROP2 surface protein expression in 647V control (black) and two 647V EV-resistant lines (purple and magenta) cell lines. Dose-response curves to (I) EV and (K) SG in 647V control (black) and two 647V EV-resistant lines (purple and magenta). BC = bladder cancer; MIBC = muscle-invasive bladder cancer; TCGA = The Cancer Genome Atlas. **** p < 0.0001 by Wilcoxon rank-sum test.
Next, we investigated TROP2 and NECTIN4 protein expression across multiple BC cell lines. We found that in NECTIN4-positive BC cells, NECTIN4 expression was more variable (median fluorescence intensity [MFI] = 1131 ± 2086 a.u.; (Fig. 2D and Supplementary Table 2), while TROP2 expression was overall higher and more uniform (MFI = 2239 ± 1354 a.u.; Fig. 2E and Supplementary Table 2). Importantly, differences in NECTIN4 and TROP2 expression corresponded to differences in ADC sensitivity. For example, in UMUC1, a NECTIN4LOW/TROP2HI luminal BC line, cells were more sensitive to SG (half-maximal inhibitory concentration [IC50] = 0.050 ± 0.021 μg/ml) than EV (IC50 = 3.1 ± 0.3 μg/ml; Fig. 2F and Supplementary Table 3). Conversely, in HT-1197, a NECTIN4LOW/TROP2MED BC line, cells were only slightly more sensitive to SG (IC50 = 1.2 ± 0.2 μg/ml) than EV (IC50 = 3.5 ± 0.4 μg/ml). Interestingly, in HT-1376, a NECTIN4HI/TROP2HI BC line, cells were more sensitive to EV (IC50 = 0.31 ± 0.25 μg/ml) than SG (IC50 = 2.8 ± 0.4 μg/ml), suggesting resistance to SG despite high surface protein levels of TROP2, potentially due to altered intracellular protein trafficking or intrinsic payload (SN-38) resistance (Supplementary Fig. 8 and Supplementary Table 3). Nonetheless, TROP2 expression is critical for SG sensitivity, as knockdown of TROP2 led to SG resistance (Supplementary Fig. 9).
Finally, to assess whether BC lines exposed to EV retain sensitivity to SG, we evolved EV resistance in vitro using a NECTIN4MED/TROP2MED BC cell line, 647V, by repeated EV exposure. The EV-resistant cell lines had decreased expression of NECTIN4 (Fig. 2G) but retained expression of TROP2 (Fig. 2H). Expression of NECTIN4 and TROP2 in additional HT-1376 and UMUC-1 EV-resistant cell lines showed similar results (Supplementary Fig. 10). While the potency of EV decreased from IC50 = 2.3 ± 0.1 μg/ml in the control to IC50 = 4.7 ± 0.7 and 5.8 ± 0.9 μg/ml in the EV-resistant cell lines (Fig. 2I and Supplementary Table 3), the potency of SG was unchanged (control IC50 = 0.015 ± 0.002; EV-resistant lines, IC50 = 0.018 ± 0.001 and 0.016 ± 0.002 μg/ml; Fig. 2J and Supplementary Table 3). Together, these data suggest that cells exposed to EV or that acquire EV resistance remain sensitive to SG, suggesting different mechanisms of resistance.
In conclusion, our study demonstrates that TROP2 mRNA and protein expression are comparably high across non-NE subtypes of BC. This contrasts with NECTIN4, which we previously showed to be enriched in luminal subtypes [7]. Interestingly, TROP2 mRNA expression exceeds NECTIN4 mRNA expression in patient cohorts and BC cell lines. Moreover, our data show that in NECTIN4LOW/TROP2HI BC cell line models, SG is more potent than EV, suggesting that TROP2 expression levels likely influence SG efficacy, in accordance with data in triple-negative breast cancer [12]. Indeed, we show that loss of TROP2 using CRISPR-mediated knockdown leads to SG resistance in BC cells. A recent study also identified a missense mutation in TROP2, which impairs TROP2 localization to the cell surface, in a breast cancer patient who developed SG resistance [13]. Whether similar mechanisms of resistance are found in BC patients awaits further study. In addition, our data in EV-resistant cell lines suggest that SG may be effective in patients previously treated with or resistant to EV, and warrant further validation in patient biopsies taken before and after ADC treatment.
Limitations of our study include extrapolating surface protein expression from transcriptomic data (although our data demonstrate a strong correlation between mRNA and protein levels, in accordance with prior studies [9,10]), utilizing primary tumor samples for most of our analysis (due to the lack of metastatic biopsy cohorts), and the lack of pre/post-ADC patient samples to confirm our findings. Although we found absent to low levels of TROP2 in NE tumors, the exact threshold of expression required to respond to SG is not known and warrants further study. Trials evaluating the efficacy of ADCs should consider tumor molecular subtyping and target protein staining to better identify patients, and our data support the use of SG in patients with non-NE BC subtypes and patients previously treated with EV.
Supplementary Material
Acknowledgments:
We would like to acknowledge the generous support of TS and Sharon Ng.
Funding/Support and role of the sponsor:
Jonathan Chou was supported by the A.P. Giannini Foundation Postdoctoral Fellowship. Kai Trepka was supported by the National Institute of General Medical Sciences with Medical Scientist Training Program Grant number T32GM141323. Martin Sjöströmwas supported by the Swedish Research Council (Vetenskapsrådet; grant number 2018–00382) and the Swedish Society of Medicine (Svenska Läkaresällskapet). Carissa E. Chu was supported by a Clinical & Translational Science Institute (CSTI) TL-1 grant. Felix Y. Feng was supported by the Benioff-Goldberg Fund for Translational Research and NIH R01 CA235741.
Footnotes
Study concept and design: Chou, Trepka, Sjöström, Chu, Feng.
Acquisition of data: Chou, Trepka, Sjöström, Chu, Zhu, Egusa, Gibb, Badura.
Analysis and interpretation of data: Chou, Trepka, Sjöström, Chu, Gibb.
Drafting of the manuscript: Chou, Trepka, Sjöström, Chu, Feng.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Chou, Trepka, Sjöström, Gibb.
Obtaining funding: Chou, Friedlander, Feng.
Administrative, technical, or material support: Contreras-Sanz, Black, Lotan, Chan, Stohr, Koshkin.
Supervision: Feng.
Other: None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euo.2021.11.005.
Financial disclosures: Jonathan Chou certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Felix Y. Feng has consulted for Astellas, Bayer, BlueEarth Diagnostics, Celgene, Clovis, EMD Serono, Genentech, Janssen, Myovant, Ryovant, and Sanofi; is a co-founder of PFS Genomics; and serves on the scientific advisory board of SerImmune. Emily A. Egusa is an employee of Decipher Biosciences, Inc. Sima P. Porten has consulted for ProTara, Merck, and Photocure. Peter C. Black has consulted for AbbVie, Astellas Pharma, Janssen Oncology, Amgen, Bayer, Merck, Sanofi Canada, Biosyent, Ferring, Roche Canada, MDxHealth, AstraZeneca, Urogen Pharma, Asieris, and Bristol-Myers Squibb, and has received research funding from Genome Dx and Sitka. Yair Lotan has consulted for Cepheid, Pacific Edge, Photocure, AstraZeneca, Merck, Fergene, and Ferring, and has received research funding from Abbott, MDxHealth, Cepheid, Pacific Edge, Genome Dx, Storz, and FKD. Terence W. Friedlander has consulted for Seattle Genetics, EMD Serono, Astra Zeneca, and Merck, and has received research funding from Seattle Genetics and Roche/ Genentech.
References
- [1].Zeng P, Chen MB, Zhou LN, Tang M, Liu CY, Lu PH. Impact of TROP2 expression on prognosis in solid tumors: a systematic review and meta-analysis. Sci Rep 2016;6:33658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol 2021;39: 2474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kamoun A, de Reynies A, Allory Y, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol 2020;77:420–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seiler R, Ashab HAD, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol 2017;72:544–54. [DOI] [PubMed] [Google Scholar]
- [5].Sjödahl G, Eriksson P, Liedberg F, Hoglund M. Molecular classification of urothelial carcinoma: global mRNA classification versus tumourcell phenotype classification. J Pathol 2017;242: 113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2018;174: 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chu CE, Sjöström M, Egusa EA, et al. Heterogeneity in NECTIN4 expression across molecular subtypes of urothelial cancer mediates sensitivity to enfortumab vedotin. Clin Cancer Res 2021;27: 5123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hsu EC, Rice MA, Bermudez A, et al. Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc Natl Acad Sci USA 2020;117:2032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Edfors F, Danielsson F, Hallström BM, et al. Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol Syst Biol 2016;12:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwanhausser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature 2011;473:337–42. [DOI] [PubMed] [Google Scholar]
- [11].Mariathasan S, Turley SJ, Nickles D, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bardia A, Tolaney SM, Punie K, et al. Biomarker analyses in the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol 2021;32:1148–56. [DOI] [PubMed] [Google Scholar]
- [13].Coates JT, Sun S, Leshchiner I, et al. Parallel genomic alterations of antigen and payload targets mediate polyclonal acquired clinical resistance to sacituzumab govitecan in triple-negative breast cancer. Cancer Discov 2021;11:2436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


