Abstract
Prostate-specific membrane antigen (PSMA) positron emission tomography (PET)/ computed tomography (CT) is an emerging imaging modality with greater sensitivity and specificity over conventional imaging for prostate cancer (PCa) staging. Using data from two prospective trials (NCT03368547 and NCT04050215), we explored predictors of overall upstaging (nodal and metastatic) by PSMA PET/CT among patients with cN0M0 National Comprehensive Cancer Network high-risk PCa on conventional imaging (n = 213). Overall, 21.1%, 8.9%, and 23.9% of patients experienced nodal, metastatic, and overall upstaging, respectively, without histologic confirmation. On multivariable analysis, Gleason grade group (GG) and percent positive core (PPC) on systematic biopsy significantly predict overall upstaging (odds ratio [OR] 2.15, 95% confidence interval [CI] 1.33–3.45; p = 0.002; and OR 1.03, 95% CI 1.01–1.04; p < 0.001). Overall upstaging was significantly more frequent among men with GG 5 disease (33.0% vs. 17.6%; p = 0.0097) and PPC ≥50% (33.0% vs 15.0%; p = 0.0020). We constructed a nomogram that predicts overall upstaging using initial prostate-specific antigen, PPC, GG, and cT stage, with coefficients estimated from a standard logistic regression model (using maximum likelihood estimation). It is internally validated with a tenfold cross-validated area under the receiver operating characteristic curve estimated at 0.74 (95% CI 0.67–0.82). In our cohort, 90% of patients who had a nomogram-estimated risk below the cutoff of 22% for overall upstaging could have been spared PSMA PET/CT as our model correctly predicted no upstaging. In other words, the predictive model only missed 10% of patients who would otherwise have benefitted from PSMA PET/CT.
Keywords: Prostate cancer, Staging, Prostate-specific membrane, antigen, Positron emission tomography/computed tomography, Conventional imaging, Overall upstaging, Percent positive core, Gleason grade, Nomogram
Patient summary:
We analyzed predictors of overall upstaging (lymph node or/ and metastasis) by prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) from conventional imaging in men with high-risk prostate cancer undergoing initial staging deemed free of disease in the lymph nodes and distant metastasis by conventional imaging techniques. We found that the pathologic grade and disease burden in a prostate biopsy are associated with upstaging. We also developed a tool that predicts the probability of upstaging according to an individual patient’s characteristics. Our study may help in defining patient groups who are most likely to benefit from the addition of a PSMA PET/CT scan.
Positron emission tomography (PET)/computed tomography (CT) with [68Ga]-labeled prostate-specific membrane antigen (PSMA) ligands has emerged as a more sensitive and specific modality for initial staging of prostate cancer than conventional bone scans or CT [1,2]. The landmark proPSMA trial randomized 302 patients to initial PSMA PET/CT or conventional imaging and found that PSMA PET/CT had greater accuracy, specificity, and sensitivity in the first-line setting [1]. When performed after conventional imaging, PSMA PET/CT led to a change in stage for 22% of patients. Notably, the trial enrolled patients with high-risk features, which included Gleason grade group (GG) 3 disease alone. According to the National Comprehensive Cancer Network (NCCN) guidelines, GG 3 disease alone is not sufficient to qualify for high-risk disease [3]. In addition, percent positive cores (PPC) on biopsy, which predicts adverse outcomes for patients with high-risk prostate cancer [4], was not available. We sought to explore predictors of nodal and metastatic upstaging among patients with NCCN high-risk prostate cancer.
The study population consisted of 213 men enrolled in two prospective clinical trials of PSMA PET/CT between December 2016 and January 2020 (NCT03368547 and NCT04050215). Patients with NCCN high-risk disease undergoing PSMA PET/CT as part of primary staging were included. Patients with known nonregional lymph node or distant metastasis, initiation of androgen deprivation therapy >3 mo before PSMA PET/CT, or prior prostate cancer treatment were excluded. To specifically evaluate upstaging afforded by PSMA PET/CT, patients with no prior abdomen/pelvis CT and exhibiting enlarged nodes according to conventional imaging criteria on the CT portion of their PSMA PET/CT examination were excluded. Similarly, patients who might have been diagnosed with M1 disease on the basis of prior abdomen/pelvis CT before PSMA PET/CT were also excluded (Supplementary Fig. 1).
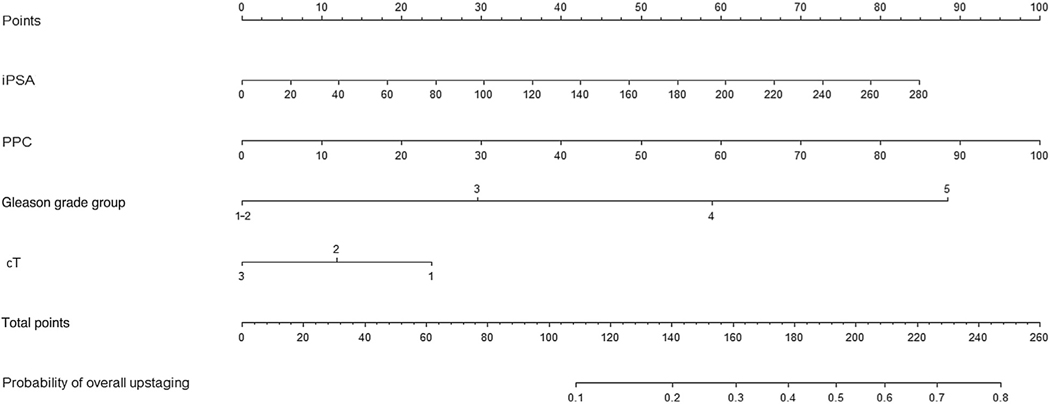
A multivariable logistic regression model for overall upstaging (nodal and metastatic upstaging) detected by PSMA PET/CT was constructed using initial prostate-specific antigen (iPSA), PPC, GG, and cT stage, with coefficients estimated from a standard logistic regression model (using maximum likelihood estimation). A nomogram was built for overall upstaging using these parameters. The model performance was internally validated using a tenfold cross-validated area under the receiver operating characteristic curve (AUC) to better assess its external prognostic ability.
Overall, 213 men with high-risk prostate cancer were included in the overall upstaging analysis (Table 1). Of these, 45/213 (21.1%), 19/213 (8.9%), and 51/213 (23.9%) experienced nodal, metastatic, and overall upstaging, respectively.
Table 1 –
Clinical and demographic characteristics for the 213 patients.
| Parameter | Result |
|---|---|
| Median age, yr (interquartile range) | 68 (63–73) |
| Percent positive cores, n (%) | |
| ≥50% | 106 (49.8) |
| <50% | 107 (50.2) |
| Median PSA, ng/mL (interquartile range) | 11.9 (7.3–26.9) |
| Gleason grade group, n (%) | |
| 1 | 0 (0) |
| 2 | 22 (10.3) |
| 3 | 16 (7.5) |
| 4 | 87 (40.8) |
| 5 | 88 (41.3) |
| cT stage, n (%) | |
| 1 | 124 (58.2) |
| 2 | 78 (36.6) |
| 3 | 11 (5.2) |
| Conventional imaging, n (%) | |
| Computed tomography abdomen/pelvis | 102 (47.9) |
| Bone scan | 127 (59.6) |
| Magnetic resonance imaging | 184 (86.4) |
| Mean number of biopsy cores, n (median) | 12.2 (12) |
| Mean number of positive cores, n (median) | 6.2 (5) |
On multivariable analysis, GG (odds ratio [OR] 2.15; 95% confidence interval [CI] 1.33–3.45; p = 0.002) and PPC (OR 1.03, 95% CI 1.01–1.04; p < 0.001) were significant predictors of overall upstaging (Supplementary Table 1). Breakdown of overall upstaging by GG and PPC ≥50% versus PPC < 50% is shown in Supplementary Figure 2. Overall upstaging was significantly more frequent among patients with PPC 50% than among those with PPC < 50% (33.0% vs 15.0%; p = 0.0020). Overall upstaging was also significantly more frequent among men with GG 5 compared to those with GG <5 disease (33.0% vs 17.6%; p = 0.0097). The nomogram that predicts the probability of overall upstaging by PSMA PET/CT based on iPSA, PPC, GG, and cT stage is displayed in Figure 1. The model had a raw AUC of 0.75 (95% CI 0.67–0.83) and a tenfold cross-validated AUC of 0.74 (95% CI 0.67–0.82; Supplementary Fig. 3C). We also performed decision curve analysis that confirmed the net benefit of using the model in realistic clinical situations (Supplementary Fig. 3B). When using a cutoff of 145 (corresponding to 22% overall upstaging risk), 90% of patients whose risk falls below the cutoff can be safely spared from PSMA PET/CT. The high negative predictive value of the model ensures that only 10% of patients who were deemed at low risk of overall upstaging by the nomogram would otherwise have benefitted from PSMA PET/CT. Note that the nomogram predicts upstaging for a patient with cN0M0 disease by conventional imaging and that PPC is solely based on systematic biopsy cores.
Fig. 1 –
Nomogram for predicting the probability of overall upstaging (nodal and metastatic) by prostate-specific membrane antigen positron emission tomography/computed tomography. The points assigned to each parameter (iPSA, PPC, Gleason grade group, and cT stage) are summed to generate a total score corresponding to the upstaging probability. A cutoff of 145 (corresponding to a 22% risk) provides 76.5% sensitivity, 66.7% specificity, 41.9% positive predictive value, and 90.0% negative predictive value. All patients had cN0M0 disease according to conventional imaging. PPC is derived from systematic biopsy cores only.
iPSA = initial prostate-specific antigen; PPC = percent positive cores.
The major finding is that PPC is a powerful predictor of increased nodal, metastatic, and any upstaging. Our study also confirms the predictive value of GG for upstaging, as previously established [5]. As PSMA PET/CT is not yet widely available and resource allocation may be a problem, our results suggest that patients with PPC ≥ 50% and GG 5 disease are the most likely to have occult nodal or metastatic disease (40.4%; 37.6% for patients with PPC ≥ 50% and GG 4–5 disease). These patients may benefit from therapeutic intensification aimed at controlling extraprostatic disease, including elective nodal radiotherapy if receiving definitive radiation [6] and the use of advanced systemic therapy agents. Indeed, early metastatic failures after definitive-intent treatment for high-grade prostate cancer may reflect occult metastases at the time of initial treatment [7]. In addition, the importance of PPC suggests that it should be included in risk stratification schemes for clinical use. It also supports the routine clinical use of systematic biopsy even when targeted biopsies are performed [8].
There are several limitations to this study. First, no histologic confirmation of PSMA PET/CT–positive lesions was obtained and therefore false-positive and -negative rates cannot be accurately estimated. However, in the proPSMA study only 20 men (23%) had data for “hard criteria” available, which includes histologic confirmation of PSMA PET/CT–identified metastasis. Second, PSMA PET/CT scans were interpreted by an expert team at a tertiary academic center, which might impact generalizability. Of note, our results are remarkably concordant with the proPSMA trial results, in which second-line PSMA PET/CT resulted in nodal upstaging in 18% of patients and metastatic upstaging in 8%. Finally, the impact of upstaging by PSMA PET/CT on clinical outcomes remains unknown and well-designed prospective studies are required to quantify this impact.
Overall, our findings indicate that PPC and GG are highly predictive of overall upstaging by PSMA PET/CT for patients with high-risk prostate cancer. Patients with PPC ≥50% and GG 4–5 disease will benefit the most from PSMA PET/CT, and also will benefit from therapeutic intensification strategies aimed at extraprostatic disease. Further studies should validate the importance of these prognostic variables and identify whether changes in treatment lead to better outcomes.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: None.
Financial disclosures: Amar U. Kishan certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Amar U. Kishan reports honoraria and consulting fees from Varian Medical Systems and honoraria from ViewRay outside the scope of the submitted work. Felix Y. Feng reports personal fees from Dendreon, EMD Serono, Janssen Oncology, Ferring, Sanofi, Bayer, Blue Earth Diagnostics, Celgene, Medivation/Astellas, and Clovis Oncology, and ownership interests in PFS Genomics outside the submitted work, and has a patent issued (EP3047037A4). The remaining authors have nothing to disclose.
Footnotes
Study concept and design: Kishan, Ma, Gafita, Grogan, Calais, Nickols.
Acquisition of data: Ma, Gafita, Shabsovich, Juarez, Thin, Armstrong, Sonni, Nguyen, Lok, Kishan.
Analysis and interpretation of data: Ma, Gafita, Grogan, Kishan.
Drafting of the manuscript: Kishan, Ma, Gafita, Grogan.
Critical revision of the manuscript for important intellectual content: Ma, Gafita, Shabsovich, Juarez, Grogan, Reiter, Rettig, Steinberg, Kupelian, Yang, Muralidhar, Chu, Feng, Savjani, Deng, Parikh, Nickols, Elashoff, Czernin, Calais, Kishan.
Statistical analysis: Gafita, Grogan, Ma, Kishan.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Kishan.
Other: None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euo.2021.01.006.
References
- [1].Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020;395: 1208–1216. [DOI] [PubMed] [Google Scholar]
- [2].Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol 2020;77:403–17. [DOI] [PubMed] [Google Scholar]
- [3].National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer. NCCN. www.nccn.org/professionals/physician_gls/pdf/prostate.pdf2019 [DOI] [PubMed] [Google Scholar]
- [4].Yang DD, Muralidhar V, Mahal BA, et al. Impact of percent positive biopsy cores on cancer-specific mortality for patients with high-risk prostate cancer. Urol Oncol 2020;38:735.e9–15. [DOI] [PubMed] [Google Scholar]
- [5].Klingenberg S, Jochumsen MR, Ulhøi BP, et al. 68Ga-PSMA PET/CT for primary NM staging of high-risk prostate cancer. J Nucl Med 2021;62:214–20. [DOI] [PubMed] [Google Scholar]
- [6].Sandler KA, Cook RR, Ciezki JP, et al. Prostate-only versus whole-pelvis radiation with or without a brachytherapy boost for Gleason grade group 5 prostate cancer: a retrospective analysis. Eur Urol 2020;77:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kishan AU, Chu FI, King CR, et al. Local failure and survival after definitive radiotherapy for aggressive prostate cancer: an individual patient-level meta-analysis of six randomized trials. Eur Urol 2020;77:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahdoot M, Wilbur AR, Reese SE, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med 2020;382:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.