Abstract
Context:
Management of locally recurrent prostate cancer after definitive radiotherapy remains controversial due to the perceived high rates of severe genitourinary (GU) and gastrointestinal (GI) toxicity associated with any local salvage modality.
Objective:
To quantitatively compare the efficacy and toxicity of salvage radical prostatectomy (RP), high-intensity focused ultrasound (HIFU), cryotherapy, stereotactic body radiotherapy (SBRT), low–dose-rate (LDR) brachytherapy, and high-dose-rate (HDR) brachytherapy.
Evidence acquisition:
We performed a systematic review of PubMed, EMBASE, and MEDLINE. Two- and 5-yr recurrence-free survival (RFS) rates and crude incidences of severe GU and GI toxicity were extracted as endpoints of interest. Random-effect meta-analyses were conducted to characterize summary effect sizes and quantify heterogeneity. Estimates for each modality were then compared with RP after adjusting for individual study-level covariates using mixed-effect regression models, while allowing for differences in between-study variance across treatment modalities.
Evidence synthesis:
A total of 150 studies were included for analysis. There was significant heterogeneity between studies within each modality, and covariates differed between modalities, necessitating adjustment. Adjusted 5-yr RFS ranged from 50% after cryotherapy to 60% after HDR brachytherapy and SBRT, with no significant differences between any modality and RP. Severe GU toxicity was significantly lower with all three forms of radiotherapeutic salvage than with RP (adjusted rates of 20% after RP vs 5.6%, 9.6%, and 9.1% after SBRT, HDR brachytherapy, and LDR brachytherapy, respectively; p ≤ 0.001 for all). Severe GI toxicity was significantly lower with HDR salvage than with RP (adjusted rates 1.8% vs 0.0%, p < 0.01), with no other differences identified.
Conclusions:
Large differences in 5-yr outcomes were not uncovered when comparing all salvage treatment modalities against RP. Reirradiation with SBRT, HDR brachytherapy, or LDR brachytherapy appears to result in less severe GU toxicity than RP, and reirradiation with HDR brachytherapy yields less severe GI toxicity than RP. Prospective studies of local salvage for radiorecurrent disease are warranted.
Patient summary:
In a large study-level meta-analysis, we looked at treatment outcomes and toxicity for men treated with a number of salvage treatments for radiorecurrent prostate cancer. We conclude that relapse-free survival at 5 years is equivalent among salvage modalities, but reirradiation may lead to lower toxicity.
Keywords: Meta-analysis, Prostate cancer, Radiorecurrent prostate cancer, Radiation therapy, Salvage therapy, Radical prostatectomy, Cryotherapy, High-intensity focused, ultrasound, Stereotactic body radiotherapy, High-dose-rate brachytherapy, Low-dose-rate brachytherapy
1. Introduction
Definitive radiotherapy and radical prostatectomy (RP) are standard of care options for clinically localized prostate cancer (PCa) [1]. Recent data indicate that approximately one-third of all PCa patients undergo radiotherapy, including nearly 40% of patients with intermediate- and high-risk disease [2,3]. A subset of patients treated with definitive radiotherapy may experience a biochemical recurrence (BCR) over time, defined by a rise in their prostate-specific antigen (PSA) level of 2 ng/mL above the postradiotherapy nadir [4]. In contrast to the management of BCR after RP, optimal management after radiotherapy remains unclear due to a lack of large prospective trials in this setting. An estimated 3–10% of patients may experience a local failure (LF) only after radiotherapy, and thus local salvage may be curative [5–7]. However, if distant metastases are present, the benefit of local salvage is unknown. Improved diagnostic studies will likely lead to enhanced detection of postradiotherapy LFs. For instance, a recent study using prostate-specific membrane antigen (PSMA) positron emission tomography (PET)/computed tomography (CT) found that approximately 30% of patients with postradiotherapy PSA rise have only local PSMA PET uptake, while >50% had evidence of regional or distant metastases [8].
Even the management of a true isolated LF after definitive radiotherapy is controversial, and consensus recommendations are limited. The natural history of biochemically recurrent PCa is highly variable. Some patients with BCR never develop distant metastases by conventional imaging or die from PCa [9]. In certain patients, however, LFs may seed distant metastases—the so-called ”second wave” theory that has underscored the rationale for local therapy intensification in PCa [10,11]. A recent individual patient-level meta-analysis of six randomized trials found that a LF after definitive radiotherapy for high- grade PCa was significantly associated with overall survival, PCa-specific survival, and distant metastasis-free survival [12]. Thus, some patients with isolated postradiotherapy LFs may benefit from local salvage treatment.
Local salvage options include RP, brachytherapy, cryotherapy, high-intensity focused ultrasound (HIFU) [13], and stereotactic body radiotherapy (SBRT) [14,15]. A major challenge in treating patients with radiorecurrent PCa are the risks of significant genitourinary (GU) and gastrointestinal (GI) toxicity associated with local salvage, which are greater than those associated with primary radiotherapy. Additionally, many centers lack the experience in treating this patient population with these myriad therapeutic options. These concerns have led to the limited use of local salvage treatments [16]. Furthermore, in comparison with the multiple phase 3 trials investigating postoperative and salvage radiotherapy trials after RP, no such trials exist after radiotherapy.
Although prior reviews have summarized various salvage options [17–19], they have not systematically analyzed all local salvage options simultaneously. The aim of this systematic review and meta-analysis was to evaluate the efficacy and toxicity following all surgical and nonsurgical local salvage therapies for radiorecurrent PCa.
2. Evidence acquisition
2.1. Search strategy
The population, intervention, control, outcome, study design (PICOS) approach [20] was used to provide a framework for the initial literature search (Supplementary Table 1). The subsequent methodology for the systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [21]. MEDLINE (via PubMed), EMBASE, the Cochrane Central Register of Controlled Trials, and guideline statements from professional organizations were queried to identify manuscripts available through November 19, 2019. The initial search strategy included the following different terms: “salvage radical prostatectomy”, “salvage prostatectomy”, “salvage” AND “brachytherapy” AND “prostate”, “salvage” AND “cryoablation” AND “prostate”, “salvage” AND “cryotherapy” AND “prostate”, “salvage” AND “high intensity focused ultrasound” AND “prostate”, “salvage” AND “SBRT” AND “prostate”, “salvage” AND “SABR” AND “prostate”, “salvage” AND “stereotactic” AND “prostate”, “salvage” AND “re-irradiation” AND “prostate”, “radiorecurrent” AND “prostate”.
2.2. Review of studies identified by the literature search
The PRISMA diagram outlining our search strategy is depicted in Supplementary Figure 1. Initial review yielded 2553 results. Further screening of manuscript abstracts revealed 257 unique primary articles for review. These were initially reviewed by a single investigator (A.U.K.) and articles were excluded if they (1) did not present primary data, (2) did not specifically report relapse-free survival (RFS) or GU or GI toxicity following local salvage after definitive radiotherapy, (3) included fewer than five patients, (4) were not written in English, or (5) did not have full text available. After the initial screening, two investigators (A.U.K. and L.F.V.) reviewed the 138 identified studies to ensure suitability. The references of these studies were reviewed carefully, allowing identification of 12 additional studies that were not identified in the original literature search.
2.3. Data extraction
2.3.1. Extracted variables
Data from the 150 included studies were extracted by two authors (L.F.V. and A.U.K.). Extracted data, where applicable, included study type (prospective or retrospective), institution of senior author, time period of study, number of patients, original disease characteristics, original treatment modality, median prior radiotherapy dose, interval to relapse or salvage, patient age at relapse or salvage, presalvage PSA, imaging workup at salvage, percent of biopsy-proven recurrences, percent of patients undergoing whole-gland salvage (focal salvage studies were included in this analysis), salvage radiotherapy dose, salvage radiotherapy fractions, salvage radiotherapy treatment volume, percent of patients undergoing androgen deprivation therapy (ADT) either before or after salvage, median follow-up, 2-yr RFS, 5-yr RFS, severe GU toxicity, and severe GI toxicity. RFS data were extracted from Kaplan-Meier curves using GetData Graph Digitizer version 2.26 [22].
2.3.2. Endpoints
The efficacy of local salvage was evaluated by extracting rates of 2- and 5-yr RFS. RFS was defined variably in each study, although it was most commonly defined as relapse free from biochemical failure, according to the Phoenix criteria [4]. The definition of failure by the American Society for Therapeutic Radiology and Oncology (ASTRO) was used in some studies, and the development of metastatic disease, initiation of ADT, or LF as determined radiographically or by physical examination, also defined relapse in other studies. If disease-free survival, failure-free survival, or biochemical control data were presented, these outcomes were considered to be equivalent to RFS. If study authors chose to stratify outcomes by study-specific subgroups and did not report overall population outcomes, RFS outcomes could not be extracted. Furthermore, if crude failure rates were reported without accompanying Kaplan-Meier curves and median follow-up did not exceed 24 mo, 2- and 5-yr RFS rates were not included.
Regarding toxicity, GU as well as GI toxicity was assessed independently. Severe toxicity was a composite outcome defined by crude rates of grade ≥3 toxicity by the Common Terminology Criteria for Adverse Events, European Organization for Research and Treatment of Cancer, Radiation Therapy Oncology Group, or Clavien toxicity criteria; as any toxicity requiring hospitalization or procedural intervention; or as any explicitly reported “severe” toxicity. If patient-reported outcomes were used, toxicity was considered severe if the survey instruments reported out as such (in the case of the International Prostate Symptom Score, severe was defined as scores between 20 and 35). If raw numbers were reported for each severe event, these numbers were summed and divided by the number of patients in the study. If percentages were reported instead, the highest percentage within each organ system domain (GU or GI) was used when evaluating the composite endpoint “any severe toxicity”. If early as well as late severe toxicity was reported and could not be summed numerically, the largest percentage of early or late toxicity was chosen. Finally, in the event that multiple severe toxicity events occurred in the same patient, these were scored as multiple events (ie, toxicity was scored on a per-event and not on a per-patient basis), since individual patient data were often not reported.
2.3.3. Statistical analysis
Meta-analyses were performed separately by treatment modality using the DerSimonian-Laird random-effect model. Prior to the analysis, the observed proportions for each study were transformed using the logit transformation (survival outcomes) or the Freeman-Tukey double arcsine transformation (toxicity outcomes) in order to stabilize the variances. Between-study heterogeneity was evaluated using the τ2 statistic, which in turn was used to calculate 95% prediction intervals. Since the analyses indicated the presence of significant heterogeneity across studies, additional random-effect meta-regression analyses were performed to explore the sources of this heterogeneity. The following individual-level study characteristics were used as potential covariates for the regression models: median age, median PSA, median time to salvage, percentage of patients with ADT, percentage of patients with whole-gland salvage, and study type (prospective vs retrospective). The regression models were performed using the logit (log odds) transformation for survival outcomes and the Freeman-Tukey double arcsine transformation for toxicity outcomes. Missing values were singly imputed using Markov Chain Monte Carlo imputation. Since regression coefficients using the Freeman-Tukey transformed values lack an intuitive interpretation, meta-regression results were back-transformed into the original (percent) scale in order to ease interpretation. The proportion of the total between study variability that was explained by the covariates in the regression models was quantified using the R2 measure. The observed proportions with each outcome and its 95% confidence intervals (CIs) were shown using forest plots separately according to treatment modality. The corresponding overall (adjusted) proportions and their 95% CIs were computed using the above random-effect regression models, allowing for any heterogeneity across study results. We then compared the average estimates with each outcome across treatment modalities after adjusting for only the significant individual study-level covariates using mixed-effect regression models, while allowing for differences in between-study variance across treatment modalities. Pairwise comparisons between each treatment modality relative to RP as the reference category were adjusted for multiple testing using the Bonferroni correction, and the null hypothesis was rejected at the p < 0.01 level for each comparison, thus controlling type 1 error at <5% within a given outcome. Publication bias was assessed via the Egger test and determined to be present if p < 0.05. In cases where a significant publication bias was observed, Egger testing was repeated after applying regression models to account for covariates. All analyses were conducted using the meta-analysis package “Metafor” in R version 4.0.2.
Stata 15.0 was used in creating mixed-effect regression models [23,24]. Mixed-effect regression models were used to determine weighted linear relationships between covariates (including the use of ADT, age, time to salvage, PSA prior to salvage, and biologically equivalent dose) and the observed percentages of patients experiencing a particular outcome (eg, recurrence-free survival), with 95% CIs. The weight of each individual study was the ratio of the number of patients analyzed in that study divided by the total number of patients over all studies used for the meta-estimate of that effect.
3. Evidence synthesis
3.1. Studies evaluated
A total of 150 studies reporting on 11 322 patients were selected for a quantitative analysis (Supplementary Table 2). Fifty-two studies evaluated RP (one prospective), 32 evaluated cryotherapy (13 prospective), 20 evaluated HIFU (seven prospective), eight evaluated SBRT (one prospective), 16 evaluated high-dose-rate (HDR) brachytherapy (four prospective), and 32 evaluated low-dose-rate (LDR) brachytherapy (two prospective). Overall, 28 independent prospective cohort studies were identified. An overview of patient and treatment details from our study population is presented in Table 1. The numbers represent weighted averages according to the number of patients in each study reporting these variables. Given heterogeneity of study reporting, mean and median were used interchangeably for all categories.
Table 1 –
Summary of patient and treatment characteristics for local salvage modalities
| Age (yr) | Whole-gland salvage (%) | Biopsy-proven recurrence (%) | Presalvage PSA (ng/mL) | Perisalvage ADT use (%) | Interval from initial treatment to recurrence or salvage (mo) | Median follow-up (mo) | Number of studies (n) | Number of patients (n) | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| RP | 65 | 100 | 99 | 6.0 | 16 | 50 | 47 | 52 | 2686 |
| Cryotherapy | 66 | 93 | 99 | 5.8 | 35 | 63 | 32 | 32 | 5153 |
| HIFU | 69 | 86 | 100 | 5.0 | 18 | 63 | 33 | 20 | 1783 |
| SBRT | 72 | 61 | 81 | 4.0 | 37 | 89 | 26 | 8 | 261 |
| HDR | 71 | 85 | 94 | 4.5 | 43 | 61 | 40 | 16 | 586 |
| LDR | 69 | 92 | 95 | 5.5 | 37 | 67 | 52 | 32 | 853 |
ADT = androgen deprivation therapy; HDR=high-dose-rate brachytherapy; HIFU = high-intensity focused ultrasound; LDR=low-dose-rate brachytherapy; PSA = prostate-specific antigen; RP = radical prostatectomy; SBRT = stereotactic body radiotherapy.
3.2. RFS after local salvage
The random-effect model–fitted 2-yr RFS estimates for each modality with adjustment for covariates at the means are shown in Table 2 and using forest plots showing individual study values as well in Figure 1A–F. Random-effect estimates for 2-yr RFS ranged from 54% (95% CI: 48–60%) with HIFU to 81% (95% CI: 74–86%) with LDR brachytherapy. The 95% prediction intervals are also shown and indicate significant amount of heterogeneity after adjustment for baseline covariates. The corresponding 5-yr RFS is also presented in Table 2, as well as individual study values in Figure 2A–E for all modalities except SBRT (as only a single study utilizing SBRT reported 5-yr RFS). Five-year RFS ranged from 50% (95% CI: 44–56%) with cryotherapy to 60% (95% CI: 52–67%) with HDR brachytherapy and SBRT. The 95% prediction intervals indicate significant heterogeneity after adjustment for baseline covariates. Significant publication bias was appreciated in HIFU, LDR brachytherapy, and SBRT studies reporting 2-yr RFS outcomes, and in HIFU studies reporting 5-yr RFS outcomes (Supplementary Table 3). After accounting for covariates, only LDR studies reporting 2-yr RFS demonstrated a significant p value for publication bias (Supplementary Table 4), with smaller studies tending to report greater 2-yr RFS.
Table 2 –
Covariate-adjusted estimates of efficacy and toxicity across salvage modalities
| 2-yr RFS (95% CI) | 5-yr RFS (95% CI) | Severe GU toxicity (95% CI) | Severe GI toxicity (95% CI) | |
|---|---|---|---|---|
|
| ||||
| RP | 69 (64–74) | 54 (49–59) | 21 (16–27) | 1.9 (0.6–3.7) |
| Number of studies | 26 | 21 | 43 | 43 |
| Number of patients | 1439 | 1488 | 1617 | 1617 |
| Heterogeneity (95% PI) | 48–84 | 34–73 | 0.1–58 | 0.0–13 |
| Cryotherapy | 68 (62–73) | 50 (44–56) | 15 (10–22) | 1.7 (1.0–2.7) |
| Number of studies | 24 | 18 | 23 | 22 |
| Number of patients | 3887 | 3616 | 2618 | 2475 |
| Heterogeneity (95% PI) | 40–87 | 28–73 | 0.0–48 | 0.2–4.1 |
| HIFU | 54 (48–60) | 53 (43–63) | 23 (17–29) | 1.6 (0.9–2.4) |
| Number of studies | 14 | 7 | 19 | 19 |
| Number of patients | 1092 | 236 | 1737 | 1737 |
| Heterogeneity (95% PI) | 36–71 | 34–71 | 4.2–49 | 0.9–2.4 |
| SBRT | 62 (47–74) | 60 (N/A) | 4.2 (0.8–9.1) | 0.0 (0.0–0.1) |
| Number of studies | 5 | 1 | 8 | 8 |
| Number of patients | 206 | 50 | 261 | 261 |
| Heterogeneity (95% PI) | 49–73 | NA | 0.0–15 | 0.0–0.1 |
| HDR | 77 (70–83) | 60 (52–67) | 8.0 (5.1–11) | 0.0 (0.0–0.2) |
| Number of studies | 14 | 7 | 16 | 15 |
| Number of patients | 456 | 350 | 586 | 571 |
| Heterogeneity (95% PI) | 55–90 | 45–73 | 2.3–16 | 0.0–0.2 |
| LDR | 81 (74–86) | 56 (48–63) | 8.1 (4.3–13) | 1.5 (0.2–3.4) |
| Number of studies | 22 | 16 | 26 | 26 |
| Number of patients | 495 | 511 | 664 | 660 |
| Heterogeneity (95% PI) | 57–93 | 33–76 | 0.0–31 | 0.0–5.7 |
CI = confidence interval; GI = gastrointestinal; GU = genitourinary; HDR = high-dose-rate brachytherapy; HIFU = high-intensity focused ultrasound; LDR = low-dose-rate brachytherapy; NA = not available; PI = prediction interval; RFS = recurrence-free survival; RP = radical prostatectomy; SBRT = stereotactic body radiotherapy.
Fig. 1 –

(A–F) Forest plots showing individual study values of 2-yr RFS estimates for various treatment modalities. CI = confidence interval; Cryo = cryotherapy; HDRBT = high-dose-rate brachytherapy; HIFU = high-intensity focused ultrasound; LDRBT = low-dose-rate brachytherapy; RFS = recurrence-free survival; RP = radical prostatectomy; SBRT = stereotactic body radiotherapy.
Fig. 2 –

(A–E) Forest plots showing individual study values of 5-yr RFS estimates for various treatment modalities. CI = confidence interval; Cryo = cryotherapy; HDRBT = high-dose-rate brachytherapy; HIFU = high-intensity focused ultrasound; LDRBT = low-dose-rate brachytherapy; RFS = recurrence-free survival; RP = radical prostatectomy.
3.3. Severe GU toxicity after local salvage
The random-effect model–fitted severe GU toxicity estimates with adjustment for covariates at the means are tabulated in Table 2 and also shown with individual study values as forest plots in Figure 3A–F. Salvage SBRT was associated with the lowest rates of severe GU toxicity at 4.2% (95% CI: 0.8–9.1%), whereas HIFU generated the highest rates of severe GU toxicity at 23% (95% CI: 17–29%). The 95% prediction intervals indicate that there was significant heterogeneity for some of the modalities after accounting for baseline covariates. No significant publication bias was appreciated across all salvage modalities (Supplementary Table 3).
Fig. 3 –

(A–F) Forest plots showing individual study values of severe GU toxicity estimates for various treatment modalities. CI = confidence interval; Cryo = cryotherapy; GU = genitourinary; HDRBT = high-dose-rate brachytherapy; HIFU = high-intensity focused ultrasound; LDRBT = low-dose-rate brachytherapy; RP = radical prostatectomy; SBRT = stereotactic body radiotherapy.
3.4. Severe GI toxicity after local salvage
The random-effect model–fitted severe GI toxicity estimates with adjustment for covariates at the means are presented in Table 2, as well as graphically with individual study values in Figure 4A–F. Overall, the rates of GI toxicity were quite low, ranging from 0.0% (95% CI: 0.0–0.2 %) with HDR brachytherapy and SBRT to 1.9% (95% CI: 0.6–3.7%) with RP. The 95% prediction intervals indicate no significant heterogeneity between studies. No significant publication bias was appreciated across all salvage modalities (Supplementary Table 3).
Fig. 4 –
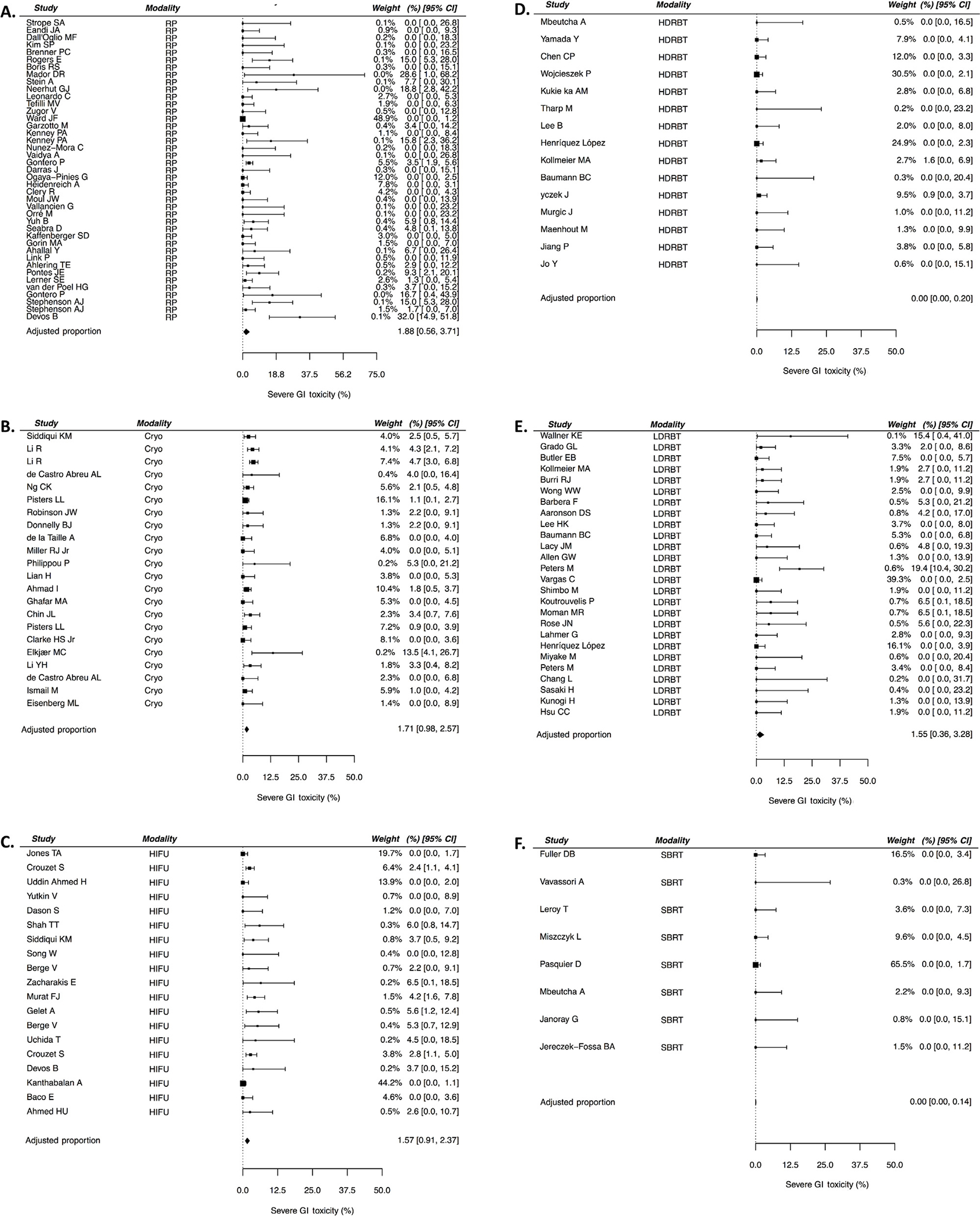
(A–F) Forest plots showing individual study values of severe GI toxicity estimates for various treatment modalities. CI = confidence interval; Cryo = cryotherapy; GI = gastrointestinal; HDRBT = high-dose-rate brachytherapy; HIFU = high-intensity focused ultrasound; LDRBT = low-dose-rate brachytherapy; RP = radical prostatectomy; SBRT = stereotactic body radiotherapy.
3.5. Meta-regression comparing salvage modalities
Upon evaluation of the effect of the various covariates on the endpoints of interest, most covariates were found to not have significant associations; subsequent meta-regression models were performed following adjustment only for the covariates found to be significantly associated with outcome, with RP as the reference (Table 3). Two-year RFS was significantly lower for HIFU than for RP after adjusting for median time to salvage. No other significant differences were found in terms of 2- or 5-yr RFS (adjusting for median PSA and percent whole-gland salvage for the latter) between modalities. With the caveat of variable follow-up, severe GU toxicity (adjusting for percent whole-gland salvage) was significantly lower with all radiotherapeutic salvage modalities than with RP, while severe GU toxicity following the other salvage modalities was no different compared to after RP. Severe GI toxicity (adjusting for median time to salvage) was significantly lower with HDR brachytherapy than with RP, but no other differences in severe GI toxicity were identified.
Table 3 –
Covariate-adjusted meta-regression comparing efficacy and toxicity between salvage modalities and radical prostatectomy
| 2-yr RFS | 5-yr RFS | Severe GU toxicity | Severe GI toxicity | |
|---|---|---|---|---|
|
| ||||
| Radical prostatectomy | ||||
| Adjusted percenta (95% CI) | 72% (66–78%) | 53% (46%–59%) | 21% (16%–26%) | 1.5% (0.4%–3.2%) |
| Odds ratio (95% CI) | 1.0 | 1.0 | NA | NA |
| p value | Reference | Reference | Reference | Reference |
| R2 (%) | 0.0 | 0.0 | 0.0 | 0.0 |
| Cryotherapy | ||||
| Adjusted percenta (95% CI) | 66% (59–72%) | 57% (49–65%) | 15% (8–23%) | 0.9% (0.3–1.8%) |
| Odds ratio (95% CI) | 0.74 (0.49–1.12) | 1.20 (0.80–1.79) | NA | NA |
| p value | 0.2 | 0.4 | 0.2 | 0.5 |
| R2 (%) | 25 | 0.0 | 8.2 | 27 |
| HIFU | ||||
| Adjusted percenta (95% CI) | 52% (45%–59%) | 46% (37%–55%) | 23% (17%–30%) | 0.8% (0.1%–2.1%) |
| Odds ratio (95% CI) | 0.42 (0.28–0.64) | 0.76 (0.48–1.21) | NA | NA |
| p value | <0.001 | 0.2 | 0.5 | 0.4 |
| R2 (%) | 0.0 | 41 | 15 | 22 |
| SBRT | ||||
| Adjusted percenta (95% CI) | 58% (46–69%) | 56% (37–73%) | 5.6% (1.4–12%) | 0.0% (0.0–1.2%) |
| Odds ratio (95% CI) | 0.52 (0.30–0.93) | 1.13 (0.50–2.58) | NA | NA |
| p value | 0.03 | 0.8 | <0.001 | 0.07 |
| R2 (%) | 55 | 4.2 | 0.00 | 0.0 |
| HDR | ||||
| Adjusted percenta (95% CI) | 77% (69–83%) | 58% (52–64%) | 9.6% (6.0–13.9%) | 0.0% (0.0–0.3%) |
| Odds ratio (95% CI) | 1.26 (0.77–2.09) | 1.25 (0.88–1.78) | NA | NA |
| p value | 0.4 | 0.2 | 0.002 | 0.003 |
| R2 (%) | 0.0 | 91 | 0.0 | 0.0 |
| LDR | ||||
| Adjusted percenta (95% CI) | 79% (72–85%) | 53% (43–63%) | 9.1% (5.2–14%) | 2.1% (0.6–4.0%) |
| Odds ratio (95% CI) | 1.49 (0.89–2.50) | 1.02 (0.63–1.67) | – | – |
| p value | 0.13 | 0.9 | 0.001 | 0.6 |
| R2 (%) | 4.3 | 5.2 | 12 | 20% |
CI = confidence interval; GI = gastrointestinal; GU = genitourinary; HDR=high-dose-rate brachytherapy; HIFU = high-intensity focused ultrasound; LDR=low-dose-rate brachytherapy; NA = not available; RFS = recurrence-free survival; SBRT = stereotactic body radiotherapy.
Significant p-values after Bonferroni correction appear in bold.
Back-transformed regression coefficients for ease of interpretation.
Adjustment for the aforementioned significant confounding variables accounted for 0.0–91% of the heterogeneity depending on the outcome and salvage modality, as indicated in Table 3.
3.6. Discussion and limitations
We report herein the first meta-analysis to comprehensively include both surgical and nonsurgical local salvage techniques for radiorecurrent PCa. Overall, we did not find evidence of large differences in 5-yr RFS outcomes for surgical, nonradiotherapeutic ablative, and radiotherapeutic salvage of radiorecurrent PCa, which ranged from 50% to 60%. For all salvage modalities, more series were available when evaluating 2-yr RFS, and based on these comparisons, HIFU resulted in significantly lower 2-yr RFS than RP. Severe GU toxicity exceeded 21% with HIFU and RP, whereas it ranged from 4.2% to 8.1% with reirradiation. Severe GI toxicity rates were <2% across all modalities. Reirradiation with HDR brachytherapy, LDR brachytherapy, or SBRT resulted in significantly lower rates of severe GU toxicity than RP, and HDR brachytherapy yielded significantly lower rates of severe GI toxicity than RP.
Identification of local salvage modalities for radio-recurrent disease with favorable efficacy and safety profiles is important in the context of managing isolated LFs, which portend a poor prognosis and are expected to be diagnosed more frequently with the advent of advanced imaging scans [8,12,25]. The controversy surrounding local salvage for radiorecurrent disease arises from several clinical considerations. First, as is the case with BCR after upfront RP, the natural history of radiorecurrent PCa is variable [9,26]. In contrast to the post-RP setting, in which emerging data support the use of genomic and transcriptomic prognostic biomarkers to guide therapy [5], no such biomarkers exist for radiorecurrent disease. Certain clinical features, such as pretreatment PSA, may be prognostic and patient selection remains a critical issue. The lack of granular data in many series on clinicopathologic features at the time of relapse, as well as at initial presentation, precluded a more detailed analysis of prognostic features in the present analysis.
Second, the uncertainty regarding whether a patient truly has an isolated LF, when juxtaposed with the perceived higher toxicity with local salvage versus systemic salvage or observation, tempers enthusiasm for local salvage [27]. It is probable that clinical features that are associated with worse RFS, such as pretreatment PSA, are simply surrogates for extraprostatic disease. Advanced imaging techniques with a high positive predictive value, such as PSMA PET/CT [8,25], will aid in treatment selection by identifying non-local failures. However, it is acknowledged that much of the evidence base supporting these imaging studies is in the postprostatectomy setting, with growing evidence in the upfront setting. Therefore, the need for post-treatment biopsy, while challenging to interpret postradiotherapy management [28], remains important given the potential for radiographic false positives and the presence of indolent disease.
Third, the integration of ADT with local salvage remains unknown. Emerging data in the postprostatectomy setting suggest a benefit of combining ADT with salvage radiotherapy [29,30], although patient selection remains the key [31]. It is also known from multiple randomized trials in the definitive radiotherapy setting that concurrent ADT has important radiosensitizing effects [27]. The role of concurrent ADT with upfront RP is less clearly associated with clinical benefits [32]. The true benefit of ADT will require randomized data to be fully understood. Given the radiosensitizing effect of ADT, it may be reasonable to consider using at least a short course of ADT with reirradiation to increase local control.
Fourth, systemic salvage with ADT may constitute an attractive option, particularly if the perceived toxicity of local salvage is high and/or the patient is thought to have occult micrometastatic disease. For instance, one registry report found that nearly 94% of patients with radio-recurrent disease receive salvage ADT and only 4.1% received local salvage [16]. Salvage ADT, although not a curative intervention, can be effective, but is associated with adverse effects and may not improve long-term clinical outcomes if given immediately upon relapse [33,34]. Moreover, randomized data from the postprostatectomy setting suggest that salvage ADT alone is inferior to salvage local therapy [35]. Given the reasonable safety and efficacy profile of local salvage reported herein, particularly with reirradiation, the decision to omit this for systemic ADT alone should be considered carefully. However, these strategies have not been compared directly.
One of the strengths of our meta-analysis is the comprehensive inclusion of both surgical and nonsurgical local salvage techniques as well as clear distinctions between the types of salvage brachytherapy (HDR or LDR brachytherapy) and external beam radiation (SBRT). Ingrosso and colleagues [17] recently conducted a systematic review and meta-analysis restricted to nonsurgical local salvage methods, and concluded that brachytherapy offered the most optimal balance between toxicity and efficacy, which is in general agreement with our conclusions. Notably, they did not differentiate between HDR and LDR brachytherapy, and included all forms of external reirradiation rather than just SBRT; they additionally did not include RP. Philippou et al [19] performed a meta-regression that included salvage RP, HIFU, cryotherapy, and brachytherapy, but similarly did not differentiate between HDR and LDR brachytherapy and included only 63 studies (vs the 150 included in the present analysis). The authors concluded that oncologic efficacy is similar, with a possibility for better GU toxicity profiles with nonsurgical salvage. Neither analysis considered the proportion of patients receiving focal therapy, although it might influence both RFS and toxicity outcomes. Other systematic analyses have focused on salvage modalities in isolation. For instance, Chade and colleagues [36] have previously performed a meta-analysis of RP alone, identifying similar oncologic efficacy to the pooled rate identified in our study.
There are several limitations to this analysis study that warrant consideration. Many of the studies included in the analysis are retrospective and of poor quality, which introduces a bias that may overestimate the effect sizes, but also motivates prospective work in this space. Since measurable differences in RFS are unlikely to translate to overall survival in the salvage setting, one might advocate selecting the local salvage treatment with the lowest toxicity until randomized data are available. Additionally, there was significant between-study variance within the same salvage modality. However, there is a dearth of prospective studies for radiorecurrent disease, and this is an unfortunate inherent limitation of meta-analyses of retrospective studies. We, however, attempted to explain and mitigate this heterogeneity by adjusting for the most likely confounding covariates including PSA, ADT use, and patient age. These covariates influenced our statistical conclusions. We conjecture that the heterogeneity of surgical and radiation planning techniques in the salvage setting, baseline patient characteristics, and selection biases inherent in retrospective studies each contributed to study outcome heterogeneity that could not be accounted for. Regarding toxicity, our definitions of severe toxicity, while arbitrary, were designed to maximize the inclusion of information reported by studies. Erectile dysfunction, however, was not considered as a toxicity category due to generally poor reporting overall as well as poor reporting of baseline potency in patients undergoing salvage treatments. All other toxicity evaluations were similarly limited by the frequent absence of baseline evaluations. While great care was taken to reduce redundant reporting of patient outcomes, there may be some overlap in particular for cryotherapy salvage, as many publications draw data from the same Cryo On-Line Data (COLD) registry [37]. As mentioned above, information on specific clinicopathologic features at diagnosis and at recurrence (eg, PSA doubling time and Gleason grade group) was not reported uniformly, precluding incorporation into the meta-regression analyses. The same was true for ADT duration, which was sparingly reported. The proportionately greater use of ADT in studies of reirradiation versus RP or HIFU might, in part, explain the lower 2-yr RFS rates with these modalities. Finally, consistent with prior meta-analyses on this topic, our central analysis included patients who received focal salvage. Finally, a publication bias was noted in multiple modalities and outcomes.
4. Conclusions
In conclusion, this meta-analysis provides pooled estimates of surgical and nonsurgical local salvage treatments for radiorecurrent PCa. Five-year RFS was similar across modalities on meta-regression, although differences in severe GU and GI toxicity appear to favor reirradiation, particularly HDR brachytherapy. Additional prospective and randomized data are required to better define how to optimally select and treat patients with isolated LFs after definitive radiotherapy.
Supplementary Material
Funding/Support and role of the sponsor:
AUK has received funding from the Prostate Cancer Foundation, the American Society of Radiation Oncology, and the Jonsson Comprehensive Cancer Center.
Footnotes
Financial disclosures: Amar U. Kishan certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eururo.2020.11.010.
References
- [1].National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer. Version I.2019; ed2019. [DOI] [PubMed]
- [2].Mahal BA, Butler S, Franco I, et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010–2015. JAMA 2019;321:704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Scherzer ND, DiBiase ZS, Srivastav SK, Thomas R, DiBiase SJ. Regional differences in the treatment of localized prostate cancer: an analysis of surgery and radiation utilization in the United States. Adv Radiat Oncol 2019;4:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–74. [DOI] [PubMed] [Google Scholar]
- [5].Zumsteg ZS, Spratt DE, Romesser PB, et al. Anatomical patterns of recurrence following biochemical relapse in the dose escalation era of external beam radiotherapy for prostate cancer. J Urol 2015;194:1624–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zelefsky MJ, Kollmeier M, McBride S, et al. Five-year outcomes of a phase 1 dose-escalation study using stereotactic body radiosurgery for patients with low-risk and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2019;104:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bolla M, Maingon P, Carrie C, et al. Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: results of EORTC trial 22991. J Clin Oncol 2016;34:1748–56. [DOI] [PubMed] [Google Scholar]
- [8].Jansen BHE, van Leeuwen PJ, Wondergem M, et al. Detection of recurrent prostate cancer using prostate-specific membrane antigen positron emission tomography in patients not meeting the phoenix criteria for biochemical recurrence after curative radiotherapy. Eur Urol Oncol 2020, Feb 19;S2588–9311(20) 30009–2. [DOI] [PubMed] [Google Scholar]
- [9].Van den Broeck T, van den Bergh RCN, Briers E, et al. Biochemical recurrence in prostate cancer: the European Association of Urology Prostate Cancer Guidelines Panel recommendations. Eur Urol Focus 2020;6:231–4. [DOI] [PubMed] [Google Scholar]
- [10].Grossman HB, Batata M, Hilaris B, Whitmore WF Jr. 125I implantation for carcinoma of prostate. Further follow-up of first 100 cases. Urology 1982;20:591–8. [DOI] [PubMed] [Google Scholar]
- [11].Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol 2002;20:3199–205. [DOI] [PubMed] [Google Scholar]
- [12].Kishan AU, Chu FI, King CR, et al. Local failure and survival after definitive radiotherapy for aggressive prostate cancer: an individual patient-level meta-analysis of six randomized trials. Eur Urol 2020;77:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate cancer. Version I.2016; ed2015. [DOI] [PubMed]
- [14].Fuller D, Wurzer J, Shirazi R, et al. Retreatment for local recurrence of prostatic carcinoma after prior therapeutic irradiation: efficacy and toxicity of HDR-like SBRT. Int J Radiat Oncol Biol Phys 2020;106:291–9. [DOI] [PubMed] [Google Scholar]
- [15].Pasquier D, Martinage G, Janoray G, et al. Salvage stereotactic body radiation therapy for local prostate cancer recurrence after radiation therapy: a retrospective multicenter study of the GETUG. Int J Radiat Oncol Biol Phys 2019;105:727–34. [DOI] [PubMed] [Google Scholar]
- [16].Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE). Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer 2008;112:307–14. [DOI] [PubMed] [Google Scholar]
- [17].Ingrosso G, Becherini C, Lancia A, et al. Nonsurgical salvage local therapies for radiorecurrent prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol 2020;3:183–97. [DOI] [PubMed] [Google Scholar]
- [18].Parekh A, Graham PL, Nguyen PL. Cancer control and complications of salvage local therapy after failure of radiotherapy for prostate cancer: a systematic review. Semin Radiat Oncol 2013;23:222–34. [DOI] [PubMed] [Google Scholar]
- [19].Philippou Y, Parker RA, Volanis D, Gnanapragasam VJ. Comparative oncologic and toxicity outcomes of salvage radical prostatectomy versus nonsurgical therapies for radiorecurrent prostate cancer: a meta-regression analysis. Eur Urol Focus 2016;2:158–71. [DOI] [PubMed] [Google Scholar]
- [20].Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York, UK: University of York; 2009. [Google Scholar]
- [21].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:Error: FPage (264) is higher than LPage (9, w64)!. [DOI] [PubMed] [Google Scholar]
- [22].Federov S GetData Graph digitizer version 2.26. 2013.
- [23].Zaorsky NG, Lee CT, Zhang E, Galloway TJ. Skin CanceR Brachytherapy vs External beam radiation therapy (SCRiBE) meta-analysis. Radiother Oncol 2018;126:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singh R, Lehrer EJ, Dahshan B, et al. Single fraction radiosurgery, fractionated radiosurgery, and conventional radiotherapy for spinal oligometastasis (SAFFRON): a systematic review and meta-analysis. Radiother Oncol 2020;146:76–89. [DOI] [PubMed] [Google Scholar]
- [25].Sonni I, Eiber M, Fendler WP, et al. Impact of (68)Ga-PSMA-11 PET/CT on staging and management of prostate cancer patients in various clinical settings: a prospective single center study. J Nucl Med 2020;61:1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martin NA-O, Chen MH, Beard CJ, et al. Natural history of untreated prostate specific antigen radiorecurrent prostate cancer in men with favorable prognostic indicators. Prostate Cancer 2014;2014:912943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nguyen PL. Optimization of the radiation management of high-risk prostate cancer. Semin Radiat Oncol 2017;27:43–9. [DOI] [PubMed] [Google Scholar]
- [28].Crook JM, Bahadur YA, Robertson SJ, Perry GA, Esche BA. Evaluation of radiation effect, tumor differentiation, and prostate specific antigen staining in sequential prostate biopsies after external beam radiotherapy for patients with prostate carcinoma. Cancer 1997;79:81–9. [PubMed] [Google Scholar]
- [29].Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 2017;376:417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carrie C, Magne N, Burban-Provost P, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol 2019;20(December 12):1740–9. [DOI] [PubMed] [Google Scholar]
- [31].Dess RT, Sun Y, Jackson WC, et al. Association of presalvage radiotherapy PSA levels after prostatectomy with outcomes of long-term antiandrogen therapy in men with prostate cancer. JAMA Oncol 2020;6:735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McClintock TR, von Landenberg N, Cole AP, et al. Neoadjuvant androgen deprivation therapy prior to radical prostatectomy: recent trends in utilization and association with postoperative surgical margin status. Ann Surg Oncol 2019;26:297–305. [DOI] [PubMed] [Google Scholar]
- [33].Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015;67:825–36. [DOI] [PubMed] [Google Scholar]
- [34].Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01–03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol 2016;17:727–37. [DOI] [PubMed] [Google Scholar]
- [35].Yokomizo A, Wakabayashi M, Satoh T, et al. Salvage radiotherapy versus hormone therapy for prostate-specific antigen failure after radical prostatectomy: a randomised, multicentre, open-label, phase 3 trial (JCOG0401). Eur Urol 2020;77:689–98. [DOI] [PubMed] [Google Scholar]
- [36].Chade DC, Eastham J, Graefen M, et al. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: a systematic review of the literature. Eur Urol 2012;61:961–71. [DOI] [PubMed] [Google Scholar]
- [37].Pisters LL, Rewcastle JC, Donnelly BJ, Lugnani FM, Katz AE, Jones JS. Salvage prostate cryoablation: initial results from the cryo on-line data registry. J Urol 2008;180:559–63, discussion 563–564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


