BACKGROUND:
Accurate staging prior to resection of pancreatic ductal adenocarcinoma (PDAC) is imperative to avoid unnecessary operative morbidity and oncologic futility in patients with occult intra-abdominal distant metastases. We aimed to determine the diagnostic yield of staging laparoscopy (SL) and to identify factors associated with increased risk of positive laparoscopy (PL) in the modern era.
STUDY DESIGN:
Patients with radiographically localized PDAC who underwent SL from 2017 to 2021 were retrospectively reviewed. The yield of SL was defined as the proportion of patients with PL, including gross metastases and/or positive peritoneal cytology. Factors associated with PL were assessed using univariate analysis and multivariable logistic regression.
RESULTS:
Of 1,004 patients who underwent SL, 180 (18%) had PL due to gross metastases (n = 140) and/or positive cytology (n = 96). Patients who had neoadjuvant chemotherapy prior to laparoscopy had lower rates of PL (14% vs 22%, p = 0.002). When the analysis was restricted to chemo-naive patients who had concurrent peritoneal lavage performed, 95 of 419 patients (23%) had PL. In multivariable analysis, PL was associated with younger (<60) age, indeterminate extrapancreatic lesions on preoperative imaging, body/tail tumor location, larger tumor size, and elevated serum CA 19-9 (all p < 0.05). Among patients with no indeterminate extrapancreatic lesions on preoperative imaging, the rate of PL ranged from 1.6% in patients with no risk factors to 42% in young patients with large body/tail tumors and elevated serum CA 19-9.
CONCLUSIONS:
The rate of PL in patients with PDAC remains high in the modern era. SL with peritoneal lavage should be considered for the majority of patients prior to resection, specifically those with high-risk features, and ideally prior to neoadjuvant chemotherapy.
In this contemporary analysis, the yield of positive staging laparoscopy (gross metastases or positive peritoneal cytology) in patients with pancreatic cancer remained high. Preoperative risk factors for positive laparoscopy included indeterminate extrapancreatic lesion on imaging, younger age, large body/tail tumor, and elevated serum CA 19-9.
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy and the third leading cause of cancer-related death in the US.1,2 More than half of patients present with distant hematogenous or peritoneal metastatic dissemination at diagnosis, and the majority of patients who undergo resection with curative intent will develop recurrence in the first 2 years after surgery, confirming the presence of occult metastases at the time of surgery.3-5 The management of PDAC continues to evolve, and recent years have seen an increase in the use of neoadjuvant therapy due to its potential to increase negative resection margin rates, address potential occult metastatic disease, and increase the rate of patients completing perioperative therapy.6,7
Accurate staging prior to consideration of resection is critically imperative to avoid the unnecessary morbidity of surgery in patients with incurable disease. Although the sensitivity of cross-sectional imaging such as CT and MRI has improved in recent years and may increase further with the addition of high-resolution metabolic imaging modalities such as PET, low-volume intra-abdominal metastatic disease continues to be missed by currently available imaging strategies.8,9 Staging laparoscopy, an invasive but low-risk procedure that allows for direct visualization, as well as cytologic evaluation of the peritoneal cavity, can aid in the detection of radiographically occult metastases. Use of staging laparoscopy for PDAC varies greatly between individual surgeons and institutions and has decreased in the past decade; in a recent study that evaluated data from the NSQIP, only 10% of patients had a staging laparoscopy prior to undergoing resection for PDAC, and this practice decreased by approximately 50% during the 8-year study period.10 Currently, at those centers that perform them, staging laparoscopy is used selectively in patients with other high-risk features, such as borderline resectable or locally advanced anatomy, markedly elevated cancer antigen (CA) 19-9, large primary tumor size, or suspicious regional lymph nodes, but there is a lack of consensus on the exact indications.11
With changing practices in the management of PDAC, the persistently high rate of early recurrence, and overall poor prognosis after seemingly curative resection, the value of staging laparoscopy needs to be re-evaluated in the modern era. With this study, we aimed to determine the diagnostic yield of staging laparoscopy in our institutional cohort and to identify factors associated with an increased risk of occult metastases identified on laparoscopy.
METHODS
Study cohort and data collection
The study was approved by the Mayo Clinic Institutional Review Board. Patients with PDAC who underwent staging laparoscopy (with or without immediate resection) at Mayo Clinic Rochester from January 2017 through December 2021 were identified. Patients with variant histologic exocrine carcinomas were excluded. Electronic medical records were retrospectively reviewed, and relevant variables were collected. Tumor location was defined as proximal if the head of the pancreas was involved and distal if only the neck, body, or tail were involved. For tumor size, serum CA 19-9, and serum CEA, the last documented value prior to laparoscopy was used. Anatomic resectability of the tumor (upfront resectable, borderline resectable, or locally advanced) was defined according to the National Comprehensive Cancer Network definition.11 Based on institutional cutoffs, laboratory values were considered elevated if serum CA 19-9 was ≥35 U/ml, serum CEA was >3.0 ng/ml, peritoneal CA 19-9 was ≥5 U/ml, or peritoneal CEA was ≥0.7 ng/ml. Patients were considered to have positive laparoscopy if gross metastatic disease was identified or if peritoneal cytology showed malignant cells. The Clavien–Dindo classification system was used to grade postoperative complications occurring within 30 days of surgery, and major complications were defined as Clavien–Dindo ≥3.12
Staging laparoscopy
Staging laparoscopy was performed at the discretion of the primary surgeon during the study period. At the current time, the majority of staging laparoscopies at our institution are performed as separate staged procedures. In this case, gross visual examination of the abdominal cavity is combined with peritoneal lavage. The protocol for peritoneal lavage consists of irrigation of the peritoneal cavity with 1,000 ml of normal saline, agitation, and retrieval of at least 700 ml. Peritoneal lavage fluid is sent for cytologic examination, tumor markers (CA 19-9 and CEA), and cell-free DNA analysis.13,14 For patients with a recent diagnosis who have not yet started neoadjuvant therapy, central venous access for administration of chemotherapy is established under the same anesthetic. The staging laparoscopy protocol evolved throughout the study period and therefore was not consistent in all subjects in the current study.
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR) and categorical variables as numbers and percentage. Differences between groups were compared using the Mann–Whitney U test for continuous variables and chi-square or Fisher’s exact tests for categorical variables. Trends over time were assessed using the Cochran-Armitage test. Missing values for serum CEA were imputed with the predictive mean matching method of multiple imputation by chained equations using the mice package in R.15,16 Variables listed in Table 1 and the primary outcome variable (positive vs negative laparoscopy) were used as predictor variables. Univariate and multivariable logistic regression was performed on 40 imputed datasets to identify factors associated with positive laparoscopy and estimates pooled using Rubin’s rules.17 Overall survival from the time of laparoscopy was estimated using the Kaplan–Meier method, and differences between subgroups were assessed using the log-rank test. Two-sided p values of less than 0.05 were considered statistically significant. All statistical calculations were performed using R (version 4.0.0).
Table 1.
Characteristics and Laboratory Values of Patients with Negative vs Positive Laparoscopy
| Characteristic | Negative laparoscopy (n = 824) | Positive laparoscopy (n = 180) |
p Value |
|---|---|---|---|
| Age at laparoscopy, y, median (IQR) | 67 (60–73) | 64 (56–72) | 0.002 |
| ≤60 y, n (%) | 211 (26) | 68 (38) | 0.001 |
| >60 y, n (%) | 613 (74) | 112 (62) | |
| Sex, n (%) | |||
| Male | 428 (52) | 96 (53) | 0.80 |
| Female | 396 (48) | 84 (47) | |
| Tumor location, n (%) | |||
| Proximal | 566 (69) | 78 (43) | <0.001 |
| Distal | 258 (31) | 102 (57) | |
| Tumor size, mm, median (IQR) | 27 (20–36) | 36 (30–48) | <0.001 |
| ≤20 mm (T1), n (%) | 239 (29) | 17 (9.4) | <0.001 |
| 21 to 40 mm (T2), n (%) | 440 (53) | 97 (54) | |
| >40 mm (T3), n (%) | 145 (18) | 66 (37) | |
| Anatomic resectability, n (%) | |||
| Resectable | 311 (38) | 40 (22) | <0.001 |
| BR/LA | 513 (62) | 140 (78) | |
| Indeterminate lesion on imaging, n (%) | |||
| No | 771 (94) | 104 (58) | <0.001 |
| Yes | 53 (6.4) | 76 (42) | |
| Neoadjuvant therapy, n (%) | |||
| No | 389 (47) | 108 (60) | 0.002 |
| Yes | 435 (53) | 72 (40) | |
| Serum CA 19-9, U/mL, median (IQR) | 57 (21–231) | 204 (50–1,186) | <0.001 |
| Normal, n (%) | 291 (35) | 35 (19) | <0.001 |
| Nonsecretor, n (%) | 53 (6.4) | 13 (7.2) | |
| Elevated, n (%) | 480 (58) | 132 (73) | |
| Serum CEA*, ng/mL, median (IQR) | 2.8 (1.7–5.1) | 3.7 (2.1–9.0) | <0.001 |
| Normal, n (%) | 283 (53) | 51 (39) | 0.004 |
| Elevated, n (%) | 250 (47) | 81 (61) | |
| Peritoneal CA 19-9*, n (%) | |||
| <5 U/mL | 277 (77) | 44 (40) | <0.001 |
| ≥5 U/mL | 82 (23) | 65 (60) | |
| Peritoneal CEA*, n (%) | |||
| <0.7 ng/mL | 335 (93) | 65 (60) | <0.001 |
| ≥0.7 ng/mL | 25 (6.9) | 44 (40) | |
Patients with missing values for serum CEA (n = 339), peritoneal CA 19-9 (n = 536), and peritoneal CEA (n = 535) were excluded from the analysis.
BR/LA, borderline resectable/locally advanced; CEA, carcinoembryonic antigen; IQR, interquartile range.
RESULTS
Demographics and clinicopathologic characteristics
From January 2017 to December 2021, 1,004 patients underwent staging laparoscopy for PDAC at Mayo Clinic Rochester. Median age was 66 years (IQR 59 to 72) and 480 patients (48%) were female. Tumor location was proximal in 644 patients (64%) and distal in 360 patients (36%). Median tumor size was 29 mm (IQR 20 to 38); 256 patients (26%) had tumors <2 cm (T1), 537 (54%) had tumors 2 to 4 cm in size (T2), and 211 (21%) had tumors >4 cm in size (T3). Upfront resectable disease was present in 351 patients (35%), and borderline resectable or locally advanced anatomy was present in 653 (65%). Indeterminate extrapancreatic lesions were noted on preoperative imaging in 129 patients (13%). Serum CA 19-9 was available for all patients and was elevated in 612 (61%), while 66 patients (6.6%) were nonsecretors. Serum CEA was available in 665 patients and was elevated in 331 (50%). Available preoperative imaging included CT scan in all patients, MRI in 723 (72%), and fluorodeoxyglucose-PET (CT or MRI) in 845 (84%). Neoadjuvant therapy had been initiated or completed prior to staging laparoscopy in 507 patients (51%).
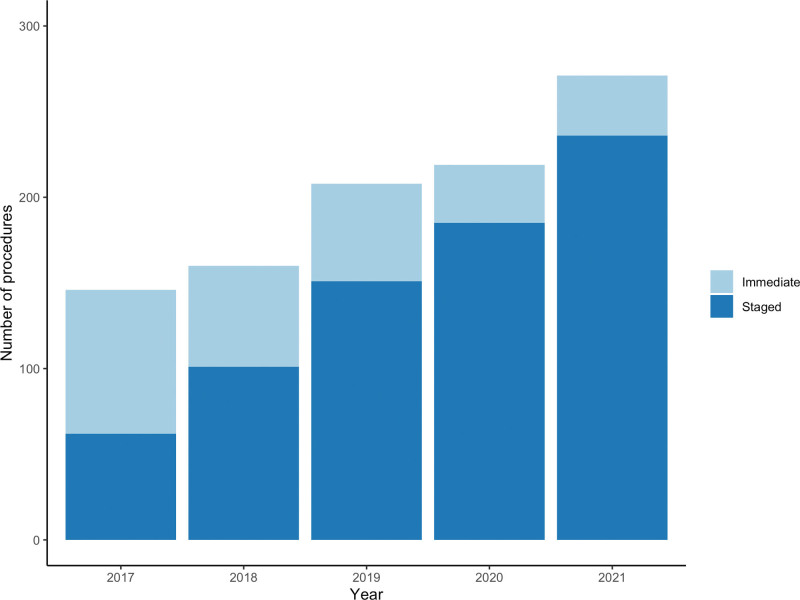
Laparoscopy was performed with plans for immediate resection under the same anesthetic in 269 patients (27%), and of those, resection was undertaken in 242 patients (90%). This practice decreased during the study period (Fig. 1), with 203 of 481 cases (42%) in the first half (January 2017 to June 2019) performed with plans for immediate resection compared to 66 of 523 cases (13%) in the second half (July 2019 to December 2021; p < 0.001). Of the 762 patients who underwent staging laparoscopy without immediate resection, postoperative complications occurred in 9 patients (1.2%), including a major complication in 1 patient (0.1%).
Figure 1.
Trend in laparoscopy performed as a separate staged procedure vs immediately before resection. Staged procedures increased significantly over time (p < 0.001).
Results of staging laparoscopy
In total, 180 patients (18%) had a positive staging laparoscopy due to gross metastatic disease (n = 140) and/or positive peritoneal cytology (n = 96). When this analysis was restricted to patients who had a staged procedure with peritoneal washings performed (n = 721), metastatic disease was confirmed in 151 patients (21%): 95 (23%) of the 419 who were treatment-naive and 56 (19%) of the 302 who had started neoadjuvant therapy. Of the entire cohort, biopsies were taken in 423 patients (42%) and confirmed gross metastatic disease in 140 (33%), including liver metastases only in 48 patients, peritoneal disease only in 81 patients, and both liver and peritoneal metastases in 11 patients. Of the 721 patients who had peritoneal washings performed, cytology was positive in 96 (13%). Among the 96 patients with positive cytology, 40 (42%) did not have any gross metastatic disease. Among the 111 patients with gross metastatic disease who also had peritoneal cytology performed, only 56 (50%) had correlative positive cytology.
Risk factors for positive laparoscopy
Characteristics and laboratory values of patients with positive laparoscopy compared to those with negative laparoscopy are shown in Table 1. Patients with positive laparoscopy were younger (median age 64 vs 67 years, p = 0.002) and were less likely to have undergone neoadjuvant chemotherapy (40% vs 53%, p = 0.002). They were more likely to have distal tumors (57% vs 31%, p < 0.001), larger (≥T3) tumors (37% vs 18%, p < 0.001), borderline resectable or locally advanced anatomy (78% vs 62%, p < 0.001), and indeterminate lesions on preoperative imaging (42% vs 6.4%, p < 0.001). They were more likely to have elevated serum CA 19-9 (73% vs 58%, p < 0.001) and serum CEA (61% vs 47%, p = 0.004). In patients who had elevated serum CA 19-9 and underwent neoadjuvant therapy (n = 385), 11 (7.9%) of 139 patients who had normalization of serum CA 19-9 had a positive staging laparoscopy, compared to 43 (17%) of 246 patients who had a persistent elevation (p = 0.015).
Peritoneal CA 19-9 and CEA were evaluated in 468 and 469 patients, respectively. Peritoneal CA 19-9 was elevated in 147 patients: 65 (60%) of the 109 patients who had a positive laparoscopy and 82 (23%) of the 359 patients who had a negative laparoscopy (p < 0.001). Peritoneal CEA was elevated in 69 patients: 44 (40%) of the 109 patients who had a positive laparoscopy and 25 (6.9%) of the 360 patients with negative laparoscopy (p < 0.001). The sensitivity, specificity, positive predictive value, and negative predictive value of peritoneal CA 19-9 and CEA are shown in Table 2.
Table 2.
Sensitivity, Specificity, and Predictive Value of Peritoneal CA 19-9 and Carcinoembryonic Antigen for Detecting Occult Intra-abdominal Metastases on Staging Laparoscopy
| Variable | Elevated peritoneal CA 19-9 | Elevated peritoneal CEA | Elevated peritoneal CA 19-9 and/or CEA |
|---|---|---|---|
| Sensitivity, % | 60 | 40 | 69 |
| Specificity, % | 77 | 93 | 76 |
| Positive predictive value, % | 44 | 64 | 46 |
| Negative predictive value, % | 86 | 84 | 89 |
The results from univariate and multivariable analysis of factors associated with positive laparoscopy are shown in Table 3. In univariate analysis, younger age, distal location, tumor size, borderline resectable, or locally advanced anatomy, the presence of indeterminate lesions on preoperative imaging, elevated serum and peritoneal CA 19-9 and CEA, and no preoperative neoadjuvant therapy were associated with an increased risk of positive laparoscopy (all p < 0.05). In multivariable analysis, younger age, distal location, increasing tumor size, the presence of indeterminate lesions on imaging, elevated serum CA 19-9, and elevated peritoneal CEA remained associated with an increased risk of positive laparoscopy (all p < 0.05). Based on these results, age ≤60 years, body/tail location, size >2 cm, and elevated serum CA 19-9 were defined as preoperative risk factors for radiographically occult metastases on laparoscopy. In patients with no indeterminate lesions on preoperative imaging, the rate of positive laparoscopy by number of risk factors is shown in Table 4 and ranged from 1.6% for patients with no risk factors to 42% for patients with all four risk factors.
Table 3.
Univariate and Multivariable Analysis of Factors Associated with Positive Laparoscopy
| Characteristic | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p Value | Odds ratio (95% CI) | p Value | |
| Age at laparoscopy | ||||
| >60 y | 1.00 (ref) | 1.00 (ref) | ||
| ≤60 y | 1.76 (1.25–2.47) | 0.001 | 1.82 (1.19–2.79) | 0.006 |
| Tumor location | ||||
| Proximal | 1.00 (ref) | 1.00 (ref) | ||
| Distal | 2.87 (2.07–4.00) | <0.001 | 2.78 (1.84–4.21) | <0.001 |
| Tumor size | ||||
| ≤20 mm (T1) | 1.00 (ref) | 1.00 (ref) | ||
| 21 to 40 mm (T2) | 3.10 (1.85–5.48) | <0.001 | 2.33 (1.25–4.32) | 0.008 |
| >40 mm (T3) | 6.40 (3.69–11.65) | <0.001 | 2.28 (1.14–4.56) | 0.019 |
| Anatomical resectability | ||||
| Resectable | 1.00 (ref) | 1.00 (ref) | ||
| BR/LA | 2.12 (1.47–3.13) | <0.001 | 1.55 (0.96–2.51) | 0.07 |
| Indeterminate lesion on imaging | ||||
| No | 1.00 (ref) | 1.00 (ref) | ||
| Yes | 10.63 (7.11–16.03) | <0.001 | 6.84 (4.19–11.17) | <0.001 |
| Serum CA 19-9 | ||||
| Normal (<35 U/mL) | 1.00 (ref) | 1.00 (ref) | ||
| Nonsecretor | 2.04 (0.98–4.03) | 0.046 | 1.69 (0.73–3.92) | 0.22 |
| Elevated (≥35 U/ml) | 2.29 (1.55–3.45) | <0.001 | 1.69 (1.02–2.81) | 0.042 |
| Serum CEA | ||||
| Normal (≤3.0 ng/mL) | 1.00 (ref) | 1.00 (ref) | ||
| Elevated (>3.0 ng/mL) | 1.66 (1.08–2.55) | 0.022 | 1.19 (0.72–1.99) | 0.49 |
| Neoadjuvant therapy | ||||
| No | 1.00 (ref) | 1.00 (ref) | ||
| Yes | 0.60 (0.43–0.83) | 0.002 | 0.72 (0.47–1.10) | 0.13 |
| Peritoneal CA 19-9 | ||||
| Normal (<5 U/mL) | 1.00 (ref) | 1.00 (ref) | ||
| Elevated (≥5 U/mL) | 2.89 (1.76–4.73) | <0.001 | 1.76 (0.95–3.27) | 0.07 |
| Peritoneal CEA | ||||
| Normal (<0.7 ng/mL) | 1.00 (ref) | 1.00 (ref) | ||
| Elevated (≥0.7 ng/mL) | 6.74 (3.72–12.21) | <0.001 | 2.94 (1.45–5.93) | 0.003 |
Data presented after multiple imputation for serum CEA, peritoneal CA 19-9, and peritoneal CEA.
BR/LA, borderline resectable/locally advanced.
Table 4.
Rate of Positive Laparoscopy among Patients with No Indeterminate Extrapancreatic Lesions on Preoperative Imaging by Number of Risk Factors
| No. of risk factors | No. of patients (% of total) | Rate of positive laparoscopy, % |
|---|---|---|
| 0 | 63 (7.2) | 1.6 |
| 1 | 203 (23) | 2.5 |
| 2 | 373 (43) | 11 |
| 3 | 203 (23) | 21 |
| 4 | 33 (3.4) | 42 |
Risk factors were defined as age ≤60 y, body/tail location, size >2 cm, and elevated serum CA 19-9.
Follow-up and survival
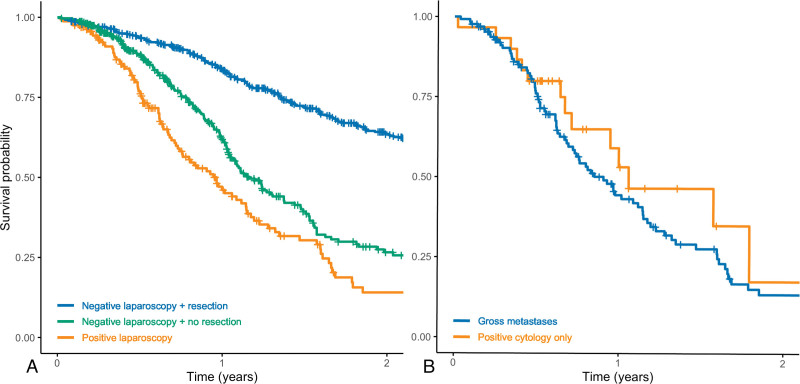
Of the 824 patients who had a negative laparoscopy, 69 patients (8.4%) were undergoing neoadjuvant therapy at last follow-up, 504 patients (61%) had undergone resection, and 251 patients (31%) were undergoing nonoperative or palliative treatment due to progression, conditional factors, or patient preference. Median overall survival from the time of staging laparoscopy was 36 months (95% CI 31 to 42) in patients with negative laparoscopy who eventually underwent resection, 14 months (95% CI 13 to 17) in patients with negative laparoscopy who did not undergo resection for whatever reason, and 12 months (95% CI 9 to 14) in patients with positive laparoscopy who did not undergo resection (p < 0.001; Fig. 2A). Of the 504 patients who underwent resection, 181 (36%) had developed recurrence at last follow-up with a median follow-up time of 19 months (IQR 11 to 33) in this group. Recurrence was locoregional (n = 26, 14%), distant (n = 135, 75%), or both (n = 20, 11%); distant recurrences involved the liver (n = 66, 43%), peritoneum (n = 37, 24%), and/or other sites (n = 63, 41%). Among patients with positive staging laparoscopy, median overall survival was 11 months (95% CI 9 to 14) in patients with gross metastatic disease and 13 months (95% CI 9 months or never reached) in patients with positive peritoneal cytology only, and this was comparable (p = 0.40; Fig. 2B).
Figure 2.
Kaplan–Meier survival curves for overall survival from staging laparoscopy. (A) All patients stratified by laparoscopy findings and whether resection was eventually performed (p < 0.001). (B) Patients with positive laparoscopy stratified by gross metastatic disease vs positive peritoneal cytology only (p = 0.40).
DISCUSSION
In this study, we describe our 5-year contemporary institutional experience with more than 1,000 consecutive staging laparoscopies for PDAC. We found that the rate of positive laparoscopy, defined as gross metastatic disease or positive peritoneal fluid cytology, was high and led to a change in management in approximately 1 in 5 patients. Additionally, we identified several factors associated with an increased risk of identifying metastatic dissemination at staging laparoscopy. These results suggest that staging laparoscopy with peritoneal lavage should be strongly considered in the majority of patients with pancreatic cancer, ideally prior to initiation of neoadjuvant therapy.
The strongest risk factor for positive staging laparoscopy was the presence of indeterminate extrapancreatic lesions on preoperative imaging. However, 12% of patients without any suspicious lesions had metastatic disease identified on staging laparoscopy, emphasizing the limited sensitivity of cross-sectional imaging even in this era of modern imaging technology and higher resolution. Other preoperative risk factors for positive staging laparoscopy included several anatomic factors known to be associated with an increased risk of metastatic dissemination, such as large tumor size and body/tail location.18,19 Additionally, young patients had higher rates of positive laparoscopy. The reason for this is unclear but may be related to differences in disease biology in this young subset of patients.20 While the rate of positive laparoscopy was low in patients who had none of these risk factors present, this applied to only a very small minority of the cohort, suggesting that the majority of patients (>80%) would benefit from undergoing staging laparoscopy. Lastly, the rate of positive laparoscopy was lower in patients who had started neoadjuvant chemotherapy, suggesting that any preoperative chemotherapy prior to staging laparoscopy may limit the sensitivity of this examination. Thus, we recommend that staging laparoscopy be performed before initiation of neoadjuvant therapy if possible, as treatment effect may mask limited metastatic disease. In our current practice, we concurrently place central line access for subsequent chemotherapy.
Over the 5-year study period, our institutional practice transitioned from frequently performing staging laparoscopy immediately prior to planned resection to almost exclusively performing separate staged procedures. This mirrors the increased use of neoadjuvant therapy, as staging laparoscopy at the time of initial diagnosis may help guide whether a patient is a candidate for neoadjuvant chemotherapy and/or chemoradiation with subsequent consideration of resection vs palliative chemotherapy only. Performing laparoscopy as a separate staged procedure also allows for diagnostic adjuncts such as peritoneal washings with cytology or peritoneal tumor markers, which take several days to analyze and report and may provide additional information that can help guide therapy. The importance of these adjuncts is evidenced by the fact that approximately half of patients with positive peritoneal cytology in the current study had no gross distant metastatic disease. Positive peritoneal cytology in the absence of gross metastases is generally considered to be equivalent to other distant metastatic dissemination, and this was confirmed by our results, which showed similar survival for patients who had positive peritoneal cytology only compared to those with gross metastatic disease.11,21
In addition to conventional serum tumor markers, we found that patients with positive laparoscopy were more likely to have elevated peritoneal fluid CA 19-9 and CEA. While the predictive capacity of peritoneal fluid tumor markers at staging laparoscopy for PDAC remains indeterminate, our experience has been that they correlate well with the presence of peritoneal metastases, with elevated peritoneal CA 19-9 and CEA found in 60% and 40%, respectively, of patients with positive laparoscopy in the current study. The significance of elevated peritoneal tumor markers in the absence of gross metastases or positive peritoneal cytology is unknown but may represent occult peritoneal tumor dissemination, although further studies are needed and are currently being performed at our center with consideration of repeat laparoscopy after neoadjuvant therapy. Additionally, our group recently published prospective trial data showing high rates of mutant cell-free KRAS DNA in peritoneal lavage fluid from patients with clinically positive laparoscopy but also in patients with clinically negative laparoscopy, suggesting that standard laparoscopic staging with gross visualization and peritoneal fluid cytologic examination may be inadequate.16 We found in this current study that only 50% of patients with gross metastases had concurrent positive cytology, suggesting that while a positive cytology result is meaningful and prognostic, negative cytology is not helpful, likely due to poor sensitivity of these assays. Therefore, the addition of biochemical (CA 19-9/CEA) and novel molecular (cell-free DNA) biomarkers may improve our current methods of peritoneal staging.
This study has several limitations inherent to its retrospective and single-center design. While staging laparoscopy is currently performed in nearly all patients considered for resection at our institution regardless of risk factors, this practice evolved during the study period, and it is possible that staging laparoscopy was preferentially performed in higher-risk patients in the earlier years of the study, although a comparison of the yield of laparoscopy throughout the study period suggests that this was not the case. Additionally, as a high-volume pancreatic surgery center, our center sees many patients with advanced lesions, which are associated with a higher risk of metastatic dissemination. As the complexity of cases seen at different institutions varies, the overall rate of positive laparoscopy may not be directly transferable to the patient populations at other centers.
CONCLUSIONS
The yield of staging laparoscopy in patients with PDAC remains significant, even in the current era of high-quality imaging, and resulted in a change in management in 1 of 5 patients in our institutional cohort. Staging laparoscopy should be considered in the majority of patients prior to resection and/or initiation of neoadjuvant therapy, specifically in patients with high-risk features such as indeterminate extrapancreatic lesions on imaging, young age, large tumor size, distal tumor location, or elevated serum tumor markers. In addition to direct visualization of peritoneal and hepatic surfaces, staging laparoscopy can facilitate the detection of occult metastases with the use of adjuncts such as peritoneal cytology, peritoneal tumor markers, and other potential peritoneal biomarkers.
Author Contributions
Conceptualization: Gudmundsdottir, Yonkus, Kendrick, Smoot, Truty
Data curation: Gudmundsdottir, Yonkus, Alva-Ruiz, Kendrick, Smoot, Warner, Starlinger, Thiels, Nagorney, Cleary, Grotz, Truty
Formal analysis: Gudmundsdottir
Investigation: Gudmundsdottir, Yonkus, Alva-Ruiz, Kendrick, Smoot, Warner, Starlinger, Thiels, Nagorney, Cleary, Grotz, Truty
Methodology: Gudmundsdottir, Yonkus, Alva-Ruiz, Warner, Starlinger, Thiels, Nagorney, Cleary, Grotz, Truty
Writing – original draft: Gudmundsdottir
Writing – review & editing: Yonkus, Alva-Ruiz, Kendrick, Smoot, Warner, Starlinger, Thiels, Nagorney, Cleary, Grotz, Truty
Supervision: Truty
Supplementary Material
Footnotes
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Cleary is a paid consultant to Ethicon.
Presented at the Western Surgical Association 130th Scientific Session, Santa Barbara, CA, November 2022.
A discussion of this article is available at http://links.lww.com/JACS/A235.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 3.Saad AM, Turk T, Al-Husseini MJ, Abdel-Rahman O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018;18:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg 2018;267:936–945. [DOI] [PubMed] [Google Scholar]
- 5.Jones RP, Psarelli EE, Jackson R, et al. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial. JAMA Surg 2019;154:1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Truty MJ, Kendrick ML, Nagorney DM, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg 2021;273:341–349. [DOI] [PubMed] [Google Scholar]
- 7.Cloyd JM, Heh V, Pawlik TM, et al. Neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomized controlled trials. J Clin Med 2020;9:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbanna KY, Jang HJ, Kim TK. Imaging diagnosis and staging of pancreatic ductal adenocarcinoma: a comprehensive review. Insights Imaging 2020;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim R, Prithviraj G, Kothari N, et al. PET/CT fusion scan prevents futile laparotomy in early stage pancreatic cancer. Clin Nucl Med 2015;40:e501–e505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paracha M, Van Orden K, Patts G, et al. Opportunity lost? Diagnostic laparoscopy in patients with pancreatic cancer in the National Surgical Quality Improvement Program database. World J Surg 2019;43:937–943. [DOI] [PubMed] [Google Scholar]
- 11.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19:439–457. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaleta EJ, Tolan NV, Ness KA, et al. CEA, AFP and CA 19-9 analysis in peritoneal fluid to differentiate causes of ascites formation. Clin Biochem 2013;46:814–818. [DOI] [PubMed] [Google Scholar]
- 14.Yonkus JA, Alva-Ruiz R, Abdelrahman AM, et al. Molecular peritoneal staging for pancreatic ductal adenocarcinoma using mutant KRAS droplet-digital polymerase chain reaction: results of a prospective clinical trial. J Am Coll Surg 2021;233:73–80.e1. [DOI] [PubMed] [Google Scholar]
- 15.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 16.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 17.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, Wiley; 198. [Google Scholar]
- 18.Liu X, Fu Y, Chen Q, et al. Predictors of distant metastasis on exploration in patients with potentially resectable pancreatic cancer. BMC Gastroenterol 2018;18:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slaar A, Eshuis WJ, van der Gaag NA, et al. Predicting distant metastasis in patients with suspected pancreatic and periampullary tumors for selective use of staging laparoscopy. World J Surg 2011;35:2528–2534. [DOI] [PubMed] [Google Scholar]
- 20.Ansari D, Althini C, Ohlsson H, Andersson R. Early-onset pancreatic cancer: a population-based study using the SEER registry. Langenbecks Arch Surg 2019;404:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrone CR, Haas B, Tang L, et al. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg 2006;10:1347–1353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.