ABSTRACT
The formation of sequential rosettes is a type of collective cell behavior recently discovered in the Caenorhabditis elegans embryo that mediates directional cell migration through sequential formation and resolution of multicellular rosettes involving the migrating cell and its neighboring cells along the way. Here, we show that a planar cell polarity (PCP)-based polarity scheme regulates sequential rosettes, which is distinct from the known mode of PCP regulation in multicellular rosettes during the process of convergent extension. Specifically, non-muscle myosin (NMY) localization and edge contraction are perpendicular to that of Van Gogh as opposed to colocalizing with Van Gogh. Further analyses suggest a two-component polarity scheme: one being the canonical PCP pathway with MIG-1/Frizzled and VANG-1/Van Gogh localized to the vertical edges, the other being MIG-1/Frizzled and NMY-2 localized to the midline/contracting edges. The NMY-2 localization and contraction of the midline edges also required LAT-1/Latrophilin, an adhesion G protein-coupled receptor that has not been shown to regulate multicellular rosettes. Our results establish a distinct mode of PCP-mediated cell intercalation and shed light on the versatile nature of the PCP pathway.
Keywords: Planar cell polarity, Multicellular rosette, Cell migration, C. elegans embryo, Lineage
Summary: A two-component polarity scheme is found to drive sequential rosette formation and resolution during tissue rearrangement in C. elegans embryos.
INTRODUCTION
Planar cell polarity (PCP) is a conserved mechanism to coordinate polarity of individual cells across a tissue (Devenport, 2014). Coordination is controlled by the asymmetric distribution of core PCP components, Van Gogh and Frizzled. These two transmembrane proteins inhibit each other intracellularly to establish polarity within a cell while attracting each other intercellularly to synchronize the orientation of polarity between cells, thus forming a polarity that is capable of propagation to many cells (Amonlirdviman et al., 2005).
Although such spatial propagation of polarity is most intuitive in static tissues, studies have shown that the PCP pathway can drive diverse modes of directional cell movement, including convergent extension to achieve global tissue shape changes in vertebrate gastrulation, neurulation and organogenesis (Butler and Wallingford, 2017; Koca et al., 2022; Williams and Solnica-Krezel, 2020), as well as the assembly of the Caenorhabditis elegans central nerve cord neurons (Shah et al., 2017a). In these cases, one mode of cell intercalation regulated by the PCP pathway is the directional formation and resolution of multicellular rosettes (Lienkamp et al., 2012), during which polarized localization of the PCP core components recruits non-muscle myosin to drive directional edge contraction. However, the PCP pathway is not the only mechanism to regulate directional formation and resolution of multicellular rosettes and tissue elongation. In Drosophila germband elongation, in which directional formation and resolution of multicellular rosettes was first discovered (Blankenship et al., 2006), the process is driven by a planar polarized distribution of Toll-like receptors (Paré et al., 2014).
The core PCP pathway is known to interact with other pathways and genes in regulating tissue polarity. In the Drosophila wing disc and eye, protocadherins Fat and Dachsous show planar polarization, which can contribute to the planar polarization of the core pathway (Ambegaonkar et al., 2012; Brittle et al., 2012). During the elongation of C. elegans ventral nerve cord, SAX-3/Robo acts in parallel to VANG-1/Van Gogh (Shah et al., 2017a). A common feature is that these pathways regulate non-muscle myosin localization (Soto, 2017).
Recently, we identified a collective cell behavior in the C. elegans embryo, termed sequential rosettes, which mediates directional cell migration through sequential formation and resolution of multicellular rosettes involving the migrating cell and its neighboring cells along the way (Wang et al., 2022). In this study, we systematically examined cell movements in the early C. elegans embryo to show that sequential rosettes are a general mechanism mediating long-range cell migration in multiple cell lineages. Furthermore, we investigated the underlying molecular mechanism regulating sequential rosettes. We show that sequential rosettes present a previously unreported mode of PCP-driven cell intercalation, where non-muscle myosin II (NMY-2) localization and edge contraction are orthogonal to the localization of the core PCP component VANG-1. We term this mode PCP-ortho and propose a two-component polarity scheme: the canonical PCP pathway with MIG-1 and VANG-1 localized to edges perpendicular to the contracting edges, and MIG-1, Dishevelled (DSH-2) and NMY-2 to the contracting edges. In addition, we identified Latrophilin (LAT-1), an adhesion G protein-coupled receptor (GPCR) that is best known for regulating axon guidance and synapse formation (Moreno-Salinas et al., 2019), as a regulator of non-muscle myosin (NMY) localization and edge contraction in PCP-ortho and sequential rosettes.
RESULTS
Sequential rosettes mediate long-range cell migration in the C. elegans embryo
In the early C. elegans embryo, there are two major cell movement motifs that are intertwined: gastrulation and left-right patterning. Gastrulation, from the 26-cell stage to the 350-cell stage, involves the internalization of many cells at multiple locations through the embryo (Harrell and Goldstein, 2011; Pohl et al., 2012). At the same time, left and right homologous lineages move to restore the superficial bilateral symmetry after the symmetry is broken at the four- to six-cell stage (Pohl and Bao, 2010; Pohl et al., 2012). Despite the technical capacity to image and track the position of every cell through this developmental time window, it is difficult to pinpoint individual cell movements because of whole-embryo rotations (Moore et al., 2013).
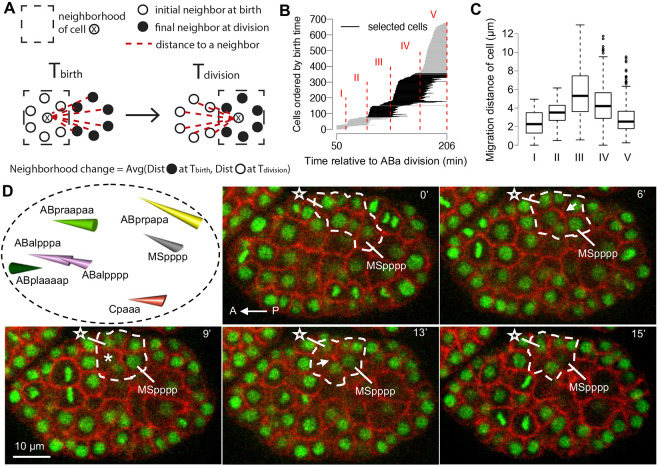
To this end, we designed a computational method based on neighborhood change to identify migrating cells (Fig. 1A; Materials and Methods). Briefly, the migration distance for each cell is defined as the average of the distances to initial neighbors immediately before the cell divides and the distances to final neighbors immediately after the cell is born. By focusing on relative movements, this approach avoids the complication of whole-embryo rotations. In particular, this method is sensitive in finding leader cells in collective movement, which appear to be common during C. elegans embryogenesis.
Fig. 1.
Sequential rosettes mediate long-range migration. (A) The computational screen for long-range migration by neighborhood change. (B) The birth and division timings of each cell from one- to 350-cell stage. Each horizontal line denotes a cell starting at birth time and ending at division time, sorted by birth time. Five stages were defined based on the timing of cell divisions (Materials and Methods). Black lines highlight stages III and IV, which are the focus of this study. (C) The migration distance of cells in each stage from panel B. The center line denotes the median, the box contains the 25th to 75th percentiles. The whiskers mark 1.5× interquartile range. (D) Top left panel, cone plot of the migration of seven cells with sequential rosettes. The base of the cone represents the position at the birth of the cell and the vertex of the cone represents the position at division of the cell. The color of cells denotes the founder lineage (see Fig. S1 for convention). Fluorescent images show the sequential rosettes during the migration of MSpppp. N=2 randomly picked embryos. The dashed line denotes cells involved in the sequential rosettes. The star denotes the most anterior cell that participates in the sequential rosettes of MSpppp. The arrows at 6 and 13 min denote the foci of rosettes. The asterisk at 9 min denotes new neighboring cell gained by MSpppp after the resolution of the first rosette. Time 0 is 140 min relative to the diamond-shaped four-cell stage. The embryo is in a ventral view with anterior to the left. Nuclei marked in green (HIS-72-GFP); plasma membrane marked in red (PH-domain of PLC1d1 fused to mCherry).
In wild-type embryos with the complete lineage traced, we divided early embryogenesis (1- to 350-cell stage, the approximate completion of gastrulation) into five stages based on synchronous cell divisions (Fig. 1B; Materials and Methods). The most pronounced migrations occurred during stages III and IV, which involved 276 cells (Fig. 1C; Fig. S1). We identified a total of 37 cells that underwent long-range migration, defined as cells that migrated a distance greater than the mean plus one standard deviation. Using images of embryos with fluorescently labeled nuclei and plasma membrane, we found that in 18 of the 37 cells the movement is mediated by the T1 process of neighbor exchange and in 12 by canonical multicellular rosettes (Fig. S2). For the remaining seven cells, the movement is mediated by sequential rosettes, each involving two or three consecutive rosettes. These cells are Cpaaa, ABalpppa, ABalpppp, ABplaaaap, ABpraapaa, ABprpapa and MSpppp (Fig. 1D). Cpaaa is the founding case of sequential rosettes that we previously identified (Wang et al., 2022). Furthermore, four of these cells (ABalpppa, ABalpppp, ABplaaaap, ABpraapaa) appear to be related to the restoration of bilateral symmetry involving the ABplaa/ABpraa lineages and their left/right (LR) counterparts (Pohl and Bao, 2010). Combined with the migration of the mu_int_R cell, which is a case of long-range migration after gastrulation identified by Sulston (Sulston et al., 1983) and shown to be mediated by sequential rosettes in our previous study (Wang et al., 2022), these results suggest that sequential rosettes are a widely used mechanism of cell migration in the C. elegans embryo.
Stereotypy of the Cpaaa sequential rosettes
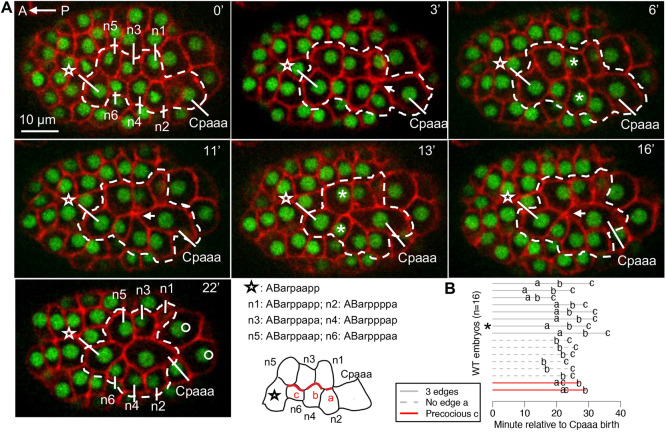
We use the Cpaaa case to elucidate the regulatory mechanism of sequential rosettes. To better define the basis of cell behaviors for phenotype analyses, we first examined the dynamics of the Cpaaa sequential rosettes in wild-type embryos. The Cpaaa sequential rosettes involve eight epidermal progenitor cells on the dorsal side of the C. elegans embryo (Wang et al., 2022). Around 170-200 min after first cell cleavage, by sequential formation and resolution of rosettes, Cpaaa intercalates from posterior to anterior into two rows of three ABarpp cells (n1/n3/n5 and n2/n4/n6) and contacts ABarpaapp before cell division of Cpaaa (Fig. 2A). After the resolution of each rosette, Cpaaa gains new neighbors, i.e., n3/4 at 6 min, n5/6 at 13 min and ABarpaapp at 22 min (Fig. 2A, asterisks and star cell). Other epidermal progenitor cells from the C lineage follow Cpaaa in the intercalation. This intercalation led by Cpaaa is essential for epidermis organization in C. elegans: the anterior and posterior regions of the dorsal epidermis, represented by ABarpaapp and Cpaaa, respectively, move into contact and eventually fuse to form the large dorsal epidermal cell hyp7; the two rows of ABarpp cells will form seam cells on the left and right side of the body.
Fig. 2.
Sequential rosettes of Cpaaa. (A) Dynamics of rosette formation and resolution in sequential rosettes of Cpaaa. The dashed line denotes cells involved in the sequential rosettes. The star denotes the most anterior cell that participates in the sequential rosettes. The arrows at 3, 11 and 16 min denote the foci of rosettes. The asterisks at 6 and 13 min denote new neighboring cells gained by Cpaaa after rosette resolution. The circles at 22 min denote other epidermal progenitor cells from the C lineage that follow Cpaaa in the intercalation. The embryo is in a dorsal view with anterior to the left. For fluorescent labels, see Fig. 1D. Time 0 is 1 min before the formation of first rosette (about 150 min relative to the diamond-shaped four-cell stage). The time stamp applies to all images of Cpaaa rosettes in this study. (B) The timings of contraction of edges in 16 wild-type embryos (randomly picked from five adult worms). Bottom left, three edges (a, b and c) are defined from posterior to anterior in the typical order of contraction. Right panel, each line represents an embryo and letters a, b and c indicate the timing of the contraction of the corresponding edge. The asterisk denotes the embryo shown in panel A. ‘Precocious c’ means the contraction of edge c is earlier than the contraction of edge b. ‘No edge a’ means the initial conformation of cells does not include edge a.
The Cpaaa sequential rosettes occur consistently in wild-type embryos, and Cpaaa always contacts ABarpaapp before its division (Fig. 2B, N=16). However, there are variations in the initial conformations of these cells and the order of edge contractions. In half the cases (8 out of 16), Cpaaa is born to contact n1 and n2; these cases involve three rosette formations and three edge contractions sequentially from posterior to anterior (namely, edge a, b and c; Fig. 2B). In 6 of 16 embryos, Cpaaa is born slightly more anteriorly and contacts n4; these cases involve two rosette formations and two edge contractions (‘No edge a’, Fig. 2B). In the least frequent cases (2 out of 16), although there are three edges in the initial conformation, the most anterior edge (edge c) contracts before the contraction of the edge in the middle (edge b) (‘Precocious c’, Fig. 2B). Overall, sequential rosettes have a highly consistent outcome despite some variations on the initial conformation and the order of contractions.
Core components of the PCP pathway regulate sequential rosettes
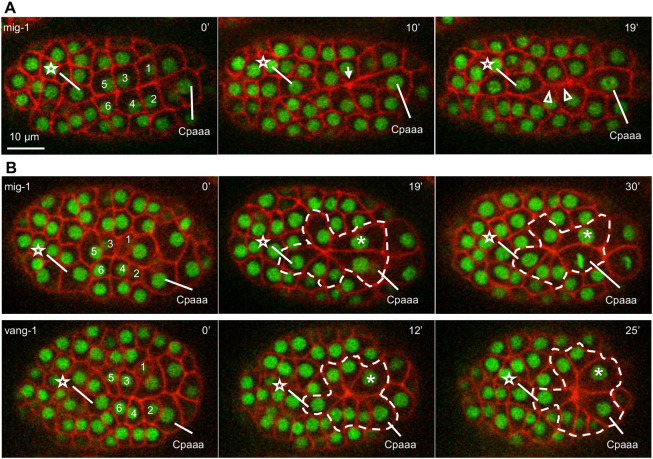
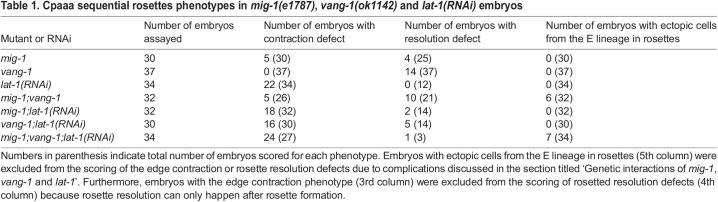
To elucidate the molecular mechanism of sequential rosettes, we first examined the core components of the PCP pathway, MIG-1 and VANG-1. Both mig-1 and vang-1 have strong expression in cells involved in the Cpaaa sequential rosettes based on single cell RNA-sequencing data (Fig. S3) (GSE126954; Packer et al., 2019). We found that loss-of-function mutations in mig-1 and vang-1 display defects in two aspects of sequential rosettes: edge contraction and resolution. Specifically, a contraction defect is defined as the failure in the contraction of at least one edge, resulting in Cpaaa not contacting ABarpaapp. A resolution defect is defined as the prolonged existence of a rosette, lasting more than 10 min instead of immediately resolving as in the wild type. In mig-1(e1787) mutants (Pan et al., 2006), 17% of embryos showed contraction defects on edge b or edge c (Fig. 3A; Table 1; N=30) and 16% embryos showed resolution defects (Fig. 3B; Table 1; N=30). In vang-1(ok1142) mutants, 38% of embryos showed resolution defects but none of them showed a contraction defect (Fig. 3B; Table 1; N=37). In all mutants examined here and below, the two rows of ABarpp cells showed wild-type configuration before the onset of sequential rosettes, in that the two rows formed an interface at the midline, with contact of ABarpaapp on the anterior and contact of Cpaaa on the posterior (Fig. S4A,B). These observations rule out the possibility that the rosette phenotypes were secondary to cell positioning defects, which may arise from potential defects in spindle regulation or adhesion. Furthermore, the rosette defects did not cause permanent defects in cell rearrangement: after cell divisions of the Cpaaa and ABarp cells, the C lineage cells were able to move in between the two rows of ABarpp cells and contact the posterior daughter cell of ABarpaapp (Fig. S5), and embryos hatched with normal morphology. The apparent correction implies that there are redundant mechanisms maintaining the robustness of morphogenesis. Together, our results show that core components of the PCP pathway regulate sequential rosettes of Cpaaa.
Fig. 3.
Core PCP genes regulate the sequential rosettes of Cpaaa. (A) Edge contraction defect in mig-1(e1787) mutants. The star and numbers denote seven ABarp cells involved in the sequential rosettes (see Fig. 2). The arrow at 10 min denotes the focus of the first rosette. The arrowheads at 19 min denote edges (b and c) with contraction defect. (B) Rosette resolution defects in mig-1(e1787) (top) and vang-1(ok1142) (bottom) mutants. The dashed line denotes cells in the prolonged rosette. The asterisks denote Caaaa in the prolonged rosette, which participates in the first rosette in some of the wild-type embryos. The star denotes ABarpaapp. See Table 1 for sample size. All embryos are in a dorsal view with anterior to the left. In all embryos, nuclei are marked by green channel and plasma membrane is marked by red channel as defined in Fig. 1D.
Table 1.
Cpaaa sequential rosettes phenotypes in mig-1(e1787), vang-1(ok1142) and lat-1(RNAi) embryos
We further examined other candidate genes. SAX-3 is known to regulate multicellular rosettes in parallel to the PCP pathway in C. elegans during ventral nerve cord (VNC) convergent extension (Shah et al., 2017a). The sax-3(ky123) mutant, which affects rosettes in the VNC, did not show defects in the Cpaaa sequential rosettes (N=11). VAB-1 is an Eph receptor regulating dorsal intercalation (Walck-Shannon et al., 2016) and ventral closure (Ghenea et al., 2005) of epidermal cells at a later stage of C. elegans embryogenesis. The vab-1(dx31) mutant did not show defects in the Cpaaa sequential rosettes (N=11). Finally, Toll-like receptors regulate multicellular rosettes during Drosophila germband extension (Paré et al., 2014). tol-1 is the sole homolog in C. elegans, but a loss-of-function allele, nr2013, did not show any defect in the Cpaaa sequential rosettes (N=13).
MIG-1 and VANG-1 localization indicate a PCP-related scheme
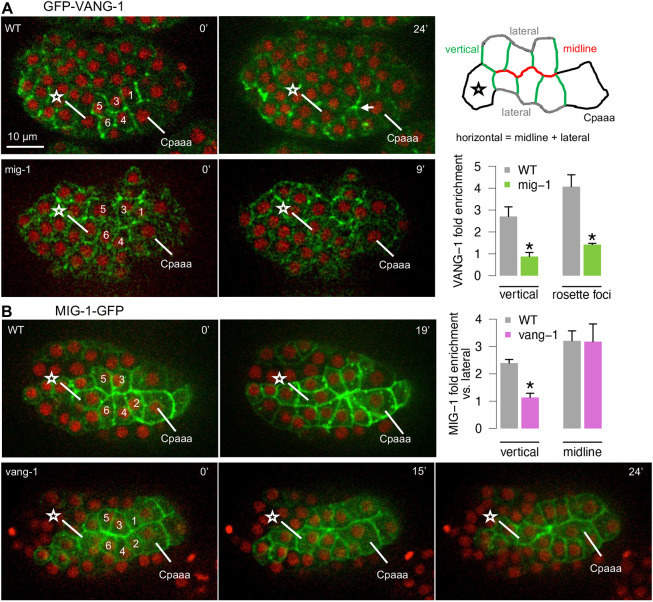
We then examined protein localization of MIG-1 and VANG-1. The contracting edges in the Cpaaa sequential rosettes coincide with the dorsal midline of the embryo and are parallel to the anterior-posterior (AP) axis. For simplicity, we refer to these edges as the midline (red line in the schematic in Fig. 4A). The edges of the ABarpp cells that are approximately perpendicular to the midline/AP axis are referred to as the vertical edges (green lines in the schematic in Fig. 4A). The edges between the ABarpp cells and surrounding cells are referred to as lateral edges (grey lines in the schematic in Fig. 4A). The midline and lateral edges collectively are referred to as horizontal edges.
Fig. 4.
Protein localization of the core PCP components during the Cpaaa sequential rosettes. (A) GFP-VANG-1 localization in wild-type and mig-1(e1787) mutants. The arrow denotes rosette focus. The star and numbers denote ABarp cells (see Fig. 2). The definitions of vertical, horizontal, lateral and midline edges are shown in the diagram on initial conformation of cells before first contraction (top right). The bar plot shows VANG-1 fold enrichment between vertical and horizontal edges, and between rosette foci and edges in rosettes. N=14 cells from three embryos. (B) MIG-1-GFP localization in wild-type and vang-1(ok1142) mutant. The bar plot shows MIG-1 fold enrichment between vertical and lateral edges, and between midline and lateral edges. N=16 cells from three embryos. Data are mean±s.e.m. *P<0.05 (two-tailed unpaired Student's t-test non-adjusted). See panel A for convention. All embryos are in a dorsal view with anterior to the left.
For VANG-1, we used a CRISPR/Cas9-mediated GFP fusion at the N terminus of the native vang-1 locus (zy60) (Shah et al., 2017a). Before the first edge contract, GFP-VANG-1 was enriched on the vertical edges, with ∼2.5-fold enrichment on the vertical edges versus horizontal edges (Fig. 4A; four embryos, 4-6 cells per embryo; see Fig. S6A for another example image). When rosettes form, GFP-VANG-1 was enriched at the foci, with an ∼4-fold enrichment compared with the edges of the rosette (four embryos, 4-6 cells per embryo). VANG-1 enrichment at rosette foci has been observed during the convergent extension of the C. elegans VNC (Shah et al., 2017a). Furthermore, VANG-1 localization to the vertical edges as well as rosette foci depends on mig-1. In mig-1(e1787) mutants, some ABarpp cells had VANG-1 localization on all edges, and foci enrichment was abolished (three embryos, 4-6 cells per embryo).
For MIG-1, we used a transgenic line expressing a functional MIG-1-GFP fusion (Ex[Pmig-1::MIG-1::GFP]) (Mizumoto and Shen, 2013). The MIG-1-GFP fusion rescued the mig-1(e1787) phenotypes and displays wild type-like sequential rosettes (N=30). Similar to GFP-VANG-1, MIG-1-GFP was enriched on vertical edges (with ∼2.5-fold enrichment, three embryos, 4-6 cells per embryo) (Fig. 4B). Interestingly, MIG-1-GFP was also enriched on contracting edges/midline, with ∼3-fold enrichment (three embryos, 4-6 cells per embryo). Proper localization of MIG-1 depends on vang-1. In vang-1(ok1142) mutants, MIG-1-GFP enrichment on the vertical edges was lost: signals were similar between vertical and lateral edges (three embryos, 4-6 cells per embryo). In contrast, MIG-1-GFP enrichment on the contracting edges/midline was unaffected by vang-1(ok1142). In all reporter strains, GFP insertion did not affect sequential rosettes dynamics as shown by the duration of sequential rosettes as well as cell positions before sequential rosettes (Fig. S4C,D).
The enrichment of VANG-1 and MIG-1 on the vertical edges as well as the mutual dependence of this localization are consistent with the canonical PCP model. Owing to limitations of the reporters, we could not determine which side of the cell-cell contact contains localized signal.
The enrichment of MIG-1-GFP on the contracting edges/midline is distinct from the canonical PCP model. To rule out potential artifacts of overexpression by the transgene, we created a CRISPR/Cas9-mediated GFP fusion at the C terminus of the native mig-1 locus. Unfortunately, the signal was too weak to be detected by imaging. However, as shown in experiments below, the midline localization of MIG-1 is most likely real and functional.
A non-canonical PCP underlies sequential rosettes
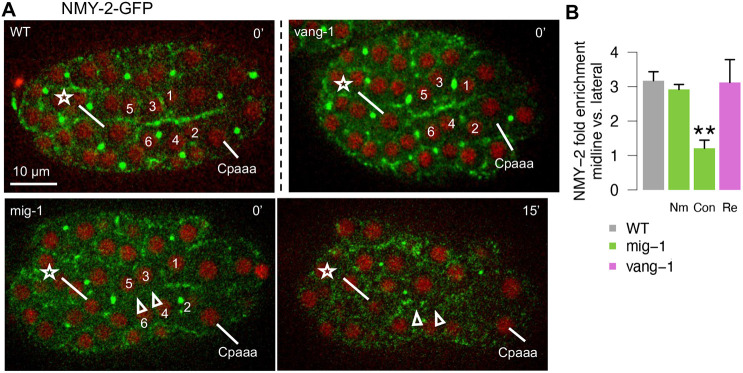
Non-muscle myosin II is known to drive edge contraction in multicellular rosettes in various tissues across species (Huebner and Wallingford, 2018). We examined the localization and regulation of NMY-2, which is one of two non-muscle myosin II heavy chains in C. elegans. Using a CRISPR/Cas9-mediated GFP fusion of the native nmy-2 locus (cp13), we found that NMY-2-GFP showed ∼3-fold enrichment on the contracting edges/midline (Fig. 5A; N=5 embryos). More specifically, despite the sequential contraction of edges, NMY-2-GFP was enriched on all midline edges 1 min before the first contraction. NMY-2 localization and the direction of edge contraction in sequential rosettes shows a crucial distinction from that in the canonical PCP model such as VNC convergent extension (Shah et al., 2017a). In both cases, VANG-1 is localized to edges that are vertical to the midline/AP axis. In sequential rosettes, NMY-2 localization and the direction of edge contraction are perpendicular to VANG-1 localization. In contrast, during VNC convergent extension NMY-2 co-localizes with VANG-1 to drive contraction of the vertical edges. Thus, sequential rosettes employ a related but distinct scheme of polarity compared with canonical PCP.
Fig. 5.
The regulation of midline NMY-2 localization in Cpaaa sequential rosettes. (A) NMY-2-GFP in sequential rosettes of Cpaaa in wild-type embryos, mig-1(e1787) and vang-1(ok1142) mutants. The arrows in mig-1(e1787) embryos denote edges with NMY-2 localization defect and contraction defect at 0 min and 15 min, respectively. The star and numbers denote ABarp cells (see Fig. 2). All embryos are in a dorsal view with anterior to the left. (B) Bar plot shows NMY-2 fold enrichment between midline and lateral edges in wild-type and mutant embryos. Nm, edges with normal contraction on midline; Con, edges with contraction defect on midline; Re, only embryos with resolution phenotype were considered for vang-1(ok1142). Data are mean±s.e.m. N=15 cells from five embryos). **P<0.01 (two-tailed unpaired Student's t-test non-adjusted).
We then asked how MIG-1 and VANG-1 may regulate NMY-2 localization. In mig-1(e1787) mutants, enrichment of NMY-2 was lost on one or more midline edges in some embryos, and the edges that lost NMY-2 enrichment displayed a contraction defect (Fig. 5A; seven out of 32 embryos contained a contraction defect, comparable with the penetrance of edge contraction defects described above). In contrast, vang-1(ok1142) mutation did not affect NMY-2 enrichment on the midline edges (Fig. 5A; 0 out of 12 embryos), consistent with the lack of edge contraction phenotypes described above.
A minimal explanation of the phenotypic difference between mig-1 and vang-1 loss-of-function mutations is that midline localization of MIG-1 is required for NMY-2 localization and edge contraction. Consistent with this explanation, we found that DSH-2, a downstream effector that binds to MIG-1 (Axelrod, 2001), was 2-fold enriched on the midline edges (Fig. S6B). This observation was based on a CRISPR/Cas9-mediated mNeonGreen fusion of the native dsh-2 locus at the N terminus (cp51) (Heppert et al., 2018), eliminating the concern of overexpression present with traditional transgene reporters. Furthermore, dsh-2 RNAi-treated embryos displayed edge contraction defects (7 out of 38 embryos) with a penetrance similar to mig-1(e1787) (Fig. S6C). These results suggest that midline enrichment of MIG-1 is required for NMY-2 midline localization and edge contraction.
The results above indicate a two-component polarity scheme underlying sequential rosettes: a canonical PCP component with MIG-1 and VANG-1 localization to the vertical edges with mutual dependence, and a midline component with NMY-2 and MIG-1 localization on the midline edges. Given that NMY-2 localization and edge contraction are orthogonal to the edge with PCP protein localization, we term this polarity scheme PCP-ortho.
LAT-1 is a regulator of PCP-ortho
Latrophilins are members of the adhesion G protein-coupled receptor (aGPCR) family, with a function best known in the vertebrate nervous system for regulating synapse formation and function as well as actin dynamics in growth cones (Moreno-Salinas et al., 2019). The C. elegans homolog, LAT-1, is known to regulate spindle orientation in the early embryo (Langenhan et al., 2009), suggesting the potential to interact with MIG-1. Furthermore, lat-1 is more highly expressed in the ABarpp cells compared with other cells (Fig. S3). Therefore, we asked whether lat-1 could regulate sequential rosettes.
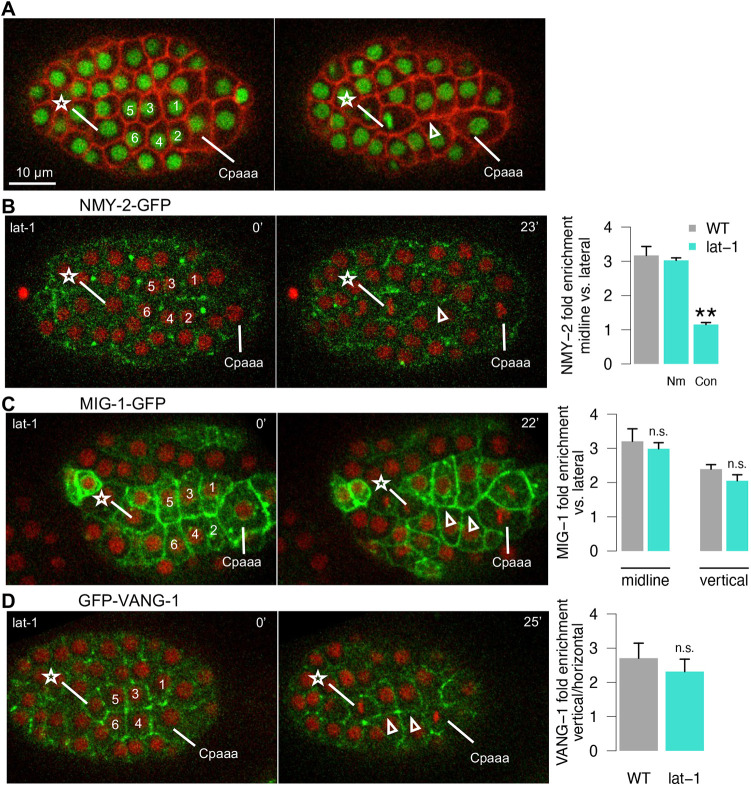
Loss of function of lat-1 is maternal lethal, therefore we used RNAi to examine lat-1 function in the Cpaaa sequential rosettes. We found that 39% of the lat-1(RNAi) embryos (N=23) showed the previously described phenotype of tilted ABal division (Langenhan et al., 2009), confirming the effect of lat-1 RNAi. However, this early defect did not affect cell position of Cpaaa or the ABarpp cells before sequential rosette formation (Fig. S4B). We found that 65% of embryos display the edge contraction defect and no embryo displays the rosette resolution defect (Fig. 6A; Table 1; N=34). We then asked whether lat-1 regulates edge contraction via NMY-2 localization. Indeed, the enrichment of NMY-2 on midline edges was abolished in lat-1 RNAi-treated embryos and the corresponding edges showed contraction defects (Fig. 6B; 8 out of 14 embryos). These results suggest that lat-1 regulates NMY-2 localization and contraction of midline edges.
Fig. 6.
The regulation of lat-1 in Cpaaa sequential rosettes. (A) Arrowhead shows the contraction defect in embryos treated with lat-1(RNAi). See Table 1 for sample size. (B) NMY-2-GFP on midline of sequential rosettes in wild-type embryos treated with lat-1(RNAi). Nm, edges with normal contraction on midline; Con, edges with contraction defect on midline. (C) MIG-1-GFP on midline and vertical edges of sequential rosettes in wild-type embryos treated with lat-1(RNAi). (D) GFP-VANG-1 on vertical edges of sequential rosettes in wild-type embryos treated with lat-1(RNAi). Data are mean±s.e.m. N=18 cells from three embryos. **P<0.01 (two-tailed unpaired Student's t-test non-adjusted). n.s., not significant. All embryos are in a dorsal view with anterior to the left. The star and numbers in all panels denote ABarp cells (see Fig. 2 for convention).
Next, we asked how lat-1 may interact with mig-1 and vang-1. We first examined whether lat-1 is required for proper localization of MIG-1 and VANG-1. To ensure that lat-1 RNAi was effective in the embryos examined, we only considered embryos with edge contraction defects in sequential rosettes, which can be determined from the fluorescent images. We found the localization of MIG-1 and VANG-1 was normal in these embryos (Fig. 6C,D; N=10 and N=9, respectively): MIG-1 was enriched on the vertical and midline edges and VANG-1 on the vertical edges, all with a fold of enrichment comparable with the wild type. These results suggest that lat-1 functions downstream of or in parallel with mig-1 and vang-1.
We then sought to examine the localization of LAT-1 by creating a CRISPR/Cas9-mediated GFP fusion at the C terminus of the native lat-1 locus. However, we were not able to generate a viable strain that could be stably maintained. Therefore, we focused the rest of the analyses on the phenotypes in double and triple loss of function of lat-1(RNAi) with mig-1 and vang-1.
Genetic interactions of mig-1, vang-1 and lat-1
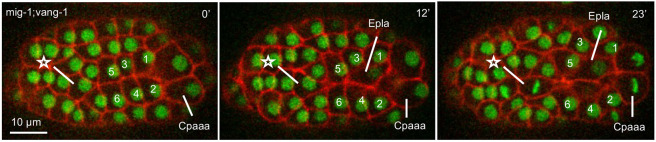
As described above, the three regulators cause two phenotypes in sequential rosettes, namely edge contraction and rosette resolution. We further found that mig-1(e1787);vang-1(ok1142) double mutants exhibited a third phenotype in which the sequential rosettes involve additional cells from the E lineage (Fig. 7; Table 1). In 6 out of 32 embryos, endodermal precursors from the E lineage, which normally lay underneath Cpaaa and the ABarp cells, were pulled by edge contraction dorsally to make contact with ABarpaapp. It is known that vang-1 is required for eight endodermal precursors (Exxx) at this stage to intercalate and form a plane (Asan et al., 2016). Further experiments are required to discern the tissue-specific gene functions and tissue interactions by which VANG-1 regulates intestinal morphogenesis. Notably, in these embryos, Cpaaa disengaged from the rosette after its initial participation. It is not clear whether the disengagement is regulated or a simple secondary consequence of excessive cell elongation and tension caused by the additional cells in the rosettes. Nonetheless, because Cpaaa no longer participates in sequential rosettes with the ABarp cells in these embryos, we excluded them from further analysis (Table 1).
Fig. 7.
An example of a rosette involving endodermal precursor in mig-1(e1787);vang-1(ok1142) double mutant. The embryo is in a dorsal view with anterior to the left. See Table 1 for sample size. See Fig. 2 for convention.
In terms of edge contraction, none of the three double loss-of-function conditions showed apparent enhancement compared with the single loss of function with stronger phenotype (Table 1; Table S1; P-value>0.05). However, the triple loss of function [mig-1(e1787);vang-1(ok1142);lat-1(RNAi)] had a significant increase of penetrance with nearly 90% of embryos showing contraction defects compared with each double loss-of-function condition (24 out of 27; Table 1; Table S1; P-value=0.0087, 0.0041 and 2.9×10−7, respectively). The implication of the apparent three-way redundancy is discussed below.
Finally, in terms of rosette resolution, in mig-1(e1787);vang-1(ok1142) double mutants, 48% of embryos showed defects (Table 1), which is slightly higher than that in vang-1(ok1142) but not statistically significant (Table S1; P-value=0.58). Furthermore, lat-1(RNAi) did not enhance resolution defects in mig-1(e1787), vang-1(ok1142) or mig-1(e1787);vang-1(ok1142) mutants (Table 1; Table S1; P-value=1). These results suggest that mig-1 and vang-1 function in the same pathway in regulating rosette resolution, whereas lat-1 is not involved. In convergent extension, VANG-1 is required for proper rosette resolution (Williams et al., 2014). Our results extend the requirement to the core PCP pathway.
DISCUSSION
Diversity in PCP-mediated tissue rearrangement
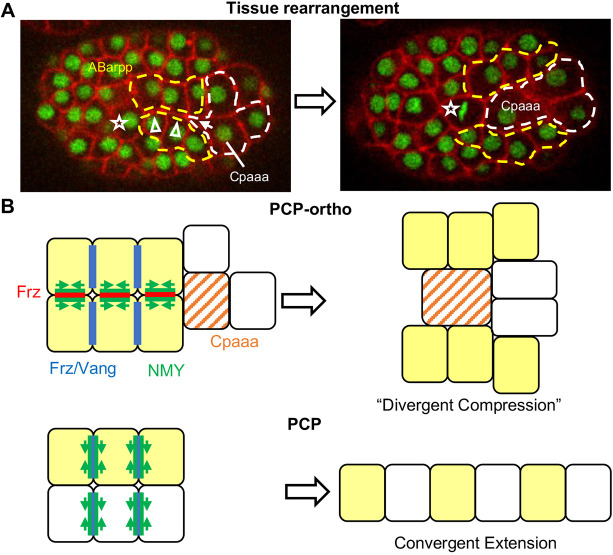
This study revealed a mode of PCP-mediated tissue rearrangement that is distinct from canonical convergent extension (Fig. 8). Specifically, NMY localization and edge contraction occur at the edges orthogonal to VANG-1 localization. The resulting tissue shape change is a widening and shortening along the AP axis, as opposed to the narrowing and lengthening in convergent extension. We term this mode PCP-ortho. Our results further suggested a polarity scheme with two components: the canonical MIG-1/VANG-1 localization to edges that are vertical to the AP axis, and MIG-1/NMY-2 localization to the midline/AP edges.
Fig. 8.
PCP-ortho model of sequential rosettes. (A) Tissue rearrangement between two rows of ABarpp cells (yellow) and the C lineage cells (white) through sequential rosettes. Additional epidermal progenitor cells in the C lineage follow Cpaaa during sequential rosettes. Star, ABarpaapp. Arrowheads indicate edges that will contract in sequential rosettes. The arrow indicates the focus of the first rosette. (B) Comparison of the PCP-ortho model and the canonical PCP model.
PCP-ortho raises intriguing questions regarding cell polarity and regulation of NMY. In multicellular rosettes formed in convergent extension, the core PCP pathway recruits NMY. What prevents NMY from following MIG-1 or VANG-1 to the vertical edges in PCP-ortho? Furthermore, what recruits NMY-2 to the midline edges? Our results offer several clues. One is the population of MIG-1 molecules on the midline edges. Given the differential localization of MIG-1 and VANG-1, it is possible that MIG-1 and VANG-1 interaction on the vertical edges distinguishes the activity of MIG-1 on the vertical versus midline edges. Another is the requirement of vang-1 for midline localization of NMY-2, which was revealed in the triple loss-of-function mig-1(e1787);vang-1(ok1142);lat-1(RNAi). This result indicates that signaling at the vertical edges acts not only in cis to repress NMY-2 localization to the vertical edges, but also in trans to promote NMY-2 localization to the midline edges. In convergent extension, PCP can also regulate directional cell crawling, in which NMY localizes to the mediolateral sides of the cell orthogonal to PCP localization. Thus, trans signaling by PCP is possible (Huebner and Wallingford, 2018; Williams and Solnica-Krezel, 2020). It is not clear what regulates the protrusive activity of NMY at the mediolateral sides in these cases of directional crawling, but in our case of sequential rosettes, MIG-1 and DSH-2 apparently put NMY in edge contraction mode. A slight complication in our data is the apparent genetic redundancy of mig-1 and vang-1, which would typically argue against a single (linear) pathway. However, given the feedback loops in canonical PCP, the apparent redundancy may not be a concern. Clear elucidation of the polarity scheme would likely require optogenetic manipulation (Shah et al., 2017b) to separate the populations of molecules in different cells and on different edges.
Our results showed that sequential rosettes belong to the class of rosettes formed through planar polarized constriction (Harding et al., 2014) along with those in vertebrate convergent extension and Drosophila germband extension (Butler and Wallingford, 2017). However, sequential rosettes show a distinct feature, namely the sequential formation and resolution of rosettes involving partially overlapping groups of cells. Sequential rosettes also revealed nuanced temporal regulation of NMY-2 contraction activity. Our analysis showed that NMY-2 is localized to all midline edges before any edge contraction, whereas contraction occurs sequentially. These results indicate a latent signal to activate NMY-2. An apparent correlation with edge contraction is the contact with the Cpaaa cell, which could provide the latent signaling to activate NMY-2. As we previously showed, cell fate of Cpaaa is required for sequential rosettes (Wang et al., 2022).
Flexibility of molecular interactions in the PCP pathway
Our study also reveals LAT-1 to be an interactor with the PCP pathway. The loss-of-function analyses suggest that lat-1 is required for NMY-2 localization to the midline edges and that it acts in parallel to or downstream of mig-1 and vang-1. In zebrafish, an aGPCR named Gpr125 (Adgra3) interacts with the PCP pathway by regulating DSH-2 localization (Li et al., 2013), and the G proteins Gα12/13 enhance the PCP mutant phenotype (Lin et al., 2005). The colocalization of zebrafish Gpr125 and DSH-2 on cell membranes (Li et al., 2013) suggests that LAT-1 might be localized on the midline of ABarpp cells, which agrees with its function in sequential rosettes. Further elucidation of lat-1 function would benefit from knowing the localization of LAT-1.
The involvement of aGPCRs in a PCP scheme shows an intriguing similarity seen in the convergent extension of the C. elegans VNC neurons, where sax-3 acts in parallel to vang-1. The PCP pathway relies on intricate feedback loops to synchronize polarity across a tissue. It is somewhat surprising that other polarity/NMY regulators can be so readily plugged into the synchronization of polarity. In particular, both LAT-1 and SAX-3 are known to function in adhesion, suggesting that their interactions with the PCP pathway could be intercellular to facilitate the synchronization of polarity across a tissue, similar to the protocadherins Fat and Dachsous in Drosophila (Ambegaonkar et al., 2012). Interestingly, many so-called axon guidance receptors are polarity/NMY regulators and are known to regulate cell intercalation and crawling in C. elegans (Asan et al., 2016; Bernadskaya et al., 2012; Chin-Sang et al., 1999; Walck-Shannon et al., 2016). It is possible that these molecules can also be incorporated into a PCP context, either in C. elegans or in other organisms, which would greatly enrich our understanding of planar cell polarity.
In terms of the flexibility of the PCP pathway, previous studies in C. elegans indicated that even the so-called core interactions between MIG-1 and VANG-1 may be partially replaced (Cravo and van den Heuvel, 2020). Regulation of spindle orientation in the early C. elegans embryo depends on polarized localization of MOM-5/Frizzled to the posterior of the dividing cell. Two features of MOM-5 resemble PCP in that synchronized direction of polarity can arise among a group of cells independent of Wnt ligands (Park et al., 2004); and that the synchronization relies on cell-to-cell relay (Bischoff and Schnabel, 2006). Vang-1 does not seem to be required in this process: although we did not characterize spindle orientation in our study, the fact that vang-1 loss of function, either as single mutant or in combination with mig-1, is viable suggests that spindle orientation is largely unaffected. Given that vang-1 is the only apparent homolog of Van Gogh in C. elegans, it is likely that a different kind of membrane protein plays the role of interacting with MOM-5 to relay polarity, either on its own or redundant to vang-1. Lat-1 is known to regulate spindle orientation in the early embryo (Langenhan et al., 2009). It remains to be seen whether and how the PCP-like scheme underlying spindle regulation is related to the PCP-ortho scheme underlying sequential rosettes.
MATERIALS AND METHODS
C. elegans strains and genetics
C. elegans were cultured at room temperature on nematode growth media (NGM) plates with OP50 bacteria as previously described (Brenner, 1974). N2 Bristol was used as the wild-type strain. See Table S2 for all strains used in this study. Genetic crosses were performed according to standard protocol (Fay, 2006). RNAi experiments were performed according to standard feeding protocol using RNAi clones from commercially available RNAi libraries (Du et al., 2015).
Plasmid construction of Pmig-1:MIG-1:GFP fusion
A 3.9 kb mig-1 promoter (Mizumoto and Shen, 2013) and mig-1 isoform a cDNA was cloned into BamHI/KpnI sites pPD95.75 by Gibson Assembly. All amplified fragments were verified by sequencing. Primer sequences: 5′-AAGCTTGCATGCCTGCAGGTCGACTCTAGAGGATCC, 3′-GGTACCGGTAGAAAAAATGAGTA.
Embryonic imaging
Live imaging was performed as previously described (Bao and Murray, 2011). In brief, about five gravid adult worms were picked to M9 buffer drop (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 ml 1 M MgSO4 per l of H2O) to wash away OP50 bacteria. The worms were then transferred to a second drop of ∼20 µl M9 buffer and cut in the middle to release embryos. We transferred 10-20 embryos at the two- to four-cell stage to 1.5 µl M9 buffer mixed with 20 µm polystyrene beads on a 24×50 mm coverslip and sealed with Vaseline under an 18×18 mm smaller coverslip. The following setup of 3D time-lapse imaging was dependent on the purpose of experiment.
For examining the movement phenotype of Cpaaa with nuclei and plasma membrane labeling, ∼20 embryos were transferred on the coverslip and arranged into two to four embryos per microscopy stage. Images were acquired on a Yokogawa CSU-X1 spinning-disk confocal microscope and Zeiss Observer Z1 microscope with Zeiss PlanApo 40×/1.3 oil objective. Embryos were imaged using 30 focal planes spaced 1 µm apart at 20°C at 60 s intervals for 4 h.
For examining the protein localization with GFP during Cpaaa movement, ∼10 embryos were transferred on the coverslip and arranged into one or two embryos per microscopy stage. Images were acquired on a spinning-disk confocal microscope comprising a Zeiss Axio Observer Z1 frame with an Olympus UPLSAPO 60× objective, a Yokogawa CSU-X1 spinning-disk unit, and two Hamamatsu C9100-13 EM-CCD cameras. Embryos were imaged using 30 focal planes spaced 1 µm apart at 20°C at 75 s intervals for 4 h.
Image analysis
Lineage analysis
Automated cell lineage tracing was implemented and curated in StarryNite and AceTree pipeline (Bao et al., 2006; Boyle et al., 2006). For embryos used for screening of cell migration, the complete lineage was curated until the 350-cell stage. For embryos used for examining movement phenotype of Cpaaa and protein localization around Cpaaa rosettes, the lineages of Cpaaa and seven ABarp cells were curated until the cell division of Cpaaa.
Intensity measurement
The intensity measurement of GFP signal was performed in Fiji software as previously described (Shah et al., 2017a). For a given cell-cell edge, a 3 pixel-wide line segment tool in Fiji was manually applied on the edge to measure the intensity. The mean per-pixel intensity was defined as the signal intensity on the edge after background subtraction, which was the median of a 75×75 pixel square in the cell cytoplasm. The mean of the signal intensity for each set of edges was calculated in each embryo. Two-tailed unpaired Student's t-test was applied to test the fold difference between edges of interest and control edges in embryos (N≥3), i.e., vertical versus horizontal, midline versus lateral, vertical versus lateral.
Computational screen of large neighborhood change
Embryonic stages based on division timings
Most cell divisions are synchronous within each founder lineage of C. elegans embryos and are progressively prolonged (Bao et al., 2008). The cell size and lifetime have a dramatic difference from one-cell to 350-cells (end of gastrulation), thus it is necessary to divide this period into stages. To this end, we searched for timepoints when massive parallel cell divisions are just finished to mark the boundary between stages (Fig. 1B). First, local maximum on the increase of cell number between ti and ti-1 (Di) was defined by Di>5 and Di>Dj, for j ∀ [i−10, i+10]. Second, the boundary between stages was defined as the first timepoint after Di with D=0. Four boundaries between five stages were found. The first boundary corresponds to the start of gastrulation (26-cell stage), indicating that the definition of boundaries is rational. We found that cells in stages III and IV have significantly larger migration distance than cells in phase I (Fig. 1C; adjusted P-value=3×10−7 and 4×10−5 by two-tailed Mann–Whitney U-test, respectively). Thus, we chose cells in stages III and IV to search for candidate cells of large neighborhood change.
Large neighborhood change
To search for large neighborhood change in candidate cells, we considered neighborhood change to exclude the effect of whole-embryo rotation. The neighborhood change for each cell is defined as the average of distances to initial neighbors when the cell is dividing and distances to final neighbors when the cell is born (Fig. 1A). Cells with neighborhood change above a threshold (mean+standard deviation) were selected and examined in images of embryos with fluorescently labeled nuclei and plasma membrane for potential T1 process, rosette and sequential rosettes.
Statistics
All statistical analyses were performed in R v3.6.3 (https://www.r-project.org/). The statistical tests used are indicated and described in the figure legends. Data distribution was assumed to be normal but this was not formally tested. No statistical method was used to predetermine sample size but our sample sizes are similar to those reported in previous publications. The experiments were not randomized. The investigators were aware of allocation during experiments and outcome assessment. The exclusion of embryos for scoring phenotypes are described in the legend of Table 1.
Supplementary Material
Acknowledgements
We thank Dr Jennifer Zallen for discussions. We thank Dr Anthony Santella for language editing.
Footnotes
Author contributions
Conceptualization: Y.X., Z.B.; Methodology: Y.X., Y.C., A.T.C.; Formal analysis: Y.X., Y.C., A.T.C.; Writing - original draft: Y.X., Z.B.; Writing - review & editing: Y.X., Z.B.; Supervision: Z.B.
Funding
This work is supported by National Institutes of Health grant R01GM097576 to Z.B. and a National Institutes of Health center grant to Memorial Sloan Kettering Cancer Center (P30CA008748). Deposited in PMC for release after 12 months.
Data availability
All relevant data can be found within the article and its supplementary information.
References
- Ambegaonkar, A. A., Pan, G., Mani, M., Feng, Y. and Irvine, K. D. (2012). Propagation of dachsous-fat planar cell polarity. Curr. Biol. 22, 1302-1308. 10.1016/j.cub.2012.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amonlirdviman, K., Khare, N. A., Tree, D. R. P., Chen, W.-S., Axelrod, J. D. and Tomlin, C. J. (2005). Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science (80-.) 307, 423-426. 10.1126/science.1105471 [DOI] [PubMed] [Google Scholar]
- Asan, A., Raiders, S. A. and Priess, J. R. (2016). Morphogenesis of the C. elegans intestine involves axon guidance genes. PLoS Genet. 12, e1005950. 10.1371/journal.pgen.1005950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod, J. D. (2001). Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15, 1182-1187. 10.1101/gad.890501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, Z. and Murray, J. I. (2011). Mounting Caenorhabditis elegans embryos for live imaging of embryogenesis. Cold Spring Harb. Protoc. 2011, pdb.prot065599. 10.1101/pdb.prot065599 [DOI] [PubMed] [Google Scholar]
- Bao, Z., Murray, J. I., Boyle, T., Ooi, S. L., Sandel, M. J. and Waterston, R. H. (2006). Automated cell lineage tracing in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 103, 2707-2712. 10.1073/pnas.0511111103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, Z., Zhao, Z., Boyle, T. J., Murray, J. I. and Waterston, R. H. (2008). Control of cell cycle timing during C. elegans embryogenesis. Dev. Biol. 318, 65-72. 10.1016/j.ydbio.2008.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadskaya, Y. Y., Wallace, A., Nguyen, J., Mohler, W. A. and Soto, M. C. (2012). UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph polarize F-actin during embryonic morphogenesis by regulating the WAVE/SCAR actin nucleation complex. PLoS Genet. 8, e1002863. 10.1371/journal.pgen.1002863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, M. and Schnabel, R. (2006). A posterior centre establishes and maintains polarity of the Caenorhabditis elegans embryo by a Wnt-dependent relay mechanism. PLoS Biol. 4, 2262-2273. 10.1371/journal.pbio.0040396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship, J. T., Backovic, S. T., Sanny, J. S. P., Weitz, O. and Zallen, J. A. (2006). Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev. Cell 11, 459-470. 10.1016/j.devcel.2006.09.007 [DOI] [PubMed] [Google Scholar]
- Boyle, T. J., Bao, Z., Murray, J. I., Araya, C. L. and Waterston, R. H. (2006). AceTree: a tool for visual analysis of Caenorhabditis elegans embryogenesis. BMC Bioinformatics 7, 275. 10.1186/1471-2105-7-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. 10.1093/genetics/77.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle, A., Thomas, C. and Strutt, D. (2012). Planar polarity specification through asymmetric subcellular localization of fat and dachsous. Curr. Biol. 22, 907. 10.1016/j.cub.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, M. T. and Wallingford, J. B. (2017). Planar cell polarity in development and disease. Nat. Rev. Mol. Cell Biol. 18, 375-388. 10.1038/nrm.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Sang, I. D., George, S. E., Ding, M., Moseley, S. L., Lynch, A. S. and Chisholm, A. D. (1999). The ephrin VAB-2/EFN-1 functions in neuronal signaling to regulate epidermal morphogenesis in C. elegans. Cell 99, 781-790. 10.1016/S0092-8674(00)81675-X [DOI] [PubMed] [Google Scholar]
- Cravo, J. and van den Heuvel, S. (2020). Tissue polarity and PCP protein function: C. elegans as an emerging model. Curr. Opin. Cell Biol. 62, 159-167. 10.1016/j.ceb.2019.11.004 [DOI] [PubMed] [Google Scholar]
- Devenport, D. (2014). The cell biology of planar cell polarity. J. Cell Biol. 207, 171-180. 10.1083/jcb.201408039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Z., Santella, A., He, F., Shah, P. K., Kamikawa, Y. and Bao, Z. (2015). The regulatory landscape of lineage differentiation in a metazoan embryo. Dev. Cell 34, 592-607. 10.1016/j.devcel.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay, D. (2006). Genetic mapping and manipulation: chapter 1 – Introduction and basics. WormBook (ed. The C. elegans Research Community). 10.1895/wormbook.1.90.1 [DOI]
- Ghenea, S., Boudreau, J. R., Lague, N. P. and Chin-Sang, I. D. (2005). The VAB-1 Eph receptor tyrosine kinase and SAX-3/Robo neuronal receptors function together during C. elegans embryonic morphogenesis. Development 132, 3679-3690. 10.1242/dev.01947 [DOI] [PubMed] [Google Scholar]
- Harding, M. J., McGraw, H. F. and Nechiporuk, A. (2014). The roles and regulation of multicellular rosette structures during morphogenesis. Development 141, 2549-2558. 10.1242/dev.101444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell, J. R. and Goldstein, B. (2011). Internalization of multiple cells during C. elegans gastrulation depends on common cytoskeletal mechanisms but different cell polarity and cell fate regulators. Dev. Biol. 350, 1-12. 10.1016/j.ydbio.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppert, J. K., Pani, A. M., Roberts, A. M., Dickinson, D. J. and Goldstein, B. (2018). A CRISPR tagging-based screen reveals localized players in wnt-directed asymmetric cell division. Genetics 208, 1147-1164. 10.1534/genetics.117.300487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner, R. J. and Wallingford, J. B. (2018). Coming to consensus: a unifying model emerges for convergent extension. Dev. Cell 46, 389-396. 10.1016/j.devcel.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koca, Y., Collu, G. M. and Mlodzik, M. (2022). Wnt-frizzled planar cell polarity signaling in the regulation of cell motility. Curr. Top. Dev. Biol. 150, 255-297. 10.1016/bs.ctdb.2022.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenhan, T., Prömel, S., Mestek, L., Esmaeili, B., Waller-Evans, H., Hennig, C., Kohara, Y., Avery, L., Vakonakis, I., Schnabel, R.et al. (2009). Latrophilin signaling links anterior-posterior tissue polarity and oriented cell divisions in the C. elegans embryo. Dev. Cell 17, 494-504. 10.1016/j.devcel.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Roszko, I., Sepich, D. S., Ni, M., Hamm, H. E., Marlow, F. L. and Solnica-Krezel, L. (2013). Gpr125 modulates Dishevelled distribution and planar cell polarity signaling. Development 140, 3028-3039. 10.1242/dev.094839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienkamp, S. S., Liu, K., Karner, C. M., Carroll, T. J., Ronneberger, O., Wallingford, J. B. and Walz, G. (2012). Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat. Genet. 44, 1382-1387. 10.1038/ng.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F., Sepich, D. S., Chen, S., Topczewski, J., Yin, C., Solnica-Krezel, L. and Hamm, H. (2005). Essential roles of Gα12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J. Cell Biol. 169, 777-787. 10.1083/jcb.200501104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto, K. and Shen, K. (2013). Two Wnts instruct topographic synaptic innervation in C. elegans. Cell Rep. 5, 389-396. 10.1016/j.celrep.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. L., Du, Z. and Bao, Z. (2013). Systematic quantification of developmental phenotypes at single-cell resolution during embryogenesis. Development 140, 3266-3274. 10.1242/dev.096040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Salinas, A. L., Avila-Zozaya, M., Ugalde-Silva, P., Hernández-Guzmán, D. A., Missirlis, F. and Boucard, A. A. (2019). Latrophilins: A neuro-centric view of an evolutionary conserved adhesion g protein-coupled receptor subfamily. Front. Neurosci. 13, 700. 10.3389/fnins.2019.00700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer, J. S., Zhu, Q., Huynh, C., Sivaramakrishnan, P., Preston, E., Dueck, H., Stefanik, D., Tan, K., Trapnell, C., Kim, J.et al. (2019). A lineage-resolved molecular atlas of C. elegans embryogenesis at single-cell resolution. Science 365, eaax1971. 10.1101/565549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, C.-L., Howell, J. E., Clark, S. G., Hilliard, M., Cordes, S., Bargmann, C. I. and Garriga, G. (2006). Multiple wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev. Cell 10, 367-377. 10.1016/j.devcel.2006.02.010 [DOI] [PubMed] [Google Scholar]
- Paré, A. C., Vichas, A., Fincher, C. T., Mirman, Z., Farrell, D. L., Mainieri, A. and Zallen, J. A. (2014). A positional Toll receptor code directs convergent extension in Drosophila. Nature 515, 523-527. 10.1038/nature13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, F. D., Tenlen, J. R. and Priess, J. R. (2004). C. elegans MOM-5/Frizzled functions in MOM-2/Wnt-independent cell polarity and is localized asymmetrically prior to cell division. Curr. Biol. 14, 2252-2258. 10.1016/j.cub.2004.12.019 [DOI] [PubMed] [Google Scholar]
- Pohl, C. and Bao, Z. (2010). Chiral forces organize left-right patterning in C. elegans by uncoupling midline and anteroposterior axis. Dev. Cell 19, 402-412. 10.1016/j.devcel.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl, C., Tiongson, M., Moore, J. L., Santella, A. and Bao, Z. (2012). Actomyosin-based self-organization of cell internalization during C. elegans gastrulation. BMC Biol. 10, 94. 10.1186/1741-7007-10-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, P. K., Tanner, M. R., Kovacevic, I., Rankin, A., Marshall, T. E., Noblett, N., Tran, N. N., Roenspies, T., Hung, J., Chen, Z.et al. (2017a). PCP and SAX-3/Robo pathways cooperate to regulate convergent extension-based nerve cord assembly in C. elegans. Dev. Cell 41, 195-203.e3. 10.1016/j.devcel.2017.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, P. K., Santella, A., Jacobo, A., Siletti, K., Hudspeth, A. J. and Bao, Z. (2017b). An in toto approach to dissecting cellular interactions in complex tissues. Dev. Cell 43, 530-540.e4. 10.1016/j.devcel.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto, M. C. (2017). Sequential rosettes drive C. elegans ventral nerve cord assembly. Dev. Cell 41, 121. 10.1016/j.devcel.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J. E., Schierenberg, E., White, J. G. and Thomson, J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119. 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- Walck-Shannon, E., Lucas, B., Chin-Sang, I., Reiner, D., Kumfer, K., Cochran, H., Bothfeld, W. and Hardin, J. (2016). CDC-42 orients cell migration during epithelial intercalation in the Caenorhabditis elegans epidermis. PLoS Genet. 12, e1006415. 10.1371/journal.pgen.1006415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Xu, Y., Wang, D., Yang, J. and Bao, Z. (2022). Hierarchical deep reinforcement learning reveals a modular mechanism of cell movement. Nat. Mach. Intell. 4, 73-83. 10.1038/s42256-021-00431-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M. L. K. and Solnica-Krezel, L. (2020). Cellular and molecular mechanisms of convergence and extension in zebrafish. Curr. Top. Dev. Biol. 136, 377-407. 10.1016/bs.ctdb.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M., Yen, W., Lu, X. and Sutherland, A. (2014). Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Dev. Cell 29, 34-46. 10.1016/j.devcel.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.