Abstract
Antibody–drug conjugates (ADCs) are a rapidly emerging therapeutic platform. The chemical linker between the antibody and the drug payload plays an essential role in the efficacy and tolerability of these agents. New methods that quantitatively assess the cleavage efficiency in complex tissue settings could provide valuable insights into the ADC design process. Here we report the development of a near-infrared (NIR) optical imaging approach that measures the site and extent of linker cleavage in mouse models. This approach is enabled by a superior variant of our recently devised cyanine carbamate (CyBam) platform. We identify a novel tertiary amine-containing norcyanine, the product of CyBam cleavage, that exhibits a dramatically increased cellular signal due to an improved cellular permeability and lysosomal accumulation. The resulting cyanine lysosome-targeting carbamates (CyLBams) are ~50× brighter in cells, and we find this strategy is essential for high-contrast in vivo targeted imaging. Finally, we compare a panel of several common ADC linkers across two antibodies and tumor models. These studies indicate that cathepsin-cleavable linkers provide dramatically higher tumor activation relative to hindered or nonhindered disulfides, an observation that is only apparent with in vivo imaging. This strategy enables quantitative comparisons of cleavable linker chemistries in complex tissue settings with implications across the drug delivery landscape.
Graphical Abstract

INTRODUCTION
Antibody–drug conjugates (ADCs) combine the specificity of monoclonal antibodies (mAbs) and the potency of small-molecule therapeutics. To date, 10 ADCs have received FDA approval and many others (>60) are in clinical trials.1–3 ADC activity generally requires lysosomal processing of a linker domain to release the active payload. Consequently, the linker component should be stable in circulation but selectively cleaved following target binding and internalization - a significant chemical challenge.4–8
To assess the state-of-the-art and to guide future linker discovery efforts, a way to quantitatively compare ADC linker chemistry in vivo would be of significant utility. ADCs are conventionally assessed by examining tumoricidal activity and toxicity profiling. While these methods are important benchmarks, they provide only indirect insights into the site and mechanism of drug release.9–11 Enzyme-linked immunosorbent assays (ELISAs) are also broadly employed but only determine the blood-pool concentration and biodistribution of the antibody component.12,13 Radiolabeling methods can provide important insights regarding mAb localization but are costly and do not directly report on the linker cleavage step.14–17
Optical imaging has the potential to provide critical insights to the ADC design and optimization process. Prior efforts using stimuli-responsive fluorophores with conventional visible wavelengths have quantified payload processing and internalization kinetics in cellular imaging experiments.18–20 However, these probes are not suitable for applications in tissue due to the poor penetration depth of wavelengths in the visible region. Complementing these efforts, approaches using always-ON near-infrared (NIR) probes have provided insights into tumor and off-target uptake.21–25 Fluorogenic turn-ON probes that use NIR wavelengths (~700–900 nm) have significant potential to provide insight into the dynamics and localization of biological phenomena in complex tissue settings. We recently developed the first class of NIR fluorogenic probes based on the heptamethine cyanine core.26 These fluorogenic cyanine carbamates (CyBams, Figure 1A) exhibit exceptional turn-ON ratios, and untargeted variants enabled in vivo imaging in a metastatic tumor model. In these studies, we hypothesized that mAb-conjugated variants could provide a real-time quantitative means to determine the site and extent of ADC linker cleavage in complex model organisms.
Figure 1.

(A) Evolution and (B) overview of CyLBam chemistry.
Here we detail the development of the first activatable mAb-targeted probes with absorbance and emission maxima beyond 700 nm. To obtain a sufficient signal for in vivo imaging, we optimized the cellular uptake and retention of the released fluorescent product of linker cleavage, the pH-sensitive norcyanine. These efforts reveal that the installation of a basic amine into the probe dramatically improves the cellular photon output, likely due to enhanced lysosomal uptake and retention. Converting the optimized norcyanine into the corresponding cyanine lysosome-targeting carbamate (CyLBam, Figure 1A,B) dramatically improves the in vitro signal and is essential for high-contrast in vivo optical imaging of mAb conjugates. Finally, we test a panel of commonly used ADC linkers in two tumor models. We find that cathepsin-cleavable linkers outperform reductively cleaved disulfide linkers with dramatic differences in tumor uptake, a distinction that is only apparent with in vivo imaging. Broadly, this approach provides a general means to assess cleavable linker efficiency and specificity, with applications from the cellular to organismal scales.
RESULTS AND DISCUSSION
Optimization of the Norcyanine Signal.
Prior to pursuing in vivo imaging of mAb conjugates, we set out to improve the cellular signal of the product of CyBam activation, the pH-sensitive norcyanine. While the previously reported sulfonated norcyanine, Sulfo-NorCy7 (Figure 2A), can provide high-contrast imaging, relatively high concentrations (20–40 μM) are required for a sufficient in vitro signal.26–29 We anticipated that this requirement might be problematic for mAb-targeted imaging because probe concentrations are intrinsically limited by antigen levels. Of note, microscopy studies indicate that the cellular signal of Sulfo-NorCy7 is predominately from the lysosome. The cellular signal is likely only due to the lysosomal fraction, given the observed pKa between 4 and 5.26 We therefore assume poor lysosomal accumulation is responsible for the modest cellular fluorescence. We hypothesized that by increasing the cellular permeability and perhaps introducing a lysosomal-targeting element, we could improve the fluorescent output.
Figure 2.
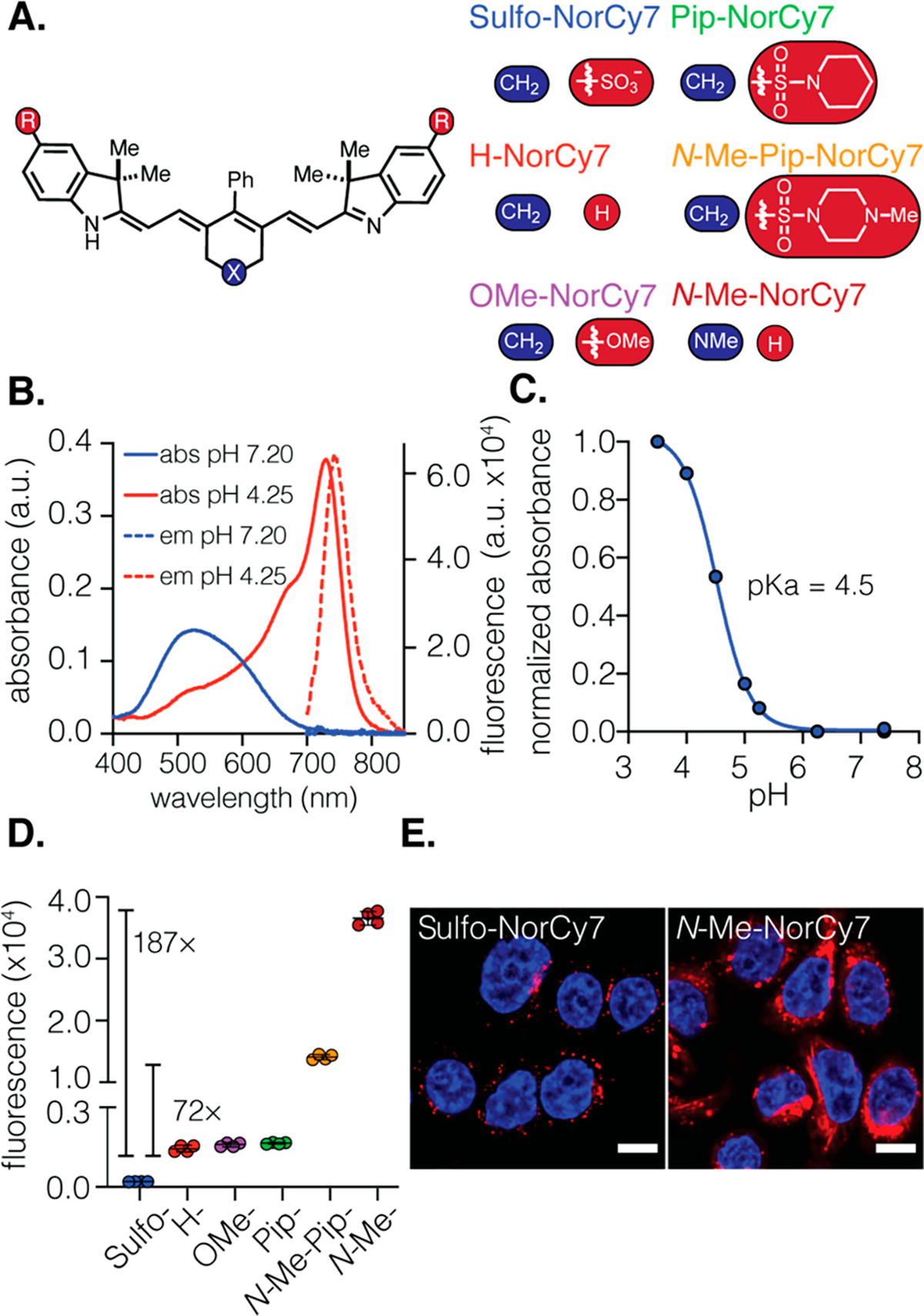
(A) Structures of norcyanines Sulfo-, H-, OMe-, Pip-, N-Me-Pip-, and N-Me-NorCy7 (X, left, blue; R, right, red). (B) Absorbance and fluorescence spectra of N-Me-NorCy7 (5 μM) in PBS pH 7.20 and acetate buffer pH 4.25 (ex. 690 nm). (C) Determination of pKa (10 μM) in buffers ranging from pH 3.5 to pH 7.4. (D) Flow cytometry quantification of the in vitro uptake of norcyanine heptamethine cyanines (5 μM) in MDA-MB-468 after 1 h of incubation. The geometric mean fluorescent intensity (± SD) of the fluorescent signal in the cells is shown (n = 4 independent experiments; ~10 000 cells counted). (E) Confocal fluorescent images (63X) of N-Me-NorCy7 and Sulfo-NorCy7 (10 μM) after 6 h of incubation with MDA-MB-468. The fluorescent signals from the probe and nucleus (Hoechst) are pseudocolored red and blue, respectively. Scale bar is 10 μm.
To approach this problem, we designed and synthesized a small panel of chemically diverse norcyanines. These compounds contained a C4′-phenyl-substituent appended to the polymethine chromophore, which we and others have found improves the synthetic accessibility, photostability, and serum stability.30–35 To test the question of cell permeability alone, we included two hydrophobic derivatives, OMe-NorCy7 and H-Nor-Cy7 (Figure 2A). Commonly used lysotracker probes contain tertiary amines, suggesting this functional group can promote lysosomal targeting.36–38 To test if this approach might apply to norcyanines, we prepared indolenine-substituted sulfonamide derivatives modified with both the hydrophobic piperidine (Pip-NorCy7) and the tertiary amine-containing N-Me piperazine (N-Me-Pip-NorCy7) substituents. We also prepared another “lysotracker-like” derivative, N-Me-NorCy7, in which the central ring system contains an N-methyl substituent. These novel norcyanines were synthesized in three or four steps, as described in the Supporting Information.
With access to this panel of probes, we investigated their photophysical properties and cellular uptake. All six probes exhibit absorbance and emission maxima above 700 nm, similar extinction coefficients and quantum yields, and pKa values between 4.4 and 5.8 (Figure 2B and C for N-Me-NorCy7, Table 1, and Figure S1). We first established that the NorCy7 series had a minimal toxicity in MDA-MB-468 and MCF-7 cells (Figure S2). Next, we quantified signal in MDA-MB-468 cells at 1, 3, and 6 h time points with flow cytometry using the APC-Cy7 channel. At all three time points, the uptake of the six norcyanines follows the rank order N-Me-NorCy7 > N-Me-Pip-NorCy7 ≫ Pip-NorCy7, OMe-NorCy7, H-Nor-Cy7 ≫ Sulfo-NorCy7 (Figures 2D, S3, and S31–33). To our delight, N-Me-NorCy7 and N-Me-Pip-NorCy7 exhibited 187- and 72-fold higher uptake, respectively, in MDA-MB-468 cells after only 1 h incubation compared to the previously used Sulfo-NorCy7. The fluorescent signal remained constant at the 1, 3, and 6 h time points, suggesting efficient uptake and high retention of tertiary amine-substituted norcyanines (N-Me-NorCy7 and N-Me-Pip-NorCy7). In contrast, the signal from Sulfo-NorCy7 increased steadily overtime and was around threefold higher after 6 h relative to the level at 1 h (Figure S3). Using confocal microscopy, we confirmed that the subcellular localization of N-Me-NorCy7 is lysosomal, with a dramatically higher signal than that of Sulfo-NorCy7 (Figures 2E, S4, and S5). The relatively modest signal of the three hydrophobic derivatives, namely Pip-NorCy7, OMe-NorCy7, and H-NorCy7, indicates that the lysotracker-mimicking tertiary amine is essential for fluorescent signal enhancement. Overall, these data indicate that the incorporation of a single basic amine dramatically enhances the cellular uptake and lysosomal localization of norcyanines. The enhanced cellular signal and synthetic accessibility led us to choose N-Me-NorCy7 to compare to the previously described Sulfo-NorCy7 in the generation of activable mAb conjugates.
Table 1.
Summary of Photophysical Properties of the NorCy7 Series of Compounds
| λmax,abs/λmax,em (nm) |
εmax (M−1 cm−1) |
pKa | ΦFd(%) | |
|---|---|---|---|---|
| Sulfo-NorCy7a | 755/775 | 73 500 | 5.2 | 8.9 |
| H-NorCy7b | 750/770 | 63 900 | 5.8 | 9.1 |
| OMe-NorCy7b | 775/790 | 56 800 | 5.5 | 1.6 |
| Pip-NorCy7c | 760/780 | 17 200 | 4.4 | 8.1 |
| N-Me-Pip-NorCy7a | 760/775 | 45 000 | 4.5 | 10 |
| N-Me-NorCy7a | 730/750 | 57 600 | 4.6 | 12 |
Acetate buffer pH 4.5.
MeOH/acetate buffer pH 4.5 (2:1).
MeOH/acetate buffer pH 3.75 (2:1).
MeOH + 0.1% formic acid for all compounds.
mAb-Targeted Imaging.
We then assessed the impact of norcyanine modification on the signal of mAb conjugates. N-Me-NorCy7 was converted to the cyanine lysosome-targeting carbamate (CyLBam) variant by attaching a cathepsin-cleavable dipeptide (valine–citrulline, VC) and a noncleavable (NC) glutaric anhydride linker substituted with a lysine-reactive NHS ester (see the SI for synthetic details). As a comparison, Sulfo-NorCy7 was converted to the corresponding CyBam molecules. The four probes were conjugated to the FDA-approved anti-epidermal growth factor receptor (EGFR) antibody panitumumab (Pan) at pH 7.4 (degree of labeling (DOL) ~ 4; Tables S1 and S28). The resulting conjugates were purified by both spin column and dialysis to ensure that no free small molecule remained. Of note, prior work from our lab and others has shown that similar conjugates maintain the in vitro and in vivo targeting properties of the parent mAb.22,5,21,39–41
We first compared the series of Pan probes in cellular assays. The four conjugates, namely Pan-VC-CyLBam, Pan-NC-CyLBam, Pan-VC-CyBam, and Pan-NC-CyBam, (Figure 3A) were tested in MDA-MB-468 (EGFR+) and MCF-7 (EGFR−) cells.42,43 The fluorescent signal emitted from Pan-VC-CyLBam was 50-fold higher than that of Pan-VC-CyBam after incubation with MDA-MB-468 cells for 24 h (Figures 3C and S34–37). Validating the role of receptor-mediated uptake, the fluorescent signal of both probes was lower in MCF-7 cells (by 7.5-fold for the CyLBam and 4.5-fold for the CyBam (Figure 3D)). As expected, the noncleavable probes Pan-NC-CyLBam and Pan-NC-CyBam did not exhibit any significant fluorescent signal in either cell line. Confocal microscopy confirmed the trends observed by flow cytometry as well as the lysosomal uptake of the released norcyanine (Figures 3B and S6–8).
Figure 3.
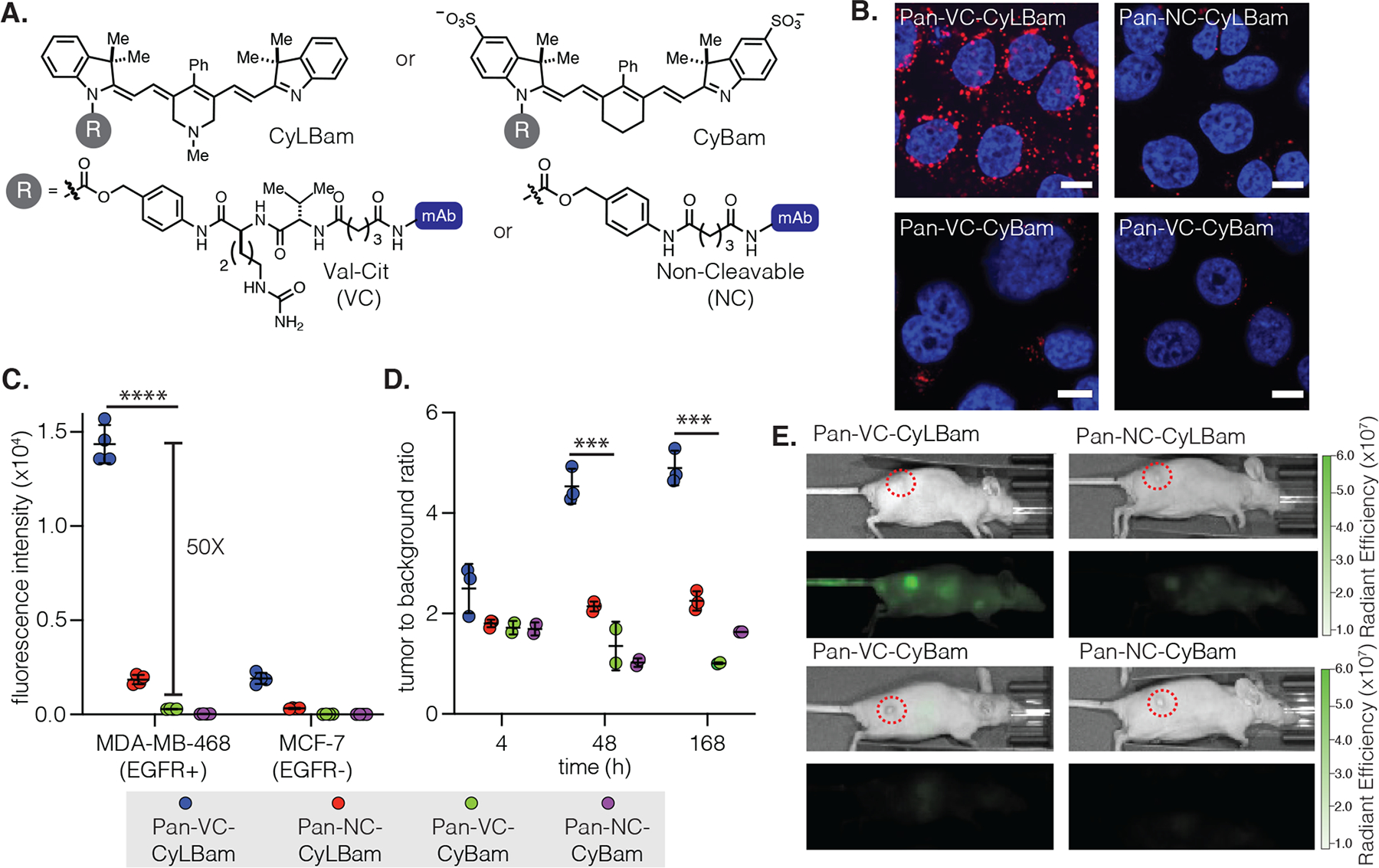
(A) Structures of CyLBam and CyBam probes and VC (val-cit) and NC (noncleavable) linkers. (B) Confocal images (63×) of Pan-VC-CyLBam, Pan-NC-CyLBam, Pan-VC-CyBam, and Pan-NC-CyBam in MDA-MB-468 (EGFR+) after 24 h of incubation. Fluorescent signals from the probe and nucleus (Hoechst) are shown in red and blue, respectively. Scale bar is 10 μm. (C) Flow Cytometry quantification of in vitro uptake in MDA-MB-468 (EGFR+) and MCF-7 (EGFR−) after 24 h of incubation. All antibody conjugates were labeled at DOL 4. The geometric mean fluorescent intensity (±SD) of fluorescent signal in the cells is shown (n = 4 independent experiments; ~10 000 cells counted). (D) Imaging of probes (200 μg; DOL 4) intravenously injected in a xenograft model of female athymic nude mice implanted with MDA-MB-468 tumors (n = 3). Tumor-to-background (TBR) ratios, the fluorescent signal of the tumor relative to an equal area in the neck, are shown at different time intervals (4, 48, and 168 h). (E) IVIS fluorescent images 48 h post-injection. Tumors are highlighted in dotted red circles. Data points are displayed as the mean ± SD. The p-values were evaluated by the Student’s t-test (***p ≤ 0.001, ****p ≤ 0.0001).
We then set out to test if the improved in vitro signal of the CyLBam conjugate would translate to high-contrast animal imaging. The conjugates (200 μg) were injected intravenously into female athymic nude mice with MDA-MB-468 xenograft tumors (25–35 mm3). The mice were imaged at 4, 24, 48, 72, and 168 h post-injection using an in vivo imaging system (IVIS) (Figures 3E and S9–14). After 24 h, a strong fluorescent signal was observed in tumors injected with Pan-VC-CyLBam, and the signal persisted over 168 h, with tumor-to-background ratios (TBRs) between 4 and 5. In contrast, the Pan-VC-CyBam group had lower TBRs (1–2) at all time points, similar to the two noncleavable probes Pan-NC-CyLBam and Pan-NC-CyBam. Significant liver signals were observed with the two probes containing the protease-cleavable linker (Pan-VC-CyLBam and Pan-VC-CyBam) at early time points (4 and 24 h), and the fluorescent signal decreased afterward (Figures S15 and S16). The selective hepatic signal of Pan-VC-CyBam and its time-dependent disappearance suggests that the released Sulfo-NorCy7 is rapidly cleared through hepatobiliary pathways. Thus, the reduced tumor signal observed with this probe likely reflects a lower tumor retention (compared to N-Me-NorCy7) and subsequent clearance. Overall, these results suggest that the improved cellular retention of N-Me-NorCy7, first observed in vitro, extends to an in vivo setting. Critically, these observations set the stage for us to evaluate a series of ADC linkers.
Quantitative Comparison of ADC Linkers.
The two most extensively studied classes of ADC linkers are disulfides, which are reductively cleaved by biological thiols, and peptide linkers, which are cleaved by lysosomal cathepsins. Significant efforts to apply disulfide linkers led to the approval and clinical testing of several agents.44 These include the first approved ADC, the acute myeloid leukemia therapy gemtuzumab ozogamicin (Mylotarg), which contains a hindered disulfide.45–47 However, some reports suggest that certain disulfides can be cleaved in circulation, leading to premature payload release and nonspecific uptake.47–50 Concurrent with these efforts, cathepsin-cleavable peptide linkers have been incorporated in four approved ADCs, including the Hodgkins lymphoma drug brentuximab vedotin (Adcetris). While these two classes of linkers have been investigated extensively, we are not aware of any reports directly comparing the relative cleavage of payloads in a solid tumor setting. To do this, we designed a small panel of probes comparing two commonly used cathepsin-cleavable peptide linkers, namely valine–citrulline Pan-VC-CyLBam (used above) and alanine–alanine, Pan-AA-CyLBam, and two disulfides, one hindered gem-dimethyl-substituted variant Pan-S,S-Me2-CyLBam and one primary disulfide Pan-S,S-CyLBam (Figure 4A and Table S2).9,51 As a control, the noncleavable probe described above Pan-NC-CyLBam, was also employed.
Figure 4.
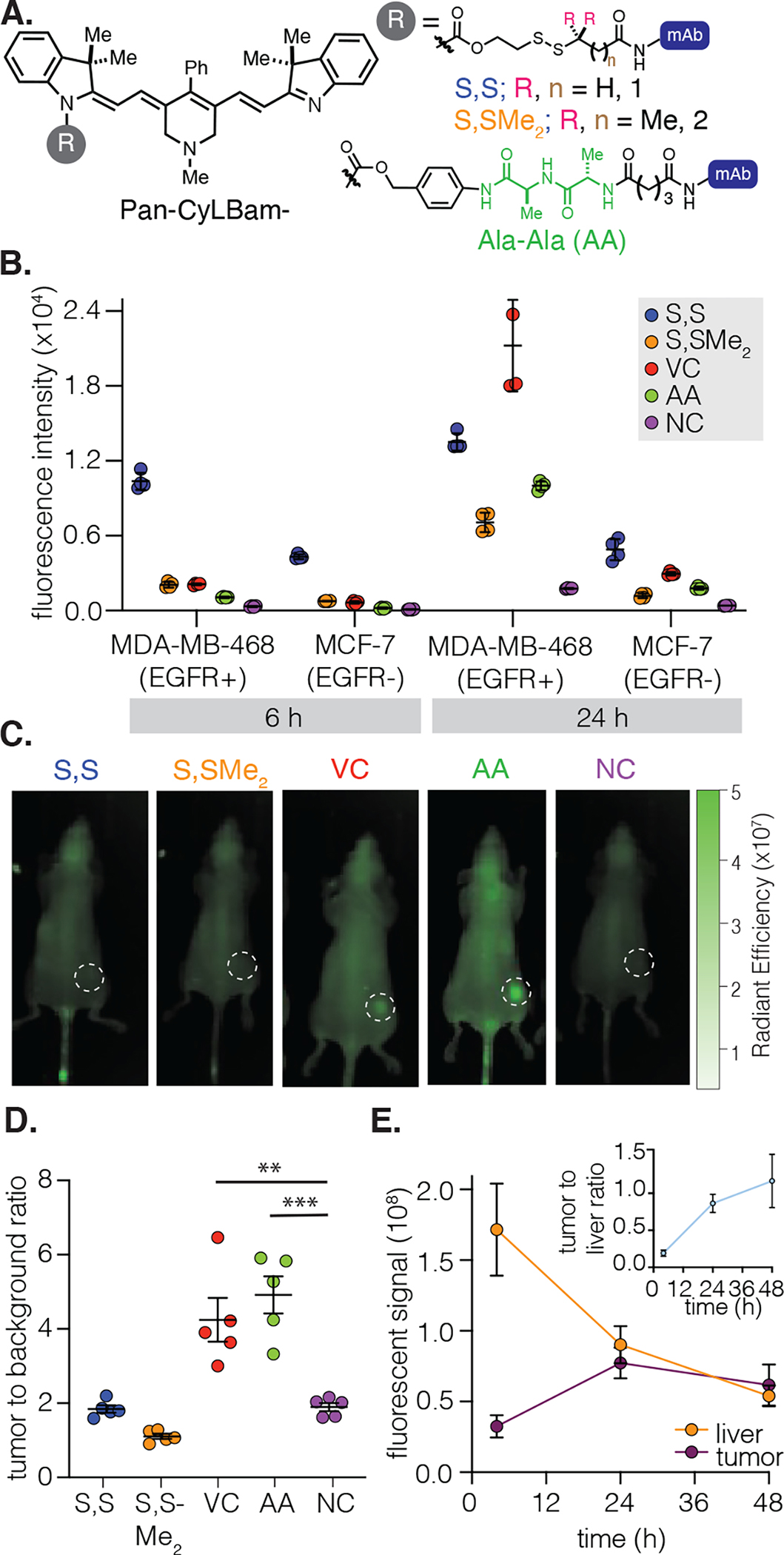
(A) Structures of Pan-AA-CyLBam, Pan-S,S-CyLBam, and Pan-S,S-Me2-CyLBam. (B) Quantification of the in vitro uptake of the five probes (50 μg; DOL 4) in MDA-MB-468 (EGFR+) and MCF-7 (EGFR−) after 24 h of incubation. The geometric mean fluorescent intensity (±SD) of the fluorescent signal in the cells is shown (n = 4 independent experiments; ~10 000 cells counted). (C) Fluorescent images following the injection of probes (100 μg; DOL 4) in female athymic nude mice (n = 5) with MDA-MB-468 tumors at the 48 h time point. Tumors are highlighted in dotted white circles. (D) Quantification of TBR 48 h post-injection. (E) Quantification of the fluorescent signal of Pan-AA-CyLBam from the liver and the tumor. The tumor-to-liver ratio (TLR) is depicted in the insert. Data points are displayed as mean ± SD. The p-values were evaluated by the Student’s t-test (**p ≤ 0.01, ***p ≤ 0.001).
We first examined the signal of the panel of conjugates in cells expressing variable levels of EGFR. The fluorescent signal from linker cleavage was quantified using flow cytometry at 6 and 24 h post-incubation (Figures 4B and S38–41). At the 6 h time point, Pan-S,S-CyLBam exhibited the highest fluorescent signal in EGFR+ MDA-MB-468 cells. The other three cleavable probes had only modest fluorescent signals after 6 h, but the signal increased substantially after 24 h. Pan-VC-CyLBam had the highest fluorescent signal among the cleavable probes after 24 h, indicating the most extensive linker cleavage. All five conjugates exhibit low levels of the probe signal at 6 and 24 h in the EGFR− cell lines (MCF-7 and MDA-MB-231, Figures 4B, S17, S42, and S43), albeit with a slightly higher signal for the Pan-S,S-CyLBam probe. Significantly, if these data were used in isolation, it would be difficult to differentiate these linkers and assess the optimal agent for further study.
With the goal of investigating differences between these linkers in an organismal context, we then harnessed the NIR spectroscopic properties of these probes for in vivo imaging. The full series of probes (100 μg dose, half the dose of the studies above), were administered to female athymic nude mice that were implanted with MDA-MB-468 tumors and imaged at regular intervals up to 48 h (Figure 4C). With the disulfide probes Pan-S,S-Me2-CyLBam and Pan-S,S-CyLBam, we did not observe significant tumor signal, and the TBR was similar to that of the noncleavable Pan-NC-CyLBam control. In contrast, strong tumor signals were observed from the cathepsin-sensitive probes Pan-VC-CyLBam and Pan-AA-CyLBam (Figures 4C and D and S19–21), with the TBR reaching as high as 4.5 after 48 h. Therefore, these studies indicate that cathepsin-cleavable linkers provide dramatically higher tumor activation relative to hindered or nonhindered disulfides. Critically, as only modest differences were observed using the cellular methods described above, this key distinction is only apparent with in vivo imaging.
In addition to tumor uptake, these data also provide significant insight into off-target cleavage pathways. As observed above, conjugates with cleavable linkers exhibit significant liver signals at early time points (4 and 24 h, Figure S18). While likely due to a combination of liver uptake/cleavage and probe clearance through hepatobiliary pathways, this approach can provide quantitative insight into off-target cleavage. With Pan-AA-CyLBam as an example, the liver signal decreases overtime but increases in the tumor (Figure 4E), which leads to an increase in the tumor-to-liver ratio (TLR) over time (Figure 4E, insert). These results are in line with prior observations that suggested significant liver linker cleavage when related cathepsin linkers were used.52,53 We also corroborated the in vivo imaging results with an ex vivo assessment, which was performed 48 h post injection. As expected, the TMR (tumor-to-muscle ratio) revealed a trend identical to that observed in vivo (Figures S22 and S23).
Finally, to test the generality of our approach, CyLBams were tested against an alternative cancer target. The cell surface protein CD276, also known as B7 homologue H3 (B7H3), is overexpressed in both tumor cells and the tumor vasculature of multiple solid tumor types.54–57 Antibodies against this promising target have been used to develop ADCs armed with either pyrrolobenzodiazepine- (PBD) or auristatin-derived (MMAE) payloads. These ADCs, which are constructed using cathepsin-cleavable linkers, exhibit potent antitumor activity.58,59 We chose to compare VC and S,S-Me2 linkers and prepared the CD276 targeting antibody m276-SL reported previously, the noncleavable NC control m276-SL conjugates, and nonbinding mAb IgG controls (DOL 4, Table S3 and Figures S29 and S30). In initial in vitro tests using a CD276+ JIMT-1 triple-negative breast cancer cell line, both cleavable m276-SL conjugates showed high levels of cleavage, and little activation was shown using the control nonbinding IgG antibody (Figures 5A, S24, S44, and S45). We then carried out a study comparing protease-cleavable m276-SL-VC-CyLBam and disulfide-cleavable m276-SL-S,S-Me2-CyLBam conjugates in JIMT-1 tumors (200–250 mm3) grown orthotopically in the mammary fat pad. As observed in the studies above, only the protease-cleavable m276-SL-VC-CyLBam conjugate exhibited a TBR greater than 5 (5.2 after 48 h), while the disulfide m276-SL-S,S-Me2-CyLBam probe exhibited a much lower tumor signal (Figures 5B and C and S25–27). These initial studies in this model serve to validate both the generality of our CyBam imaging strategy and the observation that protease-cleavable linkers outperform disulfide linkers in solid tumor models.
Figure 5.
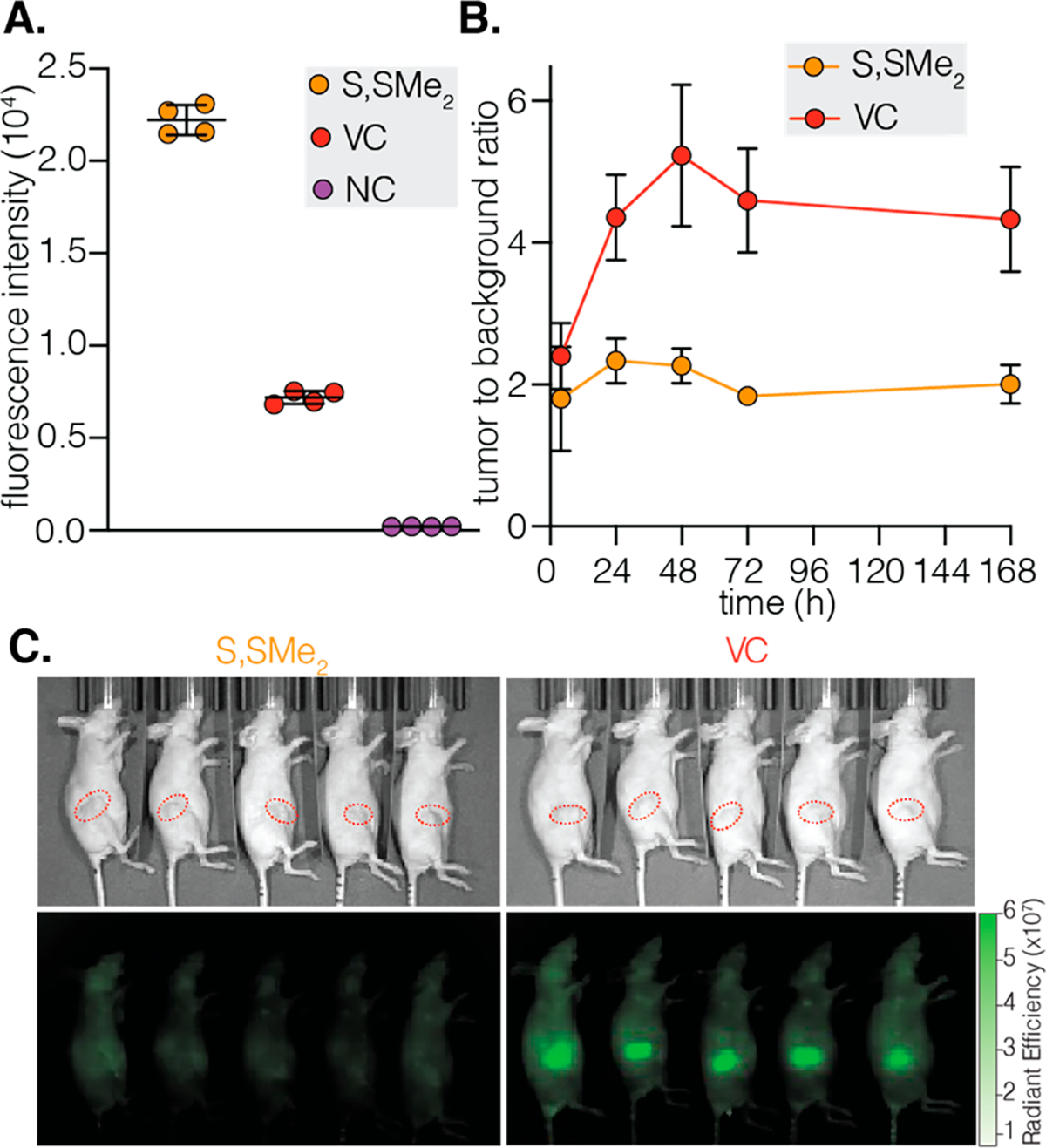
(A) Quantification of the cellular uptake of m276-SL-S,SMe2-CyLBam, m276-SL-VC-CyLBam, and m276-SL-NC-CyLBam (80 μg; DOL 4) in JIMT-1 cells following 24 h of incubation. The geometric mean fluorescent intensity (±SD) of the fluorescent signal in the cells is shown (n = 4 independent experiments; ~10 000 cells counted). (B) TBRs at different time intervals after the intravenous injection of m276-SL-S,SMe2-CyLBam and m276-SL-VC-CyLBam (100 μg; DOL ~4) in female athymic nude mice bearing orthotopic JIMT-1 tumors (n = 5). (C) Fluorescent images 72 h post-injection. Tumors are highlighted in dotted red circles.
CONCLUSIONS
The mode of action of ADCs is complex. Optimal efficacy depends on target binding, internalization, and finally lysosomal catabolism to initiate the payload release. While the linker domain plays a central role in determining the efficacy and selectivity of ADCs, it is challenging to directly assess the site and extent of linker cleavage using conventional methods.6–8 This is a critical issue because off-target cleavage of ADCs contributes significantly to toxicity.60 The optical imaging approach detailed here provides a general means to analyze linker chemistry across cellular, tissue, and body-wide scales. In this work, we identify probes suitable for mAb-targeted activatable imaging. While our previously reported sulfonated CyBams were not suitable for this application, the installation of a tertiary amine into the norcyanine framework dramatically improves cellular and in vivo signals, likely due to enhanced lysosomal uptake and retention. It is likely that these “lysotracker-like” norcyanines and the resulting CyLBam probes will have applications in other settings, including their use as activity-based sensing agents.61,62
In applying this approach for ADC applications, we assume that the fluorescent probe can serve as a useful surrogate for the payload component. Here we have shown that quantitative comparisons of linker chemistries can be obtained by varying the linker component. In addition to linker chemistry, the properties of the payload molecule (e.g., charge and hydrophobicity) are also important aspects of tumor targeting.63–66 Critically, while the norcyanine probe is a hydrophobic sp2-rich small molecule, so too are many ADC payloads.67–70 Further variations of the cyanine component could provide additional insights into the role of payload properties in tumor and normal tissue distribution, something our group has examined previously in the context of always-ON fluorophore conjugates.41,71–74
Going forward, we anticipate that CyLBam imaging could provide insights that directly inform the development of ADCs. These studies have suggested significant differences between protease-cleavable dipeptides and disulfides in terms of tumor cleavage, which is likely due to instability of these disulfides in circulation. Prior studies looking at various cysteine mutants have found the site of mAb labeling can have dramatic effects on the disulfide stability.75 This approach is well positioned to investigate these and related homogeneous labeling strategies. We also anticipate this strategy can be readily extended to analyze the impact of mAb engineering strategies.76–78 As altering the molecular weight and composition of the protein component can dramatically alter the clearance pathway, it is likely that linker cleavage dynamics will be impacted. We also anticipate applications in investigating novel linker cleavage strategies, such as those that rely on extracellular proteases. Finally, we note that this strategy can readily be translated to other targeted drug delivery strategies that rely on cleavable linkers. Efforts toward these goals, and to translate lessons from these imaging efforts into novel therapeutics, are underway.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH), NCI-CCR. It was also supported by an NCI CCR FLEX Program Synergy Award (to M.S. and B.S.C.). We also acknowledge support from the Office of the Assistant Secretary of Defense for Health Affairs through a Congressionally Directed Medical Research Program (CDRMP) Breast Cancer Research Program Grant under award no. 81WXH21-1-0109. We acknowledge Dr. James A. Kelley (National Cancer Institute) for providing the high-resolution mass spectrometry analysis. We thank Dr. Gary T. Pauly (National Cancer Institute) for assisting with LC/MS and HPLC purification. The Biophysics Resource, CCR is acknowledged for use of instrumentation. We would also like to thank Dr. Valentin Magidson, the NCI Optical Microscopy laboratory, and Dr. Jeff Carrell (CCR, Frederick Flow Cytometry Core Laboratory) for assisting with confocal microscopy and flow cytometry, respectively.
ABBREVIATIONS
- NIR
near-infrared
- ADC
antibody–drug conjugate
- mAb
monoclonal antibody
- CyBam
cyanine carbamate
- CyLBam
cyanine lysosomal carbamate
- ELISA
enzyme-linked immunosorbent assay
- PBS
phosphate buffer saline
- B7H3
B7 homologue H3
- IVIS
in vivo imaging system
- CD276
cluster of differentiation 276
- EGFR
epidermal growth factor
- abs
absorbance
- ex
excitation
- em
emission
- NA
numerical aperture
- VC
valine–citrulline
- AA
alanine–alanine
- NC
noncleavable
- Pan
panitumumab
- NHS
N-hydroxy succinimide
- DOL
degree of labeling
- TBR
tumor-to-background ratio
- TLR
liver-to-liver ratio
- TMR
tumor-to-muscle ratio
- IgG
immunoglobulin G
- MeOH
methanol
- SD
standard deviation
Footnotes
The authors declare the following competing financial interest(s): A patent describing these probes was submitted.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c10482.
Synthesis procedure and characterization of NorCy7s, CyBams, CyLBams, and key intermediate compounds; photophysical properties of NorCy7 analogs and CyLBams; and details of in vitro experiments (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.1c10482
Contributor Information
Syed Muhammad Usama, Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, Maryland 21702, United States.
Sierra C. Marker, Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, Maryland 21702, United States
Donald R. Caldwell, Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, Maryland 21702, United States
Nimit L. Patel, Small Animal Imaging Program, Frederick National Laboratory for Cancer Research, Leidos Biomedical Research Inc., Frederick, Maryland 21702, United States
Yang Feng, Tumor Angiogenesis Unit, Mouse Cancer Genetics Program (MCGP), National Cancer Institute (NCI), NIH, Frederick, Maryland 21702, United States.
Joseph D. Kalen, Small Animal Imaging Program, Frederick National Laboratory for Cancer Research, Leidos Biomedical Research Inc., Frederick, Maryland 21702, United States
Brad St. Croix, Tumor Angiogenesis Unit, Mouse Cancer Genetics Program (MCGP), National Cancer Institute (NCI), NIH, Frederick, Maryland 21702, United States.
Martin J. Schnermann, Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, Maryland 21702, United States
REFERENCES
- (1).Beck A; Goetsch L; Dumontet C; Corvaia N Strategies and Challenges for the Next Generation of Antibody-drug Conjugates. Nat. Rev. Drug Discovery 2017, 16 (5), 315–337. [DOI] [PubMed] [Google Scholar]
- (2).Chau CH; Steeg PS; Figg WD Antibody-drug Conjugates for Cancer. Lancet 2019, 394 (10200), 793–804. [DOI] [PubMed] [Google Scholar]
- (3).Nessler I; Menezes B; Thurber GM Key Metrics to Expanding the Pipeline of Successful Antibody-Drug Conjugates. Trends Pharmacol. Sci. 2021, 42 (10), 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Drago JZ; Modi S; Chandarlapaty S Unlocking the Potential of Antibody-drug Conjugates for Cancer Therapy. Nat. Rev. Clin. Oncol. 2021, 18 (6), 327–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kopp A; Thurber GM Severing Ties: Quantifying the Payload Release from Antibody Drug Conjugates. Cell Chem. Biol. 2019, 26 (12), 1631–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Su D; Zhang D Linker Design Impacts Antibody-Drug Conjugate Pharmacokinetics and Efficacy via Modulating the Stability and Payload Release Efficiency. Front. Pharmacol. 2021, 12, 687926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Dal Corso A; Pignataro L; Belvisi L; Gennari C Innovative Linker Strategies for Tumor-Targeted Drug Conjugates. Chem. - Eur. J 2019, 25 (65), 14740–14757. [DOI] [PubMed] [Google Scholar]
- (8).Tsuchikama K; An Z Antibody-drug Conjugates: Recent Advances in Conjugation and Linker Chemistries. Protein Cell 2018, 9 (1), 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lewis Phillips GD; Li G; Dugger DL; Crocker LM; Parsons KL; Mai E; Blattler WA; Lambert JM; Chari RV; Lutz RJ; Wong WL; Jacobson FS; Koeppen H; Schwall RH; Kenkare-Mitra SR; Spencer SD; Sliwkowski MX Targeting HER2-positive Breast Cancer with Trastuzumab-DM1, an Antibody-Cytotoxic Drug Conjugate. Cancer Res. 2008, 68 (22), 9280–90. [DOI] [PubMed] [Google Scholar]
- (10).Sun X; Widdison W; Mayo M; Wilhelm S; Leece B; Chari R; Singh R; Erickson H Design of Antibody-Maytansinoid Conjugates Allows for Efficient Detoxification via Liver Metabolism. Bioconjugate Chem. 2011, 22 (4), 728–35. [DOI] [PubMed] [Google Scholar]
- (11).Kovtun YV; Audette CA; Ye Y; Xie H; Ruberti MF; Phinney SJ; Leece BA; Chittenden T; Blattler WA; Goldmacher VS Antibody-drug Conjugates Designed to Eradicate Tumors with Homogeneous and Heterogeneous Expression of the Target Antigen. Cancer Res. 2006, 66 (6), 3214–21. [DOI] [PubMed] [Google Scholar]
- (12).Chang HP; Shah DK Determination of ADC Concentration by Ligand-Binding Assays. Methods Mol. Biol. 2020, 2078, 361–369. [DOI] [PubMed] [Google Scholar]
- (13).Cahuzac H; Devel L Analytical Methods for the Detection and Quantification of ADCs in Biological Matrices. Pharmaceuticals 2020, 13 (12), 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Garousi J; Orlova A; Frejd FY; Tolmachev V Imaging using radiolabelled targeted proteins: Radioimmunodetection and beyond. EJNMMI Radiopharm Chem. 2020, 5 (1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Allen KJH; Jiao R; Malo ME; Frank C; Dadachova E Biodistribution of a Radiolabeled Antibody in Mice as an Approach to Evaluating Antibody Pharmacokinetics. Pharmaceutics 2018, 10 (4), 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Fawwaz RA; Oluwole S; Srivastava S; Iga C; Wang T; Rosen JM; Hardy MA; Alderson PO The Biodistribution of Radiolabeled Antilymphocyte Monoclonal-Antibody in the Rat. Nucl. Med. Biol. 1986, 13 (1), 39–42. [DOI] [PubMed] [Google Scholar]
- (17).Westrom S; Bonsdorff TB; Abbas N; Bruland OS; Jonasdottir TJ; Maelandsmo GM; Larsen RH Evaluation of CD146 as Target for Radioimmunotherapy against Osteosarcoma. PLoS One 2016, 11 (10), e0165382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Sorkin MR; Walker JA; Kabaria SR; Torosian NP; Alabi CA Responsive Antibody Conjugates Enable Quantitative Determination of Intracellular Bond Degradation Rate. Cell Chem. Biol. 2019, 26 (12), 1643–1651. [DOI] [PubMed] [Google Scholar]
- (19).Lee BC; Chalouni C; Doll S; Nalle SC; Darwish M; Tsai SP; Kozak KR; Del-Rosario G; Yu SF; Erickson H; Vandlen R FRET Reagent Reveals the Intracellular Processing of Peptide-Linked Antibody-Drug Conjugates. Bioconjugate Chem. 2018, 29 (7), 2468–2477. [DOI] [PubMed] [Google Scholar]
- (20).Knewtson KE; Perera C; Hymel D; Gao Z; Lee MM; Peterson BR Antibody-Drug Conjugate that Exhibits Synergistic Cytotoxicity with an Endosome-Disruptive Peptide. ACS Omega 2019, 4 (7), 12955–12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cilliers C; Nessler I; Christodolu N; Thurber GM Tracking Antibody Distribution with Near-Infrared Fluorescent Dyes: Impact of Dye Structure and Degree of Labeling on Plasma Clearance. Mol. Pharmaceutics 2017, 14 (5), 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lu G; Nishio N; van den Berg NS; Martin BA; Fakurnejad S; van Keulen S; Colevas AD; Thurber GM; Rosenthal EL Co-administered antibody improves penetration of antibody-dye conjugate into human cancers with implications for antibody-drug conjugates. Nat. Commun. 2020, 11 (1), 5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Poplinger D; Bokan M; Hesin A; Thankarajan E; Tuchinsky H; Gellerman G; Patsenker L Ratiometric Fluorescence Monitoring of Antibody-Guided Drug Delivery to Cancer Cells. Bioconjugate Chem. 2021, 32, 1641. [DOI] [PubMed] [Google Scholar]
- (24).Thankarajan E; Jadhav S; Luboshits G; Gellerman G; Patsenker L Quantification of Drug Release Degree In Vivo Using Antibody-Guided, Dual-NIR-Dye Ratiometric System. Anal. Chem. 2021, 93 (23), 8265–8272. [DOI] [PubMed] [Google Scholar]
- (25).Cilliers C; Liao J; Atangcho L; Thurber GM Residualization Rates of Near-Infrared Dyes for the Rational Design of Molecular Imaging Agents. Mol. Imaging Biol. 2015, 17 (6), 757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Usama SM; Inagaki F; Kobayashi H; Schnermann MJ Norcyanine-Carbamates Are Versatile Near-Infrared Fluorogenic Probes. J. Am. Chem. Soc. 2021, 143 (15), 5674–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Keppler A; Arrivoli C; Sironi L; Ellenberg J Fluorophores for live cell imaging of AGT fusion proteins across the visible spectrum. BioTechniques 2006, 41 (2), 167. [DOI] [PubMed] [Google Scholar]
- (28).Caldwell ST; O’Byrne SN; Wilson C; Cvetko F; Murphy MP; McCarron JG; Hartley RC Photoactivated release of membrane impermeant sulfonates inside cells. Chem. Commun. (Cambridge, U. K.) 2021, 57 (32), 3917–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Yang NJ; Hinner MJ Getting across the cell membrane: an overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yang Z; Usama SM; Li F; Burgess K; Li Z A zwitterionic near-infrared dye linked TrkC targeting agent for imaging metastatic breast cancer. MedChemComm 2018, 9 (10), 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hyun H; Owens EA; Narayana L; Wada H; Gravier J; Bao K; Frangioni JV; Choi HS; Henary M Central C-C Bonding Increases Optical and Chemical Stability of NIR Fluorophores. RSC Adv. 2014, 4 (102), 58762–58768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yang C; Wang H; Yokomizo S; Hickey M; Chang H; Kang H; Fukuda T; Song MY; Lee SY; Park JW; Bao K; Choi HS ZW800-PEG: A Renal Clearable Zwitterionic Near-Infrared Fluorophore for Potential Clinical Translation. Angew. Chem., Int. Ed. 2021, 60 (25), 13847–13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lee H; Mason JC; Achilefu S Heptamethine cyanine dyes with a robust C-C bond at the central position of the chromophore. J. Org. Chem. 2006, 71 (20), 7862–5. [DOI] [PubMed] [Google Scholar]
- (34).Samanta A; Vendrell M; Das R; Chang YT Development of photostable near-infrared cyanine dyes. Chem. Commun. (Cambridge, U. K.) 2010, 46 (39), 7406–8. [DOI] [PubMed] [Google Scholar]
- (35).Usama SM; Burgess K Hows and Whys of Tumor-Seeking Dyes. Acc. Chem. Res. 2021, 54 (9), 2121–2131. [DOI] [PubMed] [Google Scholar]
- (36).Xu W; Zeng Z; Jiang JH; Chang YT; Yuan L Discerning the Chemistry in Individual Organelles with Small-Molecule Fluorescent Probes. Angew. Chem., Int. Ed. 2016, 55 (44), 13658–13699. [DOI] [PubMed] [Google Scholar]
- (37).Zhu H; Fan J; Du J; Peng X Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49 (10), 2115–2126. [DOI] [PubMed] [Google Scholar]
- (38).Choi NE; Lee JY; Park EC; Lee JH; Lee J Recent Advances in Organelle-Targeted Fluorescent Probes. Molecules 2021, 26 (1), 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Gorka AP; Nani RR; Schnermann MJ Harnessing Cyanine Reactivity for Optical Imaging and Drug Delivery. Acc. Chem. Res. 2018, 51 (12), 3226–3235. [DOI] [PubMed] [Google Scholar]
- (40).Cilliers C; Menezes B; Nessler I; Linderman J; Thurber GM Improved Tumor Penetration and Single-Cell Targeting of Antibody-Drug Conjugates Increases Anticancer Efficacy and Host Survival. Cancer Res. 2018, 78 (3), 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Usama SM; Thapaliya ER; Luciano MP; Schnermann MJ Not so innocent: Impact of fluorophore chemistry on the in vivo properties of bioconjugates. Curr. Opin. Chem. Biol. 2021, 63, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Mamot C; Drummond DC; Greiser U; Hong K; Kirpotin DB; Marks JD; Park JW Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003, 63 (12), 3154–3161. [PubMed] [Google Scholar]
- (43).Bhattacharyya S; Patel N; Wei L; Riffle LA; Kalen JD; Hill GC; Jacobs PM; Zinn KR; Rosenthal E Synthesis and biological evaluation of panitumumab-IRDye800 conjugate as a fluorescence imaging probe for EGFR-expressing cancers. MedChemComm 2014, 5 (9), 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Wang Q; Guan JK; Wan JL; Li ZF Disulfide based prodrugs for cancer therapy. RSC Adv. 2020, 10 (41), 24397–24409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Widdison WC; Wilhelm SD; Cavanagh EE; Whiteman KR; Leece BA; Kovtun Y; Goldmacher VS; Xie H; Steeves RM; Lutz RJ; Zhao R; Wang L; Blattler WA; Chari RV Semisynthetic maytansine analogues for the targeted treatment of cancer. J. Med. Chem. 2006, 49 (14), 4392–408. [DOI] [PubMed] [Google Scholar]
- (46).Liu C; Tadayoni BM; Bourret LA; Mattocks KM; Derr SM; Widdison WC; Kedersha NL; Ariniello PD; Goldmacher VS; Lambert JM; Blattler WA; Chari RV Eradication of large colon tumor xenografts by targeted delivery of maytansinoids. Proc. Natl. Acad. Sci. U. S. A. 1996, 93 (16), 8618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Kellogg BA; Garrett L; Kovtun Y; Lai KC; Leece B; Miller M; Payne G; Steeves R; Whiteman KR; Widdison W; Xie H; Singh R; Chari RV; Lambert JM; Lutz RJ Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjugate Chem. 2011, 22 (4), 717–27. [DOI] [PubMed] [Google Scholar]
- (48).Danial M; Postma A Disulfide conjugation chemistry: a mixed blessing for therapeutic drug delivery? Ther. Delivery 2017, 8 (6), 359–362. [DOI] [PubMed] [Google Scholar]
- (49).Fourie-O’Donohue A; Chu PY; Dela Cruz Chuh J; Tchelepi R; Tsai SP; Tran JC; Sawyer WS; Su D; Ng C; Xu K; Yu SF; Pillow TH; Sadowsky J; Dragovich PS; Liu Y; Kozak KR Improved translation of stability for conjugated antibodies using an in vitro whole blood assay. MAbs 2020, 12 (1), 1715705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Xie H; Audette C; Hoffee M; Lambert JM; Blattler WA Pharmacokinetics and biodistribution of the antitumor immunoconjugate, cantuzumab mertansine (huC242-DM1), and its two components in mice. J. Pharmacol. Exp. Ther. 2004, 308 (3), 1073–82. [DOI] [PubMed] [Google Scholar]
- (51).Salomon PL; Reid EE; Archer KE; Harris L; Maloney EK; Wilhelm AJ; Miller ML; Chari RVJ; Keating TA; Singh R Optimizing Lysosomal Activation of Antibody-Drug Conjugates (ADCs) by Incorporation of Novel Cleavable Dipeptide Linkers. Mol. Pharmaceutics 2019, 16 (12), 4817–4825. [DOI] [PubMed] [Google Scholar]
- (52).Dubowchik GM; Firestone RA; Padilla L; Willner D; Hofstead SJ; Mosure K; Knipe JO; Lasch SJ; Trail PA Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjugate Chem. 2002, 13 (4), 855–69. [DOI] [PubMed] [Google Scholar]
- (53).Caculitan NG; Dela Cruz Chuh J; Ma Y; Zhang D; Kozak KR; Liu Y; Pillow TH; Sadowsky J; Cheung TK; Phung Q; Haley B; Lee BC; Akita RW; Sliwkowski MX; Polson AG Cathepsin B Is Dispensable for Cellular Processing of Cathepsin B-Cleavable Antibody-Drug Conjugates. Cancer Res. 2017, 77 (24), 7027–7037. [DOI] [PubMed] [Google Scholar]
- (54).Qin X; Zhang H; Ye D; Dai B; Zhu Y; Shi G B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. OncoTargets Ther. 2013, 6, 1667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Zang X; Sullivan PS; Soslow RA; Waitz R; Reuter VE; Wilton A; Thaler HT; Arul M; Slovin SF; Wei J; Spriggs DR; Dupont J; Allison JP Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod. Pathol. 2010, 23 (8), 1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Brunner A; Hinterholzer S; Riss P; Heinze G; Brustmann H Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol. Oncol. 2012, 124 (1), 105–11. [DOI] [PubMed] [Google Scholar]
- (57).Seaman S; Stevens J; Yang MY; Logsdon D; Graff-Cherry C; St. Croix, B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell 2007, 11 (6), 539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Seaman S; Zhu Z; Saha S; Zhang XM; Yang MY; Hilton MB; Morris K; Szot C; Morris H; Swing DA; Tessarollo L; Smith SW; Degrado S; Borkin D; Jain N; Scheiermann J; Feng Y; Wang Y; Li J; Welsch D; DeCrescenzo G; Chaudhary A; Zudaire E; Klarmann KD; Keller JR; Dimitrov DS; St. Croix B Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell 2017, 31 (4), 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Kendsersky NM; Lindsay J; Kolb EA; Smith MA; Teicher BA; Erickson SW; Earley EJ; Mosse YP; Martinez D; Pogoriler J; Krytska K; Patel K; Groff D; Tsang M; Ghilu S; Wang YF; Seaman S; Feng Y; St. Croix B; Gorlick R; Kurmasheva R; Houghton PJ; Maris JM The B7-H3-Targeting Antibody-Drug Conjugate m276-SL-PBD Is Potently Effective Against Pediatric Cancer Preclinical Solid Tumor Models. Clin. Cancer Res. 2021, 27 (10), 2938–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Masters JC; Nickens DJ; Xuan D; Shazer RL; Amantea M Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Invest. New Drugs 2018, 36, 121. [DOI] [PubMed] [Google Scholar]
- (61).Bruemmer KJ; Crossley SWM; Chang CJ Activity-Based Sensing: A Synthetic Methods Approach for Selective Molecular Imaging and Beyond. Angew. Chem., Int. Ed. 2020, 59 (33), 13734–13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Scott JI; Deng Q; Vendrell M Near-Infrared Fluorescent Probes for the Detection of Cancer-Associated Proteases. ACS Chem. Biol. 2021, 16 (8), 1304–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Shao T; Chen T; Chen Y; Liu X; Chen YL; Wang Q; Zhu T; Guo M; Li H; Ju D; Wang C Construction of paclitaxel-based antibody-drug conjugates with a PEGylated linker to achieve superior therapeutic index. Signal Transduct Target Ther 2020, 5 (1), 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Buecheler JW; Winzer M; Tonillo J; Weber C; Gieseler H Impact of Payload Hydrophobicity on the Stability of Antibody-Drug Conjugates. Mol. Pharmaceutics 2018, 15 (7), 2656–2664. [DOI] [PubMed] [Google Scholar]
- (65).Singh SK; Luisi DL; Pak RH Antibody-Drug Conjugates: Design, Formulation and Physicochemical Stability. Pharm. Res. 2015, 32 (11), 3541–71. [DOI] [PubMed] [Google Scholar]
- (66).Hamblett KJ; Senter PD; Chace DF; Sun MM; Lenox J; Cerveny CG; Kissler KM; Bernhardt SX; Kopcha AK; Zabinski RF; Meyer DL; Francisco JA Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 2004, 10 (20), 7063–70. [DOI] [PubMed] [Google Scholar]
- (67).Hollander I; Kunz A; Hamann PR Selection of reaction additives used in the preparation of monomeric antibody-calicheamicin conjugates. Bioconjugate Chem. 2008, 19 (1), 358–61. [DOI] [PubMed] [Google Scholar]
- (68).Zhao RY; Wilhelm SD; Audette C; Jones G; Leece BA; Lazar AC; Goldmacher VS; Singh R; Kovtun Y; Widdison WC; Lambert JM; Chari RV Synthesis and evaluation of hydrophilic linkers for antibody-maytansinoid conjugates. J. Med. Chem. 2011, 54 (10), 3606–23. [DOI] [PubMed] [Google Scholar]
- (69).Christie RJ; Tiberghien AC; Du Q; Bezabeh B; Fleming R; Shannon A; Mao S; Breen S; Zhang J; Zhong H; Harper J; Wu H; Howard PW; Gao C Pyrrolobenzodiazepine Antibody-Drug Conjugates Designed for Stable Thiol Conjugation. Antibodies 2017, 6 (4), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Mendelsohn BA; Barnscher SD; Snyder JT; An Z; Dodd JM; Dugal-Tessier J Investigation of Hydrophilic Auristatin Derivatives for Use in Antibody Drug Conjugates. Bioconjugate Chem. 2017, 28 (2), 371–381. [DOI] [PubMed] [Google Scholar]
- (71).Luciano MP; Crooke SN; Nourian S; Dingle I; Nani RR; Kline G; Patel NL; Robinson CM; Difilippantonio S; Kalen JD; Finn MG; Schnermann MJ A Nonaggregating Heptamethine Cyanine for Building Brighter Labeled Biomolecules. ACS Chem. Biol. 2019, 14 (5), 934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Sato K; Gorka AP; Nagaya T; Michie MS; Nakamura Y; Nani RR; Coble VL; Vasalatiy OV; Swenson RE; Choyke PL; Schnermann MJ; Kobayashi H Effect of charge localization on the in vivo optical imaging properties of near-infrared cyanine dye/monoclonal antibody conjugates. Mol. BioSyst. 2016, 12 (10), 3046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Sato K; Nagaya T; Nakamura Y; Harada T; Nani RR; Shaum JB; Gorka AP; Kim I; Paik CH; Choyke PL; Schnermann MJ; Kobayashi H Impact of C4’-O-Alkyl Linker on in Vivo Pharmacokinetics of Near-Infrared Cyanine/Monoclonal Antibody Conjugates. Mol. Pharmaceutics 2015, 12 (9), 3303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Cha J; Nani RR; Luciano MP; Kline G; Broch A; Kim K; Namgoong JM; Kulkarni RA; Meier JL; Kim P; Schnermann MJ A chemically stable fluorescent marker of the ureter. Bioorg. Med. Chem. Lett. 2018, 28 (16), 2741–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Ohri R; Bhakta S; Fourie-O’Donohue A; Dela Cruz-Chuh J; Tsai SP; Cook R; Wei B; Ng C; Wong AW; Bos AB; Farahi F; Bhakta J; Pillow TH; Raab H; Vandlen R; Polakis P; Liu Y; Erickson H; Junutula JR; Kozak KR High-Throughput Cysteine Scanning To Identify Stable Antibody Conjugation Sites for Maleimide- and Disulfide-Based Linkers. Bioconjugate Chem. 2018, 29 (2), 473–485. [DOI] [PubMed] [Google Scholar]
- (76).Dorywalska M; Strop P; Melton-Witt JA; Hasa-Moreno A; Farias SE; Casas MG; Delaria K; Lui V; Poulsen K; Sutton J; Bolton G; Zhou DH; Moine L; Dushin R; Tran TT; Liu SH; Rickert M; Foletti D; Shelton DL; Pons J; Rajpal A Site-Dependent Degradation of a Non-Cleavable Auristatin-Based Linker-Payload in Rodent Plasma and Its Effect on ADC Efficacy. PLoS One 2015, 10 (7), e0132282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Liu L Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 2018, 9 (1), 15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Kelly RL; Yu Y; Sun T; Caffry I; Lynaugh H; Brown M; Jain T; Xu Y; Wittrup KD Target-independent variable region mediated effects on antibody clearance can be FcRn independent. MAbs 2016, 8 (7), 1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


