Abstract
The ABCG2 transporter plays a pivotal role in multidrug resistance, however, no clinical trial using specific ABCG2 inhibitors have been successful. Although ABC transporters actively extrude a wide variety of substrates, photodynamic therapeutic agents with porphyrinic scaffolds are exclusively transported by ABCG2. In this work, we describe for the first time a porphyrin derivative (4B) inhibitor of ABCG2 and capable to overcome multidrug resistance in vitro. The inhibition was time-dependent and 4B was not itself transported by ABCG2. Independently of the substrate, the porphyrin 4B showed an IC50 value of 1.6 μM and a mixed type of inhibition. This compound inhibited the ATPase activity and increased the binding of the conformational-sensitive antibody 5D3. A thermostability assay confirmed allosteric protein changes triggered by the porphyrin. Long-timescale molecular dynamics simulations revealed a different behavior between the ABCG2 porphyrinic substrate pheophorbide a and the porphyrin 4B. Pheophorbide a was able to bind in three different protein sites but 4B showed one binding conformation with a strong ionic interaction with GLU446. The inhibition was selective toward ABCG2, since no inhibition was observed for P-glycoprotein and MRP1. Finally, this compound successfully chemosensitized cells that overexpress ABCG2. These findings reinforce that substrates may be a privileged source of chemical scaffolds for identification of new inhibitors of multidrug resistance-linked ABC transporters.
Keywords: Cancer, Multidrug resistance, ABC transporter, ABCG2 inhibitor
1. Introduction
Multidrug resistance (MDR) mediated by ATP-binding cassette transporters (ABC transporters) is the most prevalent mechanism of resistance clinically observed [1]. Among the 48 genes in the human genome that encode ABC proteins, three ABC transporters are closely related to MDR: P-glycoprotein (P-gp/ABCB1), multidrug resistance protein 1 (MRP1/ABCC1) and breast cancer resistance protein (BCRP/ABCG2) [2]. ABCG2 transporter was discovered simultaneously in 1998 by three different laboratories. The name was coined originally based on the biological model: breast cancer resistance protein (BCRP) as a result of its identification in a multidrug resistant breast cancer (MCF7-derived) cell line; mitoxantrone resistance protein (MXR) derived of its ability to confer cell growth resistance to mitoxantrone and placenta-specific ATP-binding cassette gene (ABCP) because of its abundance in placenta [3–5].
The ABCG2 transporter exerts physiological roles related to cellular detoxification, usually found in healthy tissues such as the liver, blood-brain barrier and placenta [6,7]. In cancer cells, ABCG2 is responsible for the efflux of chemotherapeutics with unrelated chemical structures, such as mitoxantrone [8], methotrexate [9], topotecan, SN-38 [10] and photosensitizers, such as pheophorbide a [7,11]. ABCG2 is also highly expressed in cancer stem cells (CSC) called side population (SP) [12,13], which is defined by the ability to exclude hoescht 33342 [14]. The presence of ABCG2 in CSC is very intriguing, since it can protect these cells from chemotherapeutic agents [15]. This context suggests that ABCG2 inhibition may represent a way to avoid cancer recurrence.
The relationship between porphyrins and the ABCG2 transporter is well documented. ABCG2 seems to play a role in the heme biosynthesis pathway, actively transporting porphyrinic intermediates [16,17]. This interaction was first reported by Jonker (2002), which showed the protective activity of ABCG2 with dietary porphyrins, most specifically pheophorbide a [18]. Since then, pheophorbide a and other porphyrin-based photodynamic therapy (PDT) agents were reported as specific substrates of ABCG2 [11,19–23].
Some substrates of ABC transporters can also act as inhibitors. For instance, tyrosine kinase inhibitors (TKI’s) [24–26], stilbenes [27–29] and tariquidar [30–36] are substrates with intrinsic inhibitory capacity. Specifically, TKIs are known as both ABCG2/P-gp inhibitors and substrates depending on the employed concentration, where higher concentrations lead to competitive inhibition in the substrate binding site [24–26]. Tariquidar is an ABCG2 substrate at low concentrations (~100 nM), which at higher concentrations (1 μM) can compete with the substrate mitoxantrone [30,31]. Further studies reported selective and potent ABCG2 inhibitors obtained from tariquidar derivatives [32,33,35,36], with emphasis on XR9577, which showed noncompetitive behavior with pheophorbide a [34]. Another example is resveratrol, a substrate with inhibition capacity at high concentrations (30 μM). The resveratrol inhibitory potency was increased by the insertion of methoxy groups to produce analogs, making stilbenes a new class of ABCG2 selective inhibitors [27–29].
The study of substrate derivatives as potential inhibitors might be an effective strategy for the development of highly potent optimized inhibitors, taking advantages of their chemical scaffold. Porphyrins are well characterized ABCG2 substrates, and to the best of our knowledge, no previous work has screened this class of compounds as ABCG2 inhibitors. This paper describes a new porphyrin derivative as a specific ABCG2 inhibitor and characterizes its mechanism of inhibition.
2. Material and methods
2.1. Chemicals
The porphyrins tested in this work were synthetized in the Laboratory of Heterocycles and Glycoconjugates at Federal University of Paraná (Brazil). The full synthesis and characterization were described in previous publications [37–40, 54].
All chemotherapeutics, substrates and reference inhibitors were purchased from Sigma-Aldrich, Abcam and Invitrogen. Cell culture supplies were purchased from Gibco and Sigma-Aldrich. MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from VWR life sciences. Antibodies for flow cytometry Mouse anti-human CD338 Clone 5D3 and Goat anti-mouse conjugated with Phycoerythrin were purchased from BD Pharmigen (New Jersey, USA).
2.2. Cell culture
HEK293, NIH3T3, BHK21 and H460 wild type cells were cultivated in Dulbecco’s modified Eagle’s medium High Glucose supplemented with 10% of bovine fetal serum (FBS) and 50 μg/mL of gentamicin. PANC-1 wild type was cultivated in RPMI medium supplemented with 10% of FBS, 1% of penicillin and 0.25 μg/mL of amphotericin. The cells were cultivated until 80–90% of confluence and then used for experimentation. Stably transfected cells, HEK293-ABCG2, NIH3T3-ABCB1, BHK21-ABCC1 were additionally supplemented with 0.75 mg/mL of G418, 60 ng/mL of colchicine and 0.1 mg/mL of methotrexate for ABCG2, ABCB1 and ABCC1, respectively. H460MX20 and PANC-1MX100 cells were cultivated under mitoxantrone pressure (20 nM and 100 nM, respectively). The cells HEK293, NIH3T3, BHK21 and transfected cell lines were kindly provided by Dr. Attilio Di Pietro (IBCP, Lyon, France). Wild type H460, H460MX20, PANC-1 and PANC-1-MX100 were provided by Dr. Attilio Di Pietro (IBCP, Lyon, France) and Dr. Susan Bates laboratory (HIH, Bethesda, MD, USA).
2.3. Accumulation assay
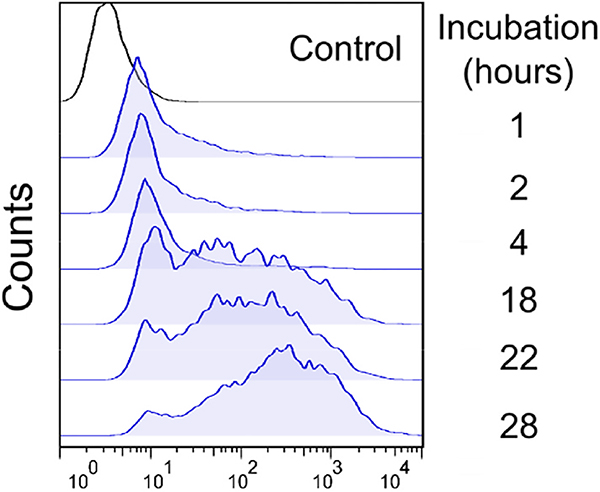
HEK293-wild type cells were seeded in a 24 wells plate (2.0 × 105 cells/well) and incubated for 24 h to attach. Then, cells were treated with porphyrin 4D for 1, 2, 4, 18, 22 and 28 h. After this period, cells were washed with PBS (300 μL), detached with trypsin and the intracellular accumulation of porphyrin 4D was analyzed by flow cytometry using a FACS Calibur (FL-3 filter) (BD Biosciences, New Jersey, USA).
2.4. Inhibition assay
The inhibition capacity was measured by flow cytometry. HEK293-ABCG2 and HEK293-wild type cells were seeded into a 24 wells plate (2.0 × 105 cells/well). After 24 h, cells were treated with porphyrins (10 μM) for 30 min and 24 h. The fluorescent substrates (mitoxantrone 10 μM or hoechst 33342 3 μM) were added as follow: for the 30 min treatment, the fluorescent substrate and porphyrin were added at the same time. For the 24 h of porphyrin treatment, the fluorescent substrate was added in the last 30 min of the treatment. After treatment, cells were washed with PBS (300 μL), detached with trypsin and analyzed by flow cytometry using FACS Calibur (BD Biosciences) Red Laser and FL-4 filter for mitoxantrone and FACS Celesta (BD Biosciences) Ultraviolet Laser and the Band-Pass 450–50 filter for hoechst 33342.
The IC50 curves were obtained with increasing concentrations of 4B (0.09 μM–10 μM, 24 h of incubation) using mitoxantrone and hoechst 33342 as substrates. Selectivity assay was performed using 10 μM of 4B incubated for 24 h and rhodamine 123 (5 μM for ABCB1) or daunorubicin (10 μM for ABCC1) as fluorescent substrates.
For the washing assay, HEK293-ABCG2 cells were treated with 4B at 10 μM for 24 h. Cells were then washed with PBS (300 μL) and kept in culture medium without 4B (300 μL) for 1, 3 and 24 h. After that, the medium was removed and mitoxantrone at 10 μM (dissolved in cell culture media) was added for 30 min, then, the cells were washed with PBS (300 μL) and detached with trypsin to perform the flow cytometry, as previously described.
The inhibition mechanism was determined by treating the cells with 4B (10, 1.65 and 0.5 μM) for 24 h. After this period, the treatment was removed and the substrate was added for 30 min (hoechst 33342 at concentrations 6, 3, 1.5 and 0.75 μM and mitoxantrone at concentrations 20, 10, 5 and 2.5 μM). The cells were washed with PBS (300 μL), detached with trypsin and analyzed by flow cytometry.
2.5. Confocal microscopy
HEK293-ABCG2 were seeded into 24 well plates (1.0 × 105 cells/well) with coverslips on the bottom and incubated for 48 h. After cell adhesion, the cells were treated with 4B (10 μM for 24 h) and hoechst 33342 (1 μM for 30 min). The coverslips were recovered, washed, and placed on microscopy slides with Glycerin. The images were taken with Confocal microscopy Nikon A1R MP + (NIKON, Tokyo, Japan) at 40x objective. The fluorescence was reached using 405 nm laser for excitation and the emission was detected using 425–475 nm filter. The images were visualized with software Nis Elements 4.20 (NIKON, Tokyo, Japan).
2.6. Cell viability assay
Cells were seeded (2.0 × 104 cell/well) into a 96 wells plate and incubated for 24 h to attachment. To evaluate the cell cytotoxicity, cells were treated with increasing concentrations of 4B (0.09 μM–100 μM) and incubated for 72 h. For chemosensitivity assay, cells were treated with 4B (10 μM) for 24 h and then SN-38 was added for additional 48 h (0.1 nm–20 μM for HEK293 cell lines, 0.5 μM and 5.0 μM for H460 and PANC-1 cell lines). After this period, the medium was removed, cells were washed with PBS (100 μL) and incubated with MTT solution (100 μL of solution 0.5 mg/mL in PBS) for 4 h. Then, the solution was removed, and the formazan crystals were dissolved with 100 μL of ethanol/DMSO (1:1). The absorbance was measured using a microplate reader at 595 nm (Bio-Rad iMark).
2.7. ATPase assay
ATPase activity was determined as previously described by Ambudkar (1998) [41]. Total membranes of High five cells overexpressing ABCG2 were used (5 μg protein/tube, final volume 100 μL). The membranes were mixed with assay buffer (50 mM Tris-HCl pH 6.8, 150 mM N-methyl-d-glucamine (NMDG)-Cl, 5 mM sodium azide, 1 mM EGTA, 1 mM ouabain, 2 mM DTT and 10 mM MgCl2) in the presence or absence of sodium orthovanadate at 0.3 mM. The membranes were treated with 4B in a range of 0.1–2.5 μM and incubated for 20 min at 37 °C with ATP (5 mM). After this period, 100 μL of 5% SDS, 400 μL of Pi solution (sulfuric acid 36.2 N, water, ammonium molybdate and antimony potassium tartarate) and 200 μL of 1% ascorbic acid were added. After 10 min, the absorbance was measured at 880 nm using Ultrospec 3100 pro spectrophotometer (Amersham Biosciences, UK).
2.8. Conformational antibody binding (5D3)
HEK293-ABCG2 were seeded at a density of 2.0 × 105 cells/well into a 24 wells plate and, after 24 h, cells were treated with 10 μM of porphyrin 4B for 10, 30 min and 24 h. Then, cells were detached with trypsin, collected with 300 μM of PBS and centrifuged (1000×g for 3 min). The pellet was resuspended in 100 μL of PBS/BSA (40 μg/mL of BSA) and the primary antibody (Mouse anti-human CD338 Clone 5D3, BD Pharmigen) was added (1:100) for 30 min at 37 °C. The cells were centrifuged (1000×g, 3 min), the supernatant removed and resuspended with 100 μL of PBS with the secondary antibody (Goat anti-mouse conjugated with Phycoerythrin, BD Pharmigen) 1:200 for 30 min at 37 °C. Then, the cells were centrifuged, the supernatant removed, and the cell pellet was resuspended in 300 μL of PBS. The analysis was performed using FACS Calibur using the blue laser (488 nm) and FL-2 filter.
2.9. Thermostability assay
Membranes from High Five insect cells overexpressing ABCG2 were incubated with assay buffer at 3 μg protein/tube in the presence or absence of 0.3 mM orthovanadate, reaching 50 μL as final volume (Assay buffer: 50 mM Tris-HCl pH 6.8, 150 mM N-methyl-d-glucamine (NMDG)-Cl, 5 mM sodium azide, 1 mM ouabain, 2 mM DTT). Then, the tests were prepared with 12.5 mM MgCl2 or 6.25 mM ATP and incubated with a temperature range from 37 to 61 °C for 10 min using a thermo-cycler C1000 Touch (Bio-Rad, Hercules, CA).
After this period, 10 μL of 25 mM ATP or 50 mM MgCl2 was added (final concentration of 5 and 10 mM respectively) followed by incubation at 37 °C for 20 min. Then, the reaction was stopped with 50 μL Pi reagent (1% ammonium molybdate, 2.5 N H2SO4 and 0.014% potassium-antimony tartarate). The tests were transferred to a 96 well plate (50 μL/well), 150 μL of 0.33% sodium ascorbate solution was added. After 15 min, the absorbance was measured using a microplate reader Spectramax iD3 (Molecular devices, San Jose, CA, USA). The sensitive activity to vanadate was calculated as the difference between the activity value in the absence and presence of vanadate at each temperature.
2.10. Molecular modelling
The system preparation and docking calculations were performed using the Schrödinger Drug Discovery suite for molecular modelling (version 2019.4). The ABCG2 crystal structure (PDB ID: 6FFC, resolution 3.56 Å [42]) was obtained from the Protein Data Bank (PDB; www.rcsb.org). The structure was chosen since it is one of the cryo-EM structures with high-resolution reported. ABCG2 structure was prepared with the Protein preparation wizard [43] to fix protonation states of amino acids residues, adding hydrogens and also fixing missing side-chain atoms. Missing loops between Asp301 – Leu328 and Gly354 – Tyr369 were generated and optimized using Prime [44]. Compounds 4B and PHEO were drawn using maestro and prepared using LigPrep [45] to generate the three-dimensional conformation, adjust protonation state to physiological pH (7.4), and calculate the partial atomic charges, with the force field OPLS3e [46].
Docking studies with the prepared ligands were performed using Glide [47,48] (Glide V7.7), with the flexible modality of Induced-fit docking with extra precision (XP), followed by a side-chain minimization step using Prime [49]. Ligands were docked within a grid around 12 Å from the centroid of the residue Glu446(A), to represent Site 1 in between the subunits. For each ligand 10 docking poses were generated. Selected docking poses were further validated by molecular dynamics simulation, where ligand stability within the proposed pocket and its interactions were evaluated. MD simulations were carried out using Desmond [50] engine with the OPLS3e force-field [47,51], which leads to improved performance in predicting protein-ligand binding affinities. Protein was embedded within a DMPC lipid pre-generated from Maestro, using the System Builder, where the membrane positioning was controlled by the orientation of the alpha-helices based on the transmembrane sequences.
The protein-membrane system was placed in a cubic box with 15 Å from the box edges to any atom of the protein, using PBC conditions, and, filled with TIP3P [52] water. Then, all systems were equilibrated by short simulations under the NPT ensemble for 5 ns implementing the Berendsen thermostat and Barostat methods. A constant temperature of 310 K and 1atm of pressure throughout the simulation using the Nose-Hoover thermostat algorithm and Martyna-Tobias-Klein Barostat algorithm, respectively. After minimization and relaxing steps, we proceeded with the production step of at least 1 μs. All MD simulations were performed at least three independent runs with randomly generated seeds. Trajectories and interaction data are available on Zenodo repository (MZ29 trajectories were previously published elsewhere [53] and can be found under the code: 10.5281/zenodo.3746123, while additional PHEO and 4B trajectory simulations are reported in 10.5281/zenodo.3988214).
For principal component analyses, the backbone of each frame was extracted and aligned using trj_selection_dl.py and trj_align.py scripts from Schrodinger. Individual simulations from all the runs were merged using trj_merge.py into a final trajectory and CMS file which was further used for the generation of the principal components. Principal components of protein C-alpha atoms were calculated using trj _essential_dynamics.py script. Most of the large moments were captured in the first two components with the first component having 32.5% and the second component having 11.10% of the total motion. Protein-ligand interactions and protein conformational changes were analyzed using the Simulation Interaction Diagram (SID) tool with standard options.
Molecular dynamics trajectories were visualized, and figures were produced using PyMol (Schrödinger LCC, version 2.2.3). The stability of the MD simulations was monitored by looking specifically at the root mean square deviation (RMSD) (Supporting information Fig. S5, represents RMSD variation along with the simulation), root mean square fluctuation (RMSF, supporting information Fig. S3) of the ligand and protein along the simulation time, our simulations were compared against ABCG2 – MZ29 previously published.
3. Results
3.1. Screening of porphyrins as inhibitors of ABCG2 transport activity
A total of 19 porphyrin derivatives (Fig. S1 and Table 1) were evaluated as potential inhibitors of ABCG2 transporter by examining their effect on mitoxantrone efflux. Stably transfected cells overexpressing ABCG2 (HEK293-ABCG2) were incubated with 10 μM of the porphyrins for 30 min. Four porphyrins showed a mild inhibition (10–30% of inhibition) of mitoxantrone efflux, and only the porphyrin 4B showed an inhibition higher than 40% (Table 1). Considering the intrinsic fluorescent property of some porphyrins, a screening with this set of compounds was performed in HEK293-wild type cells to evaluate the intracellular accumulation. The best fluorescent signal was observed with porphyrin 4D, which showed a time-dependent accumulation (Fig. 1), no other porphyrin showed relevant fluorescence (Table S2). Thus, the potential of ABCG2 inhibition for the 19 porphyrins was reevaluated after 24 h of incubation. A clear improvement on the potency of inhibition was observed to compound 4B, increasing the inhibition effect at 10 μM from 44 to 78%, confirming the time-dependent intracellular accumulation of porphyrins and consequently the inhibition effect (Table 1).
Table 1.
Porphyrin structures and the percentage of inhibition of ABCG2 transport activity.

|
||||||
|---|---|---|---|---|---|---|
| Common scaffold | ||||||
|
| ||||||
| Porphyrin | R1 | R2 = R3 = R4 | R5 | Reference | ABCG2 inhibition (%) ± SD |
|
| 30 min | 24 h | |||||
|
| ||||||
| Core |
|
−H | [32] | 14.07 ± 12.22 | −20.63 ± 3.68 | |
| 1A |
|

|
[54] | 6.95 ± 8.4 | 51.89 ± 4.75 | |
| 1B |

|
[54] | −16.43 ± 8.74 | 1.34 ± 12.36 | ||
| 1C |

|
[31,32] | −3.51 ± 3.47 | −24.65 ± 4.55 | ||
| 1C* |

|
−NO2 | [33] | −0.62 ± 5.67 | 7.61 ± 3.13 | |
| 1D |

|

|
−H | [30] | 9.83 ± 13.59 | −6.00 ± 16.33 |
| 1E |

|
7.37 ± 5.82 | 9.49 ± 17.20 | |||
| 1F |
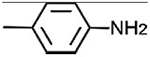
|
[30,31] | −4.45 ± 7.76 | −31.01 ± 9.60 | ||
| 1G |
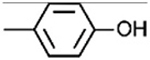
|
[30] | 23.69 ± 18.35 | −18.75 ± 0.37 | ||
| 1H |
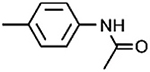
|
−7.90 ± 0.84 | −5.20 ± 0.21 | |||
| 4A |

|
[54] | −7.49 ± 11.81 | −12.20 ± 1.77 | ||
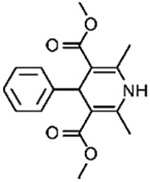
| 4B |

|
[54] | 43.99 ± 8.07 | 78.30 ± 9.33 | ||
| 4D |
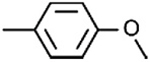
|
[32] | 19.08 ± 2.38 | −16.11 ± 0.79 | ||
| 4E |

|
[31] | 0.50 ± 3.91 | −15.58 ± 17.37 | ||
| 4I |

|
1.51 ± 6.12 | −14.86 ± 10.48 | |||
| 4J |

|
9.31 ± 5.51 | 0.71 ± 4.96 | |||
| 4K |

|
9.27 ± 0.12 | −20.46 ± 1.37 | |||
| 4L |
|
−15.48 ± 5.67 | 18.87 ± 8.56 | |||
| 4M |

|
−19.78 ± 0.03 | −4.46 ± 3.17 | |||
The inhibition of ABCG2-mediated efflux of mitoxantrone (5 μM) was assayed in HEK293-ABCG2 cells. Cells were exposed to porphyrins at 10 μM for 30 min or 24 h. The percent inhibition of ABCG2 transport was determined by flow cytometry using the reference inhibitor Ko143 (0.5 μM) as a control, which produces 100% inhibition. The data are the mean ± SD of, at least, two independent experiments.
Fig. 1.
Intracellular accumulation of porphyrin 4D along the time using HEK293-wild type cells. Cells were analyzed by flow cytometry using the blue laser (488 nm) and FL-3 filter (670 nm long pass).
The tested porphyrins can be divided in two groups: mono and tetrasubstituted. Among the monosubstituted porphyrins, the 1A structure can be highlighted since it showed 51% of inhibition after 24 h of incubation, despite the low inhibition at 30 min (around 6%). Porphyrin 1A is closely related to 1B, which bears a pyridine ring as substituent, instead of a dihydropyrine ring. However, 1B did not display considerable inhibition. In this case, the ring aromatization seems to be detrimental for the inhibition.
On the tetrasubstituted porphyrins group, porphyrin 4B showed higher inhibition than other structures at 30 min (44%) and 24 h (78% of inhibition). The related structure, 4A, did not show quantifiable inhibition.
Interestingly, on monosubstituted porphyrins, aromatization was detrimental for inhibition effect but was favorable on the tetrasubstituted structure. The opposite was observed with dihydropyridine rings, where it was favorable on monosubstituted structures and unfavorable on the tetrasubstituted scaffold.
3.2. IC50 of ABCG2 inhibition and absence of transport
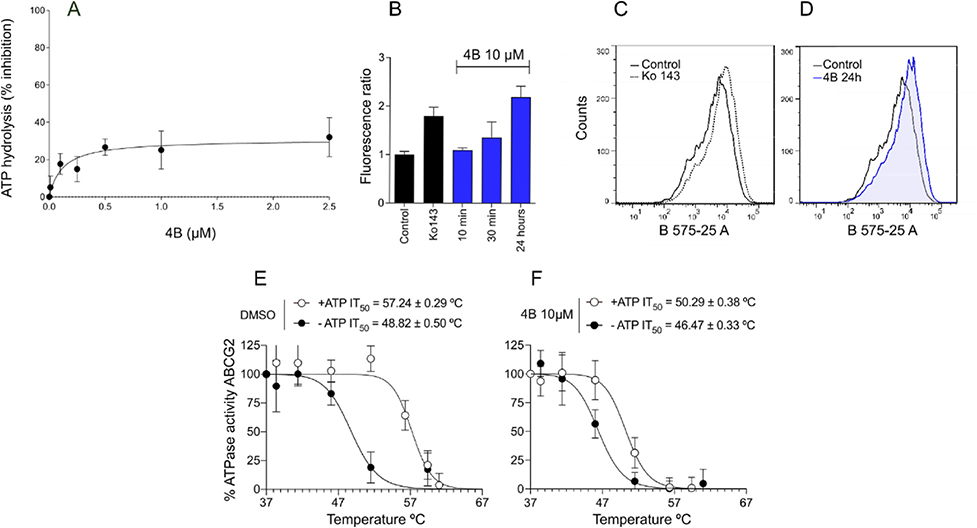
The effect of porphyrin 4B on ABCG2 function after 24 h of exposure was explored by different approaches. Porphyrin 4B was able to inhibit, with the same potency, the transport of different substrates of ABCG2, showing an IC50 of 1.63 and 1.65 μM for mitoxantrone and hoechst 33342, respectively (Fig. 2A and C). In addition, a saturating concentration of porphyrin 4B (10 μM) resulted in intracellular accumulation of mitoxantrone and hoechst 33342 similar to the reference inhibitor Ko143 (Fig. 2B and D). The intracellular accumulation of hoechst 33342 on cells treated with porphyrin 4B was also confirmed by confocal microscopy (Fig. 2E). In cells incubated only with hoechst 33342, the fluorescence was diffuse, and do not concentrate in the nucleus. However, in the presence of Ko143, the intracellular fluorescence of hoechst 33342 was higher and localized in the nucleus. The treatment of cells with 4B increased the accumulation of hoechst 33342, much like the known ABCG2 reference inhibitor Ko143, confirming that porphyrin 4B inhibits the ABCG2 transport activity independently of the substrate.
Fig. 2.
Inhibition curves of porphyrin 4B and intracellular accumulation. (A) Mitoxantrone as substrate. (B) Histogram with mitoxantrone (MTX) 10 μM (full line), Ko143 0.5 μM (dotted line) and 4B 10 μM (blue histogram). (C) hoechst 33342 as substrate. (D) Histogram with hoechst 33342 3 μM (full line), Ko143 0.5 μM (dotted line) and 4B 10 μM (blue histogram). (E) Confocal microscopy using hoechst 33342 1 μM as substrate and Ko143 0.5 μM as reference inhibitor and 4B at 10 μM. The data represent at least two independent experiments.
The cytotoxicity profile comparing the effect of drugs in parental cells and cells overexpressing ABC transporters can provide valuable information about ABCG2-mediated cross-resistance. Also, this simple approach can also demonstrate a possible collateral sensitivity effect [55]. Considering that porphyrins are classic ABCG2 substrates, potential cross-resistance to 4B was investigated using HEK293-wild type and HEK293-ABCG2 cells (Fig. 3A). The porphyrin 4B was cytotoxic at high concentrations, which allowed to verify a cross-resistance effect. The cytotoxicity pattern was similar after 72 h of treatment for both the parental and ABCG2-overexpressing cells, demonstrating an absence of cross-resistance (Fig. 3A). This finding suggests that porphyrin 4B is not transported by ABCG2.
Fig. 3.
(A) Cell viability assay performed using the MTT method. HEK293-ABCG2 and Wild type were submitted to a long-term treatment (72 h) with an increasing concentration of 4B. (B) Treatment with Ko143 (control – 30 min) porphyrin 4B (blue bars - 24 h) followed by a washing step with PBS and incubation of the cells in absence of the porphyrin for 1, 3 and 24 h. Mitoxantrone was used as substrate. The data represent at least two independent experiments.
To confirm the absence of transport, a cell washing assay was performed. HEK293-ABCG2 cells were exposed to mitoxantrone and 4B at a saturating concentration (10 μM) for 24 h. After the treatment, cells were washed with PBS to remove the porphyrin from the culture medium, and the transport assay was performed by flow cytometry after 1, 3 and 24 h. As shown in Fig. 3B, even after the removal of 4B from culture medium for 24 h, the potency of inhibition was similar to reference inhibitor Ko143, supporting the hypothesis that 4B is not transported by ABCG2 and also presents high affinity for ABCG2 (Fig. 3B).
3.3. Mechanism of inhibition
The fact that we did not find evidence of ABCG2-mediated transport of 4B suggests that the porphyrin binds to another region or site compared to substrates. To pursue this hypothesis, the type of inhibition was investigated using different concentrations of mitoxantrone or hoechst 33342 in combination with a dose response of 4B. The data showed an increasing of Vmax and Km while inhibitor concentration increased (Fig. 4A–D), suggesting a mixed type of inhibition for both substrates, which was confirmed through Hanes-Woolf plot (Fig. S2).
Fig. 4.
Kinetic behavior of ABCG2 with mitoxantrone (A) and hoechst 33342 (C). Comparison of Vmax and Km with different concentrations of 4B with mitoxantrone (B) and hoechst 33342 (D)Km and Vmax were obtained from nonlinear regression from graphs A and C using GraphPad Prism v8.0.
The ATPase activity of ABC transporters is commonly stimulated by substrates and decreased by compounds that inhibit ABCG2 trasnport activity [56,57]. ATPase activity was evaluated after treatment with increasing concentrations of 4B using total membranes from ABCG2-overexpressing High-Five insect cells. As shown in Fig. 5A, porphyrin 4B produced a mild inhibition of the ATPase activity of ABCG2 (~20%). Another common feature of ABCG2 inhibitors is increased binding of the conformation-sensitive antibody 5D3, which recognizes an epitope in the extracellular loop of ABCG2. Porphyrin 4B triggered a time-dependent increase of 5D3 binding (Fig. 5B). This increase was higher than the reference inhibitor Ko143 after 24 h of incubation (Fig. 5C and D). These data are in accordance with the time dependent inhibition observed in the initial screening (Table 1). Considering that the maximum inhibition was visualized only after 24 h of incubation, this result suggests that the porphyrin interaction causes important conformational changes to the transporter.
Fig. 5.
Allosteric changes triggered by 4B. (A) ATPase activity performed with membranes of High-Five insect cells in the presence of porphyrin 4B. (B) Conformational antibody binding in three different incubation times: 10, 30 min and 24 h with 4B at 10 μM. The data was normalized by the untreated control. (C) Histogram from the conformational antibody showing the fluorescent shift between the untreated control and cells treated with Ko143 2 μM. (D) Histogram from conformational antibody showing the untreated control and 24 h in the presence of porphyrin 4B (10 μM). (E) Thermostability assay in the absence of 4B (F) and the presence of 4B. The data represent at least two independent experiments.
To confirm allosteric conformational changes induced by 4B, a thermostability assay using total membranes from ABCG2-overexpressing High-Five insect cells was performed. In the absence of ATP, 4B does not promote significant changes on IT50 values (Fig. 5E and F). On the other hand, in the presence of ATP, porphyrin 4B decreased the IT50 value around 7 °C compared to the control (DMSO), suggesting increased ABCG2 thermostability induced by conformational changes by 4B binding.
3.4. Docking and molecular dynamics simulations
To explore potential binding modes of the porphyrin 4B and compared with the substrate pheophorbide a (PHEO), we performed molecular docking calculations in the cavity between subunits of the ABCG2 cryo-EM structure followed by long-timescale molecular dynamics (MD) simulations. Principal component analyses of our MD simulation trajectories (Fig. S3A) grouped the 4B conformations into two closely related states, in contrast to PHEO, that showed three independent states. PCA suggests a single conformational state for the inhibitor previously analyzed MZ29, however, this could be a result of shorter sampling (Fig. S3A).
Upon simulation, compound 4B has little change in position, being stabilized by the hydrogen bond interaction with Glu446(A) and water-mediated contacts with Asn436 (Fig. 6A and B and Fig. S3B). However, PHEO occupies different sites in the protein with an intermittent interaction with Glu446’s from both chains and short contacts with Arg184 (Fig. 6A, C). Porphyrin 4B and PHEO can occupy the space in between subunits establishing several different hydrophobic contacts (Fig. 6A). Interestingly, the substrate PHEO can fit in the site between subunits, near residues such as Phe439 (Fig. S3B, Fig. S4). The binding of 4B and PHEO induces conformational changes in the TMD closing the gap between subunits as observed by the smaller average distance between Phe439 and increased per residue mobility in that region, as well as the connective linker between the TMD and NBD (Figs. S3B–D).
Fig. 6.
Frequency of hydrogen bonding interactions and hydrophobic interactions (A) observed along with the molecular dynamic simulation. Each bar represents the standard error of at least three independent simulations (1 μs each, for 4B and PHEO, and 500 ns for MZ29). Snapshots of the last frames for molecular dynamic simulations of the (B) 4B inhibitor (blue) and the (C) PHEO substrate (red). ABCG2’s residues are colored according to the atom type of the interacting amino-acid residues (protein’s carbon, light grey (chain A) and pale green (chain B); oxygen, red; nitrogen, blue). Hydrogen bond interactions are represented by dashed yellow lines. MZ29 interaction profile was retrieved from the previous publication for comparison purposes.
3.5. Selectivity toward ABCG2 and chemosensitization
Despite the selectivity of porphyrins as ABCG2 substrates, the inhibition selectivity was evaluated comparing the effect of porphyrin 4B on P-gp and MRP1, using rhodamine 123 and daunorubicin as substrates, respectively. Stably transfected cells overexpressing each one of these ABC transporters were used. As shown in Fig. 7A–D, 4B selectively inhibited ABCG2 activity as it did not show any inhibition of P-gp and MRP1. This confirms that porphyrins are a class of compounds that exclusively affects the activity of ABCG2 transporter.
Fig. 7.
(A) Selectivity assay using stably transfected cell lineages for MRP1 (BHK21-ABCC1), P-gp (NIH3T3-ABCB1) and ABCG2 (HEK293-ABCG2). The cells were seeded in a 24 wells plate and treated with the porphyrin at 10 μM for 24 h. The substrates/inhibitors used for each lineage were mitoxantrone (MTX) 10 μM/Ko143 0.5 μM (ABCG2), rhodamine 123 5 μM/GF120918 1 μM (P-pg) and daunorubicin 10 μM/verapamil 30 μM (MRP1). The histogram of each condition is represented on figures B, C and D. (E) Chemo sensitization evaluation performed using HEK293-Wild type, HEK293-ABCG2 (F) lung tumor cell lineage H460-Wild type and H460-MX20 (cultivated in the presence of Mitoxantrone) (G) and pancreatic tumor cell lineage PANC-1-Wild type and PANC-1-MX100 (cultivated in the presence of Mitoxantrone). The cells were seeded and treated with the porphyrin for 24 h, following the addition of SN-38 as chemotherapy for more 48 h in increasing concentrations. The evaluation of cell viability was made by MTT assay. The data represent at least two independent experiments.
Finally, to verify the potential use of porphyrins as inhibitors in future pre-clinical models, an in vitro chemosensitization assay using transfected cells overexpressing ABCG2 was performed. Cells were treated with porphyrin 4B and the active metabolite of irinotecan (SN-38), a chemotherapeutic transported by ABCG2. Cells overexpressing ABCG2 co-treated with 4B and SN-38 displayed an EC50 value 7-fold lower than the resistant cells treated only with SN-38 (Figs. 7E and S3). In addition, the pattern observed of ABCG2 cells co-treated with 4B and SN-38 was similar to wild-type cells treated only with SN-38, demonstrating a complete phenotype reversion (Fig. 7E).
The chemosensitization effect was additionally investigated in selected cancer cell lines that overexpresses ABCG2. The mitoxantrone selected H460-MX20 lung cancer and PANC-1-MX100 pancreatic cancer cell lines were treated with SN-38 alone or in association with porphyrin 4B. Cell viability were compared with parental cell lines H460 and PANC-1, also treated with SN-38. Co-treatment of H460-MX20 and PANC-1-MX100 cells with porphyrin 4B and SN-38 chemosensitized the cells to the same level as observed with parental H460 and PANC-1 cells (Fig. 7F and G), confirming the results obtained using stably transfected cells and validating the use of 4B in cancer cells.
4. Discussion
Porphyrins and related compounds (like chlorins) have been recognized as specific ABCG2 substrates [19]. For this reason, the presence of ABCG2 in cancer cells does not impair only classical chemotherapy, but play an important role in another cancer treatment approach, the photodynamic therapy [58,59]. Here, we screened 19 porphyrins to verify their potential as specific inhibitors of ABCG2. Regarding porphyrinic substituents, the number and type displayed a counter-intuitive behavior: on the one hand, the presence of four pyridinic rings was favorable for inhibition, but a single pyridinic ring did not have any effect on ABCG2 activity. In contrast, four dihydropyridinic rings were unfavorable, and the presence of only one did promote inhibition. Considering the two best cases, the presence of four pyridinic rings was superior in inhibition than only one dihydropyridinic ring.
Porphyrin 4B inhibited the ABCG2-mediated substrate transport with an IC50 value of 1.6 μM using two different substrates, demonstrating a substrate independent inhibition (Fig. 2). Interestingly, 4B contradicts the idea of porphyrins behaving as substrates, since 4B was not transported by ABCG2 (Fig. 3A). This was also confirmed in a washout assay where no loss in transport inhibition was observed even 24 h after the removal of the inhibitor (Fig. 3B). This latter piece of data supports the idea that porphyrin 4B seems to have a high affinity toward ABCG2.
To better understand the type of inhibition produced by porphyrin 4B, a kinetic experiment was conducted. Using two different substrates, 4B showed a mixed type of inhibition (Figs. 4 and S3). The porphyrin 4B binds on the apo (substrate-free) protein and may produce conformational changes in the substrate binding site, decreasing the substrate affinity. The same type of inhibition was observed with pyridopyrimidines [42]. In contrast with some recently described ABCG2 inhibitors that stimulate the ATPase activity, such as indeno[1,2-b]indoles [60], pyridopyrimidines [42], Ulixertinib [61] acryloylphenylcarboxylates [62] and EGFR inhibitor Rociletinib [63], porphyrin 4B mildly inhibited the ATPase activity (around 20%), when compared to Ko143 and MZ29, which completely inhibits the ATPase activity of ABCG2 [43,64].
In general, ABCG2 inhibitors increase the binding of the conformation-sensitive antibody 5D3 [65–67]. Porphyrin 4B followed the same pattern, but in a time-dependent manner. This finding is in accordance with the transport inhibition results, which was also time-dependent, suggesting that conformational changes occur due to 4B binding. The thermostability assay showed a significant decrease of protein stability in the presence of 4B and ATP, since the IT50 decreased by approximately 7 °C. This is similar to the effect triggered by the indeno[1,2-b]indoles on ABCG2, where a decrease of IT50 around 5 °C was observed [60]. This result demonstrates that structural protein changes are induced by 4B binding.
Molecular modelling was then employed to compare the potential binding mode of a porphyrinic substrate, pheophorbide a (PHEO), and the porphyrinic inhibitor identified in this work. Molecular docking suggests that PHEO could bind in three different regions in the ABCG2’s central cavity (Figs. S3 and 4), while 4B displayed one single conformation. Molecular dynamics simulations, in the timescale of few microseconds (Fig. S5), supported the stability of these binding modes, showing a strong ionic interaction between the charged nitrogen atom in the center of the porphyrinic ring and the Glu446. It is noteworthy that residue Glu446 is the only negatively charged residue facing the central cavity from TMH2.
The work from Khunweeraphong et al., 2017 [68], suggests a mechanism where drugs entering central cavity would be trapped by the interaction with Glu446. They also observed that E446 K/A/D mutations disrupts the efflux of mitoxantrone, rhodamine 123 and hoechst 33342 [68,69], while retaining normal expression and membrane localization. Interestingly, the transport-incompetent E446D variant retains ATPase activity, which suggests that the side-chain size and potential interaction distances are relevant for the recognition. Our simulation data (Fig. 6 and S2,3B) suggests that 4B closely interacts with Glu446, where PHEO has a larger variation in the distance interaction, which supports its substrate behavior. Recently, Kowal et al. (2021) showed that substrate binding is more flexible than inhibitors, shifting in the binding cavity, using Cryo-EM structures [70].
When binding substrates, this increased flexibility was extended to the nucleotide-binding domains (NBDs), where multiple conformations could be observed [70]. The conformational change required for ATP hydrolysis occur in the NBDs and are conveyed to the TMDs via intracellular loop 1 (ICL1). Recent work proposes that while TMH2-E446 is involved in the trapping and recognition of inhibitors, the conformational changes in the NBDs are mediated by the Glu451 (localized in the ICL1) and therefore independent [71]. Additionally, our compounds showed an interaction with Arg184 (Fig. S4), which was more prominent with the 4B inhibitor than in the PHEO substrate. Arg184 stabilizes the ATP in closed ABCG2 conformation, as observed in the CryoEM structure (PDB ID 6HBU) [72].
Transporter selectivity is a desirable feature in the design of new inhibitors [6,73]. Selectivity can mitigate collateral effects due the interference on physiological processes and the pharmacokinetics of other drugs [74]. Porphyrin 4B was selective toward ABCG2 (Fig. 7), confirming the selective interaction of porphyrins with ABCG2. In addition, the co-treatment of stably transfected cells and drug-selected resistant cells with 4B and SN-38 successfully chemosensitized the cells to levels comparable to the parental cells, proving that porphyrin 4B is an efficient inhibitor to circumvent multidrug resistance mediated by ABCG2.
In addition to playing a role in drug resistance, ABCG2 is expressed at various barrier sites such as the gut and has been shown to limit the oral bioavailability of substrate drugs [6,73]. In mouse models, coadministration of ABCG2 inhibitors such as elacridar has been shown to increase oral bioavailability of the ABCG2 substrate topotecan [75]. Additionally, coadministration of elacridar and topotecan has been shown to increase oral bioavailability of topotecan in humans in a clinical trial [76]. Thus, ABCG2 inhibitors such as 4B may also be useful in increasing the oral bioavailability of chemotherapy agents in patients.
In summary, the conversion of substrate scaffold into inhibitors was already successfully employed; however, to the best of our knowledge, this is the first report that used a ABCG2 specific substrate core structure, the porphyrins, to identify new specific inhibitors. Interestingly, the best inhibitor, porphyrin 4B was not transported by ABCG2 and successfully reversed the MDR phenotype. These findings will support and guide the development of new ABCG2 inhibitors based on porphyrinic scaffold for future pre-clinical studies.
Supplementary Material
Acknowledgments
The authors acknowledge CENAPD at UNICAMP, Brazil, and the CSC – IT Center for Science, Finland, for the generous computational resources. We also thank Attilio Di Pietro from IBCP and Dr. Susan Bates from NCI, NIH for having provided the cell lines.
Funding
This work was supported by CNPq (grant number 400953/2016–1) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (Finance code 001). GV was supported by Fundação Araucária. RWR and SVA were supported by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Center for Cancer Research.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cbi.2021.109718.
References
- [1].Li W, Zhang H, Assaraf YG, Zhao K, Xu X, Xie J, Yang D-HH, Chen Z-SS, Overcoming ABC transporter-mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies, Drug Resist. Updates 27 (2016) 14–29, 10.1016/j.drup.2016.05.001. [DOI] [PubMed] [Google Scholar]
- [2].Stefan SM, Multi-target ABC transporter modulators: what next and where to go? Future Med. Chem. 11 (2019) 2353–2358, 10.4155/fmc-2019-0185. [DOI] [PubMed] [Google Scholar]
- [3].Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE, Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells, Cancer Res. 59 (1999) 8–13. http://cancerres.aacrjournals.org/content/59/1/8.abstract. [PubMed] [Google Scholar]
- [4].Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M, A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance, Cancer Res. 58 (1998) 5337–5339. [PubMed] [Google Scholar]
- [5].Doyle LA, Yang W, V Abruzzo L, Krogmann T, Gao Y, Rishi AK, Ross DD, A multidrug resistance transporter from human MCF-7 breast cancer cells, Proc. Natl. Acad. Sci. U.S.A. 95 (1998) 15665–15670, 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Robey RW, To KKK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE, ABCG2: a perspective, Adv. Drug Deliv. Rev. 61 (2009) 3–13, 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hira D, Terada T, BCRP/ABCG2 and high-alert medications: biochemical, pharmacokinetic, pharmacogenetic, and clinical implications, Biochem. Pharmacol. 147 (2018) 201–210, 10.1016/j.bcp.2017.10.004. [DOI] [PubMed] [Google Scholar]
- [8].Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE, The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2), J. Cell Sci. 113 (2000) 2011–2021, 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- [9].Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E, Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance, Cancer Res. 62 (2002) 5035–5040. [PubMed] [Google Scholar]
- [10].Nakatomi K, Yoshikawa M, Oka M, Ikegami Y, Hayasaka S, Sano K, Shiozawa K, Kawabata S, Soda H, Ishikawa T, Tanabe S, Kohno S, Transport of 7-Ethyl-10-hydroxycamptothecin (SN-38) by breast cancer resistance protein ABCG2 in human lung cancer cells, Biochem. Biophys. Res. Commun. 288 (2001) 827–832, 10.1006/bbrc.2001.5850. [DOI] [PubMed] [Google Scholar]
- [11].Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE, Pheophorbide a is a specific probe for ABCG2 function and inhibition, Cancer Res. 64 (2004) 1242–1246, 10.1158/0008-5472.CAN-03-3298. [DOI] [PubMed] [Google Scholar]
- [12].Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP, The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype, Nat. Med. 7 (2001) 1028–1034, 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- [13].Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H, Targeting cancer stem cell pathways for cancer therapy, Signal Transduct. Target. Ther. 5 (2020) 8, 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC, Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo, J. Exp. Med. 183 (1996) 1797–1806, 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao J, Cancer stem cells and chemoresistance: the smartest survives the raid, Pharmacol. Ther. 160 (2016) 145–158, 10.1016/j.pharmthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Desuzinges-Mandon E, Arnaud O, Martinez L, Huché F, Di Pietro A, Falson P, ABCG2 transports and transfers heme to albumin through its large extracellular loop, J. Biol. Chem. 285 (2010) 33123–33133, 10.1074/jbc.M110.139170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang P, Sachar M, Lu J, Shehu AI, Zhu J, Chen J, Liu K, Anderson KE, Xie W, Gonzalez FJ, Klaassen CD, Ma X, The essential role of the transporter ABCG2 in the pathophysiology of erythropoietic protoporphyria, Sci. Adv. 5 (2019) 1–9, 10.1126/sciadv.aaw6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jonker JW, Buitelaar M, Wagenaar E, Van der Valk MA, Scheffer GL, Scheper RJ, Plösch T, Kuipers F, Oude Elferink RPJ, Rosing H, Beijnen JH, Schinkel AH, Plosch T, Kuipers F, Elferink RPJO, Rosing H, Beijnen JH, Schinkel AH, The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria, Proc. Natl. Acad. Sci. U.S.A. 99 (2002) 15649–15654, 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Robey RW, Steadman K, Polgar O, Bates SE, ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy, Cancer Biol. Ther. 4 (2005) 187–194, 10.4161/cbt.4.2.1440. [DOI] [PubMed] [Google Scholar]
- [20].Usuda JY, Tsunoda Y, Ichinose S, Ishizumi T, Ohtani K, Lung Cancer Breast cancer resistant protein ( BCRP ) is a molecular determinant of the outcome of photodynamic therapy ( PDT ) for centrally located early lung cancer. 10.1016/j.lungcan.2009.04.002, 2010, 67,198,204. [DOI] [PubMed] [Google Scholar]
- [21].Kawai N, Hirohashi Y, Ebihara Y, Saito T, Murai A, Saito T, Shirosaki T, Kubo T, Nakatsugawa M, Kanaseki T, Tsukahara T, Shichinohe T, Li L, Hirano S, Torigoe T, ABCG2 expression is related to low 5-ALA photodynamic diagnosis (PDD) efficacy and cancer stem cell phenotype, and suppression of ABCG2 improves the efficacy of PDD, PLoS One 14 (2019) 1–15, 10.1371/journal.pone.0216503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu ZY, Wang K, Li XQ, Chen S, Deng JM, Cheng Y, Wang ZG, The ABCG2 transporter is a key molecular determinant of the efficacy of sonodynamic therapy with Photofrin in glioma stem-like cells, Ultrasonics 53 (2013) 232–238, 10.1016/j.ultras.2012.06.005. [DOI] [PubMed] [Google Scholar]
- [23].Usuda J, Ichinose S, Ishizumi T, Ohtani K, Inoue T, Maehara S, Imai K, Shima K, Ohira T, Kato H, Ikeda N, Ichinose ÃS, Ishizumi T, Ohtani K, Inoue T, Maehara S, Molecular determinants of photodynamic therapy for lung cancers, Laser Surg. Med. 599 (2011) 591–599, 10.1002/lsm.21097. [DOI] [PubMed] [Google Scholar]
- [24].Eadie LN, Hughes TP, White DL, Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib, Clin. Pharmacol. Ther. 95 (2014) 294–306, 10.1038/clpt.2013.208. [DOI] [PubMed] [Google Scholar]
- [25].Shukla S, Skoumbourdis AP, Walsh MJ, Hartz AMS, Fung KL, Wu C, Gottesman MM, Thomas CJ, V Ambudkar S, Synthesis and characterization of a BODIPY conjugate of the BCR-ABL kinase inhibitor Tasigna ( nilotinib ): evidence for transport of Tasigna and its fluorescent derivative by ABC drug transporters, Mol. Pharm. (2011) 1292–1302, 10.1021/mp2001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Inoue Y, Morita T, Onozuka M, Saito K-I, Sano K, Hanada K, Kondo M, Nakamura Y, Kishino T, Nakagawa H, Ikegami Y, Impact of Q141K on the transport of epidermal growth factor receptor tyrosine kinase inhibitors by ABCG2, Cells 8 (2019) 1–12, 10.3390/cells8070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Valdameri G, Pereira Rangel L, Spatafora C, Guitton J, Gauthier C, Arnaud O, Ferreira-Pereira A, Falson P, Winnischofer SMB, Rocha MEM, Tringali C, Di Pietro A, Methoxy stilbenes as potent, specific, untransported, and noncytotoxic inhibitors of breast cancer resistance protein, ACS Chem. Biol. 7 (2012) 322–330, 10.1021/cb200435y. [DOI] [PubMed] [Google Scholar]
- [28].Breedveld P, Pluim D, Cipriani G, Dahlhaus F, van Eijndhoven MAJ, de Wolf CJF, Kuil A, Beijnen JH, Scheffer GL, Jansen G, Borst P, Schellens JHM, The effect of low pH on breast cancer resistance protein (ABCG2)-Mediated transport of methotrexate, 7-hydroxymethotrexate, methotrexate diglutamate, folic acid, mitoxantrone, topotecan, and resveratrol in in vitro drug transport models, Mol. Pharmacol. 71 (2007) 240, 10.1124/mol.106.028167. LP – 249. [DOI] [PubMed] [Google Scholar]
- [29].Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA, Interaction of the breast cancer resistance protein with plant polyphenols, Biochem. Biophys. Res. Commun. 317 (2004) 269–275, 10.1016/j.bbrc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- [30].Kannan P, Telu S, Shukla S, Ambudkar SV, Pike VW, Halldin C, Gottesman MM, Innis RB, Hall MD, The “specific” P-glycoprotein inhibitor tariquidar is also a substrate and an inhibitor for Breast Cancer Resistance Protein (BCRP/ABCG2), ACS Chem. Neurosci. 2 (2011) 82–89, 10.1021/cn100078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE, Pheophorbide a is a specific probe for ABCG2 function and inhibition, Cancer Res. 64 (2004) 1242–1246, 10.1158/0008-5472.CAN-03-3298. [DOI] [PubMed] [Google Scholar]
- [32].Bauer S, Ochoa-Puentes C, Sun Q, Bause M, Bernhardt G, König B, Buschauer A, Quinoline carboxamide-type ABCG2 modulators: indole and quinoline moieties as anilide replacements, ChemMedChem 8 (2013) 1773–1778, 10.1002/cmdc.201300319. [DOI] [PubMed] [Google Scholar]
- [33].Ochoa-Puentes C, Bauer S, Kühnle M, Bernhardt GG, Buschauer A, König B, Kuhnle M, Bernhardt GG, Buschauer A, Konig B, Benzanilide-biphenyl replacement: a bioisosteric approach to quinoline carboxamide-type ABCG2 modulators, ACS Med. Chem. Lett. 4 (2013) 393–396, 10.1021/ml4000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pick A, Klinkhammer W, Wiese M, Specific inhibitors of the breast cancer resistance protein (BCRP), ChemMedChem 5 (2010) 1498–1505, 10.1002/cmdc.201000216. [DOI] [PubMed] [Google Scholar]
- [35].Kühnle M, Egger M, Müller C, Mahringer A, Bernhardt G, Fricker G, König B, Buschauer A, Potent and selective inhibitors of breast cancer resistance protein (ABCG2) derived from the p-glycoprotein (ABCB1) modulator tariquidar, J. Med. Chem. 52 (2009) 1190–1197, 10.1021/jm8013822. [DOI] [PubMed] [Google Scholar]
- [36].Puentes CO, Höcherl P, Kühnle M, Bauer S, Bürger K, Bernhardt G, Buschauer A, König B, Solid phase synthesis of tariquidar-related modulators of ABC transporters preferring breast cancer resistance protein (ABCG2), Bioorg. Med. Chem. Lett 21 (2011) 3654–3657, 10.1016/j.bmcl.2011.04.094. [DOI] [PubMed] [Google Scholar]
- [37].Dallagnol JCC, Ducatti DRB, Barreira SMW, Noseda MD, Duarte MER, Gonçalves AG, Synthesis of porphyrin glycoconjugates bearing thiourea, thiocarbamate and carbamate connecting groups: influence of the linker on chemical and photophysical properties, Dye, Pigment 107 (2014) 69–80, 10.1016/j.dyepig.2014.03.029. [DOI] [Google Scholar]
- [38].Slomp AM, Barreira SMW, Carrenho LZB, Vandresen CC, Zattoni IF, Ló SMS, Dallagnol JCC, Ducatti DRB, Orsato A, Duarte MER, Noseda MD, Otuki MF, Gonçalves AG, Photodynamic effect of meso - (aryl) porphyrins and meso - (1-methyl-4-pyridinium) porphyrins on HaCaT keratinocytes, Bioorg. Med. Chem. Lett 27 (2017) 156–161, 10.1016/j.bmcl.2016.11.094. [DOI] [PubMed] [Google Scholar]
- [39].Ló SMS, Ducatti DRB, Duarte MER, Barreira SMW, Noseda MD, Gonçalves AG, Synthesis of meso-tetraarylporphyrins using SeO2 as oxidant, Tetrahedron Lett. 52 (2011) 1441–1443, 10.1016/j.tetlet.2011.01.044. [DOI] [Google Scholar]
- [40].C.M.A. Alonso MGPMS, Neves AC, Tomé AMS, Silva JAS Cavaleiro, Reaction of (2-amino-5,10,15,20-tetraphenylporphyrinato)nickel(II) with quinones, Tetrahedron 61 (2005) 11866–11872, 10.1016/j.tet.2005.09.080. [DOI] [Google Scholar]
- [41].Ambudkar SV, Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells, Methods Enzymol. 292 (1998) 504–514, 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- [42].Krapf MK, Gallus J, Vahdati S, Wiese M, New inhibitors of breast cancer resistance protein (ABCG2) containing a 2,4-disubstituted pyridopyrimidine scaffold, J. Med. Chem. 61 (2018) 3389–3408, 10.1021/acs.jmedchem.7b01012. [DOI] [PubMed] [Google Scholar]
- [43].Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP, Structural basis of small-molecule inhibition of human multidrug transporter ABCG2, Nat. Struct. Mol. Biol. 25 (2018) 333–340, 10.1038/s41594-018-0049-1. [DOI] [PubMed] [Google Scholar]
- [44].Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W, Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments, J. Comput. Aided Mol. Des. 27 (2013) 221–234, 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- [45].Jacobson MP, Pincus DL, Rapp CS, Day TJF, Honig B, Shaw DE, Friesner RA, A hierarchical approach to all-atom protein loop prediction, Proteins 55 (2004) 351–367, 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- [46].Shelley JC, Cholleti A, Frye LL, Greenwood JR, Timlin MR, Uchimaya M, Epik: a software program for pK( a ) prediction and protonation state generation for drug-like molecules, J. Comput. Aided Mol. Des. 21 (2007) 681–691, 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- [47].Roos K, Wu C, Damm W, Reboul M, Stevenson JM, Lu C, Dahlgren MK, Mondal S, Chen W, Wang L, Abel R, Friesner RA, Harder ED, OPLS3e: extending Force Field Coverage for Drug-Like Small Molecules, J. Chem. Theor. Comput. 15 (2019) 1863–1874, 10.1021/acs.jctc.8b01026. [DOI] [PubMed] [Google Scholar]
- [48].Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS, Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy, J. Med. Chem. 47 (2004) 1739–1749, 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- [49].Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL, Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening, J. Med. Chem. 47 (2004) 1750–1759, 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- [50].Sherman W, Day T, Jacobson MP, Friesner RA, Farid R, Novel procedure for modeling ligand/receptor induced fit effects, J. Med. Chem. 49 (2006) 534–553, 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- [51].Bowers KJ, Chow E, Xu H, Dror RO, Eastwood MP, Gregersen BA, Klepeis JL, Kolossvary I, Moraes MA, Sacerdoti FD, Salmon JK, Shan Y, Shaw DE, Scalable algorithms for molecular dynamics simulations on commodity clusters, in: Proc. 2006 ACM/IEEE Conf. Supercomput., Association for Computing Machinery, New York, NY, USA, 2006, 10.1145/1188455.1188544, 84–es. [DOI] [Google Scholar]
- [52].Harder E, Damm W, Maple J, Wu C, Reboul M, Xiang JY, Wang L, Lupyan D, Dahlgren MK, Knight JL, Kaus JW, Cerutti DS, Krilov G, Jorgensen WL, Abel R, Friesner RA, OPLS3: a force field providing broad coverage of drug-like small molecules and proteins, J. Chem. Theor. Comput. 12 (2016) 281–296, 10.1021/acs.jctc.5b00864. [DOI] [PubMed] [Google Scholar]
- [53].Vesga LC, Kronenberger T, Tonduru AK, Kita DH, Zattoni IF, Bernal CC, Bóhorquez ARR, Mendez-Sánchez SC, V Ambudkar S, Valdameri G, Poso A, Tetrahydroquinoline/4,5-dihydroisoxazole molecular hybrids as novel inhibitors of breast cancer resistance protein (BCRP/ABCG2), ChemMedChem (2021), 10.1002/cmdc.202100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Guanaes LD, Guimarães MM, Ducatti DRB, Duarte MER, Barreira SMW, Noseda MD, Gonçalves AG, Synthesis and photophysical evaluation of meso-phenyl-1,4-dihydropyridine and pyridine-porphyrin hybrids, Chem. Heterocycl. Compd. (2021). In press. [Google Scholar]
- [55].Hall MD, Handley MD, Gottesman MM, Is resistance useless? Multidrug resistance and collateral sensitivity, Trends Pharmacol. Sci. 30 (2009) 546–556, 10.1016/j.tips.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Glavinas H, Kis E, Pál Á, Kovács R, Jani M, Vági E, Molnár É, Bánsághi S, Kele Z, Janáky T, Báthori G, von Richter O, Koomen G-JJ, Krajcsi P, ABCG2 (breast cancer resistance protein/mitoxantrone resistance-associated protein) ATPase assay: a useful tool to detect drug-transporter interactions, Drug Metab. Dispos. 35 (2007) 1533–1542, 10.1124/dmd.106.014605. [DOI] [PubMed] [Google Scholar]
- [57].Özvegy C, Litman T, Szakács G, Nagy Z, Bates S, Váradi A, Sarkadi B, Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells, Biochem. Biophys. Res. Commun. 285 (2001) 111–117, 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- [58].Müller P, Abdel SA, Zimmermann W, Wittig R, Stepp H, Abdel Gaber SA, Zimmermann W, Wittig R, Stepp H, ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells, J. Photochem. Photobiol. B Biol. 210 (2020) 111963, 10.1016/j.jphotobiol.2020.111963. [DOI] [PubMed] [Google Scholar]
- [59].Kim JH, Park JM, Roh YJ, Kim IW, Hasan T, Choi MG, Enhanced efficacy of photodynamic therapy by inhibiting ABCG2 in colon cancers, BMC Cancer 15 (2015) 1–9, 10.1186/s12885-015-1514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kita DH, Guragossian N, Zattoni IF, Moure VR, de M FG. Rego S, Lusvarghi T, Moulenat B, Belhani G, Picheth S, Bouacida Z, Bouaziz C, Marminon M, Berredjem J, Jose MB, Gonçalves S, Ambudkar V, Valdameri G, Le Borgne M, Mechanistic basis of breast cancer resistance protein inhibition by new indeno[1,2-b]indoles, Sci. Rep. 11 (2021) 1788, 10.1038/s41598-020-79892-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ji N, Yang Y, Lei Z, Cai C, Wang J, Gupta P, Ulixertinib ( BVD-523 ) antagonizes ABCB1- and ABCG2-mediated chemotherapeutic drug resistance, Biochem. Pharmacol. 158 (2018) 274–285, 10.1016/j.bcp.2018.10.028. [DOI] [PubMed] [Google Scholar]
- [62].Kraege S, Stefan K, Köhler SC, Wiese M, Optimization of acryloylphenylcarboxamides as inhibitors of ABCG2 and comparison with acryloylphenylcarboxylates, ChemMedChem 11 (2016) 2547–2558, 10.1002/cmdc.201600455. [DOI] [PubMed] [Google Scholar]
- [63].Zeng F, Wang F, Zheng Z, Chen Z, Wah To KK, Zhang H, Han Q, Fu L, Rociletinib CO (, Enhanced the efficacy of chemotherapeutic agents in ABCG2-overexpressing cancer cells in vitro and in vivo, Acta Pharm. Sin. B. 10 (2020) 799–811, 10.1016/j.apsb.2020.01.008, 1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Weidner LD, Zoghbi SS, Lu S, Shukla S, Ambudkar SV, Pike VW, Mulder J, Gottesman MM, Innis RB, Hall MD, The inhibitor Ko143 is not specific for ABCG2, J. Pharmacol. Exp. Therapeut. 354 (2015) 384–393, 10.1124/jpet.115.225482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Telbisz Á, Hegedüs C, Özvegy-Laczka C, Goda K, Várady G, Takáts Z, Szabó E, Sorrentino BP, Váradi A, Sarkadi B, Antibody binding shift assay for rapid screening of drug interactions with the human ABCG2 multidrug transporter, Eur. J. Pharmaceut. Sci. 45 (2012) 101–109, 10.1016/j.ejps.2011.10.021. [DOI] [PubMed] [Google Scholar]
- [66].Özvegy-Laczka C, Várady G, Köblös G, Ujhelly O, Cervenak J, Schuetz JD, Sorrentino BP, Koomen GJ, Váradi A, Német K, Sarkadi B, Function-dependent conformational changes of the ABCG2 multidrug transporter modify its interaction with a monoclonal antibody on the cell surface, J. Biol. Chem. 280 (2005) 4219–4227, 10.1074/jbc.M411338200. [DOI] [PubMed] [Google Scholar]
- [67].Henrich CJ, Robey RW, Bokesch HR, Bates SE, Shukla S, Ambudkar SV, Dean M, McMahon JB, New inhibitors of ABCG2 identified by high-throughput screening, Mol. Cancer Therapeut. 6 (2007) 3271–3278, 10.1158/1535-7163.MCT-07-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Khunweeraphong N, Stockner T, Kuchler K, The structure of the human ABC transporter ABCG2 reveals a novel mechanism for drug extrusion, Sci. Rep. 7 (2017) 13767, 10.1038/s41598-017-11794-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Miwa M, Tsukahara S, Ishikawa E, Asada S, Imai Y, Sugimoto Y, Single amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectants, Int. J. Cancer 107 (2003) 757–763, 10.1002/ijc.11484. [DOI] [PubMed] [Google Scholar]
- [70].Kowal J, Ni D, Jackson SM, Manolaridis I, Stahlberg H, Locher KP, Structural basis of drug recognition by the multidrug transporter ABCG2, J. Mol. Biol. 433 (2021) 166980, 10.1016/j.jmb.2021.166980. [DOI] [PubMed] [Google Scholar]
- [71].Khunweeraphong N, Kuchler K, The first intracellular loop is essential for the catalytic cycle of the human ABCG2 multidrug resistance transporter, FEBS Lett. 594 (2020) 4059–4075, 10.1002/1873-3468.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP, Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states, Nature 563 (2018) 426–430, 10.1038/s41586-018-0680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vlaming MLH, Lagas JS, Schinkel AH, Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice, Adv. Drug Deliv. Rev. 61 (2009) 14–25, 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- [74].Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F, The functions and structure of ABC transporters: implications for the design of new inhibitors of pgp and MRP1 to control multidrug resistance (MDR), Curr. Drug Targets 7 (2006) 893–909, 10.2174/138945006777709520. [DOI] [PubMed] [Google Scholar]
- [75].Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, Schinkel AH, Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan, J. Natl. Cancer Inst. 92 (2000) 1651–1656, 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- [76].Kruijtzer CMF, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC, Paul EM, Schellens JHM, Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918, J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 20 (2002) 2943–2950, 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.