Abstract
Timely and sensitive in vivo estimation of ischemic stroke-induced brain infarction are necessary to guide diagnosis and evaluation of treatments’ efficacy. The gold standard for estimation of the cerebral infarction volume is magnetic resonance imaging (MRI), which is expensive and not readily accessible. Measuring regional cerebral blood flow (rCBF) with Laser Doppler flowmetry (LDF) is the status quo for confirming reduced blood flow in experimental ischemic stroke models. However, rCBF reduction following cerebral artery occlusion often does not correlate with subsequent infarct volume. In the present study, we employed the continuous-wave near infrared spectroscopy (NIRS) technique to monitor cerebral oxygenation during 90 min of the intraluminal middle cerebral artery occlusion (MCAO) in Sprague-Dawley rats (n = 8, male). The NIRS device consisted of a controller module and an optical sensor with two LED light sources and two photodiodes making up two parallel channels for monitoring left and right cerebral hemispheres. Optical intensity measurements were converted to deoxyhemoglobin (Hb) and oxyhemoglobin (HbO2) changes relative to a 2-min window prior to MCAO. Area under the curve (auc) for Hb and HbO2 was calculated for the 90-min occlusion period for each hemisphere (ipsilateral and contralateral). To obtain a measure of total ischemia, auc of the contralateral side was subtracted from the ipsilateral side resulting in ΔHb and ΔHbO2 parameters. Infarct volume (IV) was calculated by triphenyl tetrazolium chloride (TTC) staining at 24h reperfusion. Results showed a significant negative correlation (r = −0.81, p = 0.03) between ΔHb and infarct volume. In conclusion, our results show feasibility of using a noninvasive optical imaging instrument, namely NIRS, in monitoring cerebral ischemia in a rodent stroke model. This cost-effective, non-invasive technique may improve the rigor of experimental models of ischemic stroke by enabling in vivo longitudinal assessment of cerebral oxygenation and ischemic injury.
Keywords: Middle cerebral artery occlusion (MCAO), Near infrared spectroscopy, Ischemic stroke, Optical imaging
1. Introduction
Stroke is the fifth most common cause of death and a leading cause of long-term disability among adults in the United States. Despite a concerted effort from pharmaceutical companies to develop novel therapies, more than 300 neuroprotective and thrombolytic drug candidates for ischemic stroke have failed in clinical trials between 1995 and 2015 (Chen and Wang, 2016), raising concerns regarding the efficacy of preclinical studies. Several national and international initiatives have been established, including the Stroke Preclinical Assessment Network (SPAN) (Lyden et al., 2022), to address the significant need to improve the scientific investigation of stroke. In addition, the recent successful development of thrombectomy for acute ischemic stroke has generated considerable enthusiasm to reassess neuroprotectant candidates in combination with thrombectomy.
Challenges in translational stroke research is attributable to inconsistent animal models, reliance on short-term end points, and inadequate optimization of the therapeutic time window (Fisher et al., 2009; Savitz and Fisher, 2007; Sena et al., 2010; Sutherland et al., 2012). In most animal stroke studies, infarct size is the main outcome measure and any treatment effect is evaluated by statistical comparison of infarct sizes of treatment versus placebo groups. Although parameters of experimental stroke models such as location and duration of vessel occlusion can be standardized, variability in infarct size is yet substantial (standard deviations of ~30%) due to variability of the surgical procedures and inter-animal variability of blood vessel anatomy (Leithner et al., 2015). Thus, in vivo knowledge of infarct volume before initiating a treatment is important for unbiased exclusion of outliers and reducing variability. It also prevents overestimation (Button et al., 2013) (which is abound in neuroscience publications) or underestimation of treatments’ effects. Diffusion-weighted and perfusion-weighted magnetic resonance imaging (MRI) are sensitive and specific tools for diagnosing infarction after stroke in humans (Neumann-Haefelin et al., 1999; Sorensen et al., 1996). However, MRI equipment is not generally accessible for preclinical studies, especially in smaller research institutes. Also, MRI of animals should be performed under anesthesia, which can itself be neuroprotective, producing biased outcomes (Bleilevens et al., 2013; Kitano et al., 2007); particularly, isoflurane is known to increase cerebral blood flow (CBF) and reduce the infarct size. Laser Doppler flowmetry (LDF) is the most commonly used technique for CBF measurements during the procedures of middle cerebral artery occlusion (MCAO) to ensure a proper and sustained reduction of CBF (Liu et al., 2009), but LDF measures of CBF do not correlate with the subsequent infarct outcome.
Ultimately, there is an unmet need for an affordable and easy-to-use device for accurate validation of animal models of ischemic stroke. Such a tool will enable in vivo estimation of ischemic injury and the longitudinal evaluation of treatment efficacy in preclinical models of stroke. In this study, we used a miniaturized, optical imaging tool based on Near Infrared Spectroscopy (NIRS) technique (Jöbsis, 1977) for noninvasive mapping of cerebral oxygenation in real time and quantitative in vivo estimation of ischemia-induced brain infarction. NIRS theory is simpler than that of MRI and the costs are also lower. A commercial NIRS device costs the same as a head coil for an MRI scanner. Continuous wave, LED-based NIRS used in this study is a simple, compact, noninvasive, and inexpensive device that can continuously monitor brain oxygenation during the MCAO surgery and days and/or weeks post-stroke to provide an estimate of the CBF changes. No other in vivo CBF measurement approaches are readily available for accurately monitoring the CBF and providing prognosis information about outcomes of infarct or neurological behavioral deficits. Our study shows a predictive, negative correlation (r = 0.81, p = 0.03) between cerebral oxygenation on the contralateral side and ipsilateral infarct volume. These findings suggest that LED-based NIRS has translational potential in preclinical stroke treatment studies that could improve the value of preclinical outcomes to predict efficacy in human clinical trials.
2. Materials and methods
2.1. Animal preparation
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The manuscript adheres to the ARRIVE guidelines for reporting animal experiments.
Male Sprague-Dawley (CD) rats of 9–10 weeks of age were purchased from Charles River Laboratories (Raleigh, NC) and maintained in the University Laboratory Animal Facility until experimental use. All rats were housed in group (2 per case) in a controlled temperature and humidity environment. They were maintained on a 12-h light/12-h dark cycle and provided with food and water ad libitum. At the time of experimental use, rats weighed 290–360 g.
2.2. Middle cerebral artery occlusion model
Focal cerebral ischemia was induced by transient occlusion of the left middle cerebral artery (MCA) for 90 min as described previously (Bhuiyan et al., 2017). Briefly, under an operating microscope, the left common carotid artery was exposed through a midline incision. Two branches of the external carotid artery (ECA), occipital and superior thyroid arteries, were isolated and coagulated. The ECA was dissected further distally and ligated. The internal carotid artery (ICA) was isolated and carefully separated from the adjacent vagus nerve. The extra-cranial branch of the ICA, the pterygopalatine artery, was then dissected and temporarily ligated. A 22 mm length of silicon-coated nylon filament (size 4–0, native diameter 0.19 mm; diameter with coating 0.41 ±0.02 mm; coating length 4–5 mm; Doccol Corporation, Sharon, MA) was introduced into the ECA lumen through a puncture. The silk suture around the ECA stump was tightened around the intraluminal nylon suture to prevent bleeding. The nylon suture was then gently advanced from the ECA to the ICA lumen until mild resistance was felt. For reperfusion, the suture was withdrawn 90 min after MCAO to restore blood flow (reperfusion). Body temperature was maintained for the duration of the experiment between 36.5 °C-37 °C with a heating blanket.
2.3. Neurological deficit scoring
A neurological deficit grading system (Bhuiyan et al., 2017) was used to evaluate neurological deficit in at 1, and 24 h after reperfusion. The scores are: 0, no observable deficit; 1, forelimb flexion; 2, forelimb flexion and decreased resistance to lateral push; 3, forelimb flexion, decreased resistance to lateral push, and unilateral circling; 4, forelimb flexion and partial or complete lack of ambulation.
2.4. Calculation of infarct volume
After 24 h of reperfusion, rats were anesthetized with 4% isoflurane vaporized in N2O and O2 (3:2) and decapitated. Brains were removed and 2-mm coronal slices were made with a rodent brain matrix. The sections were stained for 20 min at 37 °C with 1% 2, 3, 5-triphenyltetrazolium chloride monohydrate. Infarct volume and hemispheric swelling were measured using ImageJ software (NIH, Bethesda, MD, USA). Infarct volume was corrected for swelling as described by Swanson et al. (1990). Briefly, the sections were scanned, and the ischemic area in each section was calculated by subtracting the non-infarct area in the ipsilateral hemisphere from the total area of the contralateral hemisphere. The infarct areas were summed across all slices and multiplied by the slice thickness (2 mm) giving the total infarct volume (mm3).
2.5. NIRS principles and instrumentation
In the near infrared range (700–900 nm), water, the main ingredient of tissues in vivo, has the lowest light absorption, whereas deoxyhemoglobin (Hb) and oxy-hemoglobin (HbO2) chromophores are the main absorbers with distinctive absorption characteristics. By choosing two wavelengths in the near infrared spectrum and measuring the attenuation change at two different time points, the relative change in the concentration of Hb and HbO2 molecules can be calculated using the modified Beer-Lambert law (Cope et al., 1988):
where:
- ΔODλ is optical density, which is the change in optical intensity for the wavelength λ;
-Ib is the light intensity measured during baseline;
-It is the light intensity detected during or after a given task;
- and are the absorption coefficients of Hb and HbO2 molecules at the wavelength λ;
-ΔcHb and ΔcHbO2 are the concentration changes of Hb and HbO2 molecules due to the task;
-d is the physical distance between the light source and the photodetector;
-and DPFλ is the differential pathlength factor adjusted for the increased pathlength between the light source and the photodetector due to scattering at the wavelength λ.
When measured at two wavelengths λ1 and λ2, this equation can be solved for the change in the concentration of Hb and HbO2 molecules.
The continuous-wave NIRS system used in this study had three main components: (1) optical sensors consisting of two multiwavelength (730 and 850 nm) LED (Marubeni) and two photodiodes (PD15–22C/TR8) built on a flexible circuit substrate to conform to the rat’s head (Fig. 1); (2) an Arduino-based controller unit for operating the optical sensors at a 200Hz sampling frequency; (3) a computer running the data collection GUI created in LabVIEW for data acquisition and real-time plotting of the data. The system was calibrated using a solid phantom in accordance with pre-determined specifications.
Fig. 1. Schematic illustration of the NIRS.
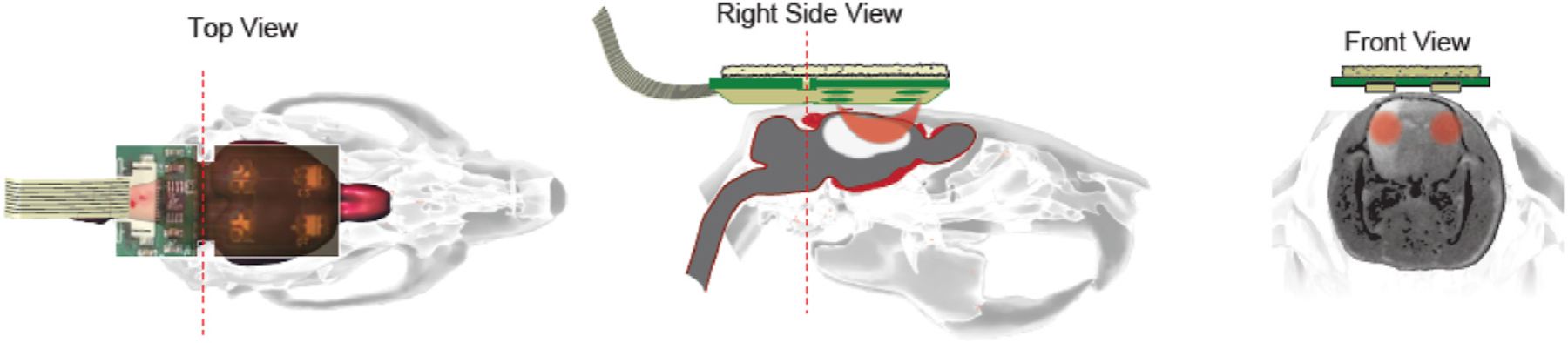
Location of the NIRS probe on the rat’s head (Top view). The photons that are emitted by the LED are capturedby the photodiode through the path (orange color banana-shaped path in Right side view). NIRS data is collected on the left and right side of the rat brain to analyze the ipsilateral and the contralateral hemisphere cerebral blood flow (Front view).
2.6. Signal processing and data analysis
Signal processing was performed in MATLAB and data analysis was conducted in IBM SPSS. Each channel was visually inspected to reject any saturated or low signals. The accepted NIRS raw data was then filtered through a lowpass filter (fc = 0.1 Hz) where external interferences were present. Area under the curve for the 90-min occlusion period for each hemisphere (ipsilateral and contralateral) were calculated by the trapz function in MATLAB.
3. Results
To obtain a measure of total ischemia during 90min occlusion, we subtracted the area under the curve of the contralateral (nonischemic) sides from the ipsilateral (ischemic) side for Hb (ΔHb) and HbO2 (ΔHbO2). Pearson correlation analysis between ΔHb and the infarct volume yielded a significant negative correlation (r = −0.81, p = 0.03); but no significant correlation was found between ΔHbO2 and the infarct volume (r = 0.17, p = 0.70) (Fig. 3). In other words, we found that the infarct volume at 24 h post-reperfusion is inversely correlated with the trans-hemispheric ischemia gradient (ipsilateral – contralateral) during occlusion: the more deoxygenated the contralateral side relative to the ipsilateral side, the larger the final infarct volume.
Fig. 3.

A significant linear correlation was found between ΔHb and infarct volume. Shown are results of correlation analysis between ΔHb, ΔHbO2 and infarct volume. Area under the curve (auc) for Hb and HbO2 in the 90-min occlusion period from Fig. 2 was calculated for each hemisphere (ipsilateral and contralateral) to obtain a measure of total ischemia for ΔHb (A) and ΔHbO2 (B) defined as auc on the contralateral side minus auc on the ipsilateral side.
The experimental design showing different time points of MCAO, NIRS, neurological scoring and TTC staining is depicted in Fig. 2A and representative traces of Hb and HbO2 for two rats with small and large infarct sizes are shown in Fig. 2B and C. A neurological deficit score of 3 was obtained in all the seven rats at 1h post-reperfusion. At 24h post-reperfusion, variable scores were obtained with worse scores for animals with larger infarct volumes (Fig. 4).
Fig. 2. Differences in hemodynamic response during and after stroke.

(A) Experimental design showing onset time points of MCAO and NIRS, neurological scoring and TTC staining data collection. (B and C) Representative tracing of continuous NIRS measurement in contralateral (CL) and ipsilateral (IL) hemisphere of rat brain during 90 min of MCAO (grey bar) and 60 min of reperfusion (pink bar). A corresponding image of TTC staining of the same rat’s brain at 24 h after reperfusion is shown. Hb: deoxyhemoglobin, HbO2: oxyhemoglobin.
Fig. 4.

Neurological deficit scores at 24h post-reperfusion was proportional to the infarct volume. The size of the circle (red) represents the relative infarct volume for each animal.
4. Discussion
In this study, we used a miniaturized continuous-wave NIRS device to monitor cerebral oxygenation parameters during transient middle cerebral artery occlusion in seven male Sprague Dawley rats. Our optical sensors allowed continuous measurement from both cerebral hemispheres before and during occlusion as well as after reperfusion. Our results indicated that the magnitude of cerebral deoxygenation on the contralateral hemisphere relative to the ipsilateral hemisphere was significantly correlated with the infarct volume.
Previous clinical and animal studies have reported that changes in the contralateral side of cerebral blood supply to the brain are important compensated mechanisms for reducing brain lesion and/or improving neurological function recovery after stroke (Horgan and Finn, 1997). For example, increased blood flow velocity of the contralateral internal cerebral artery and basilar artery in the post-stroke recovery stage was associated with better motor function in rats (Li et al., 2010). Another study identified that limited extent of native leptomeningeal collaterals affects downstream hemodynamics in Balb/C mice. Lower collateral flow during- and one day after MCAO, coincided with a greater infarct size and worse functional outcome (Kanoke et al., 2020). Following MCAO, collateral vessels undergo immense vascular restructuring and remodeling (enlargement). The middle cerebral arteries (MCAs), anterior cerebral arteries (ACAs), and posterior cerebral arteries (PCAs) anastomose at their distal ends to form the MCA-ACA and MCA-PCA collateral vessels (Okyere et al., 2020). Collateral vessels connect two arterial branches with opposing flow to allow for unidirectional retrograde blood reperfusion into the area of an occluded arterial branch (Okyere et al., 2020). Our findings further support the notion that adaptive changes of blood supply from the contralateral hemisphere compensates the ipsilateral side after the onset of stroke and leads to smaller infarct formation/or better neurological function recovery. Future study is needed with additional approaches to identify whether native leptomeningeal collaterals or other cerebral artery branches are main components for elevated HbO2 (ΔHbO2). Elevated brain edema may also link poor collateral status and infarct volume (Broocks et al., 2019).
Stroke Therapy Academic Industry Roundtable (STAIR) recommends including imaging endpoints for reducing variance and sample size (Fisher et al., 2009). To our knowledge, this is the first study to use a noninvasive optical imaging tool, namely near infrared spectroscopy (NIRS), to investigate the relationship between bilateral cerebral oxygenation and outcome in a preclinical model of ischemic stroke. We found a similar LED-based NIRS study of MCAO in rats, which aimed to validate the cerebral oxygenation parameters (i.e. Hb and HbO2) during occlusion and 1.5 h post reperfusion with the lactate/pyruvate ratio measured by microdialysis, but without any regard to outcome (Kuo et al., 2014). Unlike the fiber optic-based NIRS technology, which is significantly more expensive (>10X) and requires surgical implantation, LED-based NIRS used in this study is economical, noninvasive, and easy to apply. We showed here that continuous-wave NIRS can provide an in vivo estimate of brain infarction and early ischemic lesion volumes. Such knowledge may be used for unbiased exclusion of outliers leading to reduced variability and smaller sample sizes, which translates to lower costs of animal studies and increased time efficiency. Moreover, NIRS enables longitudinal recording of cerebral oxygenation, which will enhance the rigor of stroke research by facilitating in vivo validation of injury and reperfusion and expediting novel fields of stroke research such as spatio-temporal study of pathophysiology of infarct formation and evolution, peri-infarct depolarization (Wolf et al., 1997), real-time CBF monitoring (Culver et al., 2003), and estimation of the hypoxic state of brain cells (Kuo et al., 2014). This technology may also facilitate drug discovery for stroke recovery-enhancing drugs (e.g. monoamine agonists like amphetamines (Stroemer et al., 1998) and neurotrophic growth factors (Kawamata et al., 1997) that require prolonged assessments.
In conclusion, our results show feasibility of using a noninvasive optical imaging instrument, namely NIRS, in monitoring cerebral ischemia in rodent stroke model. This cost-effective, non-invasive technique will improve the rigor of experimental models of ischemic stroke by enabling in vivo and longitudinal assessment of ischemic injury. Our study had several caveats such as lack of end-to-end comparison with a benchmark method and absence of a novel treatment group along with a mechanistic investigation. This feasibility study helps pave the way for additional research to address these caveats.
Acknowledgements
Research reported in this publication was supported (whole or in part) by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM130443 and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number 1R43NS100174–01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of competing interest
Dr. Barati and Dr. Pourrezaei are Co-founders of Barati Medical LLC. Ardy Wong is a part-time employee of Barati Medical LLC.
Data availability
Data will be made available on request.
References
- Bhuiyan MIH, Song S, Yuan H, Begum G, Kofler J, Kahle KT, Yang SS, Lin SH, Alper SL, Subramanya AR, Sun D, 2017. WNK-Cab39-NKCC1 signaling increases the susceptibility to ischemic brain damage in hypertensive rats. J. Cerebr. Blood Flow Metabol 37 (8), 2780–2794. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleilevens C, Roehl AB, Goetzenich A, Zoremba N, Kipp M, Dang J, Tolba R, Rossaint R, Hein M, 2013. Effect of anesthesia and cerebral blood flow on neuronal injury in a rat middle cerebral artery occlusion (MCAO) model. Exp. Brain Res 224 (2), 155–164. 10.1007/s00221-012-3296-0. [DOI] [PubMed] [Google Scholar]
- Broocks G, Kemmling A, Meyer L, Nawabi J, Schön G, Fiehler J, Kniep H, Hanning U, 2019. Computed tomography angiography collateral profile is directly linked to early edema progression rate in acute ischemic stroke. Stroke 50 (12), 3424–3430. 10.1161/strokeaha.119.027062. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò, M.R., 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci 14 (5), 365–376. 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang K, 2016. The fate of medications evaluated for ischemic stroke pharmacotherapy over the period 1995–2015. Acta Pharm. Sin B 6 (6), 522–530. 10.1016/j.apsb.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope M, Delpy DT, Reynolds EO, Wray S, Wyatt J, van der Zee P, 1988. Methods of quantitating cerebral near infrared spectroscopy data. Adv. Exp. Med. Biol 222, 183–189. 10.1007/978-1-4615-9510-6_21. [DOI] [PubMed] [Google Scholar]
- Culver JP, Durduran T, Furuya D, Cheung C, Greenberg JH, Yodh AG, 2003. Diffuse optical tomography of cerebral blood flow, oxygenation, and metabolism in rat during focal ischemia. J. Cerebr. Blood Flow Metabol 23 (8), 911–924. 10.1097/01.Wcb.0000076703.71231.Bb. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, 2009. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40 (6), 2244–2250. 10.1161/strokeaha.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan NF, Finn AM, 1997. Motor recovery following stroke: a basis for evaluation, 1997/01/01 Disabil. Rehabil 19 (2), 64–70. 10.3109/09638289709166829. [DOI] [PubMed] [Google Scholar]
- Jöbsis FF, 1977. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198 (4323), 1264–1267. 10.1126/science.929199. Dec 23. [DOI] [PubMed] [Google Scholar]
- Kanoke A, Akamatsu Y, Nishijima Y, To E, Lee CC, Li Y, Wang RK, Tominaga T, Liu J, 2020. The impact of native leptomeningeal collateralization on rapid blood flow recruitment following ischemic stroke. J. Cerebr. Blood Flow Metabol 40 (11), 2165–2178. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, Dietrich WD, Schallert T, Gotts JE, Cocke RR, Benowitz LI, Finklestein SP, 1997. Intracisternal basic fibroblast growth factor enhances functional recovery and up-regulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc. Natl. Acad. Sci. U. S. A 94 (15), 8179–8184. http://www.jstor.org/stable/42828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H, Kirsch JR, Hurn PD, Murphy SJ, 2007. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J. Cerebr. Blood Flow Metabol 27 (6), 1108–1128. 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JR, Lin BS, Cheng CL, Chio CC, 2014. Hypoxic-state estimation of brain cells by using wireless near-infrared spectroscopy. IEEE Journal of Biomedical and Health Informatics 18 (1), 167–173. 10.1109/JBHI.2013.2261310. [DOI] [PubMed] [Google Scholar]
- Leithner C, Füchtemeier M, Jorks D, Mueller S, Dirnagl U, Royl G, 2015. Infarct volume prediction by early magnetic resonance imaging in a murine stroke model depends on ischemia duration and time of imaging. Stroke 46 (11), 3249–3259. 10.1161/strokeaha.114.007832. [DOI] [PubMed] [Google Scholar]
- Li L, Ke Z, Tong KY, Ying M, 2010. Evaluation of cerebral blood flow changes in focal cerebral ischemia rats by using transcranial Doppler ultrasonography. Ultrasound Med. Biol 36 (4), 595–603. 10.1016/j.ultrasmedbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhen G, Meloni BP, Campbell K, Winn HR, 2009. Rodent stroke model guidelines for preclinical stroke trials (1ST edition). J Exp Stroke Transl Med 2 (2), 2–27. 10.6030/1939-067x-2.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden PD, Bosetti F, Diniz MA, Rogatko A, Koenig JI, Lamb J, Nagarkatti KA, Cabeen RP, Hess DC, Kamat PK, Khan MB, Wood K, Dhandapani K, Arbab AS, Leira EC, Chauhan AK, Dhanesha N, Patel RB, Kumskova M, Thedens D, Morais A, Imai T, Qin T, Ayata C, Boisserand LSB, Herman AL, Beatty HE, Velazquez SE, Diaz-Perez S, Sanganahalli BG, Mihailovic JM, Hyder F, Sansing LH, Koehler RC, Lannon S, Shi Y, Karuppagounder SS, Bibic A, Akhter K, Aronowski J, McCullough LD, Chauhan A, Goh A, 2022. The stroke preclinical assessment Network: rationale, design, feasibility, and stage 1 results. Stroke 53 (5), 1802–1812. 10.1161/strokeaha.121.038047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Wittsack H.-J. r., Wenserski F, Siebler M, Seitz Rd.J., Mödder, U., Freund, H.-J., 1999. Diffusion-and perfusion-weighted MRI: the DWI/PWI mismatch region in acute stroke. Stroke 30 (8), 1591–1597. [DOI] [PubMed] [Google Scholar]
- Okyere B, Mills III WA, Wang X, Chen M, Chen J, Hazy A, Qian Y, Matson JB, Theus MH, 2020. EphA4/Tie2 crosstalk regulates leptomeningeal collateral remodeling following ischemic stroke, 02/03/ J. Clin. Investig 130 (2), 1024–1035. 10.1172/JCI131493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Fisher M, 2007. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann. Neurol 61 (5), 396–402. 10.1002/ana.21127.Mar 30 [DOI] [PubMed] [Google Scholar]
- Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR, 2010. ). Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol 8 (3), e1000344. 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen AG, Buonanno FS, Gonzalez RG, Schwamm LH, Lev MH, Huang-Hellinger FR, Reese TG, Weisskoff RM, Davis TL, Suwanwela N, Can U, Moreira JA, Copen WA, Look RB, Finklestein SP, Rosen BR, Koroshetz WJ, 1996. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 199 (2), 391–401. 10.1148/radiology.199.2.8668784, 1996/05//. [DOI] [PubMed] [Google Scholar]
- Nov Stroemer RP, Kent TA, Hulsebosch CE, 1998. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke 29 (11), 2381–2393. 10.1161/01.str.29.11.2381. discussion 2393–2385. [DOI] [PubMed] [Google Scholar]
- Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C, 2012. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int. J. Stroke 7 (5), 407–418. 10.1111/j.1747-4949.2012.00770.x. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR, 1990. A semiautomated method for measuring brain infarct volume. J. Cerebr. Blood Flow Metabol 10 (2), 290–293. 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Wolf T, Lindauer U, Reuter U, Back T, Villringer A, Einhäupl K, Dirnagl U, 1997. Noninvasive near infrared spectroscopy monitoring of regional cerebral blood oxygenation changes during peri-infarct depolarizations in focal cerebral ischemia in the rat. J. Cerebr. Blood Flow Metabol 17 (9), 950–954. 10.1097/00004647-199709000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


