Abstract
Background:
Randomized trials in pregnancy are extremely challenging, and observational studies are often the only option to evaluate medication safety during pregnancy. However, such studies are often susceptible to immortal time bias if treatment initiation occurs after time zero of follow-up. We describe how emulating a sequence of target trials avoids immortal time bias and apply the approach to estimate the safety of antibiotic initiation between 24-37 weeks’ gestation on preterm delivery.
Methods:
The Tsepamo Study captured birth outcomes at hospitals throughout Botswana from 2014-2021. We emulated 13 sequential target trials of antibiotic initiation versus no initiation among individuals presenting to care <24 weeks, one for each week from 24-37 weeks. For each trial, eligible individuals had not previously initiated antibiotics. We also conducted an analysis susceptible to immortal time bias by defining time zero as 24 weeks and exposure as antibiotic initiation between 24-37 weeks. We calculated adjusted risk ratios (RR) and 95% confidence intervals (CI) for preterm delivery.
Results:
Of 111,403 eligible individuals, 17,009 (15.3%) initiated antibiotics between 24-37 weeks. In the sequence of target trials, RRs (95% CIs) ranged from 1.04 (0.90, 1.19) to 1.24 (1.11, 1.39) (Pooled RR: 1.11 [1.06, 1.15]). In the analysis susceptible to immortal time bias, the RR was 0.90 (0.86, 0.94).
Conclusions:
Defining exposure as antibiotic initiation at any time during follow-up after time zero resulted in substantial immortal time bias, making antibiotics appear protective against preterm delivery. Conducting a sequence of target trials can avoid immortal time bias in pregnancy studies.
Keywords: immortal time bias, pregnancy, target trial emulation
Introduction
Randomized clinical trials are considered the gold standard for estimating causal effects. However, randomized clinical trials are often unethical, too expensive, or otherwise not feasible for specific research questions, and often have restrictive, homogeneous samples. These challenges are particularly relevant in the setting of evaluating medication safety and effectiveness in pregnancy, where outcomes of interest are often rare and where ethical concerns have led to pregnant people historically being systematically excluded from clinical trials. When randomized trials are not feasible, observational data and appropriate methods are necessary to attempt to estimate causal effects. However, analyses of observational data are susceptible to confounding, selection bias, and misclassification bias, especially in the setting of time-varying exposures. Immortal time bias, a bias that can be introduced when treatment initiation occurs after time zero of follow-up,1–3 is particularly common in observational studies of medication safety and effectiveness in pregnancy, where exposures are often defined as “at any time during a period”.4–12
Target trial emulation aids researchers in defining causal estimands and attempting to estimate corresponding causal effects from observational data.13 The target trial approach involves two steps. First, researchers specify the protocol of the hypothetical randomized trial (the target trial) that they would like to conduct to answer the causal question of interest if there were no resource or ethical constraints. Researchers outline key components of this target trial -- the eligibility criteria, treatment strategies, treatment assignment, start and end of follow-up, outcomes of interest, causal contrasts of interest, and analysis plan. Second, researchers use observational data to attempt to emulate each component of the target trial. Through explicitly describing and attempting to emulate each component of the target trial, many of the selection and residual confounding biases that may occur with standard observational study design and analysis can be avoided. One such common flaw is the failure to align the start of follow-up (time zero), treatment assignment, and eligibility, which can result in immortal time bias.1 Emulating a target trial requires explicitly defining time zero, time at treatment assignment, and time when eligibility criteria are met, thus reducing the likelihood of this time-related bias.
When few individuals initiate a medication at any one point in time, the target trial approach can be extended to emulate a sequence of target trials. While target trial emulation and this specific extension have been described extensively in the literature,14–20 they have rarely been applied to pregnancy studies,11,21–24 and observational studies in pregnancy that are susceptible to immortal time bias are still common in the literature. Here, we describe how to emulate a sequence of target trials to avoid immortal time bias in observational studies of medication safety and effectiveness in pregnancy. We apply this approach to identify the safety of antibiotic initiation between 24 and 37 weeks’ gestation with respect to preterm delivery, an association that has been extensively explored in the literature and that has led to conflicting results.3,10 We use data from the Tsepamo Study, which has been conducting birth outcomes surveillance throughout Botswana since 2014.
Methods
The Tsepamo Study
Tsepamo is an ongoing birth outcomes surveillance study in Botswana.25 Data are abstracted from the maternity obstetric record (a record of antenatal care) at the time of delivery from all livebirths and stillbirths at up to 18 government hospitals throughout the country. Tsepamo included eight hospitals (~45% of all births in Botswana) from August 2014-July 2018 and 18 hospitals (~72% of all births) from July 2018-August 2021. The Tsepamo study captures data on >99% of births that take place at the included sites.25,26 In Botswana, approximately 95% of births occur at a hospital.27
The maternity obstetric record captures information on demographics, past medical history, diagnoses, hospitalizations and complications during pregnancy, medications prescribed during pregnancy, HIV history, and clinical information including lab results, blood pressure, and weight measurements. All antibiotic medications prescribed during pregnancy (prior to the delivery hospitalization) are recorded in the obstetric record. According to Botswana STI treatment guidelines, typical antibiotic treatment courses last between one and 7 days.28,29 Birth outcomes including stillbirth, neonatal death, birth weight, and gestational age at delivery are recorded in the delivery record. Preterm delivery is defined as live birth or stillbirth between 24 and 37 weeks’ gestation. Gestational age at delivery is recorded by the midwife using the estimated date of delivery (EDD). EDD is calculated at the first antenatal visit using the reported last menstrual period (LMP) and confirmed by ultrasound when available. If the LMP date is unknown or suspected to be incorrect, midwives may use fundal height measurements to estimate the EDD. The Tsepamo Study has been approved by the IRB at Harvard University and by the Human Research Development Council (HRDC) in Botswana.
Emulating a target trial of antibiotic initiation at 24 weeks’ gestation
We first describe a procedure for emulating a (hypothetical) target trial to estimate the effect of antibiotic initiation at 24 weeks’ gestation.13 The protocol of the target trial is as follows. Pregnant people who present to antenatal care prior to 24 weeks’ gestation with a singleton pregnancy, and who have not used antibiotics prior to 24 weeks’ gestation are eligible for the trial and enrolled at 24 weeks’ gestation. Eligible individuals are then randomized to one of two treatment strategies: 1) initiate antibiotics between 24 and 25 weeks’ gestation or 2) do not initiate antibiotics between 24 and 25 weeks’ gestation. Individuals are then followed from baseline (24 weeks’ gestation) to the time of delivery or 37 weeks’ gestation, whichever occurs first. The primary outcome of interest is preterm delivery. The causal contrast of interest is the intention-to-treat effect (individuals who initiate antibiotics after baseline are still considered unexposed). To estimate this causal effect, we (would hypothetically) fit a log-binomial regression model to estimate the risk ratio for preterm delivery by treatment strategy.
To emulate this target trial, we use observational data from the Tsepamo Study. The eligibility criteria, treatment strategies, start and end of follow-up, outcome of interest, causal contrast of interest, and analysis plan are the same as in the target trial, with three exceptions. First, since the date of antibiotic initiation is not always known in Tsepamo, we exclude individuals who initiate antibiotics at some point during pregnancy but for whom the date of initiation is unknown (<1%). Second, because antibiotic initiation is not randomly assigned in the Tsepamo Study, the causal contrast of interest is an observational analog to the intention-to-treat effect, that is, the effect of initiating (rather than assignment to) antibiotics at 24-25 weeks’ gestation versus not initiating antibiotics at 24-25 weeks’ gestation. Third, because antibiotic initiation is not randomly assigned, we attempt to emulate the randomization component of the target trial by including the following variables measured in the pregnant individual as adjustment variables in the outcome regression model: gestational age at first antenatal visit (<12 weeks; 12-24 weeks), age (<18; 18-35; ≥35 years), education (secondary education or higher; other or unknown), employment status (salaried; other or unknown), parity (one or more children; 0 or unknown), marital status (yes; no or unknown), HIV status (yes; no or unknown), first weight in pregnancy (<50 kg; 50-80kg; ≥80kg), calendar year of delivery (2014-2016; 2017-2019; 2020-2021), delivery site (urban, rural), history of preterm delivery or stillbirth (yes; no; unknown or no prior births), and first antenatal visit syphilis screening results (positive; negative; unknown).
Because neither infection nor potential prophylactic indications are recorded in the data, we cannot consider them as inclusion criteria (as a realistic trial would have done). Therefore, the estimated effect of antibiotics would reflect a combination of clinically apparent infections and antibiotics, i.e., in this application we are not attempting to separate the effect of antibiotics from the effect of the indications on the risk of preterm delivery. We acknowledge that this trial is purely hypothetical with the goal of illustrating the methods.
Emulating a sequence of 13 target trials of antibiotic initiation during each week from 24 weeks’ through 36 weeks’ gestation
Because few individuals initiate antibiotics in any given week and because we may be interested in the effects of antibiotic initiation throughout the pregnancy period, we can emulate a sequence of target trials of antibiotic initiation during each week from 24 weeks’ through 36 weeks’ gestation (each with a 1-week enrollment period).14,23,24 The last target trial begins at 36 weeks’ gestation because individuals are no longer at risk for the outcome (preterm delivery) at 37 weeks’ gestation. For each sequential trial, eligible individuals have not previously initiated antibiotics and remain pregnant at the start of that week. For example, pregnant people who were eligible for the target trial of antibiotic initiation at 24 weeks’ gestation who did not initiate antibiotics between 24 and 25 weeks and remain pregnant at the beginning of week 25 are eligible for the target trial of antibiotic initiation at 25 weeks and enrolled at that time. For each sequential trial, we then compare those who initiate antibiotics during that week with those who do not and follow both groups from baseline until delivery or 37 weeks’ gestation, whichever occurs first. For each emulated trial, we fit a separate log-binomial regression model to estimate the risk ratio for preterm delivery by treatment strategy (initiate antibiotics at week x versus do not initiate antibiotics at week x, where x takes values from 24-36), adjusting for the same baseline maternal covariates described above.
Pooling results from all 13 target trials
In addition to obtaining risk ratios and 95% confidence intervals for each of the 13 sequential target trials, we can pool results across the 13 trials to obtain one estimate of the effect of antibiotic initiation between 24 and 37 weeks’ gestation on preterm delivery, under the assumption of no effect modification by gestational age. The estimand corresponds to a target trial in which pregnant people are identified between gestational week 24 and 36 and assigned to antibiotic initiation at that week or not, and then followed until delivery. To do so, we pool data from all 13 trials into a single model and include “trial” (taking values from 1-13 and modeled with restricted cubic splines) as an adjustment variable in our model (in addition to the same maternal covariates described above). To account for the repeated use of individuals, we used nonparametric bootstrapping with 500 samples to calculate percentile-based 95% confidence intervals.17,19
Analysis susceptible to immortal time bias
Emulating a sequence of target trials avoids immortal time bias by aligning time zero, eligibility, and treatment ascertainment. To evaluate the extent to which immortal time bias resulting from a misalignment of time zero and treatment ascertainment could bias results, we also conduct an analysis where we define the start of follow-up as 24 weeks’ gestation and define exposure as antibiotic initiation at any point between 24-37 weeks’ gestation. We estimate the risk ratio for preterm delivery by treatment strategy via a log-binomial regression model and adjust for the same set of maternal covariates described previously. Importantly, the source of bias here is not the definition of the treatment strategy itself but rather the analysis that retrospectively assigns individuals to a treatment strategy based on whether treatment is initiated after time zero, thus misclassifying the time from week 24 to antibiotic initiation as exposed.1
Additional analyses for separate causal questions
Our primary causal question of interest focuses on antibiotic initiation at or after 24 weeks’ gestation. A different causal question may focus on antibiotic initiation during different periods of pregnancy, such as prior to 24 weeks’ gestation. Since preterm delivery is defined as delivery between 24 and 37 weeks’ gestation (delivery prior to 24 weeks’ gestation is defined as spontaneous abortion), an analysis comparing antibiotic initiation versus no initiation prior to 24 weeks’ gestation would not be susceptible to immortal time bias because the treatment window occurs prior to time zero (24 weeks’ gestation). While changing the treatment ascertainment window is one solution to preventing immortal time bias, the potential for bias remains because we have still failed to align the treatment window and time zero and have now introduced the potential for selection bias by conditioning on remaining pregnant at 24 weeks’ gestation.30 For demonstrative purposes, we emulate a target trial of antibiotic initiation prior to 24 weeks’ gestation and preterm delivery as a secondary analysis. Pregnant people who present to antenatal care prior to 24 weeks’ gestation with a singleton pregnancy are eligible for the trial. Eligible individuals are then randomized to one of two treatment strategies: 1) initiate antibiotics prior to 24 weeks’ gestation or 2) do not initiate antibiotics prior to 24 weeks’ gestation. Individuals are then followed from baseline (date of presentation to care) to the time of delivery or 37 weeks’ gestation, whichever occurs first. The primary outcome of interest is preterm delivery. The causal contrast of interest is the effect of initiating antibiotic use prior to 24 weeks’ gestation versus not initiating antibiotics prior to 24 weeks’ gestation (we note here that defining causal contrasts of interest in the setting of competing events is not trivial31–33). We fit a log-binomial regression model to estimate the risk ratio for preterm delivery by treatment strategy. Emulating this target trial using observational data from Tsepamo proceeds in the same way as described above.
Results
111,403 pregnant people presented to antenatal care prior to 24 weeks’ gestation and met our eligibility criteria. Of these, 17,009 (15.3%) initiated antibiotics at some point between 24 and 37 weeks’ gestation and 94,394 (84.7%) did not; this corresponded to 17,009 initiator ‘person–trials’ and 1,290,635 non-initiator ‘person–trials’ (since non-initiators may be eligible for more than one emulated trial). In week 24, 1,371 (1.2%) individuals initiated antibiotics and 110,032 (98.8%) did not. The percent of eligible individuals initiating antibiotics in a given week was fairly stable throughout pregnancy (1.2%-1.4%). Figure 1 shows the number of eligible individuals who initiated or did not initiate antibiotics for each sequential emulated trial.
Figure 1.
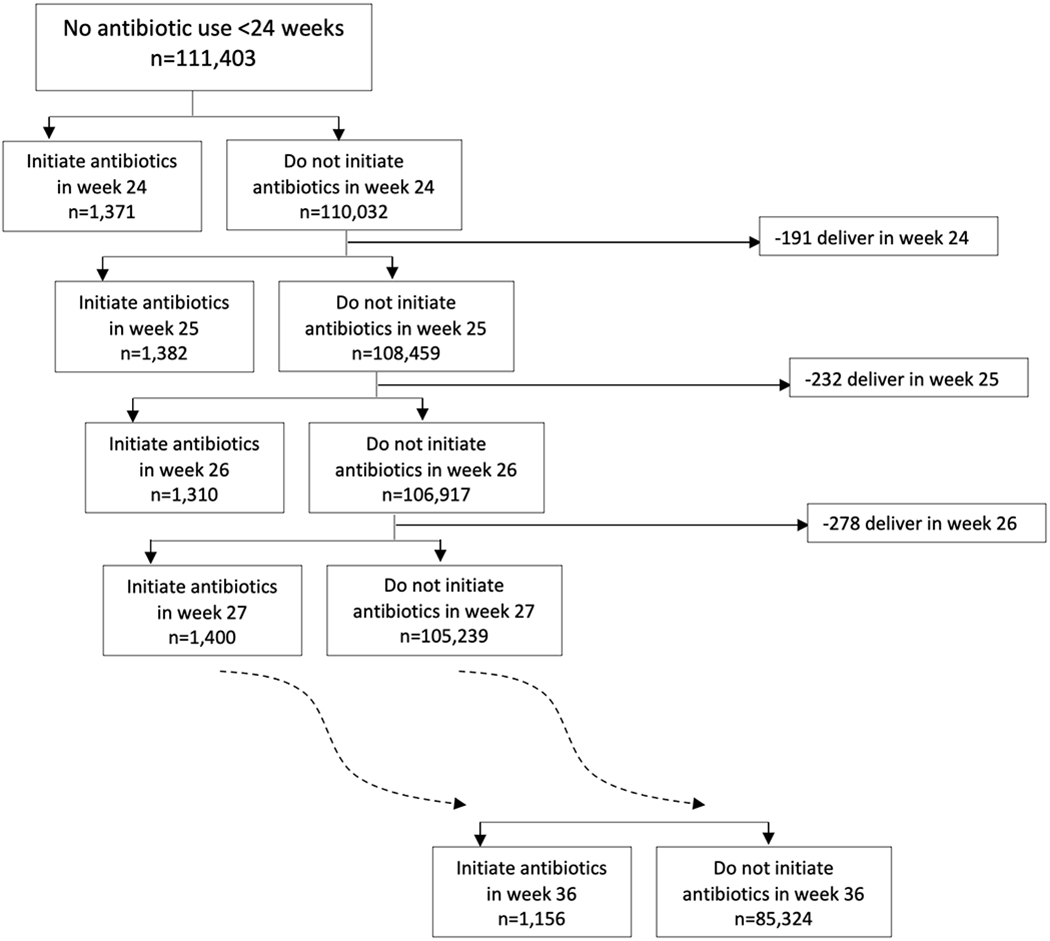
Abbreviated flow chart of target trials of antibiotic initiation at each week, 24-36 weeks’ gestation (complete flow chart is shown in eFigure 1)
Table 1 shows the types of antibiotics prescribed between 24 and 37 weeks’ gestation. The most common antibiotics were amoxicillin, azithromycin, ceftriaxone, erythromycin, and metronidazole. Antibiotic initiators and non-initiators had similar baseline characteristics but initiators were slightly more likely to deliver in earlier calendar years and at an urban delivery site (Table 2).
Table 1.
Frequency of initiating each type of antibiotic between 24 and 37 weeks’ gestation and timing of antibiotic initiation, The Tsepamo Study
| Antibiotic | Number (Percenta) |
|---|---|
| Amoxicilin | 5,353 (31.5%) |
| Azithromycin | 3,572 (21.0%) |
| Ceftriaxone | 7,699 (45.3%) |
| Cloxacillin | 289 (1.7%) |
| Cotrimoxazole | 242 (1.4%) |
| Doxycycine | 30 (0.2%) |
| Erythromycin | 3,891 (22.9%) |
| Fluconazole | 0 (0.0%) |
| Metronidazole | 7,552 (44.4%) |
| Penicillin | 1,575 (9.3%) |
| Other Antibiotic | 501 (2.9%) |
| Median (IQR) weeks at initiation | 30.1 (27.0, 33.4) |
Percentages sum to more than 100% because individuals could have initiated more than 1 type of antibiotic on the same date. IQR=interquartile range.
Table 2.
Characteristics of antibiotic initiators and non-initiators between 24 and 37 weeks’ gestation, The Tsepamo Study
| Characteristic | Non-initiators (n=1,290,635 person-trials) |
Initiators (n=17,009 person-trials) |
||
|---|---|---|---|---|
|
| ||||
| Number | Percent | Number | Percent | |
|
|
||||
| Trimester of first ANC | ||||
| First (<12 weeks) | 303,215 | 23.5% | 4,309 | 25.3% |
| Second (12-24 weeks) | 987,420 | 76.5% | 12,700 | 74.7% |
| Age | ||||
| <18 years | 47,481 | 3.7% | 565 | 3.3% |
| 18-35 years | 1,025,098 | 79.5% | 13,925 | 81.9% |
| ≥35 years | 217,682 | 16.9% | 2,514 | 14.8% |
| HIV status | ||||
| HIV-negative or unknown | 1,008,403 | 78.1% | 12,866 | 75.6% |
| Living with HIV | 282,232 | 21.9% | 4,143 | 24.4% |
| Parity | ||||
| 1 or more children | 762,121 | 59.1% | 10,122 | 59.5% |
| 0 children or missing | 528,514 | 41.0% | 6,887 | 40.5% |
| Occupation | ||||
| Salaried | 460,924 | 35.7% | 6,262 | 36.8% |
| Other, unknown, missing | 829,711 | 64.3% | 10,747 | 63.2% |
| Education | ||||
| Secondary or higher | 1,194,236 | 92.5% | 15,838 | 93.1% |
| None, primary, or missing | 96,399 | 7.5% | 1,171 | 6.9% |
| Calendar year of delivery | ||||
| 2014-2016 | 343,813 | 26.6% | 4,929 | 29.0% |
| 2017-2019 | 560,890 | 43.5% | 7,846 | 46.1% |
| 2020-2021 | 385,932 | 29.9% | 4,234 | 24.9% |
| Marital status | ||||
| Married | 148,462 | 11.5% | 1,647 | 9.7% |
| Other or missing | 1,142,173 | 88.5% | 15,362 | 90.3% |
| First weight in pregnancy | ||||
| <50 kg | 175,181 | 13.6% | 2,279 | 13.4% |
| 50-80kg | 905,070 | 70.1% | 11,918 | 70.1% |
| ≥80kg | 210,384 | 16.3% | 2,812 | 16.5% |
| Delivery site | ||||
| Urban (Gaborone or Francistown) | 483,947 | 37.5% | 7,353 | 43.2% |
| Rural (all other sites) | 806,675 | 62.5% | 9,656 | 56.8% |
| Prior preterm or stillbirth | ||||
| Yes | 58,368 | 4.5% | 894 | 5.3% |
| No | 731,816 | 56.7% | 9,691 | 57.0% |
| Unknown | 500,451 | 38.8% | 6,424 | 37.8% |
| Syphilis screening result | ||||
| Negative | 1,075,060 | 83.3% | 14,089 | 82.8% |
| Positive | 4,293 | 0.3% | 136 | 0.8% |
| Unknown | 211,282 | 16.4% | 2,784 | 16.4% |
Across the 13 emulated sequential target trials, there were 2,270 (13.4%) preterm deliveries among antibiotic initiators and 152,779 (11.8%) preterm deliveries among antibiotic non-initiators. The adjusted risk ratios (95% CIs) for preterm delivery ranged from 1.04 (0.90, 1.19) to 1.24 (1.11, 1.39). Results for the emulated target trial of antibiotic initiation at 36 weeks’ gestation were extremely unstable because there were so few events in the antibiotic initiation group and are therefore not included (we report the unadjusted results only). The pooled adjusted risk ratio (95% CI) for preterm delivery was 1.11 (1.06, 1.15).
Analysis susceptible to immortal time bias
When we defined the start of follow-up at 24 weeks and defined exposure as antibiotic initiation at any point between 24-37 weeks’ gestation, an approach that is susceptible to immortal time bias, the adjusted risk ratio (95% CI) for preterm delivery comparing antibiotic initiation to no initiation between 24-37 weeks was 0.90 (0.86, 0.94). Table 3 and Figure 2 show the results for each sequential trial of antibiotic initiation, the pooled trials, and the analysis where exposure is defined as antibiotic initiation between 24-37 weeks’ gestation.
Table 3.
Risk ratio of preterm delivery by antibiotic initiation between 24 and 37 weeks’ gestation, The Tsepamo Study
| Antibiotic Initiation | Initiation | Number of individuals | Events, number | Unadjusted Risk Ratio (95% CI) for initiation versus no initiation | Adjusteda Risk ratio (95% CI) for initiation versus no initiation |
|---|---|---|---|---|---|
|
Antibiotic initiation at:
|
|||||
| 24 weeks | No | 110,032 | 15,672 | ||
| Yes | 1,371 | 219 | 1.12 (0.99, 1.27) | 1.09 (0.97, 1.23) | |
| 25 weeks | No | 108,459 | 15,233 | ||
| Yes | 1,382 | 248 | 1.28 (1.14, 1.43) | 1.24 (1.11, 1.39) | |
| 26 weeks | No | 106,917 | 14,802 | ||
| Yes | 1,310 | 199 | 1.10 (0.96, 1.25) | 1.09 (0.96, 1.24) | |
| 27 weeks | No | 105,239 | 14,306 | ||
| Yes | 1,400 | 218 | 1.15 (1.01, 1.30) | 1.13 (1.00, 1.27) | |
| 28 weeks | No | 103,623 | 13,808 | ||
| Yes | 1,326 | 208 | 1.18 (1.04, 1.33) | 1.15 (1.02, 1.30) | |
| 29 weeks | No | 101,839 | 13,208 | ||
| Yes | 1,403 | 219 | 1.20 (1.06, 1.36) | 1.17 (1.04, 1.32) | |
| 30 weeks | No | 100,090 | 12,617 | ||
| Yes | 1,363 | 205 | 1.19 (1.05, 1.36) | 1.16 (1.03, 1.32) | |
| 31 weeks | No | 98,143 | 11,869 | ||
| Yes | 1,376 | 177 | 1.06 (0.93, 1.22) | 1.04 (0.90, 1.19) | |
| 32 weeks | No | 96,226 | 11,031 | ||
| Yes | 1,256 | 177 | 1.23 (1.07, 1.41) | 1.19 (1.04, 1.36) | |
| 33 weeks | No | 94,100 | 9,990 | ||
| Yes | 1,231 | 146 | 1.12 (0.96 1.30) | 1.10 (0.95, 1.29) | |
| 34 weeks | No | 91,797 | 8,769 | ||
| Yes | 1,227 | 145 | 1.24 (1.06, 1.44) | 1.22 (1.05, 1.42) | |
| 35 weeks | No | 88,846 | 6,923 | ||
| Yes | 1,208 | 103 | 1.09 (0.91, 1.32) | 1.07 (0.89, 1.29) | |
| 36 weeks | No | 85,324 | 4,551 | ||
| Yes | 1,156 | 6 | 0.10 (0.04, 0.22) | -- | |
| Pooled (24-36 weeks)b | No | 1,290,635 | 152,779 | ||
| Yes | 17,009 | 2,270 | 1.13 (1.09, 1.17) | 1.11 (1.06, 1.15) | |
|
Susceptible to immortal time bias:
|
|||||
| Antibiotic initiation at 24-37 weeks’ gestation | No | 94,394 | 13,621 | ||
| Yes | 17,009 | 2,270 | 0.92 (0.89, 0.96) | 0.90 (0.86, 0.94) | |
Adjusted for maternal age (<18 years; 18-35 years; >=35 years), education (secondary or higher; none or primary or unknown), occupation (salaried; non-salaried), parity (1 or more children; 0 children or missing), marital status (married; single, widowed, divorced or missing), HIV status (yes; no or unknown), delivery year (2014-2016; 2017-2019; 2020-2021), trimester of presentation to care (<12 weeks; 12-24 weeks), delivery site (Gaborone or Francistown; all others), prior preterm delivery or stillbirth (yes; no; unknown), and syphilis diagnosis at presentation to care (yes; no; unknown)
The adjusted pooled model additionally includes ‘trial’ as an adjustment variable. 95% CIs for the pooled models were obtained via bootstrapping
Figure 2.
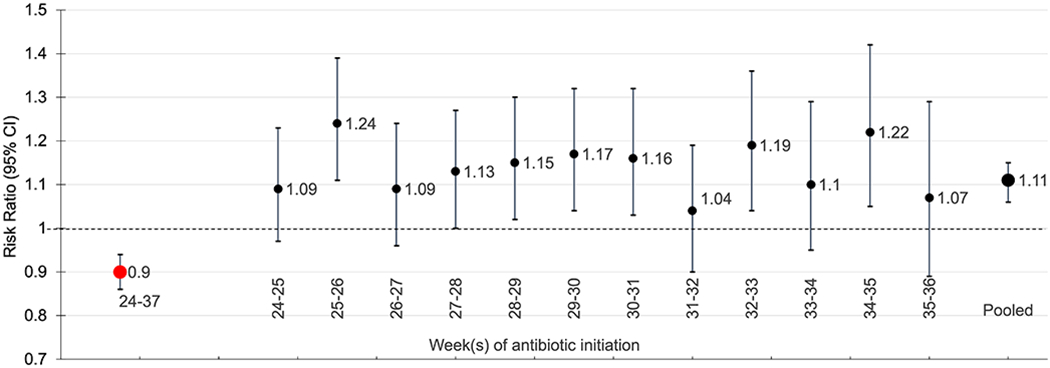
Adjusted risk ratios and 95% confidence intervals for preterm delivery comparing antibiotic initiation to no initiation between 24-37 weeks gestation (red; susceptible to immortal time bias) and by week of antibiotic initiation (black), The Tsepamo Study.
Additional analyses for separate causal questions
A total of 153,938 individuals met the eligibility criteria for the analysis of antibiotic initiation prior to 24 weeks’ gestation. Of these, 42,535 (27.6%) initiated antibiotics prior to 24 weeks’ gestation and 111,403 (72.4%) did not. The adjusted risk ratio for preterm delivery comparing antibiotic initiation versus no antibiotic initiation prior to 24 weeks’ gestation was 0.99 (0.96, 1.02), conditioning on no pregnancy loss by week 24.
Discussion
In the absence of randomized trials, observational data are necessary to evaluate medication safety and effectiveness in pregnancy. However, observational studies are often susceptible to bias if not analyzed properly. Immortal time bias is a time-related bias that occurs when treatment initiation occurs after time zero of follow-up. In this article, we describe how emulating a sequence of target trials can avoid immortal time bias and apply the approach to attempt to estimate the causal effect of antibiotic initiation between 24 and 37 weeks’ gestation and preterm delivery by conducting a sequence of target trials for each week of gestation. To demonstrate the extent to which immortal time bias could impact results, we conduct a separate analysis where we define exposure as antibiotic initiation between 24-37 weeks’ gestation. In the sequence of target trials which addressed immortal time bias, adjusted risk ratios suggested a small increased risk of preterm delivery comparing antibiotic initiation with no initiation. Because the time-period between time zero and treatment initiation is “immortal” to the outcome, defining exposure based on treatment initiation after time zero biased the risk ratio downwards and resulted in antibiotic initiation appearing protective against preterm delivery.
While we attempted to estimate a causal effect of antibiotic initiation on preterm delivery, a causal interpretation of our findings is unlikely to be warranted for two reasons. First, residual confounding by indication is likely, which threatens our ability to successfully emulate the randomization component of the target trial. While our approach by design adjusts for baseline confounding by gestational age and we were able to adjust for several demographic and clinical variables that may have been associated with antibiotic initiation and preterm delivery at the analysis stage, we were not able to adjust for the primary indications for antibiotic initiation. Maternal diagnoses of infections are not systematically measured in the Tsepamo Study and diagnosis dates are not included when they are reported. However, infections and inflammation are known to be associated with preterm delivery.34,35 We expect residual confounding would make antibiotics appear more harmful since people with infections would be more likely to be prescribed antibiotics and more likely to have a preterm delivery (“upward confounding”), and so the pooled risk ratio of 1.11 may be an overestimate of the causal effect of antibiotic initiation on preterm delivery. We expect this confounding to bias results in the same direction in the analysis where exposure is defined as antibiotic initiation at any point between 24-37 weeks’ gestation. Accordingly, the unconfounded risk ratio susceptible to immortal time bias would be even lower than the one estimated (i.e. less than 0.90). In other words, if we were able to adjust for maternal diagnoses of infection, we expect all risk ratios in Figure 2 would shift downward. The adjusted risk ratio for preterm delivery comparing antibiotic initiation versus no antibiotic initiation prior to 24 weeks’ gestation could similarly be biased due to confounding by indication, and we would expect the confounding to bias the results in the same direction. While it is interesting to consider the impact of confounding on our estimates, it is critical to note that, because we are not able to adjust for the primary indications for antibiotic initiation, our estimates are more likely to reflect the effect of clinically significant infections that require antibiotics rather than the effect of antibiotics alone. A final note related to confounding is that defining treatment initiation based on antibiotics prescribed after the delivery hospitalization could lead to reverse causation and should be carefully considered in other studies. This was not a concern in our particular study because, in the Tsepamo study, data on antibiotic prescriptions are only available prior to the delivery hospitalization.
The second reason a causal interpretation of our findings may not be warranted is that immortal time bias in our study is still possible. We chose to define our trials on the gestational week scale, meaning for each trial we defined time zero as the first day of the week and exposure as antibiotic initiation between the first and seventh day of the week. Accordingly, some amount of immortal time bias is still possible. In early pregnancy when few deliveries occur in any given week, this residual immortal time bias is likely minimal, but it could be substantial later in pregnancy when preterm delivery in a given week is less rare.39 In fact, it is likely that a one-week enrollment period for the target trial beginning at week 36 was inappropriate, and that defining trials on the day scale may have been necessary. Generally, defining trials on a shorter scale will reduce residual immortal time bias. However, the feasibility of defining trials on a given scale will depend on sample size and frequency of treatment initiation, and in some cases it may only be feasible to define trials on the month or trimester scale. We expect residual immortal time bias would make antibiotics appear less harmful, in which case the risk ratios we estimated may be underestimates of the true causal effect. While not susceptible to immortal time bias, our analysis of antibiotic initiation prior to 24 weeks’ gestation is still susceptible to selection bias because the Tsepamo Study, like many observational pregnancy studies, only captures data on births occurring at or after 24 weeks’ gestation. If antibiotic initiation prior to 24 weeks was related to miscarriage or spontaneous abortion prior to 24 weeks, our results could be biased. Selection bias due to necessarily restricting our analysis to Tsepamo delivery sites is also possible in all our analyses if antibiotic initiation were related to delivering at these sites versus other sites, but we do not believe this is the case given the representativeness of the Tsepamo delivery sites.
Even in the absence of residual confounding and immortal time bias, it is critical to note that the causal question we specified may not be the most useful for providers faced with the decision to prescribe or not prescribe antibiotics in the presence of additional information about a patient, such as a diagnosis of infection or reported symptoms of infection. A more realistic target trial would have included only participants for whom equipoise holds, either by including only those with specific infections or symptoms (a treatment trial), or by excluding those with active infection (a prophylaxis trial). Because reliable data on maternal infection is not available in Tsepamo, we would not be able to emulate the eligibility criteria for either of these target trials using the Tsepamo data. As such, our estimates are more likely to reflect the effect of clinically significant infections that require antibiotics rather than the effect of antibiotics alone.
Alternative approaches are available for avoiding immortal time bias in pregnancy safety studies. A cloning and censoring approach40 – where one copy is made of each eligible individual for each treatment strategy of interest at time zero (week 24) and then copies are prospectively censored if/when they deviate from the assigned strategy – may be an appropriate alternative that more precisely aligns time zero, eligibility, and treatment ascertainment, though this approach answers a different causal question about whether or not to initiate treatment at some point between 24 and 37 weeks after enrolling everybody at week 24 (rather than enrolling each week and initiating within one week of enrollment). In the case of antibiotics, this may not be a particularly relevant clinical question since once the decision to initiate an antibiotic regimen is made in clinical practice the regimen is typically initiated immediately (as opposed to something like cancer treatment which may be initiated at some point in the next months). Instead, the cloning and censoring method could be applied to each sequential trial (i.e., one copy is made of each eligible individual at each sequential trial’s time zero and then copies are censored throughout that one-week period if/when they deviate from each strategy). This approach may be particularly useful when concerns about residual immortal time bias are substantial but defining trials on the day scale is not feasible (due to too few individuals initiating treatment on any given day or due to concerns about measurement error for gestational age). Another approach that more precisely aligns time zero and treatment ascertainment is, each day during the study period, to match eligible pregnant individuals who initiated antibiotics on that day to eligible pregnant individuals who had not yet initiated antibiotics and were not previously matched as controls.41 However, strict matching may lead to a large proportion of the eligible population not being included in the study. An alternative strategy for preventing immortal time bias is to treat antibiotic initiation as a time-varying variable and to classify the person–time prior to antibiotic initiation as unexposed person–time.2,10 While this approach may yield an unbiased estimate of the hazard ratio for antibiotic exposure and preterm delivery, it does not allow estimation of cumulative risks or survival curves and may be biased in the setting of treatment-confounder feedback.11 Further, this approach may not correspond to well-defined treatment strategies that could be implemented in clinical practice. Finally, redefining exposure so that it occurs prior to time-zero could seem to be a simple solution to avoid immortal time bias; however, this could induce selection bias and again misaligns time of eligibility with time of exposure initiation.1
The sequential target trial approach we describe can be extended in several ways. In our study, information on preterm delivery was available for all individuals included in our analysis. This may not be the case if pregnant people are recruited into a study prospectively and some are lost to follow-up or have not yet delivered at the time of analysis. In this situation, one could conduct a time-to-delivery analysis for each sequential target trial to allow use of data from individuals until they were censored.23,24 While our analysis included the same time-fixed variables measured at the first antenatal visit in each of the 13 models (one for each target trial), the approach can be extended to adjust for time-varying variables by including the most recent measure of each time-varying variable at time zero of each sequential trial in the model for that trial.14 Finally, the approach can be extended to estimate per-protocol effects (e.g., of completing the full course of antibiotics) in addition to the intention-to-treat effect.
Conducting a sequence of target trials can avoid immortal time bias in pregnancy studies by aligning exposure initiation and start of follow-up. The sequential target trial approach also allows researchers to seamlessly investigate effect modification by gestational age and to provide risk estimates based on treatment initiation at different gestational ages, which is critical given that exposures at different periods in pregnancy could have different effects. This approach can be adapted widely in pregnancy studies of medication safety and effectiveness. However, careful attention should be given to residual immortal time bias and other types of bias.
Supplementary Material
Acknowledgments:
We thank Dr. Louisa Smith for helpful discussion and feedback on earlier drafts of this work.
Source of Funding:
National Institutes of Health NIH/NICHD K01 HD100222, NIH/NICHD R01 HD080471, NIH/NICHD K23 HD088230, NIH/NIAID K24AI131924
Footnotes
Conflicts of Interest: None declared.
Data and computing code availability:
Data and code are available upon reasonable request and approval from the necessary Institutional Review Boards in the United States and Botswana.
REFERENCES
- 1.Hernán MA, Sauer BC, Hernandez-Diaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. Journal of clinical epidemiology 2016; 79: 70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suissa S Immortal time bias in pharmaco-epidemiology. American journal of epidemiology 2008; 167(4): 492–9. [DOI] [PubMed] [Google Scholar]
- 3.Ukah UV, Aibibula W, Platt RW, Dayan N, Reynier P, Filion KB. Time-related biases in perinatal pharmacoepidemiology: A systematic review of observational studies. Pharmacoepidemiol Drug Saf 2022. [DOI] [PubMed] [Google Scholar]
- 4.Matok I, Azoulay L, Yin H, Suissa S. Immortal time bias in observational studies of drug effects in pregnancy. Birth Defects Res A Clin Mol Teratol 2014; 100(9): 658–62. [DOI] [PubMed] [Google Scholar]
- 5.Daniel S, Koren G, Lunenfeld E, Levy A. Immortal time bias in drug safety cohort studies: spontaneous abortion following nonsteroidal antiinflammatory drug exposure. Am J Obstet Gynecol 2015; 212(3): 307.e1-6. [DOI] [PubMed] [Google Scholar]
- 6.Hutcheon JA, Kuret V, Joseph KS, Sabr Y, Lim K. Immortal time bias in the study of stillbirth risk factors: the example of gestational diabetes. Epidemiology 2013; 24(6): 787–90. [DOI] [PubMed] [Google Scholar]
- 7.Schnitzer ME, Guerra SF, Longo C, Blais L, Platt RW. A potential outcomes approach to defining and estimating gestational age-specific exposure effects during pregnancy. Stat Methods Med Res 2022; 31(2): 300–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vazquez-Benitez G, Kharbanda EO, Naleway AL, et al. Risk of Preterm or Small-for-Gestational-Age Birth After Influenza Vaccination During Pregnancy: Caveats When Conducting Retrospective Observational Studies. American journal of epidemiology 2016; 184(3): 176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neophytou AM, Kioumourtzoglou MA, Goin DE, Darwin KC, Casey JA. Educational note: addressing special cases of bias that frequently occur in perinatal epidemiology. International journal of epidemiology 2021; 50(1): 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrao G, Rea F, Franchi M, Beccalli B, Locatelli A, Cantarutti A. Warning of Immortal Time Bias When Studying Drug Safety in Pregnancy: Application to Late Use of Antibiotics and Preterm Delivery. Int J Environ Res Public Health 2020; 17(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platt RW, Hutcheon JA, Suissa S. Immortal time bias in epidemiology. Current Epidemiology Reports 2019; 2019(6): 23–7. [Google Scholar]
- 12.Ukah U, Aibibula W, Platt R, Filion K. Prevalence of time-related biases in pharmacoepidemiology studies of anti-emetics, antifungal, and antibiotic medications during pregnancy: A systematic review. Abstract #11, 2020. ISPE Mid-year meeting. 2020. 10.1002/pds.5115 (accessed 17 May, 2022). [DOI]
- 13.Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American journal of epidemiology 2016; 183(8): 758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danaei G, Rodríguez LA, Cantero OF, Logan R, Hernán MA. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res 2013; 22(1): 70–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danaei G, García Rodríguez LA, Cantero OF, Logan RW, Hernán MA. Electronic medical records can be used to emulate target trials of sustained treatment strategies. Journal of clinical epidemiology 2018; 96: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Albéniz X, Hsu J, Bretthauer M, Hernán MA. Effectiveness of Screening Colonoscopy to Prevent Colorectal Cancer Among Medicare Beneficiaries Aged 70 to 79 Years: A Prospective Observational Study. Annals of internal medicine 2017; 166(1): 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Albéniz X, Hsu J, Hernán MA. The value of explicitly emulating a target trial when using real world evidence: an application to colorectal cancer screening. Eur J Epidemiol 2017; 32(6): 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caniglia EC, Rojas-Saunero LP, Hilal S, et al. Emulating a target trial of statin use and risk of dementia using cohort data. Neurology 2020; 95(10): e1322–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Avoidable flaws in observational analyses: an application to statins and cancer. Nat Med 2019; 25(10): 1601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labrecque JA, Swanson SA. Target trial emulation: teaching epidemiology and beyond. Eur J Epidemiol 2017; 32(6): 473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu YH, Yland JJ, Rinaudo P, et al. Effectiveness and safety of intrauterine insemination vs. assisted reproductive technology: emulating a target trial using an observational database of administrative claims. Fertil Steril 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caniglia EC, Zash R, Jacobson DL, et al. Emulating a target trial of antiretroviral therapy regimens started before conception and risk of adverse birth outcomes. AIDS (London, England) 2018; 32(1): 113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith LH. Bias bounds and target trials for causal inference in observational epidemiology. ProQuest Dissertations and Theses. 2021. https://www.proquest.com/docview/2564109480 (accessed 18 May 2022).
- 24.Smith LH, Dollinger CY, VanderWeele TJ, Wyszynski DF, Hernández-Díaz S. Timing and severity of COVID-19 during pregnancy and risk of preterm birth in the International Registry of Coronavirus Exposure in Pregnancy. BMC Pregnancy Childbirth 2022; 22(1): 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zash R, Jacobson DL, Diseko M, et al. Comparative Safety of Antiretroviral Treatment Regimens in Pregnancy. JAMA pediatrics 2017; 171(10): e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zash R, Holmes L, Diseko M, et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. The New England journal of medicine 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Organization WH. Botswana: WHO statistical profile. 2015. http://www.who.int/gho/countries/bwa.pdf?ua=1&ua=1 (accessed 21 October 2019). [Google Scholar]
- 28.Fennell C, Diseko M, Zash R, et al. The Impact of Syndromic Management of Vaginal Discharge Syndrome on Adverse Birth Outcomes in Botswana. Open Forum Infect Dis 2021; 8(8): ofab366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botswana Ministry of Health. Management of Sexually Transmitted Infections: Reference Manual for Health Care Workers. 2018. [Google Scholar]
- 30.VanderWeele TJ, Hernán MA. Causal Inference Under Multiple Versions of Treatment. J Causal Inference 2013; 1(1): 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu YH, Stensrud MJ, Dahabreh IJ, et al. The Effect of Prenatal Treatments on Offspring Events in the Presence of Competing Events: An Application to a Randomized Trial of Fertility Therapies. Epidemiology 2020; 31(5): 636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young JG, Stensrud MJ, Tchetgen Tchetgen EJ, Hernán MA. A causal framework for classical statistical estimands in failure-time settings with competing events. Statistics in medicine 2020; 39(8): 1199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stensrud MJ, Hernán MA, Tchetgen Tchetgen EJ, Robins JM, Didelez V, Young JG. A generalized theory of separable effects in competing event settings. Lifetime Data Anal 2021; 27(4): 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007; 25(1): 21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, Gómez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001; 15 Suppl 2: 41–56. [DOI] [PubMed] [Google Scholar]
- 36.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Annals of internal medicine 2017; 167(4): 268–74. [DOI] [PubMed] [Google Scholar]
- 37.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Website and R package for computing E-values Epidemiology 2018; 29(5): e45–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding P, VanderWeele TJ. Sensitivity Analysis Without Assumptions. Epidemiology 2016; 27(3): 368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nassar N, Schiff M, Roberts CL. Trends in the distribution of gestational age and contribution of planned births in New South Wales, Australia. PloS one 2013; 8(2): e56238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cain LE, Robins JM, Lanoy E, Logan R, Costagliola D, Hernan MA. When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. The international journal of biostatistics 2010; 6(2): Article 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021; 27(10): 1693–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are available upon reasonable request and approval from the necessary Institutional Review Boards in the United States and Botswana.


