Abstract
Background
The most frequently occurring adverse events in individuals with a transfemoral amputation treated with a bone-anchored prosthesis are soft tissue infections and stoma-related complications. These soft tissue complications are believed to be influenced by surgical technique and implant design, but little is known about the effect of changes to treatment on these events.
Questions/purposes
(1) What is the result of surgical technique and implant modifications on the incidence of soft tissue infections and stoma-related complications in transfemoral bone-anchored prosthesis users, depending on whether they had a conventional stoma and a cobalt-chrome-molybdenum (CoCrMo) osseointegration implant (treatment period 2009 to 2013) or a shallower stoma and titanium osseointegration implant (2015 to 2018)? (2) What is the incidence of serious complications, such as bone or implant infection, aseptic loosening, intramedullary stem breakage, and periprosthetic fracture?
Methods
Between 2009 and 2013, we performed osseointegration implant surgery using a conventional surgical technique and a CoCrMo implant in 42 individuals who had a lower extremity amputation experiencing socket-related problems that resulted in limited prosthesis use. We considered all individuals treated with two-stage surgery with a standard press-fit transfemoral osseointegration implant as potentially eligible for inclusion. Based on this, 100% (42) were eligible, and 5% (two of 42) were excluded because they did not provide informed consent, leaving 95% (40 of 42) for analysis. Between 2015 and 2018, we treated 79 individuals with similar indications with osseointegration implant surgery, now also treating individuals with dysvascular amputations. We used an adapted surgical technique resulting in a shallower stoma combined with a titanium implant. Using the same eligibility criteria as for the first group, 51% (40 of 79) were eligible; 49% (39 of 79) were excluded because they were treated with transtibial amputation, a patient-specific implant, or single-stage surgery and 1% (one of 79) were lost before the 2-year follow-up interval, leaving 49% (39 of 79) for analysis. The period of 2013 to 2015 was a transitional period and was excluded from analysis in this study to keep groups reasonably comparable and to compare a historical approach with the present approach. Hence, we presented a comparative study of two study groups (defined by surgical technique and implant design) with standardized 2-year follow-up. The risk factors for adverse events were similar between groups, although individuals treated with the shallow stoma surgical technique and titanium implant potentially possessed an increased risk because of the inclusion of individuals with dysvascular amputation and the discontinuation of prolonged postoperative antibiotic prophylaxis. Outcomes studied were soft tissue infections and stoma-related complications (hypergranulation or keloid formation as well as stoma redundant tissue) and bone or implant infection, aseptic loosening, implant stem breakage, periprosthetic fracture, and death.
Results
Patients treated with the shallow stoma surgical technique and titanium implant experienced fewer soft tissue infections (13 versus 76 events, absolute risk 0.17 [95% CI 0.09 to 0.30] versus 0.93 [95% CI 0.60 to 1.45]; p < 0.01), which were treated with less invasive measures, and fewer stoma redundant tissue events (0 versus five events, absolute risk 0 versus 0.06 [95% CI 0.03 to 0.14]) than patients treated with the conventional stoma surgical technique and CoCrMo implant. This was contrasted by an increased incidence of surgical site infections occurring between surgical stages 1 and 2, when no stoma was yet created, after the implementation of treatment changes (conventional surgery and CoCrMo implant versus shallow stoma surgery and titanium implant: one versus 11 events, absolute risk 0.01 [95% CI 0.00 to 0.08] versus 0.14 [95% CI 0.08 to 0.25]; p = 0.02). Patients treated with the shallow stoma surgical technique and titanium implant did not experience serious complications, although bone infections occurred (six events in 8% [three of 40] of patients) in the conventional surgery and CoCrMo implant group, all of which were successfully treated with implant retention.
Conclusion
Adaptations to surgical technique and newer implant designs, as well as learning curve and experience, have resulted in a reduced incidence and severity of soft tissue infections and stoma redundant tissue, contrasted by an increase in surgical site infections before stoma creation. Serious complications such as deep implant infection were infrequent in this 2-year follow-up period. We believe the benefits of these treatment modifications outweigh the disadvantages and currently advise surgeons to create a shallower stoma with a stable soft tissue envelope, combined with a titanium implant.
Level of Evidence
Level III, therapeutic study.
Introduction
The prevalence of extremity amputation is high. An estimated 1.6 million individuals lived with limb loss in the United States in 2005; this number is expected to more than double by 2050 [29]. This poses a major social problem because individuals who undergo lower extremity amputation have a lower quality of life than people in the general population and a higher incidence of unemployment [10, 11, 14, 26]. For centuries, socket-suspended prostheses have been used, but despite technologic advances in designs and materials, individuals still experience socket-related problems such as skin irritation, prosthetic fixation issues, and pain [12, 14, 22]. As an alternative, directly fixing the prosthesis to the residual bone via an osseointegration implant results in a modular bone-anchored prosthesis, eliminating the socket-stump interface and its associated problems [8]. Additional suggested treatment advantages are improved function, activity, and quality of life [20], but serious complications may occur, potentially resulting in pain, loss of mobility, or revision surgery [3, 4, 7, 17]. Prior studies have shown that soft tissue infections and stoma-related complications occur frequently, while serious complications such as deep implant infection are less common [5, 9, 25]. Soft tissue complications may be related to the surgeon’s experience, implant design, and surgical technique [2, 4, 5, 17].
The press-fit implant system for individuals with transfemoral amputation was introduced in 1999 and has evolved substantially since then [15, 17]. Evolutions have included changes to the implant’s alloy that seek to reduce stem fractures, different coatings of the extramedullary portion of the implant, and improvement in surgical techniques that create the stoma, aiming to reduce soft tissue irritation and subsequent soft tissue–related complications [1, 16, 17]. Juhnke et al. [17] reported on 69 individuals divided into two groups who were treated with the initial three versions of a press-fit osseointegration implant, with variable follow-up times. An absolute risk reduction of infection of 42% to 55% was reported after major device (bracket removal, bridging connector shortening, and coating of the extramedullary part) and surgical adaptations (additional subcutaneous tissue thinning and creation of a stoma < 2 cm deep). However, determining the influence of treatment changes on complication rates remained difficult in that study [17] because major implant modifications occurred between and within groups, surgical procedures were changed, and the earlier groups had more time to accrue complications. Additionally, the definition or diagnosis of infections was unclear, and it appears only infectious complications resulting in surgical interventions were reported.
The aim of the current study was to evaluate the influence of treatment modifications on complication rates, focusing on frequently occurring soft tissue infections and soft tissue complications; we compared groups with identical 2-year follow-up periods. The secondary aim was to report on overall treatment safety by reporting on serious complications.
Specifically, we asked: (1) What is the result of surgical technique and implant modifications on the incidence of soft tissue infections and stoma-related complications in transfemoral bone-anchored prosthesis users, depending on whether they had a conventional stoma and a cobalt-chrome-molybdenum (CoCrMo) osseointegration implant (treatment period 2009 to 2013) or a shallower stoma and titanium osseointegration implant (2015 to 2018)? (2) What is the incidence of serious complications, such as bone or implant infection, aseptic loosening, intramedullary stem breakage, and periprosthetic fracture?
Patients and Methods
Study Design and Setting
This was a single-institution, retrospective, comparative study of two groups (defined by surgical technique and implant design) with standardized 2-year follow-up periods. A fixed 2-year follow-up period was used to allow for comparability between groups, avoiding the bias of allowing an earlier group more time to accrue complications. Safety and functional outcome data of a portion of the groups were published earlier [6, 19, 25, 27]. We followed the STROBE guideline for observational studies [28].
Participants
Individuals with an extremity amputation experiencing difficulties with their socket prosthesis were referred to our center by orthopaedic technicians, rehabilitation physicians, or their general practitioner [13]. Eligibility for press-fit osseointegration implantation was assessed by a multidisciplinary team including a surgeon, rehabilitation physician, physiotherapist, and orthopaedic technician based on medical history, physical examination, completed questionnaires, and radiographs. Inclusion criteria were adults with an extremity amputation experiencing socket-related problems resulting in limited prosthesis use, while the exclusion criterion was the presence of severe cognitive or psychiatric disorders [18]. Amputation for peripheral vascular disease or diabetes was initially an exclusion criterion in 2009, but after an assessment of the first study confirmed that osteitis or septic implant loosening was uncommon, the indications were broadened in 2014 [13].
Between 2009 and 2013, we treated 42 individuals who had a lower extremity amputation with osseointegration implant surgery using a conventional surgical technique and CoCrMo implant. We considered all individuals treated with two-stage surgery with a Conformité Europëenne (CE)–marked transfemoral osseointegration implant as potentially eligible. Based on this, 100% (42) were eligible; 5% (two of 42) were excluded because they did not provide informed consent, leaving 95% (40 of 42) for analysis. Between 2015 and 2018, 79 individuals with a lower extremity amputation were treated with osseointegration surgery using a modified surgical technique and a titanium implant. Using the same eligibility criteria as for the first group, 51% (40 of 79) were eligible; 49% (39 of 79) were excluded because they were treated for transtibial amputation, with a patient-specific implant, or single-stage surgery and an additional 1% (one of 79 patients) were lost before the 2-year follow-up interval, leaving 49% (39 of 79) for analysis. The period of 2013 to 2015 was a transitional period during which individuals were treated with the modified surgical technique and a CoCrMo implant. Baseline characteristics are presented for this group (Supplemental Table 1; http://links.lww.com/CORR/B4), but this group was excluded from further analysis to achieve a truer comparison of a historical approach with the present approach, because only a small number of individuals were treated during this period (Fig. 1).
Fig. 1.
This flow diagram shows the participants who were included in the study.
Descriptive Data
Treatment-related differences between groups were the interval between surgical steps 1 and 2, postoperative antibiotic prophylaxis use, implant length, and dualcone adapter size (Table 1). Shortly after the transition of implant used in 2015, prolonged postoperative antibiotic prophylaxis use was discontinued, following the manufacturer’s instructions for use. Additionally, differences in implant length and dualcone adapter size were also considered to be treatment related. For the CoCrMo implant, different lengths could be used (160 mm to 180 mm), compared with only one size for titanium implants (160 mm). Differences in dualcone adaptor size are because of the modified surgical technique, because the dualcone size correlates with the depth of the stoma. Patient-related differences between groups were age at amputation and implantation and amputation etiology as treatment indications broadened with time, and older individuals and individuals with dysvascular amputations were deemed eligible for surgery. Group differences in antibiotic prophylaxis use and amputation etiology (such as an increase in dysvascular amputations) theoretically result in an increased risk of soft tissue complications for individuals treated with the adapted surgical technique and titanium implant and are expected to negatively influence potential benefits encountered after treatment adaptations.
Table 1.
Patient demographics, baseline amputation characteristics, surgical details, and implant characteristics
| Parameter | Conventional surgery and CoCrMo implant (n = 40) | Modified surgery and titanium implant (n = 39) | p value |
| Women, % (n) | 25 (10) | 36 (14) | 0.29a |
| Age in years, median (IQR) | |||
| Age at amputation | 26 (21) | 50 (38) | < 0.01b |
| Age at implantation | 48 (19) | 60 (17) | < 0.01b |
| Interval between amputation and implantation in years, median (IQR) | 12 (26) | 8 (11) | 0.14b |
| Nonsmokers, % (n) | 85 (34) | 97 (38) | 0.11c |
| Diabetes mellitus, % (n) | 0.09c | ||
| No | 98 (39) | 85 (33) | |
| Noninsulin-dependent | 3 (1) | 10 (4) | |
| Insulin-dependent | 0 (0) | 5 (2) | |
| BMI in kg/m2, mean ± SD | 26 ± 4 | 26 ± 5 | 0.96d |
| Baseline amputation characteristics | |||
| Level (per limb: n = 80), % (n) | N = 41 | N = 39 | 0.71c |
| TF | 88 (36) | 92 (36) | |
| TK | 12 (5) | 8 (3) | |
| Side (n = 80), % (n) | 0.04c | ||
| Left | 63 (25) | 41 (16) | |
| Right | 35 (14) | 59 (23) | |
| Bilateral | 3 (1) | 0 (0) | |
| Cause (per limb: n = 80), % (n) | |||
| Trauma | 76 (31) | 41 (16) | |
| Dysvascular | 0 (0) | 21 (8) | |
| Infection | 7 (3) | 15 (6) | |
| Tumor | 15 (6) | 15 (6) | |
| Congenital | 0 (0) | 3 (1) | |
| Other | 2 (1) | 5 (2) | |
| Surgical details (per implant: n = 80) | |||
| Interval in days between surgical steps 1 and 2, median (IQR) | 49 (14) | 56 (18) | 0.02b |
| Postoperative antibiotic prophylaxis, % (n) | 100 (41) | 8 (3) | < 0.01a |
| Implant characteristics (n = 80), median (IQR) | |||
| Diameter in mm | 16 (3) | 16 (2) | 0.33b |
| Length in mm | 180 (20) | 160 (0) | < 0.01b |
| Dual cone size | 5 (2) | 3 (2) | < 0.01b |
We calculated p values using aa chi-squared test, bMann-Whitney test, cFisher exact test, and dindependent-samples-t-test. TF = transfemoral; TK = through-knee amputation.
Surgical Technique
Standard two-stage osseointegration implantation was performed with a 6-week to 8-week interval between procedures for both groups, and cephazolin was administered intravenously at induction. Two surgical techniques were used, here termed “conventional” and “modified.”
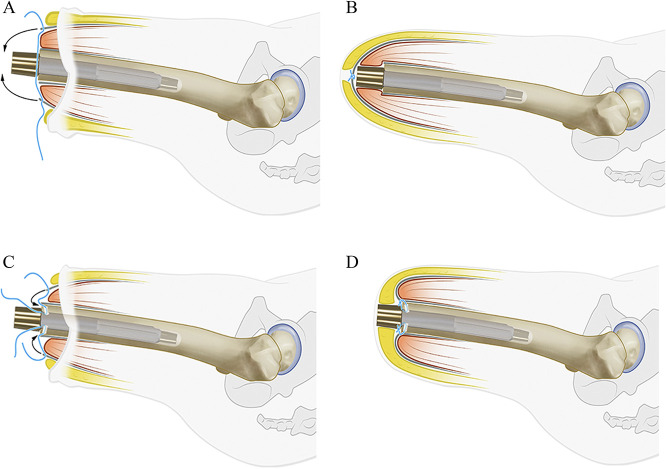
In the conventional surgical technique, used up to September 2013, the first stage of the procedure consisted of shortening the femur to an adequate length, removing neuromas and bone spurs, stepwise retrograde intramedullary reaming under radiographic guidance, and press-fit implantation of the intramedullary component. The muscle’s orientation was corrected, followed by a myoplasty, including suturing of the ventral and dorsal muscle fascia over the implant and skin closure (Fig. 2A and B) [3]. In the second stage of the procedure, the surgeon created a stoma by using a coring device to create a circular skin defect at the level of the distal osseointegration implant, and then a dualcone adapter was mounted onto the osseointegration implant.
Fig. 2.
(A) The conventional surgical technique is shown; a myoplasty was formed by suturing the fascia over the implant. (B) In the conventional surgical technique, the fascia was sutured over the implant. (C) In the adapted surgical technique, soft tissue surplus was removed and a myodesis was formed; fascia sutures were passed through the distal femur. (D) In the adapted surgical technique, the fascia was sutured onto the distal femur.
After September 2013, a modified surgical technique was used, with the following alterations to the first stage: further reduction of soft tissue surplus, removal of redundant subcutaneous fat, and formation of a myodesis by drilling burr holes in the distal femoral end, through which sutures were passed and attached to the muscle fasciae. The aim was to create a shallow stoma canal less than 2 cm thick from the tip of the bone to the skin (Fig. 2C and D) [13].
Implant Design
The implant used up to 2015 was made of a cast CoCrMo alloy (Endo-exo/Integral Leg Prosthesis, Orthodynamics) covered with a 1.5-mm-thick layer of trabecular metal to accommodate osseointegration. The distal extramedullary part was partially coated with smooth titanium niobium oxynitride (TiNbN) (Fig. 3A). According to the manufacturer’s instructions, the implant was placed without tension on the overlying skin, with a 5-cm minimum distance between the distal osseointegration implant and the skin; this was considered the conventional surgical technique.
Fig. 3.

(A) This photograph shows an anterior and transverse view of the cobalt-chrome-molybdenum implant. (B) This photograph shows an anterior and transverse view of the titanium alloy implant.
Because multiple breakages of the CoCrMo implant stem occurred by 3 years of follow-up, a new CE-marked implant was used from 2015 onward [23, 25]. This implant was forged from a titanium alloy (Ti6AL7Nb) in which the proximal half was grit blasted. It contained longitudinal flutes providing rotational stability (Osseointegration Prosthetic Limb, Permedica SPA). The distal half was coated with plasma-sprayed titanium to enhance bone-to-implant contact, and the extramedullary part was fully coated with TiNbN (Fig. 3B).
Aftercare, Rehabilitation, and Follow-up
Initially, patients received intravenous cephazolin for 5 days after the first procedure, based on the manufacturer’s instructions for use. From July 2015 onward, a change in practice occurred and only single-dose preoperative antibiotics were administered, as suggested by the manufacturer of the newly used titanium implant. These instructions were followed because early serious infection rates remained low. Rehabilitation started 1 week after stage 2, and a predefined rehabilitation program consisted of 11 weeks of outpatient physical therapy sessions, twice per week, that aimed to improve ambulation [19]. During rehabilitation, the prosthesis was gradually loaded to full bodyweight, and the use of walking aids was reduced based on the patient’s pain level [21]. Follow-up visits were scheduled at 6 months, 12 months, and 24 months postoperatively and included a radiologic examination, performance tests such as the timed-up-and-go test, and an assessment of complications.
Primary and Secondary Outcomes
Baseline amputation characteristics, surgical details, implant characteristics, and complications were retrospectively extracted from our institutional registry and from medical records. Because general practitioners have a prominent role and are the gatekeepers in the Dutch healthcare system, they were also contacted by telephone to ascertain whether any complications occurred that had been treated outside the hospital. Because no classification system encompasses all treatment-related complications, we classified complications based on an adaptation of the classification system by Al Muderis et al. [3] (Supplemental Table 2; http://links.lww.com/CORR/B5). Complications were subdivided into serious or minor complications (Table 2). Complications were soft tissue infections, stoma-related complications (hypergranulation or keloid formation as well as stoma redundant tissue), bone or implant infection, aseptic loosening, implant stem breakage, periprosthetic fracture, and death. Mechanical complications of the extramedullary components of the bone-anchored prosthesis (such as dualcone adapter body or weakpoint breakage) were outside the scope of this study because dualcone breakage was not believed to influence or be influenced by soft tissue infections or complications or the treatment changes implemented in this study and because such breakage was not considered a serious complication, since these parts can usually be replaced in an outpatient setting.
Table 2.
Simplified version of classification of soft tissue complications
| Type of adverse event | Subtype | Symptoms and signs | Treatment | Grade | Severity |
| Infectiona | Low-grade soft tissue infection | Cellulitis with signs of inflammation (redness, swelling, warmth, pain) | Local measures | 1A | Minor |
| Oral antibiotics | 1B | Minor | |||
| Parenteral antibiotics | 1C | Minor | |||
| Soft tissue surgery | 1D | Moderate | |||
| High-grade soft tissue infection | Abscess formation, purulent discharge, and/or raised level of C-reactive protein | Local measures | 2A | Minor | |
| Oral antibiotics | 2B | Minor | |||
| Parenteral antibiotics | 2C | Minor | |||
| Soft tissue surgery | 2D | Moderate | |||
| Stoma problems | Hypegranulation or keloid formation | Overgrowth of connective tissue at the stoma with absence of infection | Local measuresa | A | Minor |
| Sleeveb | B | Moderate | |||
| Soft tissue surgeryc | C | Moderate | |||
| Redundant tissue | Presence of symptomatic redundant soft tissue with absence of infection | Local measuresd | A | Minor | |
| Sleeveb | B | Moderate | |||
| Soft tissue surgerye | C | Moderate | |||
| Removal of extramedullary part of osseointegration implant | D | Moderate |
Use of Instillagel, Terra-Cotril ointment, or AgNO3.
Placement of a (protective) sleeve.
Scar tissue removal by conical excision.
Use of a stump dressing or shrinker.
Stump refashioning.
Our primary study goal was to assess the influence of surgery and implant modifications on the incidence of soft tissue infections and stoma-related complications. To achieve this, we compared the incidences of soft tissue infections and stoma-related complications in individuals treated with either a conventional surgical technique and CoCrMo implant or a modified surgical technique and titanium implant. Complications occurring between surgical stages, when the stoma is not yet formed, were evaluated separately.
Our secondary goal was to report on the incidence of serious complications such as bone or implant infection, aseptic loosening, intramedullary stem breakage, and periprosthetic fracture.
Ethical Approval
Regional ethical review board approval was obtained for this study (number 2017-3767).
Statistical Analysis
Outcomes for both groups are presented using descriptive statistics, rounded percentages with numbers, means with standard deviations, and median with interquartile range, according to data type and distribution. Differences in patient, surgery, and implant data in each group were statistically analyzed using a chi-square or Fisher exact test for categorical data. For normally and non-normally distributed continuous data, an unpaired t-test or Mann-Whitney U test was used, respectively. Complications were evaluated at the patient and event level. Group comparisons were made regarding the number of soft tissue complications (such as soft tissue infections, hypergranulation or keloid formation, soft tissue redundant tissue, and surgical site infections between surgical stages 1 and 2) per implant, leaving grading and treatment out of the equation, and were analyzed with generalized estimating equations using a negative binomial model. Absolute risks (ARs) and risk ratios (RRs) are presented. Based on clinical knowledge and considering variables with patient-related differences in distribution between treatment groups (Table 1), the following covariates were evaluated for model inclusion to adjust analyses: age at amputation, age at implantation, smoking status, sex, and presence of diabetes. However, all had p values > 0.2, and a model without covariates was fitted. The model was adjusted for the follow-up period of 2 years and for participants who underwent bilateral procedures. A two-sided p value of < 0.05 was considered statistically significant. Analyses were performed using SPSS version 23 (IBM Corp).
Results
Soft Tissue Infections and Stoma-related Complications
Soft tissue infections occurred less frequently and could be managed with less invasive measures in the group treated with the modified surgery and titanium implant than in the group treated with the conventional surgery and CoCrMo implant (13 events, AR 0.17 [95% confidence interval 0.09 to 0.30] versus 76 events, AR 0.93 [95% CI 0.60 to 1.45]) (Table 3). This resulted in an RR for soft tissue infections of 5.61 (95% CI 2.71 to 11.57; p < 0.01) for the conventional surgery and CoCrMo implant group compared with the other group (Table 4).
Table 3.
Outcomes of soft tissue complications and complications between surgical stages, as well as treatment (simplified)
| Type of adverse event | Treatment | Grade | Conventional surgery and CoCrMo implant (n = 40) | Adapted surgery and titanium implant (n = 39) | ||
| Patients, % (n) | Events | Patients, % (n) | Events | |||
| Soft tissue complications | ||||||
| Low-grade soft tissue infection | Total | 38 (15) | 27 | 21 (8) | 9 | |
| Local measures | 1A | 15 (6) | 6 | 5 (2) | 2 | |
| Oral antibiotics | 1B | 33 (13) | 19 | 15 (6) | 7 | |
| Parenteral antibiotics | 1C | 3 (1) | 1 | |||
| Surgical treatment | 1D | 3 (1) | 1 | |||
| High-grade soft tissue infection | Total | 50 (20) | 49 | 10 (4) | 4 | |
| Local measures | 2A | 35 (14) | 19 | |||
| Oral antibiotics | 2B | 30 (12) | 23 | 10 (4) | 4 | |
| Parenteral antibiotics | 2C | 5 (2) | 2 | |||
| Surgical treatment | 2D | 10 (4) | 5 | |||
| Hypergranulation or keloid | Total | 10 (4) | 4 | 10 (4) | 6 | |
| Local measures | A | 5 (2) | 2 | 10 (4) | 6 | |
| Sleeve placement | B | |||||
| Soft tissue surgery | C | 5 (2) | 2 | |||
| Stoma redundant tissue | Total | 13 (5) | 5 | |||
| Local measures | A | |||||
| Sleeve | B | |||||
| Soft tissue surgery | C | 5 (2) | 2 | |||
| Extramedullary implant removal | D | 8 (3) | 3 | |||
| Complications between surgical stages (no stoma) | ||||||
| Surgical site infection | Total | 3 (1) | 1 | 26 (10) | 11 | |
| Local measures | ||||||
| Antibiotics | 15 (6) | 7 | ||||
| Surgical treatment | 3 (1) | 1 | 10 (4) | 4 | ||
CoCrMo = cobalt-chrome-molybdenum.
Table 4.
Total complications compared between groups at 2-year follow-up
| Complication | Conventional surgery and CoCrMo implant (n = 40; 41 implants) | Modified surgery and titanium implant (n = 39; 39 implants) | p value |
| Total soft tissue infections | 76 | 13 | |
| Absolute risk (95% CI) | 0.93 (0.60 to 1.45) | 0.17 (0.09 to 0.30) | < 0.01 |
| Risk ratio Group 1 versus 2; Group 2 versus 1 (95% CI) | 5.61 (2.71 to 11.57) | 0.18 (0.09 to 0.37) | |
| Total hypergranulation or keloid formation events | 4 | 6 | |
| Absolute risk (95% CI) | 0.05 (0.02 to 0.12) | 0.08 (0.03 to 0.21) | 0.51 |
| Risk ratio Group 1 versus 2; Group 2 versus 1 (95% CI) | 0.64 (0.16 to 2.47) | 1.57 (0.41 to 6.10) | |
| Total stoma redundant tissue events | 5 | 0 | |
| Absolute risk (95% CI) | 0.06 (0.03 to 0.14) | 0 | |
| Risk ratio Group 1 versus 2; Group 2 versus 1 (95% CI) | |||
| Total complications between surgical stages | 1 | 11 | |
| Absolute risk (95% CI) | 0.01 (0.00 to 0.08) | 0.14 (0.08 to 0.25) | 0.02 |
| Risk ratio Group 1 versus 2; Group 2 versus 1 (95% CI) | 0.09 (0.01 to 0.65) | 11.55 (1.54 to 86.75) |
CoCrMo = cobalt-chrome-molybdenum.
There were no differences in the occurrence of hypergranulation or keloid formation between the conventional surgery with CoCrMo implant and the modified surgery with titanium implant groups (four events, AR 0.05 [95% CI 0.02 to 0.12] versus six events, AR 0.08 [95% CI 0.03 to 0.21]; p = 0.51). Soft tissue surgery was necessary in two individuals in the group treated with the conventional surgery and CoCrMo implant, and all events in the other group could be treated nonsurgically.
Stoma redundant tissue occurred less frequently in the group treated with the adapted surgery and titanium implant than in the group treated with the conventional surgery and CoCrMo implant (0 events, AR 0 versus five events, AR 0.06 [95% CI 0.03 to 0.14]). Soft tissue surgery and temporary removal of the extramedullary component of the implant was necessary two and three times, respectively.
All complications occurring between surgical stages, when no stoma had been created, were surgical site infections (Table 3). Surgical site infections occurred more often in the group treated with the modified surgical technique and titanium implant than in the group treated with the conventional surgical technique and CoCrMo implant (11 events, AR 0.14 [95% CI 0.08 to 0.25] versus one event, AR 0.01 [95% CI 0.00 to 0.08]). This resulted in an RR for surgical site infections of 11.55 (95% CI 1.54 to 86.75; p = 0.02) for the modified surgical technique and titanium implant group compared with the other group. Surgical site infections required us to move the date of stage 2 forward three times (three of 11 events), allowing for debridement and abscess drainage, all in the adapted surgery and titanium implant group.
Telephone consultations with general practitioners revealed that 2% (two of 89 events) and 20% (two of 10 events) of soft tissue infections and hypergranulation or keloid formation were treated outside the hospital, respectively. No other complications were treated outside the hospital.
Serious Complications
Bone infection occurred in six events in 8% (three of 40) of patients of the conventional surgery and CoCrMo implant group and was treated surgically with retention of the implant in one event. No bone infection occurred in the modified surgery and titanium implant group. No septic implant loosening, aseptic loosening with an unstable implant, intramedullary stem breakage, or periprosthetic fracture occurred in either group during the follow-up period of 2 years.
Discussion
Although studies reporting on complications in transfemoral bone-anchored prosthesis users have stated that soft tissue infections and stoma-related complications are the most frequently occurring [5, 9, 25], no prior study we know of has reported on these soft tissue complications in a detailed manner. We presented the data of transfemoral bone-anchored prosthesis users, reflecting on 10 years of clinical experience in which major changes to the implant and surgical technique were applied. We aimed to evaluate the impact of alterations in treatment, focusing on frequently occurring soft tissue complications, and to describe osseointegration implant treatment in our clinical practice. Our findings suggest that modification of the surgical technique and implant design results in decreased soft tissue infections and stoma redundant tissue, confirming the direction many osseointegration surgeons are going with relation to more stable soft tissue envelopes.
Limitations
The retrospective study design with regard to the collection of data on complications may have led to an underestimation of the number of events. However, this might have been partially addressed by contacting general practitioners, because they play a prominent role in the Dutch healthcare system and are the first point of contact when patients experience problems. Furthermore, assessment bias may have occurred because we used a self-developed system that does not grade complications based on their importance to the patient. However, in the absence of a validated all-encompassing classification system, a similar grading system has been used in other studies [3, 25]. Additionally, we focused on soft tissue complications, and no patient-reported outcome measures were collected; thus, we were not able to give insight into patient satisfaction in relation to the occurrence of complications. Nevertheless, earlier research demonstrates that most bone-anchored prosthesis users are satisfied compared with previous socket-prosthesis use, even with the occurrence of adverse events [19, 24]. Furthermore, a decrease in the incidence and severity of complications might increase patient satisfaction. Assessment bias, as well as the potential underestimation of complications, resulted in the tendency to overestimate the benefit related to treatment modifications.
Additionally, selection bias occurred because the individuals eligible for treatment were highly selected, and as such, these findings might not apply to the typical amputation practice or for individuals treated with other types of osseointegration implants. However, because most individuals undergoing osseointegration implantation are treated with standard transfemoral implants, we believe reporting these results is relevant. Selection bias also occurred because we excluded individuals treated in the transitional period from 2013 to 2015. We believe this is justified, because inclusion of a limited number of participants (n= 13), with addition of a third combination of treatment strategies, overcomplicates any potential analysis. Furthermore, the presence of multiple confounders made it impossible for us to investigate the exact influence of a single procedural change on complication rates. For example, treatment-related group differences such as discontinuing prolonged postoperative antibiotic prophylaxis may have influenced infectious outcomes. Cessation of prolonged antibiotic use may have downplayed the decrease in soft tissue infections observed, while also influencing the increase in surgical site infections between surgical stages that occurred after treatment changes. The change in implants also complicated our effort to evaluate the effect of changes to surgical technique, because changes to the coating of the extramedullary portion of the implant might also affect soft tissue complications. Obviously, for research purposes, it would be more favorable to evaluate treatment adaptations separately. However, in practice, when an implant is believed to be less safe because of the potential risk of breakage, its substitution is well founded. Another confounder is the learning curve of the surgeon because an improvement in surgical technique is expected over time, potentially making the outcomes of the latter group seem superior. This effect will most likely have been relatively small, because changes implemented to surgical technique combined with relatively small groups might have resulted in two learning curves. Lastly, the 2-year follow-up period precludes an assessment of long-term complications such as late reoperation. However, this study focused on soft tissue infections and complications, all of which predominantly occurred in the early- to midterm after treatment, as opposed to certain complications with a more long-term nature such as aseptic implant loosening or periprosthetic fractures [25].
Soft Tissue Infections and Stoma-related Complications
Patients treated with the modified surgical technique and titanium implant experienced fewer soft tissue infections (which were treated with less invasive measures) and fewer events of stoma redundant tissue. However, surgical site infections between surgical stages occurred more often in this group than in patients treated with the conventional surgical technique and CoCrMo implant. It seems that treatment adaptations to surgical technique and implant design play a beneficial role in reducing the incidence and severity of these frequently occurring soft tissue complications after the second stage of the procedure. This finding is contrasted by the increase in surgical site infections and the occasional need to expedite the second stage of surgery. We hypothesize this is caused by increased soft tissue tension over the underlying implant after surgical stage 1 because of the additional reduction of soft tissues in the modified surgical technique, leading to tissue damage or necrosis. However, the possible effect of cessation of prolonged antibiotic prophylaxis in the modified surgical technique group cannot be ruled out. Al Muderis et al. [3] reported on transfemoral bone-anchored prosthesis users and found similar results regarding the occurrence of soft tissue infections and stoma-related complications. It remains clear that soft tissue complications are the most frequently occurring, while most soft tissue infections can be successfully treated with oral antibiotics (94% in the study by Al Muderis et al. [3] versus 88% to 100% in the current study). Juhnke et al. [17] demonstrated an absolute risk reduction ([AR group 1 – AR group 2] x 100) of infection of 42% to 55% after surgical and device adaptations in individuals treated with a press-fit transfemoral osseointegration implant, comparable to our findings of 68% absolute risk reduction ([0.84 – 0.16] x 100). Furthermore, surgical intervention for soft tissue infections was not necessary in their intervention group, similar to this study. Our study thus confirmed the findings of Juhnke et al. [17], and we attempted a more methodical and systematic analysis with a fixed follow-up period and current data regarding nonsurgical treatment. Additional research is necessary to investigate the influence of solitary treatment adaptations on complications and to investigate late complications such as infection, periprosthetic fracture, and implant breakage or loosening [23, 25]. Lastly, with the increase in surgical site infections occurring between surgical stages, the potential benefit of performing single-stage surgery, thus eliminating soft tissue tension over the implant, should be investigated.
Serious Complications
Patients treated with the modified surgical technique and titanium implant did not experience serious complications in this study. Bone infections occurred in the conventional surgery and CoCrMo implant group and were successfully treated with implant retention. Because bone infection can occur as a consequence of ascending infection, treatment modifications resulting in a decrease in soft tissue infections might play a protective role. Larger studies are necessary to investigate this assumption. It remains clear, however, that the incidence of serious complications in transfemoral bone-anchored prosthesis users is low, as suggested by earlier studies focusing on treatment safety [3, 5].
Conclusion
Ongoing treatment modifications to surgical technique and implant design, as well as learning curve and experience, have resulted in a decrease in the incidence and severity of soft tissue infections and stoma redundant tissue in this procedure, contrasted by an increase in surgical site infections before stoma creation. Serious complications did not occur in the group treated with the adapted surgical technique and titanium implant. Multiple bone infections occurred in the group treated with the conventional procedure and CoCrMo implant and all were successfully treated with implant retention. Therefore, because we believe the benefits of these treatment modifications outweigh the disadvantages, we advise surgeons to create a shallower stoma with a stable soft tissue envelope combined with a titanium implant. Additional research is necessary to investigate ways to mitigate the occurrence and impact of frequently occurring soft tissue complications.
Footnotes
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from Radboud University Medical Center, Nijmegen, the Netherlands (number 2017-3767).
This work was performed at Radboud University Medical Center, Nijmegen, the Netherlands.
Contributor Information
David Reetz, Email: david.reetz@radboudumc.nl.
Nico Verdonschot, Email: nico.verdonschot@radboudumc.nl.
Marinus de Kleuver, Email: marinus.dekleuver@radboudumc.nl.
Jan Paul M. Frölke, Email: JanPaul.Frolke@radboudumc.nl.
Ruud A. Leijendekkers, Email: ruud.leijendekkers@radboudumc.nl.
References
- 1.Abdallah MN, Badran Z, Ciobanu O, Hamdan N, Tamimi F. Strategies for optimizing the soft tissue seal around osseointegrated implants. Adv Healthc Mater. 2017;6. [DOI] [PubMed] [Google Scholar]
- 2.AlMuderis M, Aschoff HH, Bosley B, Raz G, Gerdesmeyer L, Burkett B. Direct skeletal attachment prosthesis for amputee athlete: the unknown potential. Sports Engineering. 2016;19:141-145. [Google Scholar]
- 3.Al Muderis M, Khemka A, Lord SJ, Van de Meent H, Frölke JPM. Safety of osseointegrated implants for transfemoral amputees: a two-center prospective cohort study. J Bone Joint Surg Am. 2016;98:900-909. [DOI] [PubMed] [Google Scholar]
- 4.Aschoff HH, Juhnke DL. Evaluation of 10 years experience with endo-exo femur prostheses - background, data and results [in German]. Z Orthop Unfall. 2012;150:607-614. [DOI] [PubMed] [Google Scholar]
- 5.Atallah R, Leijendekkers RA, Hoogeboom TJ, Frolke JP. Complications of bone-anchored prostheses for individuals with an extremity amputation: a systematic review. PLoS One. 2018;13:e0201821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atallah R, van de Meent H, Verhamme L, Frolke JP, Leijendekkers RA. Safety, prosthesis wearing time and health-related quality of life of lower extremity bone-anchored prostheses using a press-fit titanium osseointegration implant: a prospective one-year follow-up cohort study. PLoS One. 2020;15:e0230027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atallah R, Leijendekkers RA, Hoogeboom T, van de Meent H, Frölke JP. Complications of bone-anchored prostheses for patients with an extremity amputations: a systematic review and meta-analysis. PROSPERO: International Prospective Register of Systematic Reviews. 2016;42016046722. [Google Scholar]
- 8.Brånemark R, Brånemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38:175-181. [PubMed] [Google Scholar]
- 9.Brånemark R, Hagberg K, Kulbacka-Ortiz K, Berlin O, Rydevik B. Osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective five-year follow-up of patient-reported outcomes and complications. J Am Acad Orthop Surg. 2019;27:e743-e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burger H, Marincek C. Return to work after lower limb amputation. Disabil Rehabil. 2007;29:1323-1329. [DOI] [PubMed] [Google Scholar]
- 11.Demet K, Martinet N, Guillemin F, Paysant J, Andre JM. Health related quality of life and related factors in 539 persons with amputation of upper and lower limb. Disabil Rehabil. 2003;25:480-486. [DOI] [PubMed] [Google Scholar]
- 12.Dudek NL, Marks MB, Marshall SC, Chardon JP. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663. [DOI] [PubMed] [Google Scholar]
- 13.Frölke JP, Leijendekkers RA, van de Meent H. Osseointegrated prosthesis for patients with an amputation: multidisciplinary team approach in the Netherlands. Unfallchirurg. 2017;120:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosth Orthot Int. 2001;25:186-194. [DOI] [PubMed] [Google Scholar]
- 15.Harolds J. Quality and safety in health care, part I: five pioneers in quality. Clin Nucl Med. 2015;40:660-662. [DOI] [PubMed] [Google Scholar]
- 16.Jeyapalina S, Beck JP, Bachus KN, Williams DL, Bloebaum RD. Efficacy of a porous-structured titanium subdermal barrier for preventing infection in percutaneous osseointegrated prostheses. J Orthop Res. 2012;30:1304-1311. [DOI] [PubMed] [Google Scholar]
- 17.Juhnke DL, Beck JP, Jeyapalina S, Aschoff HH. Fifteen years of experience with integral-leg-prosthesis: cohort study of artificial limb attachment system. J Rehabil Res Dev. 2015;52:407-420. [DOI] [PubMed] [Google Scholar]
- 18.Leijendekkers RA, Staal JB, van Hinte G, et al. Long-term outcomes following lower extremity press-fit bone-anchored prosthesis surgery: a 5-year longitudinal study protocol. BMC Musculoskelet Disord. 2016;17:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leijendekkers RA, van Hinte G, Frolke JP, et al. Functional performance and safety of bone-anchored prostheses in persons with a transfemoral or transtibial amputation: a prospective one-year follow-up cohort study. Clin Rehabil. 2019;33:450-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leijendekkers RA, van Hinte G, Frolke JP, van de Meent H, Nijhuis-van der Sanden MW, Staal JB. Comparison of bone-anchored prostheses and socket prostheses for patients with a lower extremity amputation: a systematic review. Disabil Rehabil. 2017;39:1045-1058. [DOI] [PubMed] [Google Scholar]
- 21.Leijendekkers RA, van Hinte G, Nijhuis-van der Sanden MW, Staal JB. Gait rehabilitation for a patient with an osseointegrated prosthesis following transfemoral amputation. Physiother Theory Pract. 2017;33:147-161. [DOI] [PubMed] [Google Scholar]
- 22.Marks LJ, Michael JW. Science, medicine, and the future: artificial limbs. BMJ. 2001;323:732-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed J, Reetz D, van de Meent H, Schreuder H, Frolke JP, Leijendekkers R. What are the risk factors for mechanical failure and loosening of a transfemoral osseointegrated implant system in patients with a lower-limb amputation? Clin Orthop Relat Res. 2022;480:722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orgel M, Schwarze F, Graulich T, et al. Comparison of functional outcome and patient satisfaction between patients with socket prosthesis and patients treated with transcutaneous osseointegrated prosthetic systems (TOPS) after transfemoral amputation. Eur J Trauma Emerg Surg. 2022;48:4867-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reetz D, Atallah R, Mohamed J, van de Meent H, Frolke JPM, Leijendekkers R. Safety and performance of bone-anchored prostheses in persons with a transfemoral amputation: a 5-year follow-up study. J Bone Joint Surg Am. 2020;102:1329-1335. [DOI] [PubMed] [Google Scholar]
- 26.Sinha R, van den Heuvel WJ, Arokiasamy P. Factors affecting quality of life in lower limb amputees. Prosthet Orthot Int. 2011;35:90-96. [DOI] [PubMed] [Google Scholar]
- 27.Van de Meent H, Hopman MT, Frolke JP. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013;94:2174-2178. [DOI] [PubMed] [Google Scholar]
- 28.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163-194. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429. [DOI] [PubMed] [Google Scholar]