To the editor,
Potential routes of SARS-CoV-2 transmission have been a matter of debate since the early phase of the current COVID-19 pandemic. Similar to other respiratory viruses, airborne droplets exhaled by an infected individual and inhaled by another susceptible person were identified as the dominant route of transmission.1 However, indirect routes of transmitting SARS-CoV-2 are still under investigation.1, 2, 3 Transmission by indirect contact has been reported for other respiratory viruses1, and therefore, it was intuitive to place great emphasis on the importance of appropriate hand disinfection during the COVID-19 pandemic4. Yet, data providing firm evidence for the presence and quantity of potentially infectious virus carried on the hands of patients infected with SARS-CoV-2 remained scarce.
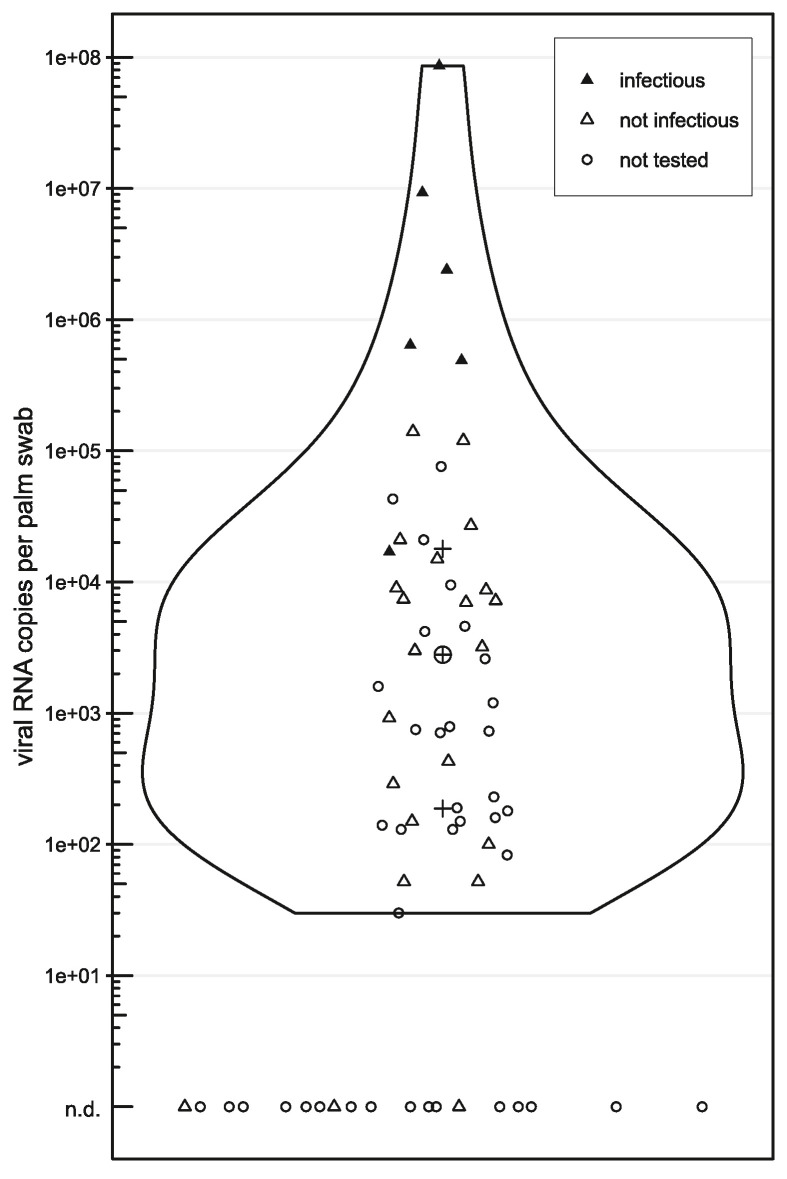
To assess the potential role of hand contamination for indirect transmission, we screened standardised palm swabs from 70 hospitalised children with SARS-CoV-2 infection for the presence and infectious capacity of the virus detectable on hands (see Supplemental Material & Methods for details). Of these, 67 palm swabs were evaluable by RT-qPCR analysis and 48 (71.6%) tested positive for SARS-CoV-2. The median viral RNA copy number detected in the transport medium of individual swabs was 2.8*103 (range 3.0*101 to 8.6*107), with thresholds of the first and third quartiles at 1.8*102 and 2.8*104, respectively (Figure 1 ). Although viral concentrations exceeding 1.0*105 copies were documented in seven samples, most swabs revealed considerably lower virus copy numbers, possibly due to sample collection beyond the peak of respiratory infection. Identification of the virus variant was possible in 34 patients and the results reflected the predominant circulation of individual SARS-CoV-2 variants in Austria at that time, as registered in the GISAID database5: between the beginning of October and mid-December, 2021, the Delta variant was detected in all patients tested, while Omicron variants were identified in all instances thereafter ().
Figure 1.
Infectious potential of hand-borne SARS-CoV-2. Violin-plot showing the distribution of SARS-CoV-2 viral loads detected by RT-qPCR screening of positive hand swabs (n=48), as specified by the scale on the ordinate. Hand swabs revealing no viral RNA (n=19) are shown at the bottom and are not included in the violin-plot (n.d. – not detected). Triangles indicate samples selected for viability testing of the virus in cell culture (n=28), and specimens with documented infectious particles are highlighted as filled triangles [▲] (n=6). Circles [○] represent samples not tested in cell culture (n=39). The plus symbols [+] mark the second and third quartiles of all viral RNA-positive hand swabs and the median is indicated by the encircled plus sign [⊕].
The viability and replication capacity of SARS-CoV-2 detected in palm swab samples were determined in cell culture using the permissive cell line Vero E6. All patient samples displaying virus concentrations exceeding 1.0*105 viral RNA copies per swab transport medium (n=7) were tested in cell culture. Moreover, 18 additional samples showing a wide range of virus concentrations and three negative samples as controls were randomly selected for further analysis by cell culture experiments (Figure 1). The cell cultures were monitored by microscopy and tested by RT-qPCR over a period of five days. The positive control, a SARS-CoV-2-negative patient sample spiked with 1.2*106 viral RNA copies per mL cell culture medium, revealed a visible cytopathic effect (CPE) even at the lowest multiplicity of infection (MOI) tested (0.0003125). Successful replication of the virus was confirmed by RT-qPCR detection of SARS-CoV-2 RNA after only one day post inoculation at the indicated MOI.
All patient samples displaying more than 5.5*105 viral genome copies per swab (n=5) revealed a clearly identifiable CPE in cell culture - four of them as early as two days post inoculation (dpi). In addition, SARS-CoV-2 RNA was detected and successfully monitored throughout the time course of the experiment in all these specimens and in one additional sample (patient No 9) with an initial viral load of 1.7*104 RNA copies per swab (Figure 1 and Supplemental Figure 1). In addition to RT-qPCR-based monitoring of virus replication, intracellular detection of viral nucleocapsids by immunofluorescence staining of Vero E6 cells was successfully performed in three patients with high initial SARS-CoV-2 concentrations on day five post inoculation (patients no 3, 22, and 46 in Supplemental Table 1).
The present study was undertaken to assess the occurrence of hand contamination with SARS-CoV-2 in children with COVID-19, with a particular focus on the infectious potential of hand-borne virus. To our knowledge, we provide the first experimental evidence supporting the notion that hand-borne SARS-CoV-2 from infected patients can display infectious potential, thus confirming the possibility of virus transmission via an indirect route. The time span between the last hand hygiene measure and collection of the palm swab samples was documented, and it appears noteworthy that two of the samples displaying positive tests in cell culture were obtained from patients whose hands had been washed only three hours prior to sample collection. This observation emphasises the role of frequent hand hygiene as a preventive measure and supports established recommendations - particularly during active infection. The observations presented should serve as a basis for further assessment of the potential role of SARS-CoV-2 transmission via virus-contaminated hands, particularly in epidemiologically critical settings, to facilitate the implementation of optimised strategies for preventing uncontrolled spread of the infection in the current pandemic and any future outbreaks.
Author contributions
Conceptualization: A.Z., T.L., J.W., M.H., J.H.; project administration: M.H., P.F.; data curation: A.Z., K.K., P.S., I.A., V.F., S.D., C.M., F.G., M.H., P.F., J.H., L.U.; formal analysis and investigation: M.H., P.F., J.H., L.U.; visualization: M.H., P.F.; funding acquisition, resources, and supervision: A.Z., T.L., J.W.; methodology: A.Z., T.L., M.H., J.H., L.U., K.O., S.P., M.F., C.W., D.S.; validation: M.H., P.F., S.P., M.F., C.W., D.S.; writing – original draft: M.H., T.L., J.H.; writing – review & editing: T.L., M.H., A.Z., F.G., K.O., J.W., V.F., P.F., P.S., I.A., K.K., C.M., S.D., L.U., S.P., M.F., D.S., C.W.
Declaration of interests
The authors declare that no competing interests exist.
Acknowledgements
This work was funded by a grant from the Medical Scientific Fund of the Mayor of the City of Vienna (Project 016). The SARS-CoV-2 viability tests were funded by the project National Institute Virology and Bacteriology (Programme EXCELES, Project No. LX22NPO5103) - Funded by the European Union - Next Generation EU.
Handling Editor: J. M. Hübschen
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2023.06.012.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Leung N.H.L. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 2021;19(8):528–545. doi: 10.1038/s41579-021-00535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bueckert M., Gupta R., Gupta A., Garg M., Mazumder A. Infectivity of SARS-CoV-2 and Other Coronaviruses on Dry Surfaces: Potential for Indirect Transmission. Materials (Basel) 2020;13(22) doi: 10.3390/ma13225211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira F., Obrova K., Haas M., et al. Intestinal Shedding of SARS-CoV-2 in Children: No Evidence for Infectious Potential. Microorganisms. 2022;11(1) doi: 10.3390/microorganisms11010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Transmission of SARS-CoV-2: implications for infection prevention precautions. 09-07-2020. https://apps.who.int/iris/handle/10665/333114 (accessed 21-05-2023).
- 5.Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob Chall. 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.