Abstract
Background
Increasing the interval between the first and second SARS-CoV-2 vaccine doses enhances vaccine immunogenicity, however the optimal timing of the third vaccine is unknown. In this study, we investigated how the time interval between the first and second (V1–V2), or second and third (V2–V3) doses affects immunogenicity after three doses of the BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine.
Methods
This is an observational cohort consisting of 360 participants enrolled in the COVID-19 Occupational Risks, Seroprevalence, and Immunity among Paramedics in Canada (CORSIP) study. Immune responses to BA.1 and other variants were measured from serum using an ACE2 competitive binding assay for surrogate SARS-CoV-2 neutralization. We fit a multiple linear regression model to estimate the independent association between both the V1–V2 and V2–V3 intervals and serum SARS-CoV-2 neutralization, while adjusting for age, sex, and the V3-to-blood collection interval. We examined vaccine dosing intervals as continuous variables and categorized them into quartiles.
Results
The mean age was 40 years, 45% were female sex (at birth), and the median BA.1 surrogate neutralization was 61% (IQR 38–77%). The multivariate analysis indicated that longer V1–V2 (β = 0.1292, 95% CI: 0.04807–0.2104) and V2–V3 (β = 0.2653, 95% CI: 0.2291–0.3015) intervals were associated with increased surrogate neutralization of BA.1. These results were consistent when examining responses against Spike from other SARS-CoV-2 strains. When categorized into V2–V3 quartiles, the first (56–231 days), and second (231–266 days) quartiles demonstrated decreased BA.1 surrogate neutralization compared to the longest V2–V3 quartile (282–329 days). There was no significant difference in surrogate neutralization between the long (266–282 days) and longest (282–329 days) V2–V3 intervals.
Conclusion
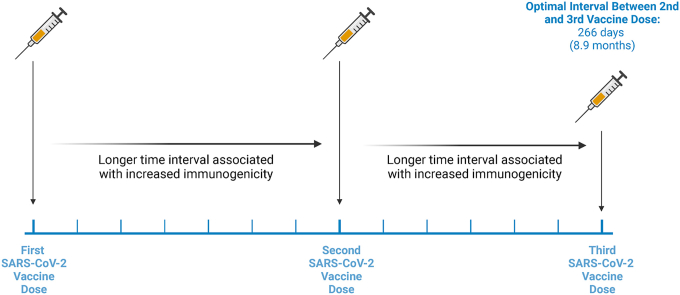
Longer intervals between first, second and third doses are independently associated with increased immunogenicity for all tested SARS-CoV-2 strains. Increasing the intervals between the second and third vaccine doses up to 8.9 months provided additive benefits increasing the immunogenicity of BNT162b2 vaccine schedules.
Keywords: Vaccine interval, Immunization, COVID, SARS-CoV-2, Surrogate neutralization
Graphical abstract
1. Introduction
The persistent worldwide circulation of SARS-CoV-2 has led to the continued emergence of variants with increased transmissibility, especially the Omicron variant [1]. This suggests that a regular vaccination regime, like that for influenza, may become standard practice. However, the optimal vaccine schedules are still an open question, with possible biannual or annual vaccine administration [2]. Widespread public vaccination programs provide an ideal opportunity for observational studies investigating the timing interval between the first three SARS-CoV-2 vaccines, which allows for inferences regarding ideal dosing intervals between future vaccines. Previous data have shown that a longer interval between the first and second dose (V1–V2 interval) of mRNA vaccines leads to greater SARS-CoV-2 immunogenicity, compared to a shorter interval [[3], [4], [5]]. However, the optimal dosing schedule for vaccine doses subsequent to the initial 2-dose series is unclear. Further, it is unclear if there is interaction between the V1–V2 and V2–V3 intervals. We sought to determine impact of both the V1–V2 and V2–V3 intervals on SARS-CoV-2 neutralization in individuals treated with three doses of the Comirnaty (BNT162b2) vaccine.
2. Materials and methods
2.1. Study cohort
Serum samples were identified from individuals enrolled in the COVID-19 Occupational Risks, Seroprevalence, and Immunity among Paramedics in Canada (CORSIP) cohort study [5,6] who had received three doses of BNT162b2 and who provided a blood sample 1 year ± 2 weeks after the first vaccine dose. We excluded: (1) samples from participants who had COVID-19; and (2) samples collected from participants vaccinated within 30 days prior to the sample blood draw. COVID-19 was defined as: individuals who had a positive polymerase chain reaction test or rapid antigen test prior to blood collection, or if the sample was reactive on an Elecsys Anti-SARS-CoV-2 nucleocapsid assay (Roche Diagnostics Corp., IND, USA); this assay has demonstrated high sensitivity and specificity for classifying preceding SARS-CoV-2 infections [7]. All samples were tested using the Elecsys Anti-SARS-CoV-2 nucleocapsid assay.
2.2. Serum testing
Serum samples were tested with the V-PLEX SARS-CoV-2 Panel 22 ACE2 Kit (Meso Scale Discovery, MD, USA). This assay measures the antibody-mediated inhibition of SULFO-TAG conjugated human ACE2 protein binding (determined by the electrochemiluminescence [ECL] signal) to the RBD region of the SARS-CoV-2 spike protein from the BA.1, B.1.351, P.1, B.1.1.7, B.1.617.2 variants and the wild type (WT) strain. First, the optimal serum dilution factor for the BA.1 spot was determined by diluting a pilot selection of serum samples at 1/20, 1/40 and 1/80 concentrations. Pilot serum samples diluted at 1/40 were the most normally distributed suggesting that the measured BA.1% surrogate inhibition values would be the least clustered at high or low values in a larger sample set at this dilution. Then, the ECL readout was used to calculate % surrogate neutralization as in the manufacturer's protocol and as previously published [8]. Briefly, % ACE2 binding inhibition = % surrogate neutralization = [1 – (average sample ECL/ECL baseline calibrator signal)] x 100.
2.3. Statistical analysis
We used Prism 9 (GraphPad Software, LLC, San Diego, CA), RStudio (RStudio, PBC, Boston, MA) and Excel (Microsoft, Redmond, WA) for data analysis. The sample size of 360 adult paramedics consisted of the entirety of individuals in our cohort who satisfied the study criteria in section 2.1. Participants were stratified by quartile interval between the first and second vaccine dose (V1–V2), and also by the interval between the second and third dose (V2–V3). Quartiles were used to ensure that all groups were the same reasonable sample size while comparing several different vaccine dosing intervals. We compared demographic data across groups categorized by V1–V2 quartiles and V2–V3 quartiles using the one-way Kruskal-Wallis test for the continuous variable of age, and the Chi-squared test for categorical variables, which were sex, ethnicity/race, educational level, smoking history, and medical history.
First, we compared surrogate neutralization between the following SARS-CoV-2 strains: BA.1, B.1.351, P.1, B.1.1.7, B.1.617.2 and WT. Then, we assessed surrogate neutralization within groups of a single strain, after categorization of the samples into four groups based on V1–V2 dosing interval quartiles. Since there existed the possibility that surrogate neutralization would be not normally distributed due to possible clustering around higher percentages of neutralization in certain variants, data normality was assessed using the Shapiro-Wilk test. As the data were not normally distributed, differences between groups were assessed with a non-parametric Kruskal-Wallis test followed by a post hoc non-parametric Dunn's test with p-values corrected using the Benjamini-Hochberg procedure for FDR (false discovery rate). The FDR value was used to compare differences between the longest BA.1 vaccine dosing interval quartile and shorter quartile groups. We repeated this analysis for the other SARS-CoV-2 variants. Any value below the FDR cut-off value of q = 0.1 was considered significant. We then repeated these analyses for groups divided into quartiles based on the V2–V3 vaccine dosing intervals.
We then performed a multiple linear regression to assess the independent association of SARS-CoV-2 surrogate neutralization with the V1–V2 interval and the V2–V3 interval, while adjusting for the V3-to-blood collection interval, age, and sex, followed by a high-powered Holm-Šídák p-value correction (α = 0.05). We fit models that did, and did not, include an intercept value in the model (as V1–V2 and V2–V3 dosing intervals of 0 days was not a possible option), and evaluated model fit with the coefficient of determination (R2). We first performed this model with vaccine dosing intervals as continuous variables, but also repeated the model with vaccine dosing intervals represented as 4-level categorical quartile-based variables (with reference to the longest vaccine dosing interval).
3. Results
3.1. Sample demographics
The study cohort consisted of a total of 360 adult paramedics (Table 1), 163 (45%) of whom reported female sex (at birth). The median V1–V2 interval was 36 days (IQR 30–44 days) while the median V2–V3 interval was 266 days (IQR 231–282 days). Table 1 shows participant demographic characteristics, grouped by both the V1–V2 and V2–V3 dosing interval quartiles. All demographic characteristics were similar between groups (p > 0.05) (Table 1).
Table 1.
Characteristics of a CORSIP cohort consisting of study participants that have received three doses of BNT16b2 stratified by V1–V2 and V2–V3 dosing interval quartile.
| Study Variables | Full Cohort |
V1–V2 Dosing Interval (days) |
V2–V3 Dosing Interval (days) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 360 |
Short (12–30) |
Moderate (30–36) |
Long (36–44) |
Longest (44–140) |
p-value |
Short (56–231) |
Moderate (231–266) |
Long (266–282) |
Longest (282–329) |
p-value |
|
| n = 90 | n = 90 | n = 90 | n = 90 | n = 90 | n = 90 | n = 90 | n = 90 | ||||
| Age (years), Mean (SD) | 40 (11) | 39.8 (10.0) | 40.3 (9.7) | 39.9 (10.6) | 40.3 (12.1) | 0.9881 | 40.3 (11.9) | 40.75 (10.6) | 39.5 (10.1) | 39.8 (9.7) | 0.8913 |
| Sex, n (%) | |||||||||||
| Female | 165 (45) | 34 (38) | 49 (54) | 37 (41) | 45 (50) | 43 (48) | 36 (40) | 44 (49) | 42 (47) | ||
| Male | 191 (54) | 56 (62) | 41 (46) | 51 (57) | 43 (48) | 0.0898 | 44 (49) | 53 (59) | 46 (51) | 48 (53) | 0.6119 |
| Prefer not to answer, n (%) | 4 (1) | 0 (0) | 0 (0) | 2 (2) | 2 (2) | 3 (3) | 1 (1) | 0 (0) | 0 (0) | ||
| Ethnicity/Race, n (%) a | |||||||||||
| White | 323 (90) | 88 (98) | 78 (87) | 77 (86) | 80 (89) | 80 (89) | 80 (89) | 82 (91) | 81 (90) | ||
| South Asian | 4 (1) | 1 (1) | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 2 (2) | ||
| Chinese | 16 (4) | 1 (1) | 7 (8) | 3 (3) | 5 (6) | 4 (4) | 5 (6) | 4 (4) | 3 (3) | ||
| Black | 3 (0.8) | 0 (0) | 2 (2) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 2 (2) | 0 (0) | ||
| Filipino | 4 (1) | 0 (0) | 1 (1) | 2 (2) | 1 (1) | 1 (1) | 1 (1) | 2 (2) | 0 (0) | ||
| Latin American | 3 (0.8) | 2 (2) | 0 (0) | 1 (1) | 0 (0) | 0.2679 | 0 (0) | 1 (1) | 0 (0) | 2 (2) | 0.9823 |
| Arab | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | ||
| Southeast Asian | 3 (0.8) | 1 (1) | 1 (1) | 0 (0) | 1 (1) | 1 (1) | 1 (1) | 0 (0) | 1 (1) | ||
| Korean | 2 (0.6) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | ||
| Japanese | 1 (0.3) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | ||
| Indigenous | 1 (0.3) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | ||
| Prefer to self-describe | 3 (0.8) | 1 (1) | 0 (0) | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (3) | ||
| Prefer not to answer | 7 (2) | 0 (0) | 0 (0) | 4 (4) | 3 (3) | 4 (1) | 2 (2) | 1 (1) | 0 (0) | ||
| Educational level, n (%) | |||||||||||
| Non-university, n (%) | 212 (59) | 55 (61) | 48 (53) | 54 (60) | 55 (61) | 57 (64) | 58 (65) | 53 (59) | 44 (49) | ||
| University Bachelor's degree, n (%) | 134 (37) | 33 (37) | 41 (46) | 30 (33) | 30 (33) | 27 (30) | 28 (31) | 35 (39) | 44 (49) | ||
| University Graduate degree (Masters or Doctorate), n (%) | 7 (2) | 1 (1) | 1 (1) | 2 (2) | 3 (3) | 0.5134 | 3 (3) | 1 (1) | 2 (2) | 1 (1) | 0.0751 |
| Prefer not to answer, n (%) | 7 (2) | 1 (1) | 0 (0) | 4 (4) | 2 (2) | 3 (3) | 3 (3) | 0 (0) | 1 (1) | ||
| Smoking History, n (%) | |||||||||||
| Cigarette use | 19 (5) | 5 (6) | 2 (2) | 8 (9) | 4 (4) | 0.3329 | 4 (4) | 9 (10) | 4 (4) | 2 (2) | 0.1173 |
| E-cigarette | 9 (3) | 3 (3) | 1 (1) | 0 (0) | 5 (6) | 6 (7) | 1 (1) | 1 (1) | 1 (1) | ||
| Medical history, n (%) | |||||||||||
| Hypertension | 31 (9) | 10 (11) | 7 (8) | 9 (15) | 5 (6) | 0.5554 | 7 (8) | 8 (9) | 8 (9) | 8 (9) | 0.9911 |
| Diabetes | 8 (2) | 1 (1) | 1 (1) | 3 (3) | 3 (3) | 0.5630 | 4 (4) | 3 (3) | 0 (0) | 1 (1) | 0.2125 |
| Asthma | 43 (12) | 15 (17) | 11 (12) | 7 (8) | 10 (11) | 0.3260 | 9 (10) | 11 (12) | 12 (13) | 11 (12) | 0.9185 |
| Chronic Lung disease | 4 (1) | 2 (2) | 0 (0) | 1 (1) | 1 (1) | 0.5678 | 1 (1) | 0 (0) | 1 (1) | 2 (2) | 0.5678 |
| Chronic Heart disease | 4 (1) | 1 (1) | 2 (2) | 1 (1) | 0 (0) | 0.5678 | 0 (0) | 1 (1) | 2 (2) | 1 (1) | 0.5678 |
| Chronic Kidney disease | 2 (0.5) | 0 (0) | 2 (2) | 0 (0) | 0 (0) | 0.1100 | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 0.1100 |
| Liver disease | 6 (2) | 1 (1) | 2 (2) | 0 (0) | 3 (3) | 0.3353 | 3 (3) | 1 (1) | 2 (2) | 0 (0) | 0.3353 |
| Malignancy | 10 (3) | 3 (3) | 1 (1) | 3 (3) | 3 (3) | 0.7448 | 4 (4) | 2 (2) | 0 (0) | 4 (4) | 0.2100 |
| Chronic blood disorder | 3 (0.8) | 0 (0) | 2 (2) | 0 (0) | 1 (1) | 0.2960 | 1 (1) | 0 (0) | 1 (1) | 1 (1) | 0.7992 |
| Immune suppressed | 11 (3) | 2 (2) | 4 (4) | 2 (2) | 3 (3) | 0.7936 | 5 (6) | 2 (2) | 1 (1) | 3 (3) | 0.3501 |
| Chronic neurol. disorder | 1 (0.3) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0.3903 | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0.3903 |
| Vaccination Interval (days), median (IQR) | |||||||||||
| V1–V2 | 36 (30–44) | 21 (21–22) | 35 (35–35) | 39 (38–42) | 98 (87–105) | <0.0001 | 95.5 (79.75–104.3) | 38 (33.75–42) | 35 (35–38) | 22 (21–35) | <0.0001 |
| V2–V3 | 265.5 (231–282) | 285 (259.8–294) | 273 (267–282) | 262.5 (255.8–275) | 203.5 (186–221.3) | <0.0001 | 203 (184.8–212.5) | 256 (249–262) | 273 (270–278) | 291 (285–300) | <0.0001 |
| V1–V3 | 305 (294–316) | 306.5 (280.8–315) | 308 (302–316) | 302 (296.8–313.3) | 294.5 (283.8–323.3) | 0.0278 | 287.5 (273–308.5) | 297.5 (288.5–300) | 308 (304.8–313) | 319 (310–331) | <0.0001 |
| V3-BC | 63 (49.25–76) | 62 (53.75–85.25) | 59 (48.75–69) | 65 (51–74) | 69 (43.75–83.25) | 0.0712 | 78 (54.5–94) | 73 (65–80) | 59.5 (51–67) | 48.5 (39–58) | <0.0001 |
Kruskal-Wallis tests were used to test for differences in the mean ages of participants and used to test for differences in vaccine intervals as they were not normally distributed. Chi-Squared tests were used to test for significant differences between categorical variables.
Some individuals identify with more than one category. SD: Standard Deviation; n: number; E, electronic; V1, first vaccine date; V2, second vaccine date; V3, third vaccine date; BC: blood collection date.
3.2. Surrogate neutralization of Omicron and other SARS-CoV-2 variants after three doses of BNT162b2
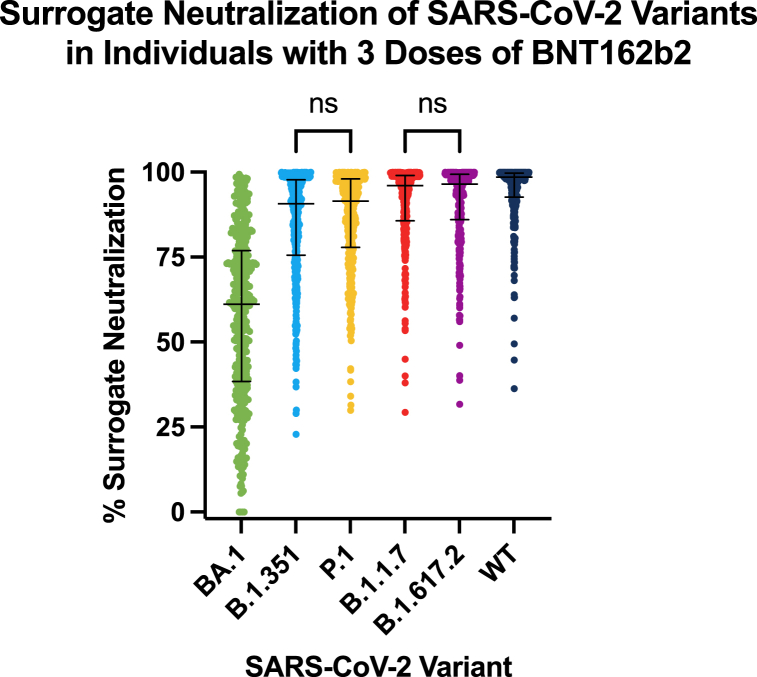
Overall, the median surrogate neutralization after three doses of BNT162b2 was 99% (IQR 93–100%) for the wild type strain, 97% (IQR 86–99%) for B.1.617.2, 96% (IQR 86–99%) for B.1.1.7, 91% (IQR 76–98%) for B.1.351, 91% (IQR 78–98%) for P.1 and 61% (IQR 38–77%) for BA.1 (Table 2). The surrogate neutralization of each variant was significantly different from one another (p < 0.0001), except for B.1.351 when compared to P.1 (p = 0.4774) and B.1.1.7 when compared to B.1.617.2 (p = 0.5930) (Fig. 1).
Table 2.
Surrogate neutralization of SARS-CoV-2 variants BA.1, B.1.351, P.1, B.1.1.7, B.1.617.2, and WT as stratified by V1–V2 and V2–V3 dosing intervals.
| SARS-CoV-2 Variant (% surrogate neutralization), median (IQR) | Full Cohort |
V1–V2 Dosing Interval (days) |
V2–V3 Dosing Interval (days) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 360 |
Short (12–30) |
Moderate (30–36) |
Long (36–44) |
Longest (44–140) |
p-value |
Short (56–231) |
Moderate (231–266) |
Long (266–282) |
Longest (282–329) |
p-value |
|
| n = 90 | n = 90 | n = 90 | n = 90 | n = 90 | n = 90 | n = 90 | n = 90 | ||||
| BA.1 | 61.14 (38.38–76.92) | 62.41 (40.66–80.06) | 64.31 (41.72–82.83) | 59.99 (40.98–75.33) | 51.35 (30.10–71.90) | 0.0312 | 47.01 (27.94–67.35) | 59.86 (36.33–73.11) | 63.12 (41.63–81.67) | 72.48 (49.85–85.38) | <0.0001 |
| B.1.351 | 90.66 (75.53–97.75) | 90.66 (75.99–98.00) | 94.16 (80.87–98.58) | 89.93 (73.46–97.69) | 87.46 (73.17–95.90) | 0.0495 | 84.14 (69.34–95.03) | 89.31 (74.03–96.57) | 92.26 (77.86–98.04) | 94.58 (83.93–99.19) | <0.0001 |
| P.1 | 91.49 (77.83–97.99) | 91.15 (78.35–98.07) | 94.83 (82.59–98.71) | 91.03 (76.76–97.82) | 88.67 (74.28–96.32) | 0.0460 | 85.20 (72.15–95.73) | 91.00 (76.72–96.33) | 93.89 (79.52–98.36) | 95.25 (85.39–99.27) | <0.0001 |
| B.1.1.7 | 96.08 (85.68–99.05) | 96.07 (85.58–99.05) | 98.00 (89.54–99.48) | 95.90 (84.67–99.00) | 94.24 (83.89–98.63) | 0.0134 | 91.19 (79.70–97.77) | 95.19 (85.60–98.59) | 97.04 (87.73–99.17) | 98.32 (92.95–99.66) | <0.0001 |
| B.1.617.2 | 96.51 (86.04–99.40) | 96.48 (86.91–99.29) | 98.12 (89.19–99.71) | 96.04 (85.95–99.34) | 94.10 (80.17–98.87) | 0.0116 | 91.37 (77.36–98.55) | 96.14 (86.64–98.87) | 97.73 (86.93–99.50) | 98.67 (92.68–99.83) | <0.0001 |
| WT | 98.52 (92.67–99.99) | 98.48 (92.62–99.73) | 99.31 (95.24–99.84) | 98.20 (92.15–99.68) | 97.35 (90.63–99.49) | 0.0039 | 96.08 (86.94–99.32) | 98.17 (92.84–99.45) | 99.22 (93.82–99.76) | 99.48 (96.78–99.90) | <0.0001 |
Kruskal-Wallis test was used to test for differences in % surrogate neutralization as they were not normally distributed.
Fig. 1.
Surrogate neutralization of SARS-CoV-2 variants in participants with three doses of BNT16b2. All variants were significantly different from one another (p < 0.0001), except for the comparisons of % surrogate neutralization for B.1.351 when compared to P.1 and B.1.1.7 when compared to B.1.617.2, which were not significantly different (ns). The median and interquartile ranges are presented for each variant.
3.3. Neutralization across SARS-CoV-2 variants with changes in V1–V2 interval
When grouped by V1–V2 quartiles (Table 1), the median V1–V2 vaccine dosing intervals (ordered by lowest to highest quartile) were 21 days, 35 days, 39 days, and 98 days. The median V2–V3 vaccine dosing intervals for these groups were 285 days, 273 days, 263 days, and 204 days. The median V3-to-blood collection intervals were 62 days, 59 days, 65 days, and 69 days and were not significantly different (p > 0.05).
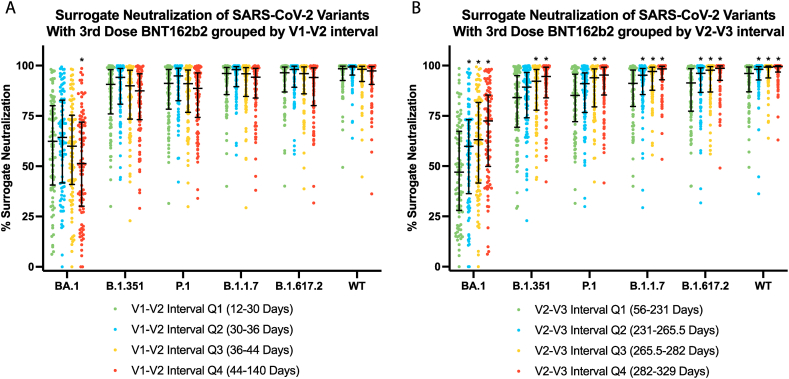
With reference to the longest quartile (44–140 days), BA.1 surrogate neutralization for the first quartile (12–30 days) was significantly higher for the Omicron strain BA.1 with a median value of 62% (IQR 41–80%) versus 51% (IQR 30–72%) (p = 0.0281, q = 0.0842) (Fig. 2A; Table 2). We did not detect a difference between the longest quartile and shortest quartile for the other variants.
Fig. 2.
Surrogate neutralization of different SARS-CoV-2 variants with dosing interval. (A) Surrogate neutralization of SARS-CoV-2 variants in individuals with three doses of BNT16b2 stratified by the interval between their first and second doses, V1–V2. BA.1 showed a statistically significant decrease in % surrogate neutralization in the V1–V2 longest quartile which represents the longest V1–V2 interval, when compared to the shortest V1–V2 interval in the first quartile. (B) Surrogate neutralization of SARS-CoV-2 variants in individuals with three doses of BNT16b2 stratified by the interval between their second and third doses, V2–V3. The two shortest V2–V3 intervals, represented by the first and second quartiles had significantly lower surrogate neutralization for all variants when compared to the longest interval for that variant. The median and interquartile ranges are presented for each variant in each group. * Denotes significant difference when compared to Q4 according to a Dunn's Test followed by a Benjamini Hochberg procedure where the FDR cut off is 0.1.
3.4. Neutralization across SARS-CoV-2 variants with changes in V2–V3 interval
When grouped by V2–V3 quartiles, the median V2–V3 vaccine dosing intervals (ordered by lowest to highest quartile) was 203 days, 256 days, 273 days, and 291 days. The median V1–V2 vaccine dosing intervals for these groups were 95.5 days, 38 days, 35 days and 22 days. The median V3-to-blood collection interval were 78 days, 73 days, 60 days, and 49 days.
With reference to the longest quartile (282–329 days), surrogate neutralization for the first (56–231 days) and second (231–266 days) quartiles were significantly lower for all strains (Fig. 2B; Table 2). There was no significant difference between the third (266–282 days) and longest V2–V3 dosing interval quartiles for any of the strains. However, there were significant differences between the V3-to-blood collection intervals.
3.5. Associations between surrogate neutralization, V1–V2 and V2–V3 intervals
The BA.1 linear regression model examining vaccine dosing intervals as continuous variables demonstrated that the goodness of fit was higher when the intercept was not included (R2 = 0.8631), and thus the fitted regression model was:
| BA.1% Surrogate Neutralization = 0.1292(V1–V2 interval) + 0.2653(V2–V3 interval) - 0.09760(V3-BC interval) - 0.2068(age) - 3.605(sex) |
The regression model was statistically significant (adjusted p-value = 0.0006). In this model, both higher V1–V2 intervals (adjusted p-value = 0.0076) and higher V2–V3 intervals (adjusted p-value = 0.0006) were associated with higher BA.1 surrogate neutralization. V3-to-blood collection interval, age and sex were not significant predictors of BA.1 surrogate neutralization (adjusted p-value >0.05). Models for the other SARS-CoV-2 variants (Table 3) demonstrate a consistent association between both the V1–V2 and V2–V3 interval and surrogate neutralization.
Table 3.
Multiple linear regression models for each SARS-CoV-2 variant.
| SARS-CoV-2 % Surrogate Neutralization (Dependent Variable) |
Independent Variables | R2 | β (95% CI) | Adjusted p-value (Holm-Šídák) |
|---|---|---|---|---|
| BA.1 | V1–V2 Interval | 0.8631 | 0.1292 (0.04807 to 0.2104) | 0.0076 |
| V2–V3 Interval | 0.2653 (0.2291 to 0.3015) | 0.0006 | ||
| V3-BC Interval | −0.09670 (−0.1938 to −0.001385) | 0.1339 | ||
| Age | −0.2068 (−0.4396 to 0.02592) | 0.1562 | ||
| Sex (Female) | −3.605 (−8.636 to 1.427) | 0.1597 | ||
| B.1.351 | V1–V2 Interval | 0.9693 | 0.2430 (0.1903 to 0.2957) | 0.0006 |
| V2–V3 Interval | 0.2898 (0.2663 to 0.3134) | 0.0006 | ||
| V3-BC Interval | 0.09856 (0.03607 to 0.1610) | 0.0063 | ||
| Age | −0.1348 (−0.2859 to 0.01637) | 0.0803 | ||
| Sex (Female) | −3.985 (−7.252 to −0.7171) | 0.0337 | ||
| P.1 | V1–V2 Interval | 0.9746 | 0.2424 (0.1939 to 0.2910) | 0.0006 |
| V2–V3 Interval | 0.2862 (0.2645 to 0.3079) | 0.0006 | ||
| V3-BC Interval | 0.1128 (0.05525 to 0.1704) | 0.0006 | ||
| Age | −0.1028 (−0.2420 to 0.03649) | 0.1476 | ||
| Sex (Female) | −3.780 (−6.790 to −0.7700) | 0.0278 | ||
| B.1.1.7 | V1–V2 Interval | 0.9851 | 0.2487 (0.2097 to 0.2878) | 0.0006 |
| V2–V3 Interval | 0.2892 (0.2718 to 0.3066) | 0.0006 | ||
| V3-BC Interval | 0.1397 (0.09337 to 0.1860) | 0.0006 | ||
| Age | −0.06297 (−0.1750 to 0.04905) | 0.2697 | ||
| Sex (Female) | −2.653 (−5.075 to −0.2319) | 0.0626 | ||
| B.1.617.2 | V1–V2 Interval | 0.9837 | 0.2411 (0.2003 to 0.2818) | 0.0006 |
| V2–V3 Interval | 0.2889 (0.2707 to 0.3071) | 0.0006 | ||
| V3-BC Interval | 0.1389 (0.09055 to 0.1872) | 0.0006 | ||
| Age | −0.05231 (−0.1701 to 0.06373) | 0.3714 | ||
| Sex (Female) | −2.863 (−5.391 to −0.3352) | 0.0523 | ||
| WT | V1–V2 Interval | 0.9917 | 0.2514 (0.2211 to 0.2817) | 0.0006 |
| V2–V3 Interval | 0.2844 (0.2709 to 0.2980) | 0.0006 | ||
| V3-BC Interval | 0.1846 (0.1487 to 0.2205) | 0.0006 | ||
| Age | −0.02443 (−0.1113 to 0.06240) | 0.5803 | ||
| Sex (Female) | −2.217 (−4.094 to −0.3402) | 0.0410 |
We fit an adjusted linear regression model examining vaccine dosing intervals as quartile-based categorical variables. With reference to the longest V1–V2 quartile, we did not detect an association between any V1–V2 vaccine dosing interval category and BA.1 surrogate neutralization. With reference to the longest V2–V3 quartile (282–329 day), the first (56–231 days; adjusted p-value = 0.0007) and second (231–266 days; adjusted p-value = 0.01) V2–V3 interval quartiles were associated with decreased BA.1 surrogate neutralization. We did not detect a difference between the third (266–282 days) and longest (282–329 days) V2–V3 interval quartiles.
4. Discussion
We examined how differences in V1–V2 and V2–V3 intervals affected antibody-mediated viral surrogate neutralization one-year after the start of a 3-dose BNT162b2 vaccination schedule. We found that an increased interval between both V1 and V2, and V2 and V3 improved 1-year immunogenicity. From our quartile-based analysis, we found that increasing V2–V3 intervals up to 266 days (just under 9 months), were associated with improved immunogenicity, which may represent practical intervals to use for booster doses.
When we stratified our cohort by V2–V3, we consistently observed increased surrogate neutralization with longer V2–V3 intervals. However, these relationships were confounded by the shorter V3-to-blood collection intervals with increasing V2-V3 intervals—given the expected surge in antibody levels post vaccination, we expect that a short interval from vaccination to blood collection can yield a higher acute immune response to the vaccine [5]. However, the multivariate regression analysis allowed to adjust for these variables independently. Our results support that longer V1–V2 and V2–V3 intervals were independently associated with increased Omicron surrogate neutralization. This was consistent when modeling results from other variants. In addition, our multivariate regression models suggested increasing benefit to wider V2–V3 vaccine dosing intervals up to 266 days. In sum, these data extend previous observations that a longer dosing interval between the first two vaccine doses increase vaccine immunogenicity, by demonstrating that increased V2–V3 dosing intervals after three BNT162b2 doses also plays a role [4,5]. These data are important may represent a clinical benefit from allowing sufficient time between booster doses.
The idea that a longer interval between exposures to an antigen, through either vaccination or infection, could lead to a more pronounced immune response is consistent with our knowledge of antibody avidity maturation processes following antigen exposure [9]. However, the exact interval and consistency of this interval across cohorts warranted investigation for SARS-CoV-2 vaccination.
Similar to previous reports, we found that neutralization of Omicron was significantly lower in our cohort, when compared to other SARS-CoV-2 variants and the wild type [10]. In addition, our findings that a longer interval between the second and third BNT162b2 doses led to higher surrogate neutralization were consistent with other studies examining the V2–V3 interval in mRNA-treated vaccinees [11]. Our results were also similar to reports for the ZF2001 protein subunit vaccine, also known as Zifivax, which is authorized for use in China, Uzbekistan, Indonesia, and Colombia [12]. Serum collected from individuals with an interval of 3 months (∼90 days) to 6 months (∼180 days) between second and third doses of ZF2001 had higher BA.1 neutralization when compared to serum collected from individuals with an interval of 1 month (∼30 days) between second and third doses [[13], [14], [15]].
The limitations to this study include its observational nature, limiting direct casual inference about the effect of extended vaccine intervals. Another limitation is a possible saturation effect at higher percentages of surrogate neutralization, especially for the WT strain and strains more closely related to the WT. This could result in loss of finer resolution between surrogate neutralization values once they approach 100%. However, despite this limitation, it was still possible to resolve differences in vaccine dosing intervals due to the differing proportion of these higher surrogate neutralization values between groups and variants.
Given its resilience [16], it is probable that a regular vaccine regimen against SARS-CoV-2 may be implemented to combat its ongoing persistence and mutation. Our data demonstrates that longer intervals between the first, second and third vaccine doses results in overall improved vaccine immunogenicity 1 year after initiation of the vaccine series. Moreover, increasing the V2–V3 dosing interval up to 266 days may represent a reasonable booster dose schedule. However, the immediate risk of infection to an individual should also be regarded when considering an extended dosing interval to potentially increase immunogenicity.
5. Conclusions
Among triple BNT162b2 vaccines, longer intervals between both the first and second dose, and second and third, doses, are independently associated with increased immunogenicity. Increased intervals between the second and third vaccines up to 266 days demonstrated increased immunogenicity, which may represent an appropriate interval for booster vaccination.
Institutional review board statement
The CORSIP study was approved by the University of British Columbia (H2O-03620) and University of Toronto (40435) research ethics boards.
Informed consent statement
Informed written consent was collected from all participants.
Author contribution statement
Martin Adam Prusinkiewicz: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sadaf Sediqi and Ying Jie Li: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
David M. Goldfarb and Michael Asamoah-Boaheng: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Nechelle Wall: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Pascal M. Lavoie and Brian Grunau: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Brian Grunau reports financial support was provided by Government of Canada. Brian Grunau reports financial support was provided by Michael Smith Foundation for Health Research. Mohammad E. Karim reports financial support was provided by Michael Smith Foundation for Health Research. Michael Asamoah-Boaheng reports financial support was provided by Michael Smith Foundation for Health Research. Martin Prusinkiewicz reports financial support was provided by Canadian Institutes of Health Research.
Acknowledgements
We acknowledge the contributions of all the paramedics who participated as well as all participating paramedic services and unions. In addition, we acknowledge members of the BC Children's Hospital Biobank for their contributions. We thank Liam Golding and Bahaa Abu Raya for their suggestions regarding optimization of the MSD assay.
References
- 1.Mohapatra R.K., Kandi V., Sarangi A.K., Verma S., Tuli H.S., Chakraborty S., Chakraborty C., Dhama K. The recently emerged BA.4 and BA.5 lineages of Omicron and their global health concerns amid the ongoing wave of COVID-19 pandemic – correspondence. Int. J. Surg. 2022;103 doi: 10.1016/j.ijsu.2022.106698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly S.L., le Rutte E.A., Richter M., Penny M.A., Shattock A.J. COVID-19 vaccine booster strategies in light of emerging viral variants: frequency, timing, and target groups. Infect. Dis. Ther. 2022;11:2045–2061. doi: 10.1007/s40121-022-00683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne R.P., Longet S., Austin J.A., Skelly D.T., Dejnirattisai W., Adele S., Meardon N., Faustini S., Al-Taei S., Moore S.C., Tipton T., Hering L.M., Angyal A., Brown R., Nicols A.R., Gillson N., Dobson S.L., Amini A., Supasa P., Cross A., Bridges-Webb A., Reyes L.S., Linder A., Sandhar G., Kilby J.A., Tyerman J.K., Altmann T., Hornsby H., Whitham R., Phillips E., Malone T., Hargreaves A., Shields A., Saei A., Foulkes S., Stafford L., Johnson S., Wootton D.G., Conlon C.P., Jeffery K., Matthews P.C., Frater J., Deeks A.S., Pollard A.J., Brown A., Rowland-Jones S.L., Mongkolsapaya J., Barnes E., Hopkins S., Hall V., Dold C., Duncan C.J.A., Richter A., Carroll M., Screaton G., de Silva T.I., Turtle L., Klenerman P., Dunachie S., Abuelgasim H., Adland E., Adlou S., Akther H.D., Alhussni A., Ali M., Ansari M.A., v Arancibia-Cárcamo C., Bayley M., Brown H., Chalk J., Chand M., Chawla A., Chinnakannan S., Cutteridge J., de Lara C., Denly L., Diffey B., Dimitriadis S., Drake T.M., Donnison T., Dupont M., Eyre D., Fairman A., Gardiner S., Gilbert-Jarmillo J., Goulder P., Hackstein C.-P., Hambleton S., Haniffa M., Haworth J., Holmes J., Horner E., Jämsén A., Johnson S., Jones C., Kasanyinga M., Kelly S., Kirk R., Knight M.L., Lawrie A., Lee L., Lett L., Lillie K., Lim N., Mehta H., Mentzer A.J., O'Donnell D., Ogbe A., Pace M., Payne B.A.I., Platt G., Poolan S., Provine N., Ramamurthy N., Robinson N., Romaniuk L., Rongkard P., Sampson O.L., Simmons B., Spegarova J.S., Stephenson E., Subramaniam K., Thaventhiran J., Thomas S., Travis S., Tucker S., Turton H., Watson A., Watson L., Weeks E., Wilson R., Wood S., Wright R., Xiao H., Zawia A.A.T. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184:5699–5714.e11. doi: 10.1016/j.cell.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grunau B., Goldfarb D.M., Asamoah-Boaheng M., Golding L., Kirkham T.L., Demers P.A., Lavoie P.M. Immunogenicity of extended mRNA SARS-CoV-2 vaccine dosing intervals. JAMA. 2022;327:279–281. doi: 10.1001/jama.2021.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunau B., Asamoah-Boaheng M., Lavoie P.M., Karim M.E., Kirkham T.L., Demers P.A., Barakauskas V., Marquez A.C., Jassem A.N., O'Brien S.F., Drews S.J., Haig S., Cheskes S., Goldfarb D.M. Clinical Infectious Diseases; 2021. A Higher Antibody Response Is Generated with a 6- to 7-Week (Vs Standard) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Dosing Interval; p. ciab938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunau B., Golding L., Prusinkiewicz M.A., Asamoah-Boaheng M., Armour R., Marquez A.C., Jassem A.N., Barakauskas V., O'Brien S.F., Drews S.J. Microbiol Spectr; 2022. Comparative 6-Month Wild-type and Delta-Variant Antibody Levels and Surrogate Neutralization for Adults Vaccinated with BNT162b2 versus mRNA-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ainsworth M., Andersson M., Auckland K., Baillie J.K., Barnes E., Beer S., Beveridge A., Bibi S., Blackwell L., Borak M., Bown A., Brooks T., Burgess-Brown N.A., Camara S., Catton M., Chau K.K., Christott T., Clutterbuck E., Coker J., Cornall R.J., Cox S., Crawford-Jones D., Crook D.W., D'Arcangelo S., Dejnirattsai W., Dequaire J.M.M., Dimitriadis S., Dingle K.E., Doherty G., Dold C., Dong T., Dunachie S.J., Ebner D., Emmenegger M., Espinosa A., Eyre D.W., Fairhead R., Fassih S., Feehily C., Felle S., Fernandez-Cid A., Fernandez Mendoza M., Foord T.H., Fordwoh T., Fox McKee D., Frater J., Gallardo Sanchez V., Gent N., Georgiou D., Groves C.J., Hallis B., Hammond P.M., Hatch S.B., Harvala H.J., Hill J., Hoosdally S.J., Horsington B., Howarth A., James T., Jeffery K., Jones E., Justice A., Karpe F., Kavanagh J., Kim D.S., Kirton R., Klenerman P., Knight J.C., Koukouflis L., Kwok A., Leuschner U., Levin R., Linder A., Lockett T., Lumley S.F., Marinou S., Marsden B.D., Martinez J., Martins Ferreira L., Mason L., Matthews P.C., Mentzer A.J., Mobbs A., Mongkolsapaya J., Morrow J., Mukhopadhyay S.M.M., Neville M.J., Oakley S., Oliveira M., Otter A., Paddon K., Pascoe J., Peng Y., Perez E., Perumal P.K., Peto T.E.A., Pickford H., Ploeg R.J., Pollard A.J., Richardson A., Ritter T.G., Roberts D.J., Rodger G., Rollier C.S., Rowe C., Rudkin J.K., Screaton G., Semple M.G., Sienkiewicz A., Silva-Reyes L., Skelly D.T., Sobrino Diaz A., Stafford L., Stockdale L., Stoesser N., Street T., Stuart D.I., Sweed A., Taylor A., Thraves H., Tsang H.P., Verheul M.K., Vipond R., Walker T.M., Wareing S., Warren Y., Wells C., Wilson C., Withycombe K., Young R.K. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect. Dis. 2020;20:1390–1400. doi: 10.1016/S1473-3099(20)30634-4. 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sancilio A.E., D'Aquila R.T., McNally E.M., Velez M.P., Ison M.G., Demonbreun A.R., McDade T.W. A surrogate virus neutralization test to quantify antibody-mediated inhibition of SARS-CoV-2 in finger stick dried blood spot samples. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-94653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis V., Glick B. Research note: anamnestic response of neonatal chickens to sheep red blood cells as influenced by the number of weeks between the first and second injections. Poultry Sci. 1988;67:855–857. doi: 10.3382/ps.0670855. 10.3382/ps.0670855. [DOI] [PubMed] [Google Scholar]
- 10.Kurhade C., Zou J., Xia H., Cai H., Yang Q., Cutler M., Cooper D., Muik A., Jansen K.U., Xie X., Swanson K.A., Shi P. Neutralization of Omicron BA.1, BA.2, and BA.3 SARS-CoV-2 by 3 doses of BNT162b2 vaccine. Nat. Commun. 2022;13:3602. doi: 10.1038/s41467-022-30681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel R.R., Painter M.M., Lundgreen K.A., Apostolidis S.A., Baxter A.E., Giles J.R., Mathew D., Pattekar A., Reynaldi A., Khoury D.S., Gouma S., Hicks P., Dysinger S., Hicks A., Sharma H., Herring S., Korte S., Kc W., Oldridge D.A., Erickson R.I., Weirick M.E., McAllister C.M., Awofolaju M., Tanenbaum N., Dougherty J., Long S., D'Andrea K., Hamilton J.T., McLaughlin M., Williams J.C., Adamski S., Kuthuru O., Drapeau E.M., Davenport M.P., Hensley S.E., Bates P., Greenplate A.R., Wherry E.J. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell. 2022;185:1875–1887.e8. doi: 10.1016/j.cell.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai L., Gao L., Tao L., Hadinegoro S.R., Erkin M., Ying Z., He P., Girsang R.T., Vergara H., Akram J., Satari H.I., Khaliq T., Sughra U., Celi A.P., Li F., Li Y., Jiang Z., Dalimova D., Tuychiev J., Turdikulova S., Ikram A., Flores Lastra N., Ding F., Suhardono M., Fadlyana E., Yan J., Hu Z., Li C., Abdurakhmonov I.Y., Gao G.F. Efficacy and safety of the RBD-dimer–based covid-19 vaccine ZF2001 in adults. N. Engl. J. Med. 2022;386 doi: 10.1056/NEJMoa2202261. 2097–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X., Li D., Ruan W., Chen Z., Zhang R., Zheng A., Qiao S., Zheng X., Zhao Y., Dai L., Han P., Gao G.F. Effects of a prolonged booster interval on neutralization of omicron variant. N. Engl. J. Med. 2022;386:894–896. doi: 10.1056/NEJMc2119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X., Zheng A., Li D., Zhang R., Sun H., Wang Q., Gao G.F., Han P., Dai L. Neutralisation of ZF2001-elicited antisera to SARS-CoV-2 variants. Lancet Microbe. 2021;2:e494. doi: 10.1016/S2666-5247(21)00217-2. 10.1016/S2666-5247(21)00217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao X., Zhang R., Qiao S., Wang X., Zhang W., Ruan W., Dai L., Han P., Gao G.F. Omicron SARS-CoV-2 neutralization from inactivated and ZF2001 vaccines. N. Engl. J. Med. 2022;387:277–280. doi: 10.1056/NEJMc2206900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y., Yisimayi A., Jian F., Song W., Xiao T., Wang L., Du S., Wang J., Li Q., Chen X., Yu Y., Wang P., Zhang Z., Liu P., An R., Hao X., Wang Y., Wang J., Feng R., Sun H., Zhao L., Zhang W., Zhao D., Zheng J., Yu L., Li C., Zhang N., Wang R., Niu X., Yang S., Song X., Chai Y., Hu Y., Shi Y., Zheng L., Li Z., Gu Q., Shao F., Huang W., Jin R., Shen Z., Wang Y., Wang X., Xiao J., Xie X.S. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022 doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.