Abstract
Over the last decade or so, there has been a paradigm shift in the oncologic care of patients with a range of solid tumour and haematologic malignancies, away from traditional cytotoxic chemotherapy and towards personalized cancer treatments, using both targeted therapy and immunotherapy. This shift has contributed to the remarkable and sustained increase in the number of cancer survivors and the longevity of patients with a cancer diagnosis. This review will focus on the cardiovascular effects of immune checkpoint inhibitors and will present a background on immune checkpoint inhibition for cancer, the epidemiology, potential mechanisms, the potential insights into cardiovascular biology, and a diagnostic and therapeutic approach to potential cases. Our understanding of the cardiovascular effects of immune checkpoint inhibitors needs to improve. However, the evolution necessarily needs to be rapid. Initial observations noted that immune checkpoint inhibitor therapy can lead to a fulminant myocarditis. Recent reports have expanded the effect of immune checkpoint inhibitor therapy on the cardiovascular system to include an increase in cardiac dysfunction without myocarditis, arrhythmias, venous thromboembolic disease, accelerated atherosclerosis, and atherosclerosis-related cardiovascular events. The association between immune checkpoint inhibitor therapy and an increase in these cardiovascular events is not only limited to events occurring within the first few weeks after starting therapy but can also include events that occur months to years after therapy. The latter observation is especially of relevance in those treated with adjuvant or neoadjuvant therapy. There needs to be a shift from recognition of an increase in cardiovascular events to currently approved immune checkpoint inhibitor therapies to understanding the mechanisms that lead to adverse cardiovascular effects, understanding who is at risk, and understanding what we can do about it.
Keywords: Immune checkpoint inhibitors, Myocarditis, Atherosclerosis, Cancer
Graphical Abstract
Graphical Abstract.
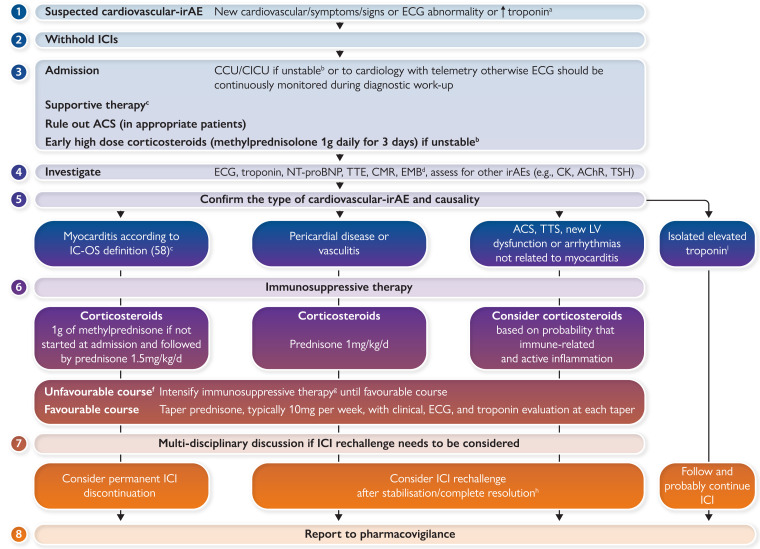
Proposed eight-point-based management of patients with a suspicion for a cardiovascular immune-related adverse event. aTroponin testing may be positive if Troponin I or T is >99th percentile of the upper reference limit. Concomitant myositis may result in significant elevations of CK, CK isoforms, and even Troponin T. In patients with pre-therapeutic troponin elevation, a 50% increase of the level may be used as a cut-off, but no evidence currently supports this recommendation. bHaemodynamic instability, heart failure requiring non-invasive or invasive ventilation, complete or high-grade heart block, and/or significant ventricular arrhythmia. cArrhythmias, conduction abnormalities, acute coronary syndromes, stroke, thromboembolic events, and heart failure should be managed urgently according to the international guidelines. dEndomyocardial biopsy should be performed especially in unstable patients who cannot undergo urgent CMR and in patients with uncertain diagnosis. eSee Table 2. fDefined as hemodynamic instability or electrical instability or increasing troponin or decreasing left ventricular ejection fraction. gThere are no data to recommend a standardized initial treatment strategy for the intensification of immunosuppressive therapy. For now, case series and case reports have shown the potential efficacy of anti-thymocyte globulin, intravenous immunoglobulin, plasma exchange, mycophenolate mofetil, tacrolimus, tocilizumab, abatacept, alemtuzumab, and tofacitinib. The decision regarding optimal therapy must be multidisciplinary, involving specialists in immunology and organ rejection. Infliximab was also proposed but was not incorporated into the algorithm because of its contraindication in acute heart failure. iTroponin elevation without cardiovascular signs/symptoms and negative investigations including EMB. ACS, acute coronary syndrome; CCU, coronary care unit; CK, creatine kinase; AchR, acetylcholine receptor antibodies; CMR, cardiac magnetic resonance; EMB, endomyocardial biopsy; irAE, immune-related adverse event; ICI, immune checkpoint inhibitor; NT-proBNP, N-terminal-pro-brain-natriuretic-peptide; SMB, skeletal muscle biopsy; TSH, thyroid-stimulating hormone; TTE, transthoracic echocardiography; TTS, takotsubo syndrome.
Introduction
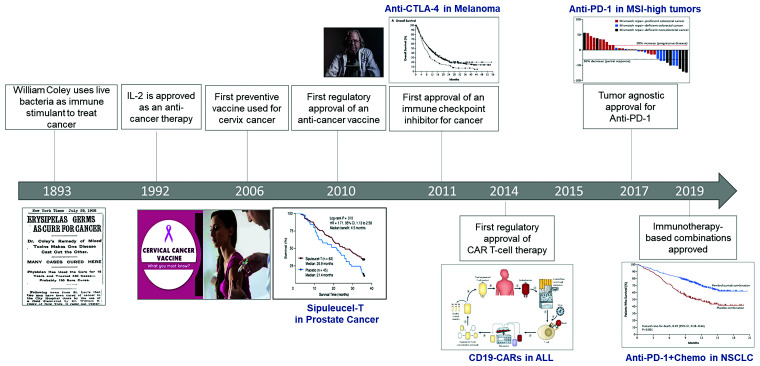
Leveraging the immune system for the treatment of cancer has been on a path of scientific discovery since at least the 1800s (Figure 1).1 While several immunotherapeutic strategies have made important and impactful contributions to cancer medicine such as cancer vaccines and interleukin therapy, it is only in recent years that an exponential progress in immunotherapeutics has resulted in a sea change in cancer treatment. In the last 10 years, several pre-clinical discoveries have led to a rapid drug development and resultant approvals for cancer immunotherapies in >20 different cancer indications. The two classes of immunotherapeutic agents that have dominated these approvals and are now widely available in the clinic include immune checkpoint inhibitors (ICIs) and CAR T-cell therapies.
Figure 1.
Timeline for immunotherapy in cancer.
The first ‘immune checkpoint’ molecule, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), was discovered in the laboratory of Leach et al. in 1996.2 In a seminal experiment, they identified that an antibody to CTLA-4 in a murine model could induce tumour rejection in a syngeneic mouse.2 This led to a first-in-human Phase I trial of CTLA-4 blockade (MDX-010) with responses seen in melanoma and ovarian cancer, rapid studies in melanoma in the Phase II setting, and a subsequent FDA approval based on the landmark Phase III study on the humanized anti-CTLA-4 monoclonal antibody ‘ipilimumab’ (Bristol Myers Squibb, NJ, USA) for metastatic melanoma.3–5 In these studies, several important observations were made which led to an appreciation that immune checkpoint blockade was a unique therapeutic strategy for cancer. The traditional response kinetics used to assess anti-cancer therapies, based on their ability to induce tumour shrinkage, did not appear to apply to immunotherapy.6 This class of agents appeared to have a unique ‘immune-related’ response kinetics, where tumour lesions could increase in size before they shrink and new lesions to even transiently develop, leading to a unique response criterion developed specifically for immunotherapy. Additionally, patients who benefitted from therapy appear to sustain a prolonged benefit that could span years—a phenomenon known as the ‘tail of the curve’.6 This is postulated to represent immunologic memory in the field of cancer immunotherapy. Now, 10 years since the approval of ipilimumab for metastatic melanoma,7 Dr Allison has won a Nobel Prize for his discovery of CTLA-4, and ipilimumab has changed the landscape of melanoma management. It is now combined with a second ICI called nivolumab [anti-programmed death 1 (anti-PD-1)], and the combination has resulted in an unprecedented 5-year survival of >5 years for metastatic melanoma, calling us to reimagine what ‘cure’ may look like in the age of cancer immunotherapy.
The second immune checkpoint molecule to be described was anti-PD-1, and its principal ligand PD-L1 was first described by the other Nobel Prize Winner Dr Tasuko Honjo and colleagues in 2000.8 This discovery was supported by murine data again by Iwai, Honjo, and Colleagues which identified that anti-PD-1-deficient mice led to inhibition of tumour growth, as well as in immunocompetent wild-type mice treated with anti-PD-L1 antibodies.9,10 These data gave rise to Phase I trials of anti-PD-1 and anti-PD-L1 monoclonal antibodies which demonstrated activity across tumour types, including non-small-cell lung cancer (NSCLC), melanoma, and kidney cancer.11 Rapid clinical development in these tumour types and others have led to the approval and use of single agent anti-PD-(L)1 in >15 cancer indications across solid tumour and haematologic malignancies.12 The combination of anti-PD-1 and CTLA-4 blockade principally with the combination of ipilimumab and nivolumab is also licensed in several indications (advanced NSCLC, melanoma, and kidney cancer), and is moving into early stage disease settings as both adjuvant and neoadjuvant approaches. Importantly, there have also been approvals for anti-PD-1 therapy that are tumour agnostic, and based on the molecular features of tumours, such as those that harbour a large number of non-synonymous mutations, for example, microsatellite instable-high colorectal and endometrial cancers.13 These underscore the hypothesis that anti-tumour immunity may be stimulated by a genomically complex tumour that generates a more diverse immunologic response.
During approval of ICIs, it was anticipated that leveraging the immune system through this approach would lead to a wide spectrum of side effects, termed immune-related adverse events (irAEs), due to the suppression of immune inhibitory functions.14 These irAEs occur in 70–90% of patients and all organs can be affected.14 A high-grade of severity is noted in 10–15% and clinical manifestations usually start within the first few weeks to months after the onset of treatment but can occur at any time, even late after cessation of therapy.15 The incidence and severity is higher with combination immunotherapy (e.g. anti-PD-1/PD-L1 and CTLA-4 combination). The other risk factors for irAEs are not well known because patients potentially at risk have been excluded from large clinical trials. Thus, for example, the safety of ICIs in patients with autoimmune disease, recipients of solid-organ transplant, or haematopoietic stem-cell transplant is uncertain.14
Epidemiology of immune checkpoint inhibitor–associated cardiovascular diseases
Cardiovascular irAEs are more common than were initially reported in early ICI clinical trials.12 The lower reporting of cardiovascular irAEs in some oncology clinical trials when compared with real-world data is not specific to clinical trials in cancer patients with ICI therapies. Myocarditis is the most commonly considered cardiovascular irAE with ICI therapy. In initial pharmacovigilance reporting, myocarditis was noted in 0.06% of patients treated with single agent ICI therapy and 0.27% of patients treated with combination ICI.16 Real-world data and more recent clinical trials have confirmed a higher rate of myocarditis.17 For example, in a prospective observational surveillance study where patients underwent serial troponin measurements, an incidence rate of 1.4% for myocarditis was reported.18 Similarly, in a retrospective study of ICI myocarditis patients, an incidence rate of 1.14% was reported,19 and in a recent randomized clinical trial comparing anti-PD-1 therapy to combined anti-PD-1 and lymphocyte-activation gene 3 (LAG-3) therapy where patients underwent serial troponin measurements over the first 2 months, an incidence rate 0.6% for myocarditis for single agent ICI therapy and 1.7% for combination was reported.20 Beyond myocarditis, other cardiovascular irAEs have been increasingly described such as pericardial disease, vasculitis, acute coronary syndromes, thromboembolic events, as well as arrhythmias, and left ventricular (LV) dysfunction [including takotsubo syndrome (TTS)] without evidence of myocarditis.21–28 With such a broader definition of cardiovascular irAEs, a recent meta-analysis of 51 clinical trials reported a rate of cardiovascular irAEs of 3.1% for ICI monotherapies and 5.8% for ICI combination therapies.29 The timing of cardiovascular irAEs was felt to be mostly acute but a recent nationwide cohort study showed patients treated with ICI had a two- to five-fold increased risk of developing a cardiac event both early (within 6 months) and late (>6 months) after initiation of treatment.27 These results were significant after adjustment for age, sex, and date of cancer.27
Immune checkpoint inhibitor–associated myocarditis
Myocarditis is the most common considered cardiovascular irAE, with an incidence rate presented above that ranges from 0.5 to 1.7% of patients.20 However, the prognosis with myocarditis related to ICI therapy is significantly worse than that of other types of myocarditis with mortality rates ranging from 25 to 50% over a short period of follow up,19,20 in comparison with a mortality rate of 4% during a median follow up of 4.7 years for non-ICI myocarditis.30 This poor prognosis results from either fatal cardiovascular complications (arrhythmias, heart failure) or the interruption of effective cancer treatment. Immune checkpoint inhibitor myocarditis typically presents early after starting treatment. For example, Mahmood et al.19 reported a median time from the first ICI dose to the onset of myocarditis of 34 days and 81% of cases presented within 3 months of the first dose. Later, myocarditis cases have been also described up to several months after starting ICI therapy31; however, a caveat is that in some, it is unknown whether they are late diagnosis of myocarditis that had begun much earlier, delayed development of myocarditis, or a cardiomyopathy related to persistent systemic inflammation. The LV ejection fraction (LVEF) is impaired in <50% of patients,32 with the most common complications with ICI myocarditis including atrioventricular conduction disorders, ventricular/supraventricular arrhythmias, and heart failure.33 Non-cardiovascular irAEs occur simultaneously in 30 to 50% of patients; among these, myositis or myasthenia gravis occur in 20–25%, and are associated with a worse prognosis. Several other predictors of poor prognosis have been identified (Table 1).19,34–42
Table 1.
Potential factors associated with a poor prognosis in immune checkpoint inhibitor–associated myocarditis
| Clinical factors |
|
| Electrocardiogram |
|
| Echocardiography |
|
| Cardiac magnetic resonance imaging |
T1 >mean value ±2 standard deviations of the site norm
|
| Serum biomarkers |
|
| Endomyocardial biopsy |
|
| Management |
|
CMR, cardiac magnetic resonance; EMB, endomyocardial biopsy; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; |GLS|, global longitudinal strain absolute value; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction.
Other cardiovascular immune-related adverse events
Early post-marketing data showed that pericardial diseases accounted for 0.36% of all reported irAEs.25 However, the exact incidence of pericarditis and pericardial effusion related to ICI is unclear, particularly because they may also be the consequence of cancer progression or other cancer therapies such as thoracic radiotherapy or conventional cytotoxic chemotherapy. In a large matched case–control cohort study, the incidence rate of ICI-associated pericardial diseases was 1.57 events per 100 person-years after a median follow up of 193 days in cancer patients with ICI when compared with 0.14 events per 100 person-years after a median of 1841 days in cancer controls and the adjusted risk of pericardial disease was more than four-fold higher in patients treated with ICI.23 The occurrence of pericardial disease with ICI therapy has a high mortality with a case fatality rate of 21%.25
Data have suggested vascular toxicity may occur in patients treated with ICI. These vascular toxicities include vasculitis, atherosclerosis-related events, and arterial/venous thrombosis.22,24,25,43 A pharmacovigilance analysis found that ICI therapy was associated with a higher reporting of vasculitis disorders, especially temporal arteritis. Thus, vasculitis accounted for 0.26% of all reported irAEs but the exact incidence in patients treated with ICI remains unknown. In that report, death occurred in 6% of patients with ICI-associated vasculitis.25 In a case series, large vessel vasculitis including giant-cell arteritis, aortitis, and primary angiitis of the nervous system were the most common forms of vasculitis reported with ICIs. No related death was observed in this study.43
Besides vasculitis, there are evolving clinical data, basic on solid scientific plausibility, that ICIs may accelerate atherosclerosis and promote acute coronary syndromes through changes in plaque composition.28 In a matched case–control study, ICI treatment was associated with a three-fold higher risk for atherosclerosis-related cardiovascular events compared with cancer patients who did received ICIs. These findings were confirmed in a second case-crossover analysis, in which the 2-year incidence rate of atherosclerotic cardiovascular event was 1.37 per 100 person-years before ICI treatment and 6.55 per 100 person-years after ICI treatment onset (hazard ratio, 4.78).22 In an imaging analysis, the investigators also found that the rate of progression of total aortic plaque volume was more than three-fold higher with ICIs and was partially attenuated by concurrent use of statins or corticosteroids.22 These checkpoints targeted for cancer therapy play also a key role in thrombosis, and a higher risk of venous thromboembolic events has been reported in patients treated with ICI.24 Finally, LV dysfunction and arrhythmias without obvious myocarditis have been found in patients treated with ICI. Takotsubo syndrome is a particular form of LV dysfunction, which has been significantly highly reported in a pharmacovigilance analysis of ICI patients.26 In the absence of myocarditis, the pathophysiology of this effect remains uncertain, but stress induced by a non-cardiovascular irAE or metabolic changes induced by ICI are plausible hypotheses. The analysis of data concerning these arrhythmias in patients treated with ICI is complex, as there are several possible mechanisms, some of which are linked.27
Potential mechanisms of immune checkpoint inhibitor–associated cardiovascular diseases
The pathophysiology of cardiovascular irAEs remains poorly understood. Given the involvement of immune checkpoints in many aspects of cardiovascular homeostasis, the mechanisms are probably multiple and inter-related.44 Specifically, immune checkpoints are essential in cardiac cell self-tolerance, the process that prevents the immune system from targeting normal organs.45 In addition, they are involved in regulating the systemic inflammatory response but also within the atheromatous plaque.46
Johnson et al.16 were the first to perform an in-depth post-mortem study of two ICI myocarditis patients with associated myositis during treatment of melanoma with nivolumab plus ipilimumab. They reported necrosis and infiltration of CD4+, CD8+ T cells, and macrophages in the myocardium and conduction system, which were comparable with those seen in acute cellular rejection of cardiac transplants. In contrast, no B cells and autoantibodies were found. Similar T-cell clones were observed in the infiltrated myocardium, skeletal muscle, and tumours. Moreover, PD-L1 was expressed on the membrane of injured myocytes.16 Pre-clinical models have highlighted the complexity of the immune response in the development of immune-related cardiovascular diseases47; in mouse models, genetic deletion of both alleles of Pdcl1 (encoding for anti-PD-1) and a single of Ctla4 was associated with recapitulation of the immune-mediated myocardial and pericardial disease phenotypes.48 Progression of atherosclerosis has also been reported with ICIs.22 There is robust scientific plausibility to support a pathology effect of specific ICIs on atherosclerosis.46,49,50 Specifically, anti-PD-1 inhibits the activation of proatherogenic T cells in plaques and short-term ICI therapy in hyperlipidaemic mice leads to a marked increase in CD4+ and CD8+ T cells in the plaque, a four-fold increase in the plaque necrotic core, and increased apoptosis in the plaque, all indicators of increased plaque vulnerability.50,51 However, the effect of ICI on coronary plaque is highly dependent on the ICI target and some ICI therapies may reduce coronary plaque. For example, magrolimab is being tested in several cancer types and inhibits CD-47, an immune checkpoint on macrophages.52 In animal studies, CD-47 is increased in atherosclerosis plaque and inhibition of CD-47 leads to a reduction in atherosclerosis plaque.53 In a single clinical study, magrolimab reduced the 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) signals in the aorta of patients with cancer.54 Thus, it is possible that some ICIs, such as those that target CD-47, will reduce progression of atherosclerosis or reduce plaque vulnerability. Additional insights into the mechanisms involved in the cardiovascular toxicities with ICI therapies come from other animal models. It is recognized that some patients on an ICI can develop LV dysfunction in the absence of myocarditis. In a mouse melanoma model, anti-PD-1 administration led to early CD4+ and CD8+ myocardial infiltration, LV dysfunction, and broad expression of PD-L1 on cardiac endothelial cells.55 This occurred without evidence of myocarditis. Interestingly, LV dysfunction was not detected in mice receiving ICI therapy but without tumour transplantation. Proteome and lipidome analysis revealed significant changes affecting cellular energy generation, suggesting a global and slow effect of ICIs on the heart, which might explain non-myocarditis-related myocardial dysfunction.55 In this latter model, blockade of tumour necrosis factor alpha (TNF-α) prior to starting ICI therapy preserved LV function without attenuating the anti-cancer efficacy of anti-PD1 therapy.55 All these data have led to the development of several pathophysiological hypotheses (Figure 2).
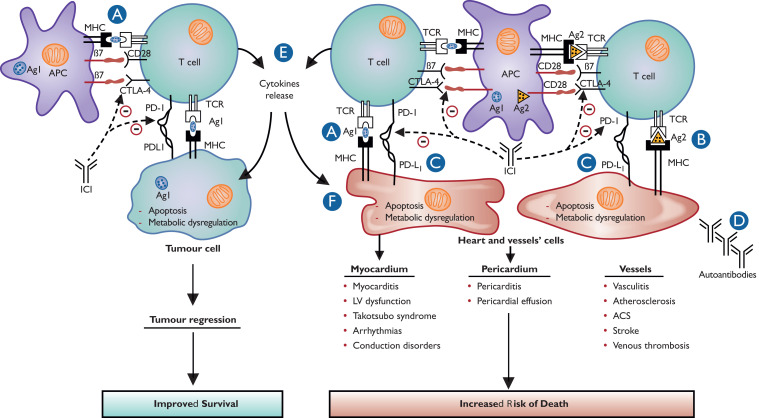
Figure 2.
Proposed pathophysiology and mechanisms of cardiovascular immune-related adverse events. (A) T-cell targeting cardiovascular cells might result from cross-reactivity of tumour and cardiovascular tissues antigens. Tumour and cardiovascular antigens might be similar or with common epitopes. (B) T-cell targeting cardiovascular cells might result from important immune reaction and decrease in cell self-tolerance, which can lead to self-antigen recognition, especially if they had been modified by previous cardiovascular injury. (C) The use of immune checkpoint inhibitor in the context of programmed death ligand 1 overexpression on cardiovascular cells might also contribute to T-cell mediated myocardial, vascular, and pericardial injury related to T-cell infiltration. This overexpression of programmed death ligand 1 is protective in processes such as myocardial ischaemia reperfusion or atherosclerosis. (D) Though autoantibodies targeting cardiac troponin were only detected in pre-clinical models, the hypothesis that activation of the immune system might upregulate pre-existing autoantibodies, especially in predisposed patients, is not completely excluded. (E) The release of pro-inflammatory cytokines secondary to an immune response dysregulated by immune checkpoint inhibitors may cause cytokine release syndrome resulting in myocardial injury. However, cytokine release syndrome is rarely observed with immune checkpoint inhibitor therapy and is more frequent with CAR T-cell therapy. (F) Dysregulation of myocardial metabolism induced by smouldering inflammation is a possible mechanism of myocardial dysfunction after immune checkpoint inhibitor administration.
Diagnostic and therapeutic strategies for cardiovascular immune-related adverse events
Cardiovascular irAEs—myocarditis—represent one of the most serious complications of ICI treatment because it can rapidly lead to death if not managed promptly. Furthermore, they have long-term implications for the continuation of cancer treatment. This cardiovascular event has unique characteristics and should be known to all practitioners incharge of cancer patients. The relatively low level of evidence supporting guidelines has resulted in variable diagnostic and therapeutic strategies.12,56 Whatever the suspected cardiovascular irAE, we propose an eight-point-based management, which is based partly on published papers and also on opinion (Graphical abstract). Regardless of the approach, the decisions should involve cardiologists, oncologists, and other specialists depending on the patient’s condition working together in a ‘cardio-oncology team’.
Clinical presentation and early management
Cardiovascular irAE should always be suspected in the presence of any new cardiovascular symptoms/signs, as well as electrocardiogram (ECG) changes or troponin elevation in asymptomatic patients. Severe fulminant cases can present with cardiogenic shock, complete atrioventricular heart block, intractable ventricular arrhythmias, or cardiac arrest. Symptoms of non-cardiovascular irAE are sometimes prominent like those of myositis or myasthenia gravis.15 Thus, patients may present with diplopia, myalgias, muscle weakness, or breathing difficulties.19 Although an acute coronary syndrome should be considered in all patients,57 myocarditis should be the primary suspected diagnosis to be considered because it is the most common acute cardiovascular irAE and has a very high fatality rate.57
For those with suspected myocarditis, ICI therapy should be immediately withheld, and the patient admitted to a cardiology department with telemetry. Given the potential severity and rapid evolution of myocarditis, unstable patients should be admitted to coronary or intensive care unit and for unstable patients’ high doses of corticosteroids (1000 mg of methylprednisolone) must be initiated without the investigation results being expected. Indeed, high initial dose and early initiation of corticosteroids were associated with improved cardiac outcomes with myocarditis.37 In parallel with the investigations carried out to clarify the diagnosis, supportive treatment of complications (arrhythmias, heart failure, and tamponade) is essential. Investigations should include ECG, serum cardiac biomarkers, transthoracic echocardiography (TTE), cardiac magnetic resonance (CMR) imaging, coronary imaging, and eventually endomyocardial biopsy (EMB).19,32,35,36,38,39,42 For those with other cardiovascular irAEs, the management is less clear. For example, for a patient who presents with LV dysfunction in the absence of myocarditis, it is unclear if corticosteroids or cessation of the ICI is the correct approach. Similarly, for a patient with an atherosclerosis-related cardiovascular event such as a myocardial infarction, most would not hold the ICI or initiate corticosteroids but advice on this is not based on rigorous study.
Electrocardiogram
Electrocardiogram is usually abnormal, but a normal result does not rule out cardiovascular irAEs, especially myocarditis.19,42 A recent multicentre retrospective registry compared the ECG findings obtained in patients before their ICI exposure and within 3 days of their admission for myocarditis.42 During myocarditis, ECG showed significant elevated heart rate, prolonged QRS, prolonged QTc, and decreased in QRS voltage. The incidence of left bundle branch block, conduction disorders, and repolarization abnormalities were significantly increased vs. baseline.42 Throughout hospitalization, patients experienced fascicular, bundle, and/or heart blocks with second-degree heart block in 7.5%, complete heart block in 17%, and life-threatening ventricular arrhythmia in 15%. In this work, pathological Q waves and decreased in QRS voltage were independently associated with 30-day all-cause mortality,42 and in a second similar study, a prolonged QRS duration with ICI myocarditis conferred an increased risk of cardiac events.35
Serum cardiac biomarkers
Troponin is the more relevant serum biomarker in the diagnosis of non-ICI myocarditis. In a small series of ICI myocarditis patients, a troponin elevation was noted in 94% of patients.19 In contrast, this rate was only 46% in Escudier et al.21 This difference may result from lack of standardization of the diagnosis of myocarditis and thus the inclusion in the latter study of patients with cardiac dysfunction but without myocardial necrosis. Importantly, if elevated troponin levels are detected, other potential causes for troponin elevation should be considered, such as myocardial infarction, pulmonary embolism, severe renal insufficiency, myocardial injury due to hyperthyroidism, anaemia, or other cancer therapy–related toxicity. The magnitude of the troponin increase in ICI myocarditis may be prognostic, where troponin at admission, peak, and at discharge has been shown to be predictive of major adverse events in myocarditis patients.19,58 Importantly, cardiac Troponin T may increase in patients with inflammatory myopathies including myositis, in the absence of cardiac involvement and without a concomitant increase in cardiac Troponin I. This is observed with all generations of Troponin T assays and may be related to re-expression of cardiac Troponin T within regenerating skeletal muscle tissue.59 It is of added importance with ICI therapy, as an immune myositis is noted in 20–25% of patients with myocarditis. Thus, when both assays are available, preference should be given to high-sensitivity Troponin I assays. In addition, there is a considerable value to the availability of a baseline/pre-ICI troponin value in patients scheduled to receive ICI. This baseline value may provide a critical comparator in the assessment and surveillance of patients with an elevated baseline level. Finally, in the experience of this team, even among patients with a favourable clinical course, troponin can remain elevated for several weeks or months after having started to decrease following the initiation of immunosuppressive therapy. The long half-life of ICIs could explain this situation and thus the persistence of smouldering myocarditis. The prognostic impact of this observation is not known.
Echocardiography
An echocardiogram is the first-line non-invasive imaging test in patients with suspicion of cardiovascular irAE and a normal LVEF does not rule out the diagnosis of myocarditis.19,32 In myocarditis, wall motion abnormalities can be observed but the LVEF is preserved in >60% of patients and rarely severely reduced.32 Awadalla et al.32 showed a reduction in global longitudinal strain (GLS) with myocarditis and the GLS value was lower than control patients treated with ICI, independent of LVEF. Moreover, lower GLS was strongly associated with major adverse cardiac events. However, recent data suggest that GLS may also be reduced after an ICI in the absence of myocarditis. In an elegant combined basic and physiology study, Michel and colleagues55 showed a reduction in GLS in mice and patients treated with an ICI without evidence of myocarditis, likely due to a ‘natural’ immune cell infiltration into the heart after starting an ICI.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance is an optimal non-invasive imaging modality for the diagnosis of myocarditis and applies criteria based on the updated 2018 Lake Louise criteria.60 These criteria identify myocardial abnormalities, including global or regional non-ischaemic injury and oedema. In addition, in non-ICI myocarditis, CMR provides prognostic value based on late gadolinium enhancement (LGE) presence, location, extent, and pattern.30 However, CMR may have a lower sensitivity in ICI myocarditis. Two retrospective studies36,38 reported that: (i) LGE and elevated T2-weighted inversion recovery (qualitative oedema) were present at admission, respectively, in only 48 and 28% of patients with a preserved LVEF, whereas they are present in approximatively 80 and 27% in patients with non-ICI myocarditis30,60; (ii) abnormal native T1 and T2 values were reported in 78 and 43% of patients, respectively; (iii) the presence of LGE increased from 21.6% when CMR was performed within 4 days of admission to 72% when CMR was performed on Day 4 of admission or later; (iv) in biopsy-proved cases, the presence of the two main updated 2018 Lake Louise criteria had a sensitivity of only 52%, whereas it is around 90% in non-ICI myocarditis.49 Several limitations should be noted including the lack of a cancer patient control group. Recently, a case–control study also reported a lower rate of LGE and the lower sensitivity of the 2018 Lake Louise criteria in ICI myocarditis when compared with viral myocarditis.34 The findings on the CMR may still have prognostic value. For example, the native T1 value in the septum and septal LGE were identified as predictors of major adverse events in ICI myocarditis.34
Positron emission tomography–based imaging
Myocarditis is an inflammatory disease associated with an increase in glucose uptake. However, data suggest a limited to no role for FDG-PET/computed tomography (CT) in the evaluation of ICI myocarditis.61 In an elegant study of 34 patients, there was no difference in the myocardial FDG-PET signal between biopsy confirmed myocarditis patients and those without myocarditis on biopsy.61 There may be a role for other PET tracers in the diagnosis of ICI myocarditis, with novel targets that include fibroblast activation protein and the somatostatin receptor,62,63 but more research is needed.
Coronary imaging
Exclusion of coronary ischaemia, with coronary imaging (invasive angiography or CT) or stress testing, should be performed in appropriate patients.57 However, the presence of bystander significant coronary artery disease should not exclude the possibility of myocarditis, thus the other diagnostic tests must be performed.
Endomyocardial biopsy
An EMB is the gold standard for the diagnosis of myocarditis but is not commonly employed because of its potential complications and variable sensitivity (∼70%).64 The diagnosis of myocarditis is confirmed when lymphocytic infiltration with myocyte necrosis is detected. Data on the characteristic finding on pathology with EMB in ICI myocarditis are relatively consistent. It is an inflammatory infiltrate rich in T cells and similar in appearance to cardiac transplant rejection.39 There may also be data linking the pathological appearance and the outcomes with ICI myocarditis. In a single centre study of 18 patients with ICI myocarditis, the 4 patients with low grades of inflammation were able to continue ICI without immunosuppressive therapy.39 Given the low sensitivity of CMR, the potential severity of myocarditis and the therapeutic consequences, most experts recommend that an EMB be performed when the patient is too unstable to undergo CMR or the diagnosis is uncertain after non-invasive imaging.
Immune checkpoint inhibitor–associated myocarditis diagnostic criteria
The impact on making the correct diagnosis of ICI myocarditis is considerable. Under-diagnosis may lead to a lack of or a delay in the initiation of corticosteroid therapy and a higher rate of cardiovascular events in the short term.37 Over-diagnosis may lead to permanent discontinuation of ICI, withholding of cancer therapy and progression of cancer. Recently, the International Cardio-Oncology Society (IC-OS) has defined diagnostic criteria that can be used in clinical practice.57 However, data are evolving rapidly and these criteria do not address how to consider and manage patients with biopsy findings that do not fulfil the IC-OS definition of major biopsy criteria. Specifically, in the largest pathological series of ICI myocarditis, an intermediate grade was noted where cardiac inflammation was present without cardiomyocyte necrosis.39 These are patients who present with a constellation of cardiac symptoms where the concern for ICI myocarditis was high, and included a newly elevated troponin, a new decline in LVEF, or a magnetic resonance imaging consistent with myocarditis. However, as these patients do not have myocyte necrosis on the pathological samples reviewed, this criterion alone on biopsy is not sufficient for the diagnosis and other criteria would be required in addition. The edit is also suggested to provide guidance for those where the sampling may be of inadequate quality, the biopsy sample is small, or a smaller number of biopsy samples is taken. Therefore, this review proposes an edited version of the IC-OS diagnostic criteria that expands the diagnostic criteria to include patients with myocardial inflammation on the biopsy but without clear evidence of myocyte necrosis as a minor criteria (Table 2).39
Table 2.
Modified International Cardio-Oncology Society 2021 consensus for the diagnosis of immune checkpoint inhibitor myocarditis
| Either a pathohistological diagnosis: |
| Multifocal inflammatory cell infiltrates with overt cardiomyocyte loss by light microscopy on cardiac tissue samples |
| Or a clinical diagnosisa,b: |
| A troponin elevation (new, or significant change from baseline) with one major criterion or a troponin elevation (now, or significant change from baseline) with two minor criteria after exclusion of acute coronary syndrome or acute infectious myocarditis based on clinical suspicion |
| Major criterion |
|
| Minor criteria |
|
|
|
|
|
|
Both Troponin I and Troponin T can be used; however, Troponin T may be falsely elevated in those with concomitant myositis.
The clinical diagnoses should be confirmed with cardiac MRI or endomyocardial biopsy if possible and without causing delays of treatment.
In a patient who is clinically unwell, treatment with immunosuppression should be promptly initiated while awaiting further confirmatory testing.
Investigations for vasculitis
In case of suspected vasculitis, PET, CT, brain magnetic resonance imaging, and arterial biopsy should be considered according to the involved vessel location.
Therapy for immune checkpoint inhibitor–associated cardiovascular immune-related adverse events
In case of a confirmed myocarditis, intravenous (methylprednisolone 1000 mg/day) followed by long-term oral corticosteroids should be prescribed (prednisone, 1–2 mg/kg/day).65 The optimal length of these therapies or the speed of taper in unknown, but it is reasonable to continue the treatment until resolution of symptoms and normalization or near-normalization of troponin, LVEF, and conduction abnormalities, which is usually >6–12 weeks. The persistence of an elevated troponin likely reflects some ongoing myocardial injury and may be due to the long half-life (>20 days for anti-PD-1) or an immune fingerprint of the therapy (>1 year). It is reasonable to recommend a clinical, ECG, and troponin assessment before each corticosteroid dose reduction, and in some, a TTE. However, evolving data suggest that high-dose corticosteroids may adversely impact cancer outcomes.66 Additionally, up to 50% of patients remain refractory or corticosteroid resistant.67,68 For corticosteroid-refractory or fulminant myocarditis with clinical instability, intensified immunosuppressive therapy should be considered with mycophenolate mofetil, tacrolimus, anti-thymocyte globulin, intravenous immunoglobulin, infliximab, abatacept, belatacept, or alemtuzumab.65 These options may be combined with plasmapheresis to reduce circulating levels of ICI and cytokines. However, the potential value of any of these strategies has not been demonstrated in prospective well-designed trials and such trials are needed urgently. In an ICI myocarditis mouse model, blockade of TNF-α prior to starting an ICI preserved LV function without attenuating the anti-cancer efficacy of anti-PD1 therapy.55 However, in a retrospective study, patients who received infliximab (anti-TNF-α) were more likely to die from cardiovascular causes.68 Thus, there are conflicting recommendations in the guidelines on the use of infliximab in these patients.15,65
For non-myocarditis cardiovascular irAEs, data are limited to guide management of immunosuppressive treatment.15 Intermediate-to-high doses of corticosteroids (equivalent prednisone 1–2 mg/kg/day oral or i.v.) should be given in cases of pericardial disease, arrhythmias, conductive disorders, LV dysfunction (including TTS, when evidence of myocardial inflammation), and vasculitis until recovery. However, this recommendation should be adapted to the determination of whether ICI therapy is responsible for the cardiovascular diseases.
Rechallenging with immune checkpoint inhibitor therapy after cardiovascular immune-related adverse events
There are limited to no studies testing the safety of rechallenging ICI after cardiovascular irAEs.39 Rechallenging ICI treatment after irAEs depends on the severity and organs involved. Given the risk and the potential fatality of a recurrence, some experts suggest that ICI rechallenge should be avoided in any case of ICI myocarditis even after recovery. However, some suggest rechallenging with an alternate ICI approach may be reasonable after consideration of the index case, a multidisciplinary discussion, and the availability of cardiac monitoring.
Surveillance of patients treated with immune checkpoint inhibitors
There are no data supporting the value of a routine screening approach of cardiovascular irAEs in patients treated with ICIs and, as a result, there is some variability between institutions. In a recent trial comparing avelumab (PD-L1 inhibitor) plus axitinib [vascular endothelial growth factor (VEGF) inhibitor] vs. sunitinib (VEGF inhibitor) in patients with advanced renal cell carcinoma, routine cardiac investigations including serial cardiac troponin testing in asymptomatic patients were not useful for early detection of cardiovascular irAEs, including myocarditis.69 Nevertheless, patients in the combination arm who had higher baseline Troponin T values were at higher risk of all cardiac events.69 Since the clinical suspicion of myocarditis is usually made by oncologists during patient monitoring, a strategy that can be available in all oncology departments, easy to perform, and easy for a non-cardiologist to analyse is the goal. A baseline assessment of cardiovascular symptoms/signs, ECG, and troponin should be performed for each patient prior to receiving an ICI. All patients on ICIs should undergo a routine serial assessment for symptoms and signs of cardiovascular irAEs. However, there appears to be no value to the routine use of an ECG and troponin among patients without potential symptoms or signs of a cardiovascular irAE on an ICI.69 A routine on ICI treatment surveillance strategy combining ECG and troponin would have the advantage of a higher sensitivity but may lead to unnecessary interruptions of cancer therapy especially among patients with a modestly asymptomatic (isolated) troponin elevation.18
Summary
Our understanding of the effects of various immunotherapeutic approaches on the cardiovascular structure, cardiovascular function, and cardiovascular events is early, evolving, and relatively limited. Rigorous studies are required. Additionally, it should be acknowledged how these cancer therapies can provide unique windows into cardiovascular biology and provide actionable insights that will be relevant to patients without cancer. For a cardiologist, keeping up with the advances in oncology care is an amazing and rewarding full-time job with a goal of mirroring the improvement in cancer outcomes with the shared goal of parallel shifts in cardiac outcomes.
Contributor Information
Franck Thuny, Aix-Marseille University, University Mediterranean Center of Cardio-Oncology, Unit of Heart Failure and Valvular Heart Diseases, Department of Cardiology, North Hospital, Assistance Publique - Hôpitaux de Marseille, Centre for CardioVascular and Nutrition Research (C2VN), Inserm 1263, Inrae 1260, Marseille, France.
Jarushka Naidoo, Sidney Kimmel Comprehensive Cancer Center, John Hopkins University, Baltimore, MD, USA; Beaumont Hospital and RCSI University of Health Sciences, Dublin, Ireland.
Tomas G Neilan, Division of Cardiology and Department of Radiology, Cardiovascular Imaging Research Center (CIRC), Massachusetts General Hospital, Boston, MA, USA; Cardio-Oncology Program, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Funding
T.G.N. is supported by a gift from A. Curt Greer and Pamela Kohlberg and from Christina and Paul Kazilionis, the Michael and Kathryn Park Endowed Chair in Cardiology, and a Hassenfeld Scholar Award. T.G.N. is also supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute grants [R01HL130539, R01HL137562, K24HL150238, R01HL159187].
References
- 1.Dobosz P, Dzieciatkowski T. The intriguing history of cancer immunotherapy. Front Immunol 2019;10:2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res 2009;15:6446–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 2020;11:3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov 2022;21:509–528. [DOI] [PubMed] [Google Scholar]
- 8.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996;8:765–772. [DOI] [PubMed] [Google Scholar]
- 9.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol 2004;17:133–144. [DOI] [PubMed] [Google Scholar]
- 11.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity: JACC: CardioOncology primer. JACC CardioOncol 2021;3:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 15.Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol 2021;39:4073–4126. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waliany S, Neal JW, Reddy S, Wakelee H, Shah SA, Srinivas S, et al. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. JACC CardioOncol 2021;3:137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutierrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 2022;386:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 2017;136:2085–2087. [DOI] [PubMed] [Google Scholar]
- 22.Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 2020;142:2299–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong J, Drobni ZD, Zafar A, Quinaglia T, Hartmann S, Gilman HK, et al. Pericardial disease in patients treated with immune checkpoint inhibitors. J Immunother Cancer 2021;9:e002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong J, Drobni ZD, Alvi RM, Murphy SP, Sullivan RJ, Hartmann SE, et al. Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur J Cancer 2021;158:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ederhy S, Dolladille C, Thuny F, Alexandre J, Cohen A. Takotsubo syndrome in patients with cancer treated with immune checkpoint inhibitors: a new adverse cardiac complication. Eur J Heart Fail 2019;21:945–947. [DOI] [PubMed] [Google Scholar]
- 27.D'Souza M, Nielsen D, Svane IM, Iversen K, Rasmussen PV, Madelaire C, et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J 2021;42:1621–1631. [DOI] [PubMed] [Google Scholar]
- 28.Cautela J, Rouby F, Salem JE, Alexandre J, Scemama U, Dolladille C, et al. Acute coronary syndrome with immune checkpoint inhibitors: a proof-of-concept case and pharmacovigilance analysis of a life-threatening adverse event. Can J Cardiol 2020;36:476–481. [DOI] [PubMed] [Google Scholar]
- 29.Rubio-Infante N, Ramirez-Flores YA, Castillo EC, Lozano O, Garcia-Rivas G, Torre-Amione G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail 2021;23:1739–1747. [DOI] [PubMed] [Google Scholar]
- 30.Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017;70:1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolladille C, Ederhy S, Allouche S, Dupas Q, Gervais R, Madelaine J, et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer 2020;8:e000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awadalla M, Mahmood SS, Groarke JD, Hassan MZO, Nohria A, Rokicki A, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol 2020;75:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol 2018;19:e447–e458. [DOI] [PubMed] [Google Scholar]
- 34.Cadour F, Cautela J, Rapacchi S, Varoquaux A, Habert P, Arnaud F, et al. Cardiac MRI features and prognostic value in immune checkpoint inhibitor-induced myocarditis. Radiology 2022;303:512–521. [DOI] [PubMed] [Google Scholar]
- 35.Zlotoff DA, Hassan MZO, Zafar A, Alvi RM, Awadalla M, Mahmood SS, et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J Immunother Cancer 2021;9:e002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thavendiranathan P, Zhang L, Zafar A, Drobni ZD, Mahmood SS, Cabral M, et al. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol 2021;77:1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation 2020;141:2031–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J 2020;41:1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palaskas NL, Segura A, Lelenwa L, Siddiqui BA, Subudhi SK, Lopez-Mattei J, et al. Immune checkpoint inhibitor myocarditis: elucidating the spectrum of disease through endomyocardial biopsy. Eur J Heart Fail 2021;23:1725–1735. [DOI] [PubMed] [Google Scholar]
- 40.Drobni ZD, Zafar A, Zubiri L, Zlotoff DA, Alvi RM, Lee C, et al. Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor-associated myocarditis. J Am Heart Assoc 2020;9:e018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Champion SN, Stone JR. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod Pathol 2020;33:99–108. [DOI] [PubMed] [Google Scholar]
- 42.Power JR, Alexandre J, Choudhary A, Ozbay B, Hayek S, Asnani A, et al. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation 2021;144:1521–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol 2018;37:2579–2584. [DOI] [PubMed] [Google Scholar]
- 44.Baik AH, Oluwole OO, Johnson DB, Shah N, Salem JE, Tsai KK, et al. Mechanisms of cardiovascular toxicities associated with immunotherapies. Circ Res 2021;128:1780–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lichtman AH. The heart of the matter: protection of the myocardium from T cells. J Autoimmun 2013;45:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med 2019;25:1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291:319–322. [DOI] [PubMed] [Google Scholar]
- 48.Wei SC, Meijers WC, Axelrod ML, Anang NAS, Screever EM, Wescott EC, et al. A genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov 2021;11:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Investig 2007;117:2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bu DX, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, Lederer J, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol 2011;31:1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poels K, van Leent MMT, Reiche ME, Kusters PJH, Huveneers S, de Winther MPJ, et al. Antibody-mediated inhibition of CTLA4 aggravates atherosclerotic plaque inflammation and progression in hyperlipidemic mice. Cells 2020;9:1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med 2018;379:1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016;536:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jarr KU, Nakamoto R, Doan BH, Kojima Y, Weissman IL, Advani RH, et al. Effect of CD47 blockade on vascular inflammation. N Engl J Med 2021;384:382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michel L, Helfrich I, Hendgen-Cotta UB, Mincu RI, Korste S, Mrotzek SM, et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J 2022;43:316–329. [DOI] [PubMed] [Google Scholar]
- 56.Thuny F, Alexandre J, Salem JE, Mirabel M, Dolladille C, Cohen-Solal A, et al. Management of immune checkpoint inhibitor-induced myocarditis: the French Working Group's Plea for a pragmatic approach. JACC CardioOncol 2021;3:157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J 2022;43:280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puzanov I, Subramanian P, Yatsynovich YV, Jacobs DM, Chilbert MR, Sharma UC, et al. Clinical characteristics, time course, treatment and outcomes of patients with immune checkpoint inhibitor-associated myocarditis. J Immunother Cancer 2021;9:e002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hughes M, Lilleker JB, Herrick AL, Chinoy H. Cardiac troponin testing in idiopathic inflammatory myopathies and systemic sclerosis-spectrum disorders: biomarkers to distinguish between primary cardiac involvement and low-grade skeletal muscle disease activity. Ann Rheum Dis 2015;74:795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 61.Ederhy S, Devos P, Pinna B, Funck-Brentano E, Abbar B, Fenioux C, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging for the diagnosis of immune checkpoint inhibitor-associated myocarditis. Arch Cardiovasc Dis 2022;115:114–116. [DOI] [PubMed] [Google Scholar]
- 62.Finke D, Heckmann MB, Herpel E, Katus HA, Haberkorn U, Leuschner F, et al. Early detection of checkpoint inhibitor-associated myocarditis using 68Ga-FAPI PET/CT. Front Cardiovasc Med 2021;8:614997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boughdad S, Latifyan S, Fenwick C, Bouchaab H, Suffiotti M, Moslehi JJ, et al. 68Ga-DOTATOC PET/CT to detect immune checkpoint inhibitor-related myocarditis. J Immunother Cancer 2021;9:e003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J 2007;28:3076–3093. [DOI] [PubMed] [Google Scholar]
- 65.Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9:e002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018;36:2872–2878. [DOI] [PubMed] [Google Scholar]
- 67.Wang C, Lin J, Wang Y, Hsi DH, Chen J, Liu T, et al. Case series of steroid-resistant immune checkpoint inhibitor associated myocarditis: as comparative analysis of corticosteroid and tofacitinib treatment. Front Pharmacol 2021;12:770631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cautela J, Zeriouh S, Gaubert M, Bonello L, Laine M, Peyrol M, et al. Intensified immunosuppressive therapy in patients with immune checkpoint inhibitor-induced myocarditis. J Immunother Cancer 2020;8:e001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rini BI, Moslehi JJ, Bonaca M, Schmidinger M, Albiges L, Choueiri TK, et al. Prospective cardiovascular surveillance of immune checkpoint inhibitor-based combination therapy in patients with advanced renal cell cancer: data from the phase III JAVELIN renal 101 trial. J Clin Oncol 2022;40:1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]