Abstract
Aims
Perioperative myocardial infarction/injury (PMI) following non-cardiac surgery is a frequent cardiac complication. Better understanding of the underlying aetiologies and outcomes is urgently needed.
Methods and results
Aetiologies of PMIs detected within an active surveillance and response programme were centrally adjudicated by two independent physicians based on all information obtained during clinically indicated PMI work-up including cardiac imaging among consecutive high-risk patients undergoing major non-cardiac surgery in a prospective multicentre study. PMI aetiologies were hierarchically classified into ‘extra-cardiac’ if caused by a primarily extra-cardiac disease such as severe sepsis or pulmonary embolism; and ‘cardiac’, further subtyped into type 1 myocardial infarction (T1MI), tachyarrhythmia, acute heart failure (AHF), or likely type 2 myocardial infarction (lT2MI). Major adverse cardiac events (MACEs) including acute myocardial infarction, AHF (both only from day 3 to avoid inclusion bias), life-threatening arrhythmia, and cardiovascular death as well as all-cause death were assessed during 1-year follow-up. Among 7754 patients (age 45–98 years, 45% women), PMI occurred in 1016 (13.1%). At least one MACE occurred in 684/7754 patients (8.8%) and 818/7754 patients died (10.5%) within 1 year. Outcomes differed starkly according to aetiology: in patients with extra-cardiac PMI, T1MI, tachyarrhythmia, AHF, and lT2MI 51%, 41%, 57%, 64%, and 25% had MACE, and 38%, 27%, 40%, 49%, and 17% patients died within 1 year, respectively, compared to 7% and 9% in patients without PMI. These associations persisted in multivariable analysis.
Conclusion
At 1 year, most PMI aetiologies have unacceptably high rates of MACE and all-cause death, highlighting the urgent need for more intensive treatments.
Study registration
Keywords: Myocardial injury, Non-cardiac surgery, Major cardiac events, Perioperative care, Risk factors, Risk prediction
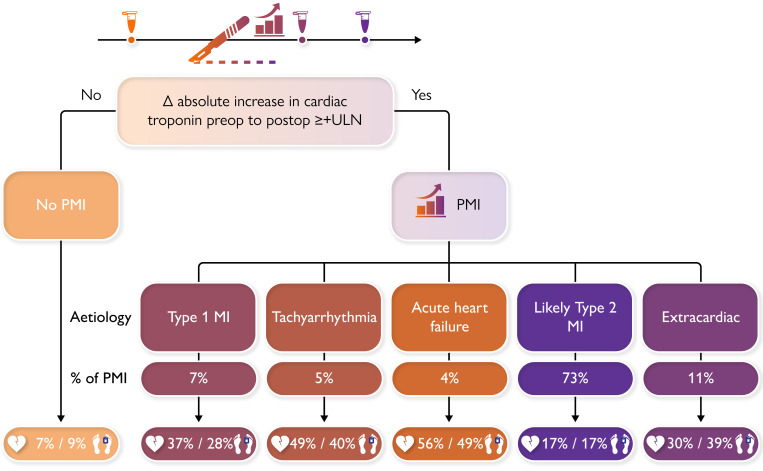
Structured Graphical Abstract
Structured Graphical Abstract.
At 1 year, most PMI aetiologies have unacceptably high rates of major adverse cardiac event and all-cause death, highlighting the need for more comprehensive management strategies, taking different aetiologies, their incidence, and their associated outcomes into consideration. PMI was defined as an absolute increase in cTn from pre-operative to post-operative concentrations of at least the upper limit of normal of the used assay (for high sensitivity cTnT this corresponds to an increase of at least +14 ng/L, e.g. from 10 ng/L pre-operatively to ≥24 ng/L post-operatively). cTn, cardiac troponin; PMI, perioperative myocardial infarction/injury.
See the editorial comment for this article ‘Potential risks of non-cardiac peri-operative myocardial infarction/injury’, by K. Thygesen and A.S. Jaffe, https://doi.org/10.1093/eurheartj/ehac768.
Introduction
Perioperative myocardial infarction/injury (PMI) is increasingly recognized as a frequent perioperative cardiac complication following major non-cardiac surgery and an important contributor to post-operative mortality.1–5 Due to potent analgesia administered in the perioperative period, most PMIs present without typical ischaemic symptoms and are therefore missed in routine clinical practice in the absence of active surveillance.1–3,6,7 As the mortality associated with asymptomatic PMI was found to be comparable to that associated with symptomatic PMI,1–3 active surveillance for PMI with pre-operative and post-operative measurements of cardiac troponin (cTn) embedded into routine clinical care is increasingly advocated to allow early detection and management.8–11 Recently, the European Society of Cardiology has issued a class IB recommendation for such active surveillance for PMI.11
Previous studies implementing active surveillance for PMI have suggested that PMI is not a homogeneous disease but is a heterogeneous syndrome with multiple different underlying aetiologies, including type 1 myocardial infarction (T1MI) caused by plaque rupture, type 2 myocardial infarction (T2MI) caused by a supply-demand mismatch, tachyarrhythmia, acute heart failure (AHF), and primarily extra-cardiac PMI due to e.g. severe sepsis or pulmonary embolism (PE).1–3,12–15 Better understanding of the underlying aetiology is a pre-requisite for targeted preventive and/or therapeutic interventions for PMI in the individual patient.8,16,17 Centrally adjudicating the aetiology of PMI, we recently observed unexpected differences in incidence and short-term mortality at 30 days in a pilot study, further highlighting the clinical importance of detailed phenotyping.15 However, little is known about long-term outcomes associated with the different PMI aetiologies.2
Therefore, we aimed to evaluate major adverse cardiac events (MACEs) and all-cause mortality associated with different centrally adjudicated PMI aetiologies within 1 year in a large prospective multicentre study.
Methods
This study was pre-specified within the basel incidence, patient characteristics, outcome and possible strategies to improve outcome of perioperative myocardial injury after non-cardiac surgery: 1-year follow-up (BASEL-PMI) study programme (NCT02573532) and approved by the local ethics committees. We adhered to the strengthening the reporting of observationalstudies in epidemiology reporting recommendations.18
Population
We prospectively included consecutive patients undergoing major inpatient non-cardiac surgery (with an expected post-operative stay of ≥2 days) at three hospitals (University Hospital Basel, Cantonal Hospital Aarau, both in Switzerland, and Instituto do Coracao, InCor, Universidade de Sao Paulo, Brazil) who were eligible for the institutional active PMI surveillance and response programme for high-risk patients undergoing major inpatient non-cardiac surgery and provided written general consent to registration in a dedicated prospective database.1
Patients were screened if they were considered at increased mortality risk, defined as ≥65 years of age, OR ≥45 years with history of coronary artery disease (CAD), peripheral arterial disease, or stroke, undergoing inpatient non-cardiac surgery with a planned post-operative stay of ≥24 h. Plasma concentrations of cTn [high-sensitivity-cTnT (hs-cTnT) in Basel and Sao Paulo, sensitive cTnI (s-cTnI) in Aarau; details in Supplementary material online] were measured within 30 days prior to surgery and on post-operative days 1 and 2 as part of the active surveillance, as well as if clinically indicated following surgery. Twelve-lead electrocardiogram (ECG) was performed on the day PMI was detected and whenever indicated clinically.
For this analysis focusing on long-term outcome, patients were included only once at first enrolment. We excluded patients who did not meet active surveillance criteria (<45 years, < 24 h hospital stay, surgery involving a cardiac surgeon), who had their surgery cancelled, or whose PMI aetiology was not adjudicable (see below).
Adjudication of PMI aetiology
PMI was prospectively defined as an absolute increase of ≥14 ng/L for hs-cTnT/≥45 ng/L for s-cTnI (the 99th percentile of each assay) above the pre-operative concentration (or between two post-operative concentrations if the pre-operative measurement was missing) within 3 days following surgery (e.g. for hs-cTnT an increase of at least 14 ng/L, e.g. from 10 ng/L pre-operatively to ≥24 ng/L post-operatively). PMI was defined solely by the (hs)s-cTn criterion, independent of symptoms or ECG changes.
PMI aetiology was centrally adjudicated by two independent experts based on all clinical information obtained during index hospitalization, including ECG, serial laboratory measurements including cTn and haemoglobin, monitoring of vital signs in the peri-operative and intra-operative period, as well as echocardiography, cardiac stress testing, and coronary angiography if performed. The sequence and extent of cardiac imaging performed in the individual patient was defined by the clinical cardiologist in charge of the PMI work-up. In cases of disagreement between the two adjudicating reviewers, consensus was sought and found by discussion with a third reviewer. A total of nine reviewers did adjudication (C.P., D.M.G., G.L.B., R.H., K.W., B.C., F.A.C., K.A., C.M.), and four reviewers did third reviews (C.P., D.M.G., B.C., C.M.). PMI were hierarchically classified according to the dominant trigger for myocardial injury or infarction, to reflect clinical management pathways8,15:
-
(1)
extra-cardiac if caused by a primarily extra-cardiac disease such as severe sepsis, stroke, PE, or blunt or surgical cardiac trauma;
-
(2)
cardiac, further subtyped into T1MI, tachyarrhythmia, or AHF;
-
(3)
cardiac, likely T2MI (lT2MI) if there was absence of the causes mentioned above (1, 2) with documented or suspected type 2 trigger (e.g. severe hypotension, anaemia, hypoxia, sinus tachycardia).
Extra-cardiac PMI was adjudicated if there was post-operative evidence of: (i) septic shock, severe sepsis, or an uncontrolled infection with criteria of severe immune response syndrome without requirement of bacteria or fungi growth in blood cultures; if infection was already present pre-operatively and on adequate antimicrobial therapy, infection was seen as controlled and not adjudicated as a cause of PMI. Evidence of infection alone (e.g. pneumonia, prosthetic joint infection, or urinary tract infection) was not sufficient for extra-cardiac adjudication; (ii) PE with right heart strain; (iii) or cardiac trauma by injury or during surgery (defined as requiring opening of pericardium).
T1MI was adjudicated when there was (i) evidence of coronary plaque rupture on coronary angiography or a coronary stenosis was severe and led to coronary revascularization; (ii) contraindications or patient refusal to undergo coronary angiography despite high suspicion of T1MI (ST-segment elevation, pronounced ST-segment depression, typical angina pectoris, substantially elevated cTn with new regional wall motion abnormalities, initiation of dual antiplatelet therapy by the clinical team), T1MI could also be adjudicated without coronary angiographic evidence; and (iii) evidence of acute myocardial infarction with thrombosis on autopsy.15
Tachyarrhythmia was adjudicated if there was evidence of a tachyarrhythmia (not sinus tachycardia) with ≥120 bpm prior to the first elevated cTn measurement, either on 12-lead ECG irrespective of documented duration or on a continuous three-lead ECG with clinical documentation and considered relevant by the attending team.
AHF was adjudicated in cases with signs of congestion (dyspnoea, peripheral oedema, pleural effusion, rales), use of i.v. diuretics improving symptoms, or substantially elevated natriuretic peptides,19,20 without evidence of T1MI or tachyarrhythmia >120 bpm.
The term lT2MI was used for cases without evidence for any extra-cardiac or other cardiac aetiology to indicate that although T2MI was clearly the most likely aetiology, T1MI may also have been present in a small minority given the absence of intracoronary imaging including e.g. near-infrared spectroscopy and optical coherence tomography (OCT).11,15,21
Patients were excluded from this analysis if adjudication of PMI aetiology was not possible, due to (i) only one cTn measurement available; (ii) missing pre-operative cTn concentration and post-operative cTn concentrations elevated above the 99th percentile without a dynamic change; (iii) pre-operatively elevated cTn values which were falling peri-operatively, e.g. following pre-operative T1MI or recent cardiac surgery (defined as drop of > −50 ng/L hs-cTnT or > −200 ng/L s-cTnI), as all three situations create the inability to distinguish between acute elevations and chronic injury.
Endpoints
The primary endpoints were the occurrence of MACE and all-cause death following different PMI aetiologies within 1 year. Deaths were classified as cardiovascular or non-cardiovascular according to recent guidelines.22 MACE was defined as a composite endpoint of acute myocardial infarction, AHF, life-threatening arrhythmia, and cardiovascular death. Follow-up began after surgery on the day of surgery (patients who died intra-operatively were not included). To avoid definitional bias, in patients with PMI classified as T1MI or AHF, the index PMI was not counted as an endpoint event, instead follow-up period for follow-up acute myocardial infarction or AHF began after post-operative day 3. A composite of MACE and all-cause death was a secondary endpoint.
To optimize event-to-noise ratio for time-to-event and prognostic analyses, 120-day occurrence of MACE and all-cause death were chosen as secondary endpoint and used for prognostic and time-to-event analyse as a prior study suggested a vulnerable period of 120 days following non-cardiac surgery.23 Definitions of further variables assessed are shown in the Supplementary material online.
During follow-up, patients were contacted after 1 year by mail or telephone, and local death registries and electronic health records at participating institutions were checked. In case of suspicion of an outcome event either during telephone, mailing or review of the clinical charts, study personnel requested reports from the general practitioners, treating facilities, and/or death registries. Patients lost to follow-up were censored at the last contact with the study team, a hospital, or their general practitioner, and for this analysis excluded.
In two centres, multiple events were assessed and death and up to a maximum number of five distinct MACE events (irrespective of the type of event) could be entered into the database, allowing for up to six MACE events possible per patient.
Statistical analysis
We described baseline and perioperative factors for all PMI aetiologies in comparison to patients without PMI. The analysis of association of PMI aetiologies with first MACE within 1 year was done univariable using Kaplan–Meier estimates with 95% confidence interval (CI), with non-PMI patients as comparator. To further test the association, we performed multivariable Cox proportional hazards analysis for first MACE and all-cause mortality in patients with PMI adjusted for: age, sex, European Society of Cardiology (ESC)/ European Society of Anaesthesiology (ESA) surgery risk (low, medium, high risk of cardiac events),24 revised cardiac risk index (RCRI) class,25 urgency of surgery, post-operative complications (sepsis, stroke, bleeding), centre, and surgical speciality. Finally, we calculated the total amount of MACE occurring in each patient stratified according to PMI aetiology. The uni- and multivariable analyses were repeated for all-cause death at 1 year.
As a previous pilot study suggested that lT2MI seems to be the most common PMI aetiology and overall has only a modest increase in short-term mortality,15 and as its optimal management is particularly ill defined, we derived a risk model for MACE and all-cause death at 120 days using logistic regression analysis to help risk stratify these patients.
To identify risk factors and allow risk stratification in lT2MI, we pre-specified that only perioperative variables should be included into the model, to (i) create a model with added value in the perioperative setting beyond risk stratification according to baseline variables and (ii) avoid biased inference on the impact of baseline characteristics, as the population of this study was enriched in comorbidities due to the inclusion criteria, especially in patients 45–64 years of age. Prognostic modelling was done for MACE plus all-cause mortality at 120 days.23 Based on the number of events and the consensus of requiring 10 events per independent variable compared in regression models, we could include all pre-specified perioperative variables.26 We constructed a logistic binary regression model including variables available at time of clinical evaluation: additional symptoms or ECG criteria required according to the Universal Definition of Myocardial Infarction8 (details in Supplementary material online), absolute increase in cTn (categorized according to level of absolute increase 1 to ≤2 times the 99th percentile, ≥ 2 to <4 times, and ≥4 times), urgency of procedure, perioperative bleeding (drop in haemoglobin >30 g/L or deemed relevant for PMI by adjudicator), and ESC/ESA surgery risk (low, medium, high risk of cardiac events).24 No model simplification was done. We constructed a calibration plot and calculated the area under the receiver-operating characteristic curve (AUC) and Brier score. For internal validation, we calculated the predicted probabilities and classified patients into low-risk (predicted event rate <10%), intermediate risk (10%–20%), and high-risk (>20%) and compared the predicted with the observed event rate. Finally, we compared the results to the RCRI, American Society of Anaesthesiology (ASA) class, and age via AUC and explored whether combining the prognostic model with RCRI or ASA class added prognostic value using binary logistic regression, followed by AUC analysis.
Sensitivity analysis for different MACE definition
We did a sensitivity analysis using a more conservative definition of MACE, incorporating only cardiovascular mortality and acute myocardial infarction (Supplementary material online).
Statistical analysis was done in SPSS v26 and R v4.2.0 (‘Survival’, ‘survminer’, ‘rms’, ‘pROC’).
Results
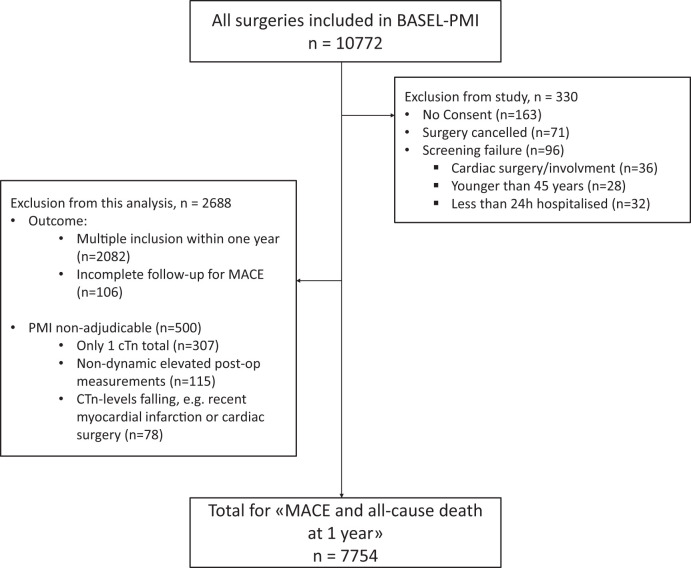
A total of 10 772 patients were enrolled between May 2014 and June 2018, of which 7754 patients were eligible for this analysis (Figure 1). Baseline characteristics and outcome of those patients excluded due to unadjudicable PMI aetiology (n = 500) are shown in Supplementary material online, Table S1.
Figure 1.
Patient flow. AMI, acute myocardial infarction; cTn, cardiac troponin; MACE, major adverse cardiac event; lT2MI, likely type 2 myocardial infarction; PMI, perioperative myocardial infarction/injury.
Characteristics of PMI aetiologies
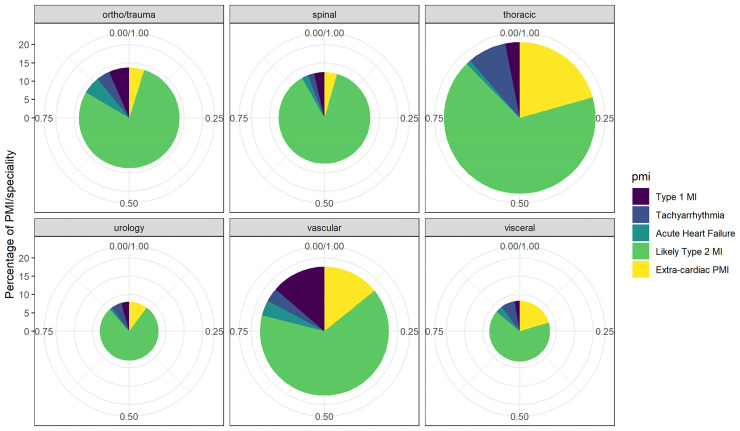
PMI occurred in 1016/7754 patients (13.1%), of which 109/1016 (10.7%) were centrally adjudicated as primarily extra-cardiac (Table 1). PMI were adjudicated as due to T1MI in 71/1016 patients (7.0%), tachyarrhythmia in 47/1016 patients (4.6%), and AHF in 39/1016 (3.8%). Among patients adjudicated to have T1MI, coronary angiography within 7 days was performed in 42/71 patients, within 30 days in 47/71 patients, and adjudication without coronary angiography occurred in 24 patients (Supplementary material online, Table S2). The remaining 750/1016 (73.8%) were adjudicated as lT2MI. Distribution across surgical specialities can be seen in Figure 2.
Table 1.
Baseline characteristics split according to aetiology of perioperative myocardial infarction/injury
| Number | No PMI | Type 1 MI | Tachyarrhythmia | Acute heart failure | Likely type 2 MI | Extra-cardiac PMI, sepsis | Extra-cardiac PMI, other |
|---|---|---|---|---|---|---|---|
| 6738 | 71 | 47 | 39 | 750 | 60 | 49 | |
| Age (median) | 73 (68–79) | 77 (71–82) | 77 (71–82) | 80 (77–83) | 76 (70–82) | 74 (67–79) | 70 (66–76) |
| Sex, male | 3676 (55%) | 53 (75%) | 30 (64%) | 20 (51%) | 417 (56%) | 40 (67%) | 28 (57%) |
| Coronary artery disease | 1704 (25%) | 43 (60%) | 18 (38%) | 25 (65%) | 300 (40%) | 21 (35%) | 14 (28%) |
| History of myocardial infarction | 825 (12%) | 25 (35%) | 10 (21%) | 17 (44%) | 159 (21%) | 13 (22%) | 10 (20%) |
| Peripheral artery disease | 1342 (20%) | 35 (49%) | 10 (21%) | 12 (31%) | 192 (26%) | 16 (27%) | 22 (45%) |
| History of stroke/transient ischaemic attack | 475 (7%) | 14 (20%) | 3 (6%) | 2 (5%) | 77 (10%) | 8 (13%) | 8 (16%) |
| Chronic heart failure | 589 (9%) | 16 (23%) | 16 (34%) | 22 (56%) | 124 (17%) | 14 (23%) | 13 (27%) |
| Atrial fibrillation | 1007 (15%) | 10 (14%) | 27 (57%) | 19 (49%) | 167 (22%) | 18 (30%) | 12 (25%) |
| Moderate to severe valvular disease | 384 (6%) | 13 (18%) | 10 (21%) | 13 (33%) | 87 (12%) | 4 (7%) | 8 (16%) |
| Corrected valvular disease | 232 (3%) | 1 (1%) | 3 (6%) | 4 (10%) | 38 (5%) | 5 (8%) | 0 (0%) |
| Diabetes mellitus, non-insulin dependent | 983 (15%) | 11 (16%) | 8 (17%) | 12 (31%) | 108 (14%) | 10 (17%) | 7 (14%) |
| Diabetes mellitus, insulin dependent | 486 (7%) | 13 (18%) | 5 (11%) | 6 (15%) | 97 (13%) | 11 (18%) | 8 (16%) |
| Chronic kidney disease III+ | 761 (11%) | 17 (25%) | 8 (17%) | 10 (26%) | 132 (18%) | 9 (15%) | 14 (29%) |
| Chronic haemodialysis | 93 (1%) | 3 (5%) | 1 (2%) | 2 (5%) | 39 (5%) | 4 (7%) | 8 (16%) |
| Hypertension | 4356 (65%) | 54 (76%) | 37 (79%) | 30 (77%) | 548 (73%) | 36 (60%) | 35 (71%) |
| Obstructive pulmonary disease | 978 (15%) | 10 (14%) | 13 (28%) | 8 (21%) | 127 (17%) | 8 (14%) | 6 (12%) |
| Surgery due to malignancy | 1045 (16%) | 4 (6%) | 9 (19%) | 2 (5%) | 115 (15%) | 3 (5%) | 8 (16%) |
| Pre-operative anaemia | 2092 (31%) | 37 (52%) | 20 (43%) | 27 (69%) | 366 (49%) | 44 (73%) | 25 (51%) |
Figure 2.
Distribution of adjudicated aetiology of perioperative myocardial infarction/injury in different surgical specialities. The size of the circle indicates the overall percentage of perioperative myocardial infarction/injury, and the different colours indicate the adjudicated aetiologies of perioperative myocardial infarction/injury following surgery in the respective specialities.
Baseline characteristics differed among the pre-specified PMI aetiologies, with e.g. known CAD being more common in T1MI and AHF vs. all other PMI aetiologies (Table 1). Surgical characteristics can be found in Supplementary material online, Table S3.
In 260/1016 (25%) of patients with PMI, additional criteria required for the diagnosis of spontaneous AMI were present, e.g. ischaemic symptoms or dyspnoea in 143/1016 (14%), again with major differences among the PMI aetiologies (Supplementary material online, Table S2).
In 34/1016 patients (3%), PMI was adjudicated without a pre-operative cTn value using change between post-operative values.
Follow-up
Follow-up was complete in 7754/7833 patients (99%) with a median length of 388 days. At least one MACE occurred in 684/7754 patients at 1 year (8.8%). A total of 818/7754 patients died within 1 year (10.5%), with death occurring during the index hospitalization in 154/817 patients. The composite of MACE or all-cause death occurred in 1160/7754 patients (15.0%).
MACE after PMI according to aetiology
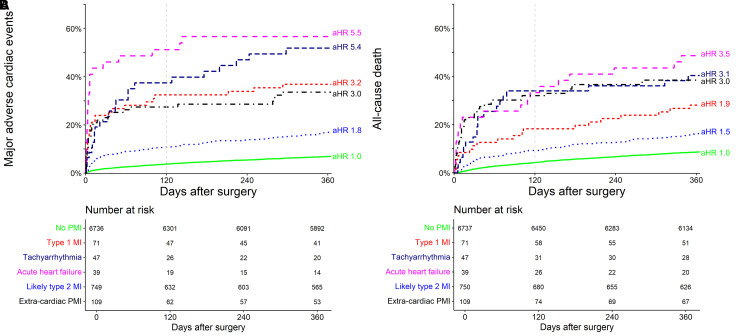
All PMI aetiologies showed substantially increased 1-year MACE rates vs. patients without PMI (7%, 95% CI 6%–8%), and there was a stark difference within PMI aetiologies (Table 2, Figure 3A): extra-cardiac PMI, T1MI, tachyarrhythmia, and AHF were associated with very high rates of MACE [30% (95% CI 20–47), 37% (95% CI 24–47), 49% (95% CI 34–75), and 56% (95% CI 38–70), respectively], and lT2MI was associated with high rates of MACE (17%, 95% CI 22%–28%). The associations were confirmed in multivariable analysis with adjusted hazard ratios (aHRs) of 3.0 (2.0–4.5), 3.2 (95% CI 2.1–4.8), 5.4 (3.5–8.4), and 5.5 (3.5–8.7) for extra-cardiac PMI, T1MI, tachyarrhythmia, and AHF and 1.8 (1.5–2.2) for lT2MI (Table 3).
Table 2.
Outcome following perioperative myocardial infarction/injury
| n | No PMI | Type 1 MI | Tachy- arrhythmia |
Acute heart failure | Likely type 2 MI | Extra-cardiac PMI, sepsis | Extra-cardiac PMI, other |
|---|---|---|---|---|---|---|---|
| 6738 | 71 | 47 | 39 | 750 | 60 | 49 | |
| MACE at 1 year | 456 (7%) [6%–8%] |
26 (37%) [24%–47%] |
23 (49%) [34%–75%] |
22 (56%) [38%–70%] |
124 (17%) [14%–20%] |
18 (30%) [20%–47%] |
15 (31%) [17%–45%] |
| ȃAcute myocardial infarction | 88 (1%) | 5 (7%) | 2 (4%) | 3 (8%) | 21 (3%) | 1 (2%) | 0 (0%) |
| ȃAcute heart failure | 235 (4%) | 16 (23%) | 11 (23%) | 17 (44%) | 61 (8%) | 10 (17%) | 3 (6%) |
| ȃLife-threatening arrhythmia | 52 (1%) | 3 (4%) | 4 (9%) | 2 (5%) | 18 (2%) | 5 (8%) | 2 (4%) |
| ȃCardiovascular death | 169 (3%) | 16 (23%) | 12 (26%) | 11 (28%) | 52 (7%) | 4 (7%) | 11 (23%) |
| All-cause death within 1 year | 591 (9%) [8%–9%] |
20 (28%) [17%–38%] |
19 (40%) [25%–53%] |
19 (49%) [30%–62%] |
127 (17%) [14%–20%] |
21 (35%) [22%–46%] |
21 (43%) [27%–55%] |
| ȃNon-cardiovascular death | 422 (6%) | 4 (6%) | 7 (15%) | 8 (21%) | 75 (10%) | 17 (29%) | 10 (21%) |
| MACE or all-cause death at 1 year | 834 (12%) [12%–13%] |
30 (42%) [29%–53%] |
27 (57%) [41%–69%] |
25 (64%) [45%–76%] |
188 (25%) [22%–28%] |
32 (53%) [39%–64%] |
24 (49%) [23%–63%] |
Number and percentage of MACEs, all-cause death or ‘MACE and all-cause death’ occurring 1 year in patients with different aetiologies of PMI, shown with [95% confidence interval] derived from survival analysis for primary and secondary endpoint. MACE is a composite endpoint; therefore, total number of individual components is greater than that of the composite endpoint.
MACE, major adverse cardiac events; MI, myocardial infarction; PMI, perioperative myocardial infarction/injury.
Figure 3.
Major adverse cardiac events (part A) and all-cause death (part B) within 1-year stratified according to adjudicated aetiology of perioperative myocardial infarction/injury after non-cardiac surgery, including adjusted hazard ratios from multivariable Cox proportional hazards analysis [adjusted for age, sex, ESC/ESA surgery risk (low, medium, high risk of cardiac events),24 revised cardiac risk index class,25 centre, surgical speciality, urgency of surgery, and post-operative complications (sepsis, stroke, bleeding)]. Grey dashed line denotes 120 day. MI, myocardial infarction.
Table 3.
Adjusted outcome following perioperative myocardial infarction/injury
| Variable | Adjusted hazard ratio, 1 year MACE |
P-value | Adjusted hazard ratio, 1 year all-cause death |
P-value |
|---|---|---|---|---|
| PMI aetiology—no PMI | Reference | Reference | Reference | Reference |
| ȃType 1 myocardial infarction | 3.2 (2.1–4.8) | <0.001 | 1.9 (1.2–3.0) | 0.008 |
| ȃTachyarrhythmia | 5.4 (3.5–8.4) | <0.001 | 3.1 (2.0–5.0) | <0.001 |
| ȃAcute heart failure | 5.5 (3.5–8.7) | <0.001 | 3.5 (2.2–5.6) | <0.001 |
| ȃLikely type 2 myocardial infarction | 1.8 (1.5–2.2) | <0.001 | 1.5 (1.3–1.9) | <0.001 |
| ȃExtra-cardiac PMI | 3.0 (2.0–4.5) | <0.001 | 3.0 (2.1–4.3) | <0.001 |
| Age, per year | 1.1 (1.0–1.1) | <0.001 | 1.1 (1.1–1.1) | <0.001 |
| Sex, male | 1.0 (0.9–1.1) | 0.681 | 1.0 (1.0–1.1) | 0.343 |
| ESC/ESA surgical risk <1% | Reference | Reference | Reference | Reference |
| ȃESC/ESA surgical risk 1%–5% | 0.8 (0.6–1.0) | 0.04 | 0.9 (0.7–1.0) | 0.11 |
| ȃESC/ESA surgical risk >5% | 1.4 (1.1–1.9) | 0.019 | 1.5 (1.2–2.0) | <0.001 |
| Urgency: elective | Reference | Reference | Reference | Reference |
| ȃUrgency: < 24 h | 1.3 (1.0–1.6) | 0.047 | 2.0 (1.6–2.5) | <0.001 |
| ȃUrgency: 2–7 days | 1.6 (1.3–2.0) | <0.001 | 2.1 (1.8–2.6) | <0.001 |
| Revised cardiac risk index class I | Reference | Reference | Reference | Reference |
| ȃRevised cardiac risk index class II | 2.0 (1.6–2.5) | <0.001 | 1.4 (1.1–1.6) | 0.002 |
| ȃRevised cardiac risk index class III | 3.2 (2.5–4.1) | <0.001 | 2.0 (1.6–2.5) | <0.001 |
| ȃRevised cardiac risk index class IV | 5.4 (4.1–7.1) | <0.001 | 2.9 (2.3–3.7) | <0.001 |
| Surgery due to malignancy | 0.9 (0.6–1.1) | 0.298 | 4.6 (3.7–5.7) | <0.001 |
| Post-operative stroke | 1.7 (1.0–3.1) | 0.071 | 1.6 (0.9–2.8) | 0.095 |
| Post-operative sepsis | 2.9 (2.1–4.0) | <0.001 | 3.4 (2.6–4.4) | <0.001 |
| Post-operative bleeding | 0.9 (0.8–1.2) | 0.615 | 0.8 (0.6–1.0) | 0.014 |
| Centre, Basel | Reference | Reference | Reference | Reference |
| ȃCantonal hospital Aarau | 1.1 (0.9–1.3) | 0.425 | 1.0 (0.9–1.3) | 0.642 |
| ȃUniversity Hospital Sao Paulo | 1.4 (0.9–2.1) | 0.105 | 1.1 (0.7–1.7) | 0.595 |
| Surgical speciality, orthopaedic/trauma | Reference | Reference | Reference | Reference |
| ȃVisceral | 0.7 (0.5–0.9) | 0.009 | 0.7 (0.5–0.9) | 0.002 |
| ȃVascular | 0.9 (0.7–1.2) | 0.467 | 1.1 (0.8–1.4) | 0.699 |
| ȃThoracic | 1.0 (0.7–1.5) | 0.909 | 1.0 (0.7–1.4) | 0.958 |
| ȃSpinal | 0.9 (0.6–1.2) | 0.401 | 0.8 (0.6–1.1) | 0.154 |
| ȃUrology | 0.9 (0.7–1.3) | 0.623 | 0.6 (0.5–0.9) | 0.003 |
| ȃOther | 1.6 (1.0–2.5) | 0.058 | 1.3 (0.9–2.0) | 0.155 |
Multivariable Cox proportional hazards model for occurrence of MACEs and all-cause death after different aetiologies of PMI at 1 year, showing adjusted hazard ratios with (95% confidence interval).
ESC/ESA, European Society of Cardiology/European Society of Anaesthesiology; MACEs, major adverse cardiac events; PMI, perioperative myocardial infarction/injury.
Similar association was shown for MACE at 120 days (Supplementary material online, Table S4 and Figure S1), with numerically higher aHR in multivariable analysis (Supplementary material online, Table S5) compared to 1-year associations, but lower power due to less events. The time from surgery to MACE within 120 days differed substantially among the PMI aetiologies (Supplementary material online, Table S4), from a median of 3 days following AHF to 13 days following lT2MI, and 26 days in patients without PMI. When exploring the total amount of MACE stratified according to PMI aetiology, multiple events occurred in 3%–23% of patients (Supplementary material online, Table S4) compared to 1% in patients without PMI. The association of PMI aetiologies with MACE was stronger within the first 120 days compared to 1 year (Supplementary material online, Figure S1, Tables S5 and S6).
All-cause mortality after PMI
All PMI aetiologies showed increased 1-year all-cause death rates vs. patients without PMI (9%, 95% CI 8%–9%), and there was a stark difference within PMI aetiologies (Table 2, Figure 3B): extra-cardiac PMI, T1MI, tachyarrhythmia, and AHF were associated with high rates of death [38% (95% CI 29–47), 28% (95% CI 17–38), 40% (95% CI 25–53), and 49% (95% CI 30–62), respectively], and lT2MI was associated with high rates of death (17%, 95% CI 14–20). The association was confirmed in multivariable analysis (Table 3).
Prognostic model for lT2MI
MACE or all-cause death within 120 days occurred in 117/750 patients with lT2MI (Supplementary material online, Table S4). The initial and final logistic prognostic model for 120-day MACE or death is shown in Table 4. Presence of chest pain or dyspnoea, an absolute increase in cTn of >2 × 99th percentile compared to baseline, high-risk surgery according to the ESC/ESA surgical risk score, and non-elective surgery were associated with increased MACE, while bleeding was associated with a lower risk of MACE. Internal validation found a good fit of predicted and observed event rate following bootstrapping of 1000 iterations (Supplementary material onilne, Figure S2) and a Brier score of 0.16.
Table 4.
Risk prediction model
| Variables (initial model) | Adjusted odds ratio 120 day MACE or death |
Beta-coefficient | P-value |
|---|---|---|---|
| Chest pain or dyspnoea | 2.2 (1.2–4.1) | 0.799 | 0.01 |
| New or presumably new ECG changes | 1.3 (0.7–2.5) | 0.279 | 0.375 |
| Troponin delta +1 to <2x 99th percentile | Reference | Reference | |
| ȃTroponin delta +2–4x 99th percentile | 2.2 (1.3–3.5) | 0.77 | 0.002 |
| ȃTroponin delta +>4x 99th percentile | 1.7 (1.0–2.9) | 0.529 | 0.051 |
| ESC/ESA surgical risk <1% | Reference | Reference | |
| ȃESC/ESA surgical risk 1%–5% | 1.1 (0.6–2.0) | 0.071 | 0.816 |
| ȃESC/ESA surgical risk >5% | 2.8 (1.4–5.6) | 1.024 | 0.005 |
| Urgency: elective | Reference | Reference | |
| ȃUrgency: < 24 h | 2.6 (1.5–4.4) | 0.938 | <0.001 |
| ȃUrgency: 2–7 days | 2.4 (1.4–4.0) | 0.863 | <0.001 |
| Post-operative bleeding | 0.5 (0.3–0.8) | −2.565 | 0.005 |
Derivation of risk prediction model for MACEs and all-cause death within 120 days following perioperative myocardial infarction/injury likely due to type 2 myocardial infarction using logistic binary regression model, incorporating perioperative variables available at time of detection of likely due to type 2 myocardial infarction-perioperative myocardial infarction/injury.
ECG, electrocardiogram; ESA, European Society of Anaesthesiology; ESC, European Society of Cardiology; MACE, major adverse cardiac event.
The prognostic model showed a moderate AUC of 0.71 (95% CI 0.66–0.76, Table 5). In comparison to the maximum RCRI (available in 747/750 cases), ASA class (available in 718/750 cases), and absolute hs-cTnT-delta (available in 564/750 cases), the prognostic model showed the numerically highest AUC (Table 5), statistically different to ASA class and hs-cTnT-delta (P = 0.003), and comparable to the RCRI (P = 0.195). Combination of the prognostic model with the ASA class or the RCRI further increased the AUC significantly compared to the RCRI or ASA class alone (model+ASA 0.75, 95% CI 0.71–0.80, P < 0.001; model+RCRI 0.75, 95% CI 0.70–0.80, P < 0.001). After categorization by the prognostic model, ‘very high-risk’ lT2MI-PMI showed a rate of MACE or death of 31% (comparable to PMI of T1MI), while the ‘low-risk’ group showed rates of 7% (comparable to patients without PMI) (Supplementary material online, Figure S3).
Table 5.
Model performance
| AUC (with 95% CI) | P-value | |
|---|---|---|
| lT2MI-PMI prognostic model | 0.71 (0.66–0.76) | |
| Perioperative hs-cTnT deltaa | 0.60 (0.53–0.66) | 0.003b |
| RCRI | 0.66 (0.61–0.72) | 0.195b |
| ASA class | 0.63 (0.58–0.68) | 0.008b |
| RCRI + prognostic model | 0.75 (0.70–0.80) | <0.001c |
| ASA class + prognostic model | 0.75 (0.71–0.80) | <0.001c |
AUCs for the derived risk model for lT2MI-PMI aetiology compared to perioperative hs-cTnT delta, RCRI, and ASA class, as well as combination of RCRI and ASA with the risk model; AUC calculated in 718 cases.
ASA, American Society of Anaesthesiology; AUC, areas under the receiver operating characteristics curve; hs-cTnT, high-sensitivity cardiac troponin; lT2MI-PMI, perioperative myocardial infarction/injury of likely type 2 myocardial infarction; RCRI, revised cardiac risk index.
hs-cTnT delta only available in 564 cases.
P-value compared to AUC of lT2MI prognostic model.
P-value compared to AUC of RCRI or ASA class.
Discussion
This large prospective international multicentre study centrally adjudicated the aetiology of PMI detected during active surveillance in high-risk patients undergoing major non-cardiac surgery, and meticulously observed MACE during long-term follow-up. We report six major findings:
First, all PMI aetiologies were associated with an increased risk of MACE and death within 1 year compared to patients without PMI. In fact, for most PMI aetiologies morbidity and mortality was unacceptably high within the first year following non-cardiac surgery. Second, the MACE and death rate differed substantially among PMI aetiologies: 5 of 10 patients with PMI due to AHF or tachyarrhythmia developed at least one MACE within 1 year, vs. 4 of 10 with T1MI, and 1.5 in 10 in lT2MI. Similarly, 5 of 10 patients with PMI due to AHF and 4 of 10 with PMI due to tachyarrhythmia died within 1 year vs. 3 of 10 with T1MI and 1 in 6 in lT2MI (Structured Graphical Abstract). Third, these associations persisted after multivariable adjustments. Fourth, median time from PMI to subsequent MACE differed between aetiologies. This resulted in very short windows of opportunity for treatment in AHF and T1MI vs. tachyarrhythmia and lT2MI. Fifth, following PMI patients were at elevated risk also for recurrent MACE, further highlighting the association of PMI with increased morbidity. Sixth, for patients with the most common PMI aetiology, lT2MI, a simple prognostic model was derived and optimized to predict MACE occurring within the first 4 months, the vulnerable post-operative period,15 using perioperative factors to aid in their risk stratification and management.
These findings extend and corroborate observations made in previous work aiming to better characterize the incidence and outcomes of the different aetiologies underlying PMI.1–3,12–15 In a pilot study including 4475 high-risk patients undergoing major non-cardiac surgery, short-term mortality at 30 days was 12% (aHR 3.3) for T1MI, 18% (aHR 4.6) for tachyarrhythmia, 28% (aHR 9.7) for AHF, 28% (aHR 6.4) for extra-cardiac, and 4.7% (aHR 1.9) for lT2MI.15 During long-term follow-up, we could observe an attenuation of the association of PMI aetiologies with MACE from 0–120 days compared to 121–365 days. While the outcomes diverged early and strongly during the first 120 days, the association weakened with increased follow-up duration, indicating that PMI is an acute event and not merely a marker of increased cardiovascular risk. However, PMI also occurs more frequently in patients with greater cardiovascular disease burden,27 hence the curves diverge more slowly but steadily also after 120 days (Supplementary material online, Figure S1).
Identifying the underlying aetiology seems of paramount importance given the availability of evidence-based therapies for AHF, tachyarrhythmia, and T1MI.19,28 These data also corroborate insights from previous pilot studies indicating that T1MI is likely not the main driver of perioperative cardiovascular complications.12,13,16,29–31 T1MI due to coronary plaque rupture or plaque erosion represented only 7% of all PMIs detected during PMI screening. Accordingly, the dominant aetiologies and pathophysiologies of PMI differ markedly from those of spontaneous MI.8 In the era of active surveillance for PMI low rates T1MI should be expected, as also highlighted by a recent study using active surveillance showing evidence of thrombosis in only 13.3% (4/30) of patients with perioperative myocardial infarction.14 The main aetiology of PMI seems to be lT2MI. However, our data highlight that when T1MI is encountered, the time frame for intervention seems significantly shorter than in many other PMI cases.
These findings also highlight that the prior concept of myocardial injury following non-cardiac surgery (MINS),1–3 that focussed on T1MI, T2MI, and CAD but excluded AHF and tachyarrhythmia, the two post-operative cardiac complications with the highest rate of MACE and 1-year mortality must be seen in the broader context if screening and response systems shall improve perioperative outcomes.
First evidence suggests that patients with MINS, which corresponds to T1MI and the subgroup of lT2MI by the definition from the vascular events In noncardiac surgery patients cohort evaluation study (VISION) study as ‘myocardial injury of ischaemic origin’,3,32 might profit from medical optimization, e.g. aspirin and statins,6 or anticoagulation with dabigatran 110 mg.32 In the perioperative period bleeding risk needs to be considered thoroughly.
Different patterns of morbidity and mortality were found in different PMI, with the cardiovascular complications of MACE being predominant in cardiac PMI, while non-cardiovascular death was the main event in patients with extra-cardiac PMI and patients without PMI, further highlighting the importance of distinguishing the aetiologies when considering the risk-benefit ratio of additional treatment options. Importantly, while relative more non-cardiac mortality occurred in extra-cardiac PMI, the still carried a relevant burden of cardiovascular complications.
Use of our derived prognostic model could allow stratification of lT2MI into very high-risk patients with unacceptably high MACE rates vs. low-risk patients with associated MACE rates similar to patients without PMI. Following successful external validation, the tool could aid in the identification of patients that would benefit from cardiac imaging, fast treatment escalation, and more intense follow up. Importantly, the score should be used on top of baseline characteristics of the patient (as summarized in the pre-operatively done RCRI or ASA risk score). Interestingly, in patients with PMI of lT2MI evidence of perioperative bleeding was associated with lower occurrence of MACE and death within 120 days. This is potentially due to bleeding and the resulting anaemia being usually a rapidly reversible cause of supply-demand mismatch. Also, lower delta cTn values were associated with lower risk of MACE and death, highlighting an increased risk with higher cTn deltas.
Finally, our analysis again confirmed that the vast majority of patients with PMI did not experience typical ischaemic symptoms, further highlighting the need for active surveillance in order to reliably detect these events.1,2,6
The pre-operative cTn concentration is not only the reference point to assess perioperative changes, but was also shown in previous pilot studies to have predictive value for prediction of fatal or non-fatal cardiac complication of non-cardiac surgery.33,34
Limitations
First, there is no universally accepted definition of PMI. The absolute cTn change criteria used to define PMI in this study was to a certain extent arbitrary. While the cTn cut-off criteria for spontaneous MI (99th percentile of healthy individuals) also is arbitrary, it is widely accepted. Our definition for PMI not requiring any other additional criterion required in spontaneous myocardial infarction is supported by recent data from VISION,8 a recent american heart association/american college of cardiology statement,35 and the recent ESC guidelines,11 but still requires approval by other expert groups. Second, this study included patients at increased cardiovascular risk undergoing major non-cardiac surgery. The rate of MACE and mortality associated with PMI occurring in lower risk patients and patients undergoing minor outpatient surgery remains unknown.8,9,11 Third, central adjudication of PMI aetiology by two independent cardiologists was based on all available clinical, ECG, laboratory, and cardiac imaging data as obtained by the clinical team during the PMI work-up as part of routine clinical care within the active surveillance and clinical response programme. Thereby, the timing and extent of cardiac imaging was determined by the cardiologist in charge of the individual patient and not by a uniform study protocol. While this resulted in appropriate granularity for most classifications, it did not include near-infrared-spectroscopy and/or OCT intracoronary imaging,11,14,15,21 the current invasive gold-standard methodology for the differentiation of T1MI due to plaque rupture or plaque erosion from T2MI due to supply demand mismatch or rather uncommon coronary pathologies including spontaneous coronary dissection. This could have led to the misclassification of smaller number of patients with PMI due to T1MI as lT2MI, and thereby blurring of the differences in outcomes among the different PMI aetiologies.14 As the enormous differences in outcomes observed supports the argument that misclassifications were uncommon. Still, future studies implementing more extensive and systematic cardiac imaging for PMI work-up including e.g. intracoronary OCT are warranted to provide data with even higher granularity for the central adjudication of the PMI aetiology. Fourth, patients might present with different pathologies simultaneously, e.g. sepsis and atrial fibrillation. To tackle this, the adjudication was done hierarchically. Fifth, also during follow-up the extent of cardiac imaging for work-up of suspicious symptoms including acute chest pain was determined by the local cardiologist, and not the study protocol. Thereby, future studies with standardized and systematic cardiac imaging during follow-up have the potential to provide even higher granularity of follow-up data. Sixth, the derived prognostic model for lT2MI requires external validation before it should be considered for clinical use.
Conclusion
At 1 year, most PMI aetiologies have unacceptably high rates of MACE and all-cause death, highlighting the urgent need for more intensive treatments.
Supplementary Material
Acknowledgements
Additional BASEL-PMI Investigators and contributors to this manuscript BASEL-PMI Investigators: Reka Hidvegi1,9, Michael Freese1, Ketina Arslani1, Samantha Weder1, Silvia Maiorano1, Katharina Rentsch13, Andreas Buser14, Sandra Mitrovic13, Ivo Strebel1, Esther Seeberger5, Didier Lardinois15, Stefan Schaeren16, Rebecca Meister1 and Mirjam Pargger1
13Department of Laboratory Medicine, University Hospital Basel, University Basel, Switzerland; 14Department of Hematology, University Hospital Basel, University Basel, Switzerland; 15Department of thoracic surgery, University Hospital Basel, University Basel, Switzerland; and 16Department of Spinal Surgery, University Hospital Basel, University Basel, Switzerland.
Contributor Information
Christian Puelacher, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland; Department of Internal Medicine, University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland.
Danielle M Gualandro, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland; Department of Cardiology, Unidade de Medicina Interdisciplinar em Cardiologia, Instituto do Coração (InCor), Universidade de Sao Paulo, Sao Paulo, Brazil.
Giovanna Lurati Buse, Department of Anaesthesiology, University Hospital Dusseldorf, Dusseldorf, Germany.
Andreas Lampart, Department of Anaesthesiology, University Hospital Basel, University Basel, Basel, Switzerland.
Daniel Bolliger, Department of Anaesthesiology, University Hospital Basel, University Basel, Basel, Switzerland.
Luzius A Steiner, Department of Anaesthesiology, University Hospital Basel, University Basel, Basel, Switzerland.
Mario Grossenbacher, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland.
Katrin Burri-Winkler, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland; Department of Anaesthesiology, University Hospital Basel, University Basel, Basel, Switzerland.
Hatice Gerhard, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland.
Elisabeth A Kappos, Department of Plastic, Reconstructive, Aesthetic and Hand Surgery, University Hospital Basel, Basel, Switzerland.
Olivier Clerc, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland.
Laura Biner, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland.
Zaza Zivzivadze, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland.
Christoph Kindler, Department of Anaesthesiology, Cantonal Hospital Aarau, Aarau, Switzerland.
Angelika Hammerer-Lercher, Department of Laboratory medicine, Cantonal Hospital Aarau, Aarau, Switzerland.
Miodrag Filipovic, Department of Anaesthesiology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland.
Martin Clauss, Department of Orthopaedic and Trauma Surgery, University Hospital Basel, Basel, Switzerland; Center for Musculoskeletal Infections, University Hospital Basel, Basel, Switzerland.
Lorenz Gürke, Department of Vascular Surgery, University Hospital Basel, Basel, Switzerland.
Thomas Wolff, Department of Vascular Surgery, University Hospital Basel, Basel, Switzerland.
Edin Mujagic, Department of Vascular Surgery, University Hospital Basel, Basel, Switzerland.
Murat Bilici, Department of Orthopaedic and Trauma Surgery, University Hospital Basel, Basel, Switzerland.
Francisco A Cardozo, Department of Cardiology, Unidade de Medicina Interdisciplinar em Cardiologia, Instituto do Coração (InCor), Universidade de Sao Paulo, Sao Paulo, Brazil.
Stefan Osswald, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland.
Bruno Caramelli, Department of Cardiology, Unidade de Medicina Interdisciplinar em Cardiologia, Instituto do Coração (InCor), Universidade de Sao Paulo, Sao Paulo, Brazil.
Christian Mueller, Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University of Basel, Petersgraben 4, 4031 Basel, Basel-Stadt, Switzerland.
for the BASEL-PMI Investigators:
Reka Hidvegi, Michael Freese, Ketina Arslani, Samantha Weder, Silvia Maiorano, Katharina Rentsch, Andreas Buser, Sandra Mitrovic, Ivo Strebel, Esther Seeberger, Didier Lardinois, Stefan Schaeren, Rebecca Meister, and Mirjam Pargger
Supplementary data
Supplementary data is available at European Heart Journal online.
Data availability
Data are available on reasonable request following an embargo phase until main results of the BASEL-PMI study are published.
Funding
This work was supported by the Swiss National Science foundation (C.M.); the Swiss Heart Foundation (C.M.); Roche, Switzerland (C.P., C.M.); Abbott (C.M.), AstraZeneca (C.M.), the FAPESP (Sao Paulo Research Foundation) (D.M.G.); the Cardiovascular Research Foundation Basel (C.M.); and the Forschungsrat des Kantonsspitals Aarau (C.K.). The funders had no role in the design, data collection, statistical analysis, writing of this manuscript, or decision to publish.
References
- 1.Puelacher C, Lurati Buse G, Seeberger D, Sazgary L, Marbot S, Lampart A, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018;137:1221–1232. 10.1161/CIRCULATIONAHA.117.030114 [DOI] [PubMed] [Google Scholar]
- 2.Botto F, Alonso-Coello P, Chan MT V, Villar JC, Xavier D, Srinathan SK, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014;120:564–578. 10.1097/ALN.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 3.Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MT V, Srinathan SK, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017;317:1642–1651. 10.1001/jama.2017.4360 [DOI] [PubMed] [Google Scholar]
- 4.Landesberg G, Mosseri M, Zahger D, Wolf Y, Perouansky M, Anner H, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001;37:1839–1845. 10.1016/S0735-1097(01)01265-7 [DOI] [PubMed] [Google Scholar]
- 5.Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003;42:1547–1554. 10.1016/j.jacc.2003.05.001 [DOI] [PubMed] [Google Scholar]
- 6.Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011;154:523–528. 10.7326/0003-4819-154-8-201104190-00003 [DOI] [PubMed] [Google Scholar]
- 7.Devereaux PJ, Chan MT V, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012;307:2295–2304. 10.1001/jama.2012.5502 [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–269. 10.1093/eurheartj/ehy462 [DOI] [PubMed] [Google Scholar]
- 9.Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017;33:17–32. 10.1016/j.cjca.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 10.Gualandro DM, Yu PC, Caramelli B, Marques AC, Calderaro D, Fornari LS, et al. 3rd guideline for perioperative cardiovascular evaluation of the Brazilian society of cardiology. Arq Bras Cardiol 2017;109:1–104. 10.5935/abc.20170140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halvorsen S, Mehilli J, Cassese S, Hall TS, Abdelhamid M, Barbato E, et al. 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J 2022;43:3826–3924. 10.1093/eurheartj/ehac270 [DOI] [PubMed] [Google Scholar]
- 12.Gualandro DM, Campos CA, Calderaro D, Yu PC, Marques AC, Pastana AF, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012;222:191–195. 10.1016/j.atherosclerosis.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 13.Duvall WL, Sealove B, Pungoti C, Katz D, Moreno P, Kim M. Angiographic investigation of the pathophysiology of perioperative myocardial infarction. Catheter Cardiovasc Interv 2012;80:768–776. 10.1002/ccd.23446 [DOI] [PubMed] [Google Scholar]
- 14.Sheth T, Natarajan MK, Hsieh V, Valettas N, Rokoss M, Mehta S, et al. Incidence of thrombosis in perioperative and non-operative myocardial infarction. Br J Anaesth 2018;120:725–733. 10.1016/j.bja.2017.11.063 [DOI] [PubMed] [Google Scholar]
- 15.Puelacher C, Gualandro DM, Lurati Buse G, Bolliger D, Marbot S, Kindler C, et al. Etiology of peri-operative myocardial infarction/injury after noncardiac surgery and associated outcome. J Am Coll Cardiol 2020;76:1910–1912. 10.1016/j.jacc.2020.08.043 [DOI] [PubMed] [Google Scholar]
- 16.Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation 2009;119:2936–2944. 10.1161/CIRCULATIONAHA.108.828228 [DOI] [PubMed] [Google Scholar]
- 17.Howell SJ, Brown OI, Beattie WS. Aetiology of perioperative myocardial injury: a scientific conundrum with profound clinical implications. Br J Anaesth 2020;125:642–646. 10.1016/j.bja.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 19.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 20.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019;21:715–731. 10.1002/ejhf.1494 [DOI] [PubMed] [Google Scholar]
- 21.Bularga A, Hung J, Daghem M, Stewart S, Taggart C, Wereski R, et al. Coronary artery and cardiac disease in patients with type 2 myocardial infarction: a prospective cohort study. Circulation 2022;145:1188–1200. 10.1161/CIRCULATIONAHA.121.058542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials. Circulation 2015;132:302–361. [DOI] [PubMed] [Google Scholar]
- 23.Sazgary L, Puelacher C, Lurati Buse G, Glarner N, Lampart A, Bolliger D, et al. Incidence of major adverse cardiac events following non-cardiac surgery. Eur Heart J Acute Cardiovasc Care 2020;10:550–558. 10.1093/ehjacc/zuaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristensen SD, Knuuti J, Saraste A, Anker S, Botker HE, De HS, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014;35:2383–2431. 10.1093/eurheartj/ehu282 [DOI] [PubMed] [Google Scholar]
- 25.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of Major noncardiac surgery. Circulation 1999; 100:1043–1049. 10.1161/01.CIR.100.10.1043 [DOI] [PubMed] [Google Scholar]
- 26.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol 2001;54:979–985. 10.1016/S0895-4356(01)00372-9 [DOI] [PubMed] [Google Scholar]
- 27.Gueckel J, Puelacher C, Glarner N, Gualandro DM, Strebel I, Zimmermann T, et al. Patient- and procedure-related factors in the pathophysiology of perioperative myocardial infarction/injury. Int J Cardiol 2022;353:15–21. 10.1016/j.ijcard.2022.01.015 [DOI] [PubMed] [Google Scholar]
- 28.Collet J, Thiele H, Barthe O, Folliguet T, Gale CP, Kingdom U, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 29.Helwani MA, Amin A, Lavigne P, Rao S, Oesterreich S, Samaha E, et al. Etiology of acute coronary syndrome after noncardiac surgery. Anesthesiology 2018;128:1084–1091. 10.1097/ALN.0000000000002107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollis RH, Holcomb CN, Valle JA, Smith BP, DeRussy AJ, Graham LA, et al. Coronary angiography and failure to rescue after postoperative myocardial infarction in patients with coronary stents undergoing noncardiac surgery. Am J Surg 2016;212:814–822.e1. 10.1016/j.amjsurg.2016.07.023 [DOI] [PubMed] [Google Scholar]
- 31.Parashar A, Agarwal S, Krishnaswamy A, Sud K, Poddar KL, Bassi M, et al. Percutaneous intervention for myocardial infarction after noncardiac surgery: patient characteristics and outcomes. J Am Coll Cardiol 2016;68:329–338. 10.1016/j.jacc.2016.03.602 [DOI] [PubMed] [Google Scholar]
- 32.Devereaux PJ, Duceppe E, Guyatt G, Tandon V, Rodseth R, Biccard BM, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018;391:2325–2334. 10.1016/S0140-6736(18)30832-8 [DOI] [PubMed] [Google Scholar]
- 33.Weber M, Luchner A, Seeberger M, Manfred S, Mueller C, Liebetrau C, et al. Incremental value of high-sensitive troponin T in addition to the revised cardiac index for peri-operative risk stratification in non-cardiac surgery. Eur Heart J 2013;34:853–862. 10.1093/eurheartj/ehs445 [DOI] [PubMed] [Google Scholar]
- 34.Chew MS, Puelacher C, Patel A, Hammarskjöld F, Lyckner S, Kollind M, et al. Identification of myocardial injury using perioperative troponin surveillance in major noncardiac surgery and net benefit over the revised cardiac risk Index. Br J Anaesth 2022;128:26–36. 10.1016/j.bja.2021.10.006 [DOI] [PubMed] [Google Scholar]
- 35.Ruetzler K, Smilowitz NR, Berger JS, Devereaux PJ, Maron BA, Newby LK, et al. Diagnosis and management of patients with myocardial injury after noncardiac surgery: a scientific statement from the American heart association. Circulation 2021;144:e287-e305. 10.1161/CIR.0000000000001024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request following an embargo phase until main results of the BASEL-PMI study are published.