Abstract
Introduction:
Digoxin is indicated for the management of heart failure with reduced ejection fraction and atrial fibrillation. Despite stronger guideline recommendations for other pharmacologic and device therapies, digoxin retains a role in select patients unable to tolerate or refractory to standard therapies. Contemporary utilization of and costs related to digoxin in the United States remain uncharacterized.
Methods:
We utilized the Medical Expenditure Panel Survey to estimate trends in digoxin use and expenditures across the United States from 2010 to 2017. The Medical Expenditure Panel Survey is an overlapping panel survey that interviews households in the United States to ascertain their healthcare utilization and expenditures. Complex sampling procedures allow for nationally representative estimates of utilization and expenditures. We report the number of digoxin users and expenditures across key subgroups in 2-year increments from 2010 to 2017.
Results:
The number of digoxin users in the United States declined by 47% from 766 users per 100,00 adults in 2010–2011 to 402 users per 100,000 adults in 2016–2017. While digoxin use declined among women and self-identified White adults, adults living at or below the federal poverty level and those who self-identified as Asian or Hispanic represent an increasing proportion of overall digoxin users. While nationwide digoxin expenditures declined by 26% from 2010–2011 to 2012–2013, they peaked at $260.3 million in 2014–2015 and remained elevated at $188.7 million in 2016–2017.
Conclusions:
Despite a nationwide trend towards declining use, digoxin remains prevalent amongst people of Asian and Hispanic descent in the United States. After a spike in cost in 2013, digoxin prices have yet to return to pre-spike levels. The role of digoxin in contemporary heart failure and arrhythmia management will continue to evolve as additional randomized and observational analyses become available.
Introduction
Clinicians have used digitalis-based therapies, including digoxin, to increase left ventricular contractility and attenuate adverse cardiac remodeling in patients with systolic heart failure for two centuries.1 While subsequent advances have introduced safer and more effective therapies into the management of heart failure with reduced ejection fraction, digoxin remains a treatment option for patients who remain symptomatic despite guideline-directed medical and device therapy as well as for those with advanced heart failure and intolerance to the hemodynamic effects of standard heart failure medications.2,3 Due to its ability to control the ventricular rate, digoxin also has a unique role in the management of atrial arrhythmias among patients with structural heart disease.4
Observational studies have identified potentially increased risks of mortality with digoxin use.5–7 Nevertheless, the use of digoxin at baseline exceeded that of sacubitril-valsartan in a recent clinical trial that demonstrated the efficacy and safety of sodium-glucose co-transporter-2 (SGLT2) inhibitors in heart failure.8 While much attention has focused rightly on the uptake of novel therapies, relatively little has been paid to the deprescribing of potentially inappropriate therapies to limit polypharmacy, decrease patient financial burden and minimize the potential for adverse effects.
Yet contemporary patterns of digoxin utilization in the United States remain unclear due to the lack of studies with a nationally representative sample that combines demographic and clinical characteristics with medication use data. Such analyses may identify gaps in guideline-recommended patient management while quantifying the economic resources allocated towards particular therapies. We analyzed digoxin use and expenditure trends in the Medical Expenditure Panel Survey (MEPS), a nationally representative longitudinal panel survey of households in the United States that focuses specifically on prescription medication use and expenditures.
Methods
Study Design
We performed an 8-year retrospective cohort study of the non-institutionalized adult population in the United States from 2010 to 2017 using the MEPS. MEPS is a nationwide overlapping panel survey of the non-institutionalized civilian population across a broad range of ages and demographic groups, as well as their medical providers and employers, in the United States. Each panel samples participating households over 5 rounds during a 2.5-year period. Households are randomly selected from the National Health Interview Survey. Heads of households complete a computer-based interview to provide the requested information for all household members. Participants in the survey are weighted relative to their representation in the United States population. Information on the medical and financial aspects of an individual’s health provided by the head of household is supplemented or replaced by such information from hospitals, physician offices, pharmacies and other medical providers where possible with permission from the participants. All data are available for public use and can be accessed at https://www.meps.ahrq.gov/mepsweb/.
Study Population and Variables
The study population included individuals who were 18 years or older with a person-weight of greater than zero. We gathered the following demographic information which were self-reported by individuals in MEPS: age at the end of the calendar year, gender, race, ethnicity, family income level and insurance type (Supplemental Table 1). Participants self-identified race and ethnicity. Family income level was classified according to the federal poverty level as poor (<100% of the federal poverty level), near poor (100% to <125%), low income (125% to <200%), middle income (200% to <400%), and high income (≥400%).
In response to open-ended questions, participants reported any physical or mental health conditions within the previous year and professional medical coders converted response into International Classification of Diseases (ICD)-9 codes for years 2010–2015 and ICD-10 codes for years 2016–2017 (Supplemental Table 1). In addition, the survey asks directly about certain conditions designated as priority by the Agency for Healthcare Research and Quality (this analysis included the following priority conditions: high blood pressure, ischemic heart disease, stroke, emphysema, chronic bronchitis, high cholesterol, diabetes and asthma). We used collapsed three-digit ICD codes for the present analysis as fully specified codes lack sufficient accuracy due to the self-reported nature of medical conditions.9 We reviewed forward and backward mapping from the Centers for Medicare and Medicaid Services general equivalence mappings (https://www.cms.gov/Medicare/Coding/ICD10/2018-ICD-10-CM-and-GEMs) for each ICD-9 and ICD-10 to ensure consistency between the two coding systems.
We identified digoxin users by searching through professionally coded generic drug name entries for “digoxin” in the pharmacy data. Manual review of the MEPS prescription data identified entries labeled as “digoxin” but associated with a National Drug Code for metformin. These entries were excluded from the analysis. We defined a digoxin user as any individual who filled at least one prescription for digoxin during the survey period. Expenditures reflect the actual payments made and any discounts or write-offs provided. The name of the medication, strength, dosage form and the associated charges were verified or supplied by the dispensing pharmacy where possible.
Outcomes
We estimated the number of digoxin users and expenditures on digoxin in the non-institutionalized, civilian adult population of the United States in pooled 2-year increments from 2010 to 2017. We performed additional analyses to estimate the number of digoxin users in select subgroups, third-party and out-of-pocket expenditures and digoxin use and expenditures. Subgroups of interest were age (18 to 65 years, >65 to 80 years, >80 years), race, ethnicity, insurance type and self-reported heart failure or cardiac dysrhythmia.
Validation Study
We used the Medicare and Medicaid Drug Spending Dashboards as an external control to corroborate or refute our main results from the MEPS (https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Information-on-Prescription-Drugs). We estimated digoxin use and expenditures from 2013 to 2018 based upon data availability in these databases. We calculated the annual number of Medicare digoxin beneficiaries (beneficiary data are unavailable for the Medicaid database), the annual number of Medicaid digoxin prescription claims and annual digoxin expenditures.
Statistical Analysis
All standard errors were estimated using Taylor series linearization and accounted for sample weights and the complex design of MEPS (stratification, clustering, multiple stages of selection, disproportionate sampling and non-response). We estimated the associations between demographics (age, sex, race/ethnicity), socioeconomic status (family income, insurance status), self-reported medical conditions (heart failure, cardiac dysrhythmia, myocardial infarction, angina, stroke, diabetes, dyslipidemia, hypertension, chronic kidney disease, asthma or chronic obstructive pulmonary disease) and survey year with digoxin use in a multivariable logistic regression model. All analyses were performed using Stata version 15.1 (Stata Corp, College Station, TX).
Results
Overall Study Participant Characteristics
The overall cohort of included MEPS respondents represented between 233 and 248 million non-institutionalized adults in the United States annually. The mean age in this cohort was 47 years and 52% were women, 13% were self-reported Black individuals, 6% were self-reported Asian individuals and 15% were self-reported Hispanic individuals (Supplemental Table 2). Households with family income greater than 400% of the federal poverty level comprised 39% of MEPS respondents in 2010 as compared to 44% in 2017. The proportion of respondents with private or public insurance increased from 2010 to 2017 and only 8% of 2016–2017 MEPS respondents were uninsured. The proportion of participants who reported heart failure among eligible MEPS respondents increased from 0.65% in 2010–2011 to 0.81% in 2014–2015 before declining to 0.67% in 2016–2017. The proportion of participants who reported any cardiac dysrhythmia followed a similar pattern with an increase from 2.2% in 2010–2011, a peak of 2.6% in 2014–2015 and a decline to 2.4% in 2016–2017.
Trends in Digoxin Use and Expenditures in the Non-Institutionalized Adult Population in the United States between 2010 and 2017
The number of digoxin users in the United States decreased from 766 users per 100,000 adults in 2010–2011 to 402 users per 100,000 adults in 2016–2017 (Figure 1). Overall, digoxin use declined between 2010–2011 and 2016–2017 across most demographics and across insurance types (Figure 2).
Figure 1.
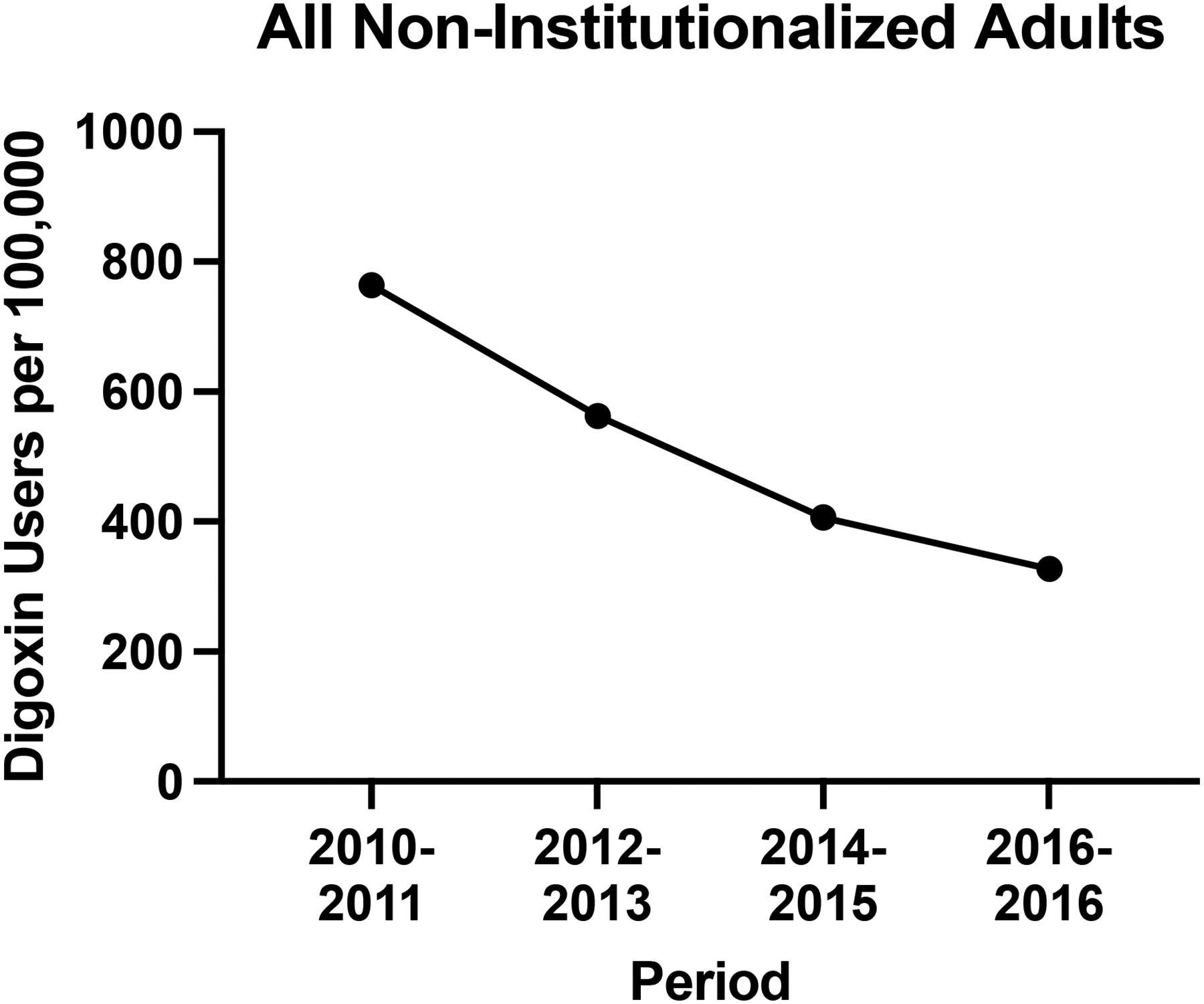
Trends in Digoxin Use in the United States from 2010–2011 to 2016–2017
Figure 2.
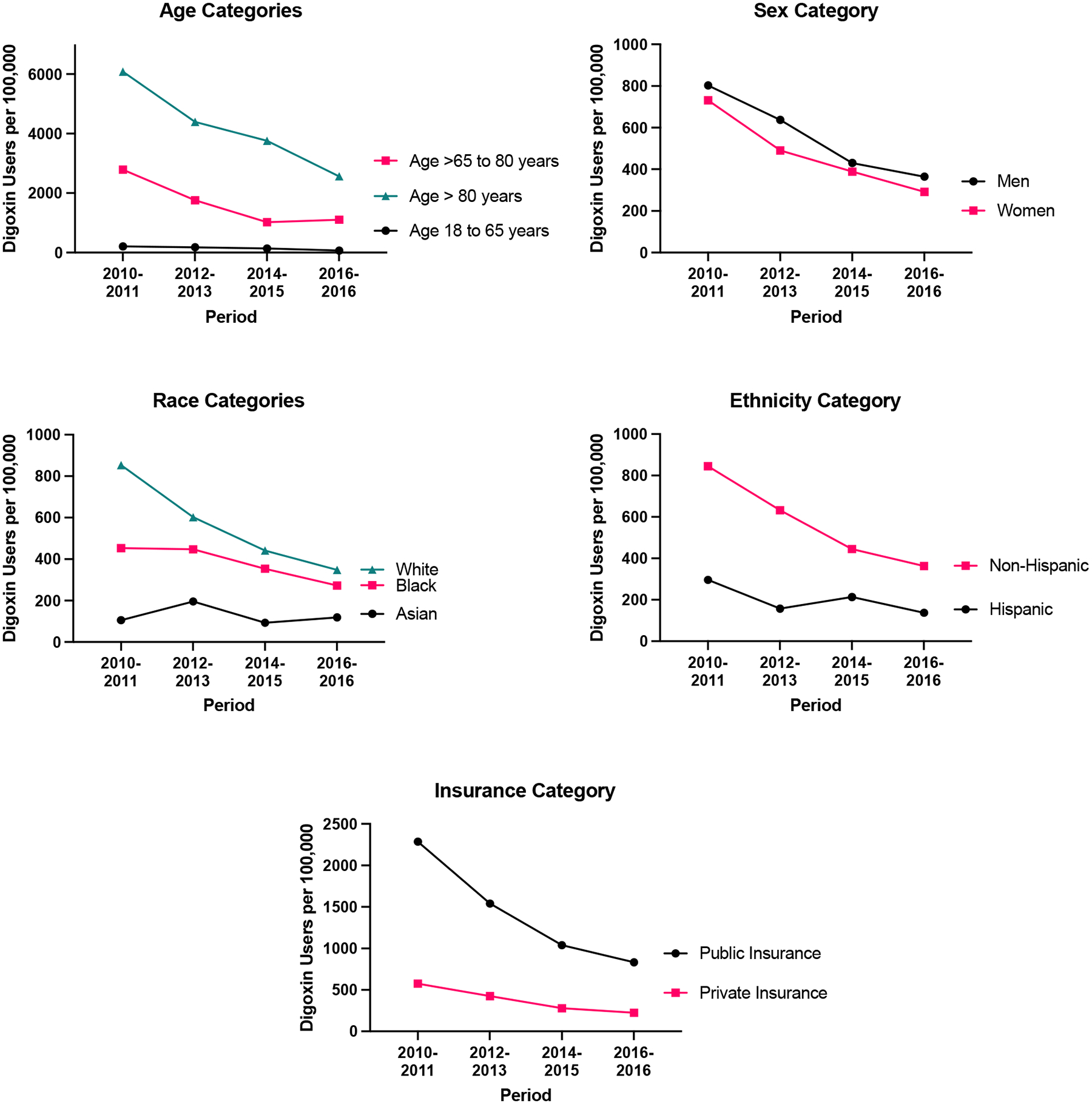
Digoxin Use Across Subgroups of Adults in the United States from 2010–2011 to 2016–2017
When examining demographic subgroups of interest, the mean age of digoxin users increased from 73 years in 2010–2011 to 74 years in 2016–2017. By contrast, the proportion of female digoxin users and White digoxin users declined from 731 users per 100,000 adults to 291 users per 100,000 adults and from 854 users per 100,000 adults to 348 adults per 100,000 adults, respectively, between 2010–2011 and 2016–2017. Among Asian and Hispanic individuals, however, digoxin use remained stable over the same time period (Figure 2). Use of digoxin among Black individuals was stable between 2010–2011 and 2012–2013 before declining thereafter. Of note, adults living at or below the federal poverty level represented a growing proportion of digoxin users (9.4% in 2010–2011 and 15.8% in 2016–2017).
The percentage of digoxin users who reported having heart failure or a cardiac dysrhythmia increased from 12% and 43% in 2010–2011 to 15% and 50% in 2016–2017, respectively. Among adults who self-reported heart failure, digoxin use decreased between 2010–2011 and 2016–2017 from 0.21 million users to 0.12 million users. The use of digoxin among adults with a self-reported cardiac dysrhythmia declined from 0.76 million users in 2010–2011 and to 0.40 million users in 2016–2017. The number of digoxin users who reported neither heart failure nor a cardiac dysrhythmia also decreased (0.84 million in 2010–2011 and 0.34 million in 2016–2017).
Total nationwide digoxin expenditures decreased from $111.3 million in 2010–2011 to $82.2 million in 2012–2013 before tripling to $260.3 million in 2014–2015 and then declining to $188.7 million in 2016–2017. Increases in digoxin expenditures were absorbed largely by third-party payers, who accounted for 41% ($45.7 million) of digoxin expenditures in 2010–2011 and 36% ($29.4 million) in 2012–2013 compared to 81% ($211.5 million) in 2014–2015 and 67% ($125.6) in 2016–2017. Out-of-pocket digoxin expenditures varied between $48.8 million and $65.6 million between 2010–2011 and 2016–2017.
Digoxin Use and Expenditures in the Medicare and Medicaid Drug Utilization Databases between 2013 and 2018
The number of Medicare digoxin beneficiaries declined from 352 per 100,000 overall Medicare beneficiaries in 2013 to 143 per 100,000 overall beneficiaries in 2018 (Figure 3). Between 2013 and 2018, the nationwide number of Medicaid digoxin prescription claims declined from 83 per 100,000 overall Medicaid claims to 45 per 100,000.
Figure 3.
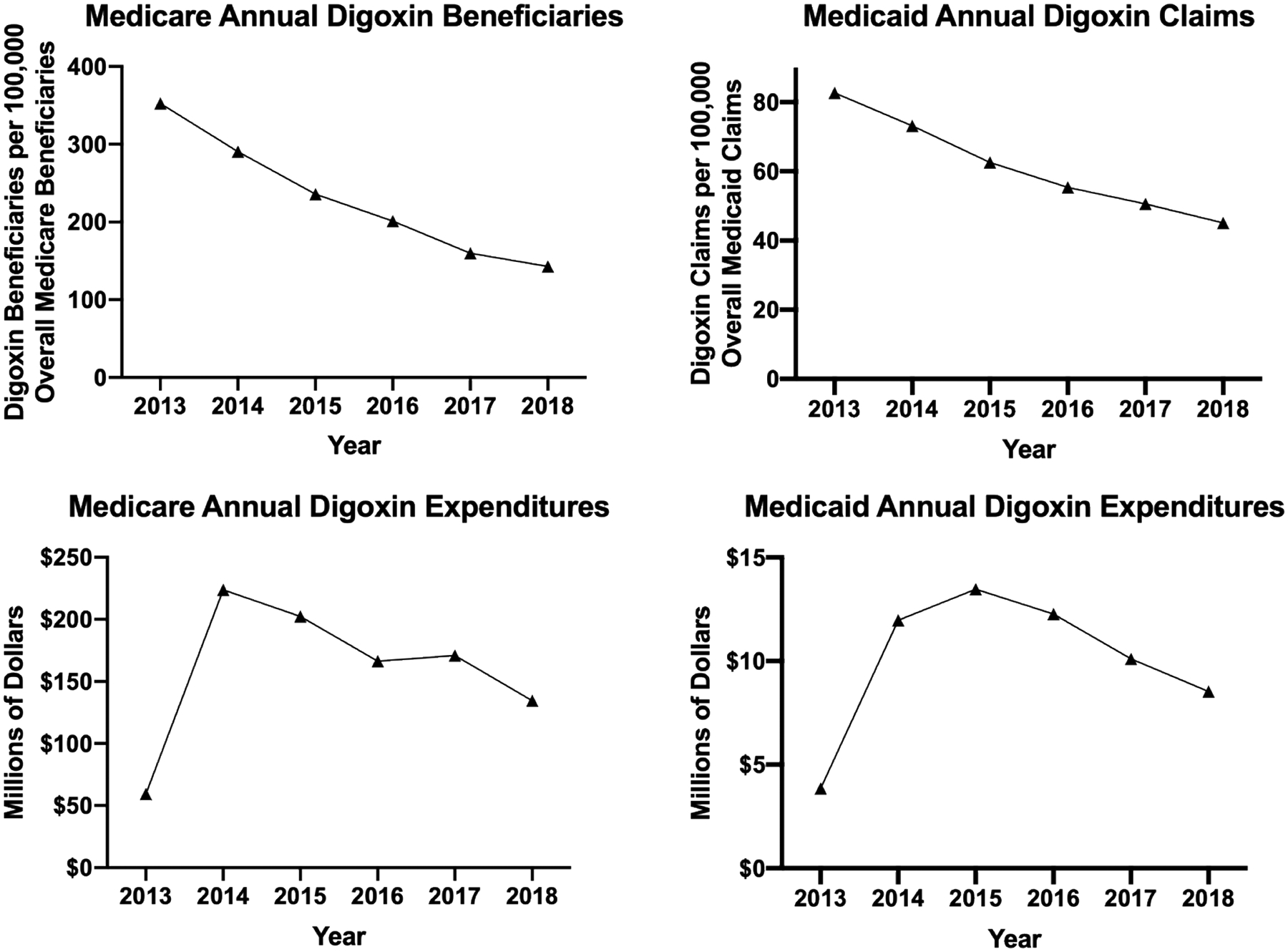
Digoxin Use and Expenditures in the Medicare and Medicaid Drug Utilization Databases from 2013 to 2018
Total Medicare annual digoxin spending increased by 377% between 2013 ($59.3 million) and 2014 ($223.8 million) and then decreased gradually through 2018 ($134.6 million). Total Medicaid annual digoxin expenditures increased from $3.9 million in 2013 to a peak of $13.5 million in 2015 before decreasing to $8.5 million in 2018.
Predictors of Digoxin Use
In a multivariable analysis, the odds of adults aged 65 to 80 years and adults aged 80 years or older reporting digoxin use compared to those aged 18 to 65 years old were 4.03-times higher (95% confidence interval [CI]: 2.97–5.45; P<.001) and 7.83-times higher (95% confidence interval: 5.49–11.15; P<.001), respectively. The odds of digoxin use were 21% lower in women compared to men (odds ratio [OR]: 0.79; 95% CI: 0.64–0.97). The odds of digoxin use were lower in survey years 2012–2013, 2014–2015 and 2016–2017 compared to 2010–2011 (Table 2). In addition, individuals with heart failure, a cardiac dysrhythmia, myocardial infarction, diabetes, and hypertension had higher odds of digoxin use (Table 2). The odds of digoxin use were lower in individuals with chronic renal failure (OR: 0.18; 95% CI: 0.04–0.87; P=.032).
Table 2.
Associations of participant characteristics with digoxin use
| Characteristics | Odds ratio (95% confidence interval) | P-value |
|---|---|---|
| Age | ||
| 18 to 65 years | Reference | |
| >65 to 80 years | 4.03 (2.97–5.45) | <.001 |
| >80 years | 7.83 (5.49–11.15) | <.001 |
| Gender | ||
| Men | Reference | |
| Women | 0.79 (0.64–0.97) | 0.026 |
| Self-Reported Race/Ethnicity | ||
| Hispanic | 0.75 (0.56–1.02) | 0.07 |
| Non-Hispanic White | Reference | |
| Non-Hispanic Black | 0.98 (0.71–1.34) | 0.88 |
| Non-Hispanic Asian | 1.16 (0.89–1.50) | 0.27 |
| Non-Hispanic Other | 0.68 (0.27–1.72) | 0.42 |
| Family Income (% federal poverty level) | ||
| Poor (<100%) | 1.20 (0.87–1.66) | 0.26 |
| Near Poor (100–125%) | 1.37 (0.91–2.07) | 0.13 |
| Low (125 to 200%) | 1.08 (0.78–1.50) | 0.63 |
| Middle (200–400%) | 1.08 (0.86–1.37) | 0.52 |
| High (≥400%) | Reference | |
| Private Insurance Coverage | ||
| Yes | Reference | |
| No | 1.26 (1.00–1.59) | 0.05 |
| Index Year | ||
| 2010–2011 | Reference | |
| 2012–2013 | 0.76 (0.60–0.95) | 0.017 |
| 2014–2015 | 0.45 (0.34–0.59) | <.001 |
| 2016–2017 | 0.39 (0.30–0.51) | <.001 |
| Comorbidities (Yes vs. No) | ||
| Heart failure | 4.85 (3.23–7.29) | <.001 |
| Cardiac dysrhythmia | 13.33 (10.34–17.17) | <.001 |
| Myocardial infarction | 4.00 (2.98–5.37) | <.001 |
| Angina | 1.18 (0.79–1.77) | 0.43 |
| Stroke | 1.21 (0.83–1.76) | 0.32 |
| Diabetes mellitus | 1.60 (1.26–2.05) | <.001 |
| Dyslipidemia | 1.11 (0.84–1.47) | 0.44 |
| Hypertension | 1.50 (1.12–2.02) | 0.007 |
| Chronic renal failure | 0.18 (0.04–0.87) | 0.032 |
| Asthma or chronic obstructive pulmonary disease | 1.32 (0.99–1.75) | 0.054 |
Discussion
Digoxin use declined among adults in the United States between 2010 and 2017. While such declines appear consistent across many subgroups, digoxin use among self-identified Asian, Black and Hispanic individuals remained stable or declined minimally. Despite the availability of a generic formulation, annual digoxin expenditures increased over the same time period, peaking at $260.3 million in 2014–2015. Since digoxin has a less favorable risk-to-benefit ratio than alternative therapies, these data provide an overall encouraging sign that clinicians have begun to move away from this therapy and identify opportunities to further scale back digoxin use.
The observed decrease in digoxin use represents a continuation of trends that began as early as 2005. In the Get With The Guidelines registry, the proportion of hospitalized patients with heart failure with reduced ejection fraction who received a discharge prescription for digoxin decreased from over 30% in 2005 to 10% in 2014.10 Between 2007 and 2014, the number of prescription claims for digoxin in a commercial prescription database representative of adults aged 65 years or older in the United States decreased.11 This present analysis of the Medical Expenditure Panel Survey updates these prior observations of digoxin use through the year 2017 and broadens our understanding of digoxin use in the United States using a nationally representative sample. Indeed, the Medical Expenditure Panel Survey was designed to support this exact type of analysis.
Several possible explanations may account for the overall declining use of digoxin. Newer therapies have surpassed digoxin in both efficacy and safety. Whereas digoxin reduces heart failure hospitalizations, mineralocorticoid receptor antagonists, sacubitril-valsartan12 and sodium glucose co-transporter-2 inhibitors8 decrease cardiovascular death or heart failure hospitalizations and can be implemented without therapeutic drug monitoring. Recent observational analyses have raised concern for possible increased mortality with digoxin use in heart failure and atrial fibrillation, although bias cannot be excluded.5–7 In addition, the 2013 price increase for both generic and brand digoxin may have contributed to re-evaluating and deprescribing digoxin.13 This increase was confirmed in our analysis of individual-level data in MEPS and aggregated Medicare and Medicaid data. The price changes were likely attributable to a reduction in the number of worldwide generic digoxin manufacturers, which allowed the remaining manufacturers to dictate higher prices.
Factors that associated with digoxin use in our study were older age and potential indications for digoxin use, such as a history of a cardiac dysrhythmia or heart failure. Additionally, a history of chronic kidney disease associated with a lower odds of digoxin use. These associations suggest that the self-reported medical history for these conditions had adequate positive predictive value. These results agree with a recent analysis of the Swedish Heart Failure registry.6
The declining use of digoxin has occurred amongst steady, albeit slow, growth in the use of sacubitril-valsartan14 and the discovery that sodium-glucose co-transporter-2 inhibitors provide incremental protection against heart failure events. Nevertheless, baseline use of digoxin (19%) exceeded that of sacubitril-valsartan (11%) in the 4744-patient heart failure clinical trial that demonstrated the efficacy and safety of sodium-glucose co-transporter-2 inhibitors in heart failure.8 While the rationale for and circumstances surrounding digoxin use among adults in the Medical Expenditure Panel Survey are unavailable, the present analysis urges clinicians and policymakers to question digoxin’s place in therapy outside of select patients.
Digoxin serves as a case-study of the shortcomings of the current prescription medication reimbursement model in the United States. The price of brand and generic prescription medications, including digoxin, has risen steadily in the United States over the past decade.15 Moreover, medication prices often vary considerably between neighboring pharmacies and price transparency is limited.16 The growing expenditures associated with digoxin use should warn clinicians, patients and policymakers of the need to consider cost for generic and brand medications. The availability of affordable medications acquires added importance in light of the increasing prevalence of guideline-recommended polypharmacy in people with cardiovascular disease.
We anticipate further changes in the use of digoxin in the near future. The ongoing DIGIT-HF trial (EudraCT 2013-005326-38) will investigate the efficacy and safety of an alternate digitalis formulation, digitoxin, on a background of contemporary standard of care in 2,190 patients with heart failure with reduced ejection fraction.17 The RATE-AF found similar effects on symptom burden between digoxin and bisoprolol in patients with permanent atrial fibrillation, but fewer adverse events among digoxin-treated patients.18
This study has certain strengths and limitations. The Medical Expenditure Panel Survey allows nationally representative estimates of medical and pharmacy use and expenditures in the United States. We corroborated our findings using Medicare and Medicaid drug spending data. Heads of household respond to open-ended questions about their own medical conditions and those of other household members that were bothersome or led to healthcare utilization in the previous year. Reported conditions may lack accuracy and detail and MEPS conditions cannot be considered exhaustive. Left ventricular ejection fraction and similar parameters were not measured as part of the MEPS study. A large proportion of digoxin users in this study failed to report a clear indication for digoxin use, such as heart failure or a cardiac dysrhythmia. The limited awareness of heart failure and heart failure therapies among the public may have contributed to under-reporting of heart failure as an indication for digoxin use among MEPS respondents.
Conclusions
Despite a nationwide trend towards declining use, digoxin remains prevalent amongst people of Asian and Hispanic descent in the United States. After a spike in cost in 2013, digoxin prices have yet to return to pre-spike levels. The role of digoxin in contemporary heart failure and arrhythmia management will continue to evolve as additional randomized and observational analyses become available.
Supplementary Material
Table 1.
Characteristics of Digoxin Users in the United States from 2010 to 2017
| Characteristic | 2010–2011 | 2012–2013 | 2014–2015 | 2016–2017 |
|---|---|---|---|---|
| Number of Adults (millions) | 1.78 | 1.34 | 0.99 | 0.81 |
| Age, years | 72.6 (0.9) | 71.8 (1.2) | 73.1 (1.2) | 74.2 (0.9) |
| Women | 49.3% | 45.3% | 49.2% | 46.1% |
| Race | ||||
| Asian | 0.7% | 2% | 1.5% | 2.4% |
| Black | 7.2% | 9.9% | 11.3% | 11.2% |
| White | 91.3% | 87.4% | 87.2% | 85.4% |
| Ethnicity | ||||
| Hispanic | 5.7% | 4.2% | 8.1% | 6.8% |
| Non-Hispanic | 94.3% | 95.8% | 91.9% | 93.2% |
| Family Income (% federal poverty level) | ||||
| Poor (<100%) | 9.4% | 11.0% | 15.1% | 15.8% |
| Near Poor (100–125%) | 8.8% | 6.6% | 7.0% | 5.0% |
| Low (125 to 200%) | 19.3% | 18.1% | 14.1% | 18.6% |
| Middle (200–400%) | 30.1% | 34.2% | 31.2% | 25.0% |
| High (≥400%) | 32.4% | 30.1% | 32.6% | 35.5% |
| Insurance | ||||
| Medicare | 82.4% | 77.9% | 79.1% | 90.0% |
| Medicaid | 9.5% | 9.2% | 13.9% | 12.3% |
| Private | 52.9% | 54.2% | 48.9% | 57.1% |
| None | 2.4% | 1.8% | 1.8% | 0.7% |
| Medical Conditions | ||||
| Heart Failure | 12.0% | 14.0% | 15.6% | 14.6% |
| Cardiac dysrhythmia | 42.6% | 43.2% | 46.6% | 50.0% |
| Myocardial infarction | 24.2% | 28.0% | 25.5% | 30.3% |
| Angina | 9.8% | 11.2% | 8.3% | 10.1% |
| Stroke | 13.0% | 11.3% | 9.3% | 9.0% |
| Diabetes mellitus | 35.1% | 35.0% | 39.4% | 25.5% |
| Dyslipidemia | 60.5% | 62.1% | 56.4% | 57.7% |
| Hypertension | 71.9% | 70.7% | 79.4% | 67.8% |
| Chronic kidney disease | 0% | 0% | 0.6% | 0.9% |
| Asthma or Chronic Obstructive Pulmonary Disease | 19.3% | 15% | 22.8% | 28.0% |
Funding:
Dr. Lauffenburger is supported in part by a career development award (K01HL141538) from the NIH. Dr. Vaduganathan is supported by a KL2/Catalyst Medical Research Investigator Training Award from Harvard Catalyst (NIH/NCATS Award UL1TR002541). Dr. Buckley is supported by a Mentored, Patient-Oriented Research Career Development Award from the NIH (K23HL150311), a Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology and a Khoury Innovation Award from the Brigham and Women’s Hospital.
Conflicts of Interest:
Dr. Vaduganathan receives research grant support from Amgen, serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics and Relypsa, and participates on clinical endpoint committees for studies sponsored by Galmed, Novartis and the NIH. All other authors have nothing to disclose.
References
- 1.Silverman ME. William Withering and an account of the foxglove plant. Clinical Cardiology 1989;12:415–8. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal 2016;37:2129–200m.27206819 [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 5.Vamos M, Erath JW, Hohnloser SH. Digoxin-associated mortality: A systematic review and meta-analysis of the literature. European Heart Journal 2015;36:1831–8. [DOI] [PubMed] [Google Scholar]
- 6.Kapelios CJ, Lund LH, Benson L, et al. Digoxin use in contemporary heart failure with reduced ejection fraction: an analysis from the Swedish Heart Failure Registry. Eur Heart J Cardiovasc Pharmacother 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Y, Kotalczyk A, Wang Y, Lip GYH. Digoxin use and clinical outcomes in elderly Chinese patients with atrial fibrillation: a report from the Optimal Thromboprophylaxis in Elderly Chinese Patients with Atrial Fibrillation (ChiOTEAF) registry. Europace 2022. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. New England Journal of Medicine 2019:NEJMoa1911303-NEJMoa. [DOI] [PubMed] [Google Scholar]
- 9.Machlin S, Cohen J, Elixhauser A, Beauregard K, Steiner C. Sensitivity of household reported medical conditions in the medical expenditure panel survey. Med Care 2009;47:618–25. [DOI] [PubMed] [Google Scholar]
- 10.Patel N, Ju C, Macon C, et al. Temporal Trends of Digoxin Use in Patients Hospitalized With Heart Failure. Analysis From the American Heart Association Get With The Guidelines-Heart Failure Registry. JACC: Heart Failure 2016;4:348–56. [DOI] [PubMed] [Google Scholar]
- 11.Angraal S, Nuti SV, Masoudi FA, et al. Digoxin Use and Associated Adverse Events Among Older Adults. American Journal of Medicine 2019;132:1191–8. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJV, Packer M, Desai AS, et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. New England Journal of Medicine 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 13.Rapid Price Increases for Some Generic Drugs Catch Users by Surprise - The New York Times.
- 14.Sumarsono A, Vaduganathan M, Ajufo E, et al. Contemporary Patterns of Medicare and Medicaid Utilization and Associated Spending on Sacubitril/Valsartan and Ivabradine in Heart Failure. JAMA Cardiol 2019;5:336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alpern JD, Stauffer WM, Kesselheim AS. High-cost generic drugs - Implications for patients and policymakers. New England Journal of Medicine 2014;371:1859–62. [DOI] [PubMed] [Google Scholar]
- 16.Hauptman PJ, Goff ZD, Vidic A, Chibnall JT, Bleske BE. Variability in retail pricing of generic drugs for heart failure. JAMA Internal Medicine 2017;177:126–8. [DOI] [PubMed] [Google Scholar]
- 17.Bavendiek U, Berliner D, Dávila LA, et al. Rationale and design of the DIGIT-HF trial (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure): a randomized, double-blind, placebo-controlled study. European Journal of Heart Failure 2019;21:676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotecha D, Bunting KV, Gill SK, et al. Effect of Digoxin vs Bisoprolol for Heart Rate Control in Atrial Fibrillation on Patient-Reported Quality of Life: The RATE-AF Randomized Clinical Trial. JAMA 2020;324:2497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


