Abstract
INTRODUCTION
Tranexamic acid (TXA) is an antifibrinolytic agent widely used in surgery to decrease bleeding and reduce the need for blood product transfusion. The role of TXA in urology is not well-summarized. We conducted a systematic review of studies reporting outcomes of TXA use in urological surgery.
METHODS
A comprehensive search was conducted from the following databases: PubMed, Embase, Cochrane Library, and Web of Science. Two reviewers performed title and abstract screening, full-text review, and data collection. Primary outcomes included estimated blood loss (EBL), decrease in hemoglobin, decrease in hematocrit, and blood transfusion rates. Secondary outcomes included TXA administration characteristics, length of stay, operative time, and postoperative thromboembolic events.
RESULTS
A total of 26 studies consisting of 3261 patients were included in the final analysis. These included 11 studies on percutaneous nephrolithotomy, 10 on transurethral resection of prostate, three on prostatectomy, and one on cystectomy. EBL, transfusion rate, hemoglobin drop, operative time, and length of stay were significantly improved with TXA administration. In addition, the use of TXA was not associated with an increased risk of venous thromboembolism (VTE ). The route, dosage, and timing of TXA administration varied considerably between included studies.
CONCLUSIONS
TXA use may improve blood loss, transfusion rates, and perioperative parameters in urological procedures. In addition, there is no increased risk of VTE associated with TXA use in urological surgery; however, there is still a need to determine the most effective TXA administration route and dose. This review provides evidence-based data for decision-making in urological surgery.
INTRODUCTION
Tranexamic acid (TXA) is a synthetic lysine derivative with antifibrinolytic properties that is used in the management of traumatic and surgical bleeding.1,2 It exerts its primary mechanism of action through its competitive interactions with the lysine binding sites on plasminogen to inhibit plasmin formation and fibrin degradation, thereby suppressing fibrinolysis, promoting hemostasis, and reducing blood loss.3 TXA has found widespread use in the medical management of heavy menstrual bleeding, postpartum hemorrhage, coagulopathy disease, and trauma;1 however, its hemostatic properties have also led to the use of TXA being explored in the surgical setting.
Aside from the direct negative consequences of surgical bleeding, excessive blood loss during surgery can indirectly impact patient morbidity and mortality.4 In the intraoperative setting, excessive bleeding can impair surgeon visibility, increasing the risk of tissue injury, prolonged operative times, and further bleeding. Postoperatively, the need for blood product transfusion can lead to rare but harmful immunological and infectious adverse events.5,6 Certain patient populations, such as those with religious objections to blood transfusions and patients preparing for renal transplant, my place special interest in avoiding blood transfusions. 7 Finally, in cases of severe bleeding, patients may require additional procedures, including angioembolization and reoperation, to limit bleeding, which can further complicate recovery. Overall, the potential of TXA to limit these adverse events makes its use an attractive prospect for surgeons of all specialties.
One major concern that tempers widespread surgical use of TXA is its potential to promote thrombosis.8,9 For example, Myers et al found that the administration of TXA in trauma patients was associated with an increased risk of venous thromboembolism (VTE );10 however, other studies have found no such association between TXA use and VTE in the setting of trauma.11–13 In the context of surgery, most studies have shown no association between TXA use and thrombotic adverse events.14,15 Even in oncological surgery, in which the patient population is at a heightened risk of VTE, studies suggest no increase in thrombotic events.16,17
The utility of TXA in urology remains open-ended, with multiple ongoing randomized controlled trials (RCTs) investigating its application in a variety of urological surgeries. This may be due to the relatively low risk of clinically significant bleeding associated with common urological procedures.18,19 In order to characterize the use of TXA in urology, we aimed to conduct a systematic review of studies reporting outcomes of TXA use in urological surgery.
METHODS
This review was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Intervention and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISM A).20 Prior to implementing our search strategy, this study was prospectively registered in PROS PERO (CRD 42021231304).
Search strategy
A comprehensive literature search of medical databases was conducted for studies assessing the postoperative outcomes of patients receiving TXA and urological surgery. The search strategy was developed in consultation with a medical librarian and is outlined in Supplementary Table 1 (available at cuaj.ca). The literature search was conducted on January 13, 2021, and databases searched included Ovid MEDLINE, Embase, CENTRAL, and Web of Science. We also performed a manual search on PubMed and Google Scholar and reviewed references of included articles to identify any published or unpublished studies that may have been missed in the initial search. The inclusion criteria were any English-language comparative study examining blood loss and transfusion rate after TXA administration in adults undergoing urological surgery.
Data extraction
Studies identified via the search strategy were independently screened by two reviewers using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Conflicts were resolved by a third reviewer.
Two reviewers, independently and in duplicate, performed title and abstract screening, full-text review, and data collection. Primary outcomes included estimated blood loss (EBL) in mL, rates of blood transfusion, and postoperative drop in hemoglobin in g/dL. Secondary outcomes included TXA administration characteristics, perioperative outcomes, and postoperative complications.
Statistical analysis
Extracted study data were summarized using descriptive statistics and analyzed using RevMan (Review Manager v5.4, The Cochrane Collaboration, London, U.K.). Meta-analysis of RCTs was carried out using a random effects model and resulting mean differences (MD ) for continuous variables and risk ratios (RR ) for dichotomous variables were presented with 95% confidence intervals (CI). Heterogeneity was assessed using a χ2 test with N-1 degrees of freedom, with α=0.05 for statistical significance. The I2 test was used to evaluate variability across studies, with an I2 value ≥50% indicating high heterogeneity. Subgroup analysis was performed according to procedure type. Missing data were excluded from analysis. A p-value of <0.05 was considered statistically significant.
Risk of bias was evaluated with the Cochrane risk-of-bias tool for RCTs,21 and the methodological index for non-randomized studies (MINORS ) tool for non-randomized studies.22 The maximum MINORS score is 16 for non-comparative studies and 24 for comparative studies, with higher scores indicating lower risk of bias. For this review, a study’s risk of bias was categorized as high (MINORS score of 0–8 for non-comparative studies and 0–12 for comparative studies), moderate (score of 9–12 for non-comparative studies and 13–18 for comparative studies), or low (score of 13–16 for non-comparative studies and 19–24 for comparative studies). The average MINORS score was presented as mean ± standard deviation.
RESULTS
Study identification
The initial database search retrieved 21 111 articles. After removal of duplicates, abstract review, full-text review, and application of inclusion and exclusion criteria, a total of 15 studies, published between 2004 and 2020, were identified for inclusion.23–37 Our manual search identified an additional 11 studies in our manual search and subsequently included in our study.38–47 Figure 1 summarizes the search in a PRISM A flow diagram.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Study and population characteristics
Of the 26 included studies, 19 were RCTs, five were retrospective cohort studies, and two were prospective cohort studies. Of these studies, 10 evaluated transurethral resection of prostate (TURP), three evaluated radical prostatectomy (RP), 12 evaluated percutaneous nephrolithotomy (PCNL ), and one evaluated radical cystectomy (RC). Risk of bias for included RCTs is outlined in Supplementary Figure 1 (available at cuaj.ca). The average MINORS score for the seven included nonrandomized studies was 17.9±2.3, indicating moderate risk of bias.
The pooled population included 3261 patients, with 1578 patients receiving TXA and 1683 patients acting as controls. The average age of included patients was 43.6 years and there was no significant difference in age between groups (MD 0.15 years, 95% CI −0.83–1.13, p=0.02, I2 = 48%) (Supplementary Figure 2; available at cuaj.ca). Most (73%) patients in the included studies were male. A summary of study characteristics, primary outcomes, and secondary outcomes is depicted in Table 1.
Table 1.
Primary and secondary outcomes of included studies
| Group characteristics | Primary outcomes | Secondary outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Author (year) | Surgical procedure | Study type | Groups | Sample size (n) | Mean age ± SD (years) | Male (n) | EBL ± SD (mL) | Blood transfusions (n) | Change in hemoglobin ± SD (g/dL) | Length of stay ± SD (days) | Operative time ± SD (minutes) |
|
| |||||||||||
| Alfredo et al (2020) | PCNL | RCT | TXA | NR | NR | NR | NR | NR | NR | NR | NR |
| Placebo | NR | NR | NR | NR | NR | NR | NR | NR | |||
|
| |||||||||||
| Bansal et al (2017) | PCNL | RCT | TXA | 200 | 33±14 | 116 | 155±47 | 10 | 1.7±0.9 | 2.1±0.9 | 69±3 |
| Placebo | 200 | 35±15 | 109 | 213± 8 | 25 | 2.7±1.2 | 3.4±1.4 | 88±29 | |||
|
| |||||||||||
| Barbilian et al (2017) | PCNL | Retrospective cohort | TXA | 23 | NR | NR | NR | 1 | 1.1±NR | NR | 73 |
| No | TXA | 24 | NR | NR | NR | 6 | 2.4±NR | 76 | |||
|
| |||||||||||
| Cauni et al (2017) | PCNL | Retrospective cohort | TXA | 51 | NR | NR | NR | NR | 1.1±NR | NR | 72±NR |
| Control | 53 | NR | NR | NR | NR | 2.3±NR | NR | 83±NR | |||
|
| |||||||||||
| Iskakov et al (2016) | PCNL | Retrospective cohort | TXA | 82 | 47±1.4 | 35 | NR | 2 | 1.1±0.02 | 9.1±0.3 | 107±5.4 |
| Control | 82 | 46±1.5 | 47 | NR | 10 | 2.3±0.4 | 10±0.5 | 119±5.3 | |||
|
| |||||||||||
| Jhanwar et al (2016) | PCNL | Retrospective cohort | TXA | 96 | 34±10 | 54 | NR | 6 | 1.0±0.3 | 2.9±0.5 | 44±4.2 |
| No TXA | 102 | 35±11 | 58 | NR | 15 | 1.4±0.4 | 3.3±0.4 | 53±5.2 | |||
|
| |||||||||||
| Kumar et al (2013) | PCNL | RCT | TXA | 100 | 38±11 | 58 | NR | 2 | 1.4±NR | 2.7±1.1 | 48±NR |
| No TXA | 100 | 40±12 | 54 | NR | 11 | 2.3±NR | 4.7±3.1 | 71±NR | |||
|
| |||||||||||
| Mohammadi et al (2019) | PCNL | RCT | TXA | 30 | 41±13 | 25 | 298±95 | NR | NR | 4.3±0.6 | NR |
| Placebo | 30 | 42±13 | 21 | 500±121 | NR | NR | 4.5±0.6 | NR | |||
|
| |||||||||||
| Mohammadi Sichani et al (2018) | PCNL | RCT | TXA | 60 | 42±14 | 76.65 | 751±523 | NR | 1.5±NR | 4.3±NR | NR |
| Placebo | 60 | 43±14 | 75 | 825±526 | NR | 2.7±NR | 4.1±NR | NR | |||
|
| |||||||||||
| Prakash et al (2017) | PCNL | Prospective cohort | TXA | 69 | NR | NR | NR | 3 | 1.1±NR | NR | NR |
| No TXA | 72 | NR | NR | NR | 18 | 2.4±NR | NR | NR | |||
|
| |||||||||||
| Rashid et al (2018) | PCNL | RCT | TXA | 25 | 48±14 | 16 | 74±60 | 1 | 0.5 ± 0.4 | NR | 48 ± 18 |
| Placebo | 25 | 49±16 | 17 | 117±88 | 3 | 1.0±0.5 | NR | 62±16 | |||
|
| |||||||||||
| Siddiq et al (2017) | PCNL | RCT | TXA | 120 | 41±NR | 82 | NR | 4 | 1.3±NR | 4.0±NR | 85±NR |
| Placebo | 120 | 40±NR | 72 | NR | 12 | 1.6±NR | 4.0±NR | 90±NR | |||
|
| |||||||||||
| Zaid et al (2016) | RC (open) | Retrospective cohort | TXA | 103 | 69±NR | 91 | 650±NR | 32 | NR | NR | 278±NR |
| No TXA | 200 | 69±NR | 161 | 650±NR | 115 | NR | NR | 302±NR | |||
|
| |||||||||||
| Balik et al (2020) | RP (robotassisted) | RCT | TXA | 50 | 64±5.9 | 50 | 93±NR | NR | 2.3±NR | NR | NR |
| Placebo | 50 | 655.7 | 50 | 97±NR | NR | 2.4±NR | NR | NR | |||
|
| |||||||||||
| Crescenti et al (2011) | RP (open) | RCT | TXA | 100 | 64±7.4 | 100 | 1103±501 | 34 | 2.9±NR | 9.0±4.3 | 166±44 |
| Placebo | 100 | 64±7.8 | 100 | 1335±687 | 55 | 3.1±NR | 9.0±4.3 | 159±40 | |||
|
| |||||||||||
| Pourfakhr et al (2016) | RP (open) | RCT | TXA | 93 | 68±9.9 | 93 | 340±NR | 0 | 1.9±1.0 | NR | 75±NR |
| Placebo | 93 | 65±8.9 | 93 | 515±NR | 5 | 2.0±1.3 | NR | 80±NR | |||
| Abdullah et al (2012) | TURP | RCT | TXA | 26 | NR | 26 | NR | NR | 1.2±NR | NR | NR |
| Placebo | 26 | NR | 26 | NR | NR | 1.9±NR | NR | NR | |||
|
| |||||||||||
| Karkhanei et al (2020) | TURP | RCT | TXA | 35 | 66±7.9 | 35 | NR | 0 | 0.3±NR | NR | 54±16 |
| Placebo | 35 | 70±9.7 | 35 | NR | 3 | 1.2±NR | NR | 121±48 | |||
|
| |||||||||||
| Khan et al (2017) | TURP | RCT | TXA | 35 | 65 | 35 | NR | NR | 1.3±NR | 1.0±NR | 49±NR |
| No TXA | 35 | 62 | 35 | NR | NR | 1.3±NR | 1.0±NR | 49±NR | |||
|
| |||||||||||
| Kumsar et al (2011) | TURP | RCT | TXA | 20 | 67 | 20 | NR | NR | 0.7 ± NR | 3.0 ± NR | 47±NR |
| No TXA | 20 | 65 | 20 | NR | NR | 1.0±NR | 3.0±NR | 64±NR | |||
|
| |||||||||||
| Meng et al (2019) | TURP | RCT | TXA | 30 | 71±5.4 | 30 | 102±11 | NR | 1.4±NR | 15.9±5.2 | 102±8.9 |
| Placebo | 30 | 71±8.5 | 30 | 304±25 | NR | 2. ±NR | 13.9±3.9 | 90±5.2 | |||
|
| |||||||||||
| Mirmansouri et al (2016) | TURP | RCT | TXA | 40 | NR | 40 | NR | 4 | NR | NR | NR |
| No TXA | 40 | NR | 40 | NR | 12 | NR | NR | NR | |||
|
| |||||||||||
| Pravin et al (2016) | TURP | RCT | TXA | 40 | 57±6.1 | 40 | 12±8.5 | 1.3±1.3 | NR | NR | |
| No TXA | 40 | 57±5.4 | 40 | 141± | 12 | 1.1±0.2 | NR | NR | |||
|
| |||||||||||
| Rani et al (2018) | TURP | RCT | TXA | 30 | 67±5.3 | 30 | 145±13 | 0 | 0.8± 0.4 | 3.0±NR | 50±5.3 |
| Placebo | 30 | 64±4.7 | 30 | 198±18 | 0 | 1.5±0.4 | 3.0±NR | 50±4.2 | |||
|
| |||||||||||
| Rannikko et al (2004) | TURP | RCT | TXA | 70 | 71±NR | 70 | 128±NR | 6 | 1.2±NR | 3.0±NR | 36±NR |
| No TXA | 66 | 68±NR | 66 | 250±NR | 5 | 1.7±NR | 3.0±NR | 48±NR | |||
|
| |||||||||||
| Vezhaventhan et al (2018) | TURP | Prospective cohort | TXA | 50 | NR | 50 | NR | 1 | NR | NR | NR |
| No TXA | 50 | NR | 50 | NR | 1 | NR | NR | NR | |||
EBL: estimated blood loss; NR: not recorded; PCNL: percutaneous nephrolithotomy; RC: radical cystectomy; RCT: randomized controlled trial; RP: radical prostatectomy; SDL: standard deviation; TURP: transurethral resection of prostate; TXA: tranexamic acid.
Tranexamic acid characteristics
The methods of TXA administration, including the route, dosage, and timing, varied considerably between studies and types of procedure (Table 2). The most common route of administration was intravenous injection alone, which was used in 17 studies. Administration involving oral TXA alone was used in only one study. In three studies, initial preoperative administration of TXA was via the intravenous route and subsequent TXA administration was oral. Four articles involved local administration of TXA; Pourfakhr et al29 sprayed TXA dissolved in normal saline directly onto the surgical site, while three studies included TXA in the irrigation fluid.
Table 2.
Tranexamic acid administration characteristics of included studies
| Study details | TXA characteristics | ||||
|---|---|---|---|---|---|
| Author | Year | Surgery | Study design | Dose | Route |
| Alfredo et al | 2020 | PCNL | RCT | 1g preop | IV |
| Bansal et al | 2017 | PCNL | RCT | 1g in irrigation fluid intraop | Irrigation |
| Barbilian et al | 2017 | PCNL | Retrospective Cohort | 1g intraop, 1g 12h postop | IV |
| Cauni et al | 2017 | PCNL | Retrospective Cohort | 1g intraop, 1g 12h postop | IV |
| Iskakov et al | 2016 | PCNL | Retrospective Cohort | 10 mL preop | IV |
| Jhanwar et al | 2016 | PCNL | Retrospective Cohort | 1g preop | IV |
| Kumar et al | 2013 | PCNL | RCT | 1g preop, 5 00mg q8h x3 doses | IV, then oral |
| Mohammadi et al | 2019 | PCNL | RCT | 1g preop, 1g q8h x48h | IV, then oral |
| Mohammadi Sichani et al | 2018 | PCNL | Prospective Cohort | 1g preop, 1g 12h postop | IV |
| Prakash et al | 2017 | PCNL | RCT | 1g preop | IV |
| Rashid et a | 2018 | PCNL | RCT | 1g preop | IV |
| Siddiq et al | 2017 | PCNL | RCT | 1g over 12 hours, 1g orally x 7 days | IV, then oral |
| Zaid et al | 2016 | RC | Retrospective Cohort | 10 mg/kg preop, 2 mg/kg/h intraop | IV |
| Balik et al | 2020 | RP | RCT | 1.5 g preop | IV |
| Crescenti et al | 2011 | RP | RCT | 500 mg preop, 250 mg/h intraop | IV |
| Pourfakhr et al | 2016 | RP | RCT | 500 mg preop | Spray |
| Abdullah et al | 2012 | TURP | RCT | 500 mg in irrigation fluid intraop | Irrigation |
| Karkhanei et al | 2020 | TURP | RCT | 15 mg/kg preop, 1 mg/kg/h intraop and until 5h postop | IV |
| Khan et al | 2017 | TURP | RCT | 1g preop | IV |
| Kumsar et al | 2011 | TURP | RCT | 10 mg/kg preop | IV |
| Meng et al | 2019 | TURP | RCT | 1g preop | IV |
| Mirmansouri et al | 2016 | TURP | RCT | 15 mg/kg preop, 1 mg/kg/h intraop and until 5h postop | IV |
| Pravin et al | 2016 | TURP | RCT | 500 mg preop, 500 mg immediately postop | IV |
| Rani et al | 2018 | TURP | RCT | 15–30 mg/kg preop | Irrigation |
| Rannikko et al | 2004 | TURP | RCT | 2 g preop, 2 g TID x 2 days | Oral |
| Vezhaventhan et al | 2018 | TURP | Prospective Cohort | 10 mg/kg preop, 10 mg/kg q8h x 2 doses | IV |
IV: intravenous; PCNL: percutaneous nephrolithotomy: RC: radical cystectomy; RCT: randomized controlled trial; RP: radical prostatectomy; TURP: transurethral resection of prostate.
In 19 studies, all patients received the same dose of TXA, ranging from 0.5–1.5 g, while five used a weight-based dosing regimen, ranging from 10–15 mg/kg intravenously; similarly, Rani et al administered 15–30 mg/ kg of TXA dissolved in irrigation fluid.33 Three papers administered an IV TXA infusion intraoperatively, at rates ranging from 1–2 mg/kg/h.
In regard to the timing of TXA administration, 10 studies involved a single intravenous administration of TXA immediately prior to surgery, with the dose ranging from 1–1.5 g. Twelve studies included an initial loading dose of TXA given preoperatively, with subsequent maintenance doses being given throughout surgery and up to one week postoperatively.
Blood loss and transfusion rate
Overall, TXA use was associated with decreased EBL (MD −102.59 mL, 95% CI −157.77 to −47.40, p<0.00001, I2 = 99%) (Figure 2A) and hemoglobin decrease (MD −0.48 g/dL, 95% CI −0.80 to −0.16, p<0.00001, I2=96%) (Figure 2B) in patients undergoing urological surgery. Subgroup analysis demonstrated that, in PCNL, the use of TXA was associated with decreased EBL (MD −93.37 mL, 95% CI −157.78 to −28.96, p<0.00001, I2=88%) and hemoglobin drop (MD −0.077 g/dL, 95% CI −1.20 to −0.34, p<0.00001, I2=98%). In contrast, patients receiving TXA and undergoing TURP also demonstrated reduced blood loss (MD −94.53 mL, 95% CI −182.37 to −6.68, p<0.00001, I2=100%) but showed no difference in hemoglobin decrease (MD −0.022 g/dL, 95% CI −0.74 to 0.30, p=0.0002, I2=88%).
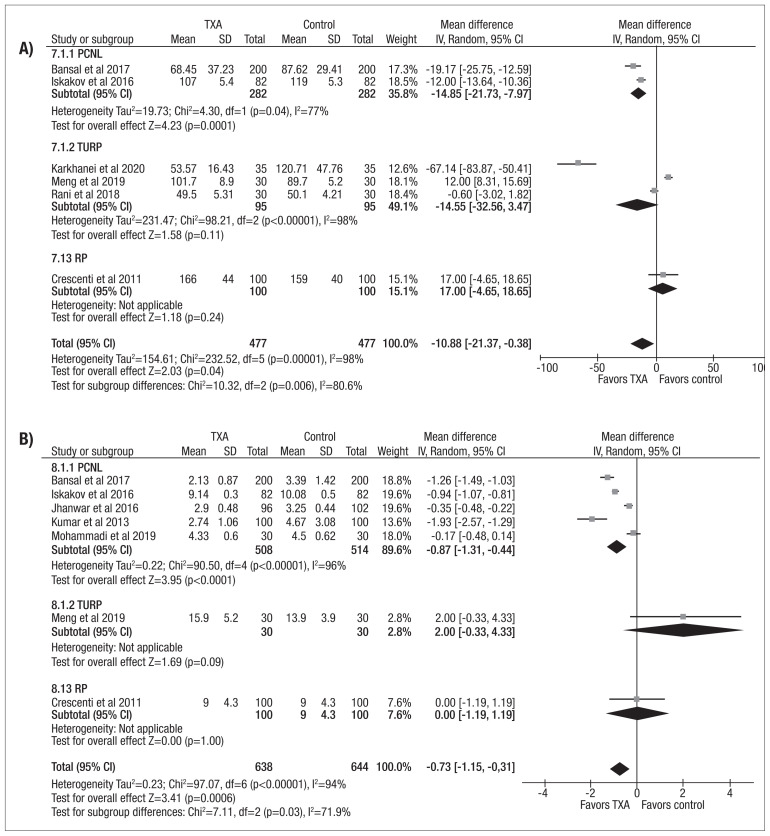
Figure 2.
Forest plot comparing (A) estimated blood loss, and (B) decrease in hemoglobin between patients undergoing urological surgery who did or did not receive tranexamic acid. CI: confidence interval; PCNL: percutaneous nephrolithotomy; RP: radical prostatectomy; TURP: transurethral resection of prostate.
TXA administration during urological surgery was also associated with reduced risk of requiring transfusion (RR 0.46, 95% CI 0.36–0.59, p=0.30, I2=14%) (Figure 2C). In our subgroup analysis, this finding was preserved in PCNL (RR 0.31, 95% CI 0.21–0.46, p=0.88, I2=0%) but not in TURP (RR 0.56, 95% CI 0.25–1.25, p=0.33, I2=13%) or RP (RR 0.40, 95% CI 0.08–2.04, p=0.18, I2=44%).
Figure 2C.
Forest plot comparing transfusion rate between patients undergoing urological surgery who did or did not receive tranexamic acid. CI: confidence interval; PCNL: percutaneous nephrolithotomy; RP: radical prostatectomy; TURP: transurethral resection of prostate.
The most common method of TXA administration was at least 1 g IV, which was the regimen used in 14 of our included papers. In order to investigate whether differences in administration method may have impacted our results, we performed a sensitivity analysis by removing all papers that did not use this regimen from our meta-analysis. We found that the association between TXA administration and decreased EBL (MD −131.5 mL, 95% CI −211.61 to −51.40, p<0.00001, I2=93%), reduced hemoglobin decrease (MD −0.52 g/dL, 95% CI −0.67 to −0.37, p<0.09, I2=58%), and decreased transfusion risk (RR 0.34, 95% CI 0.21–0.56, p<0.35, I2=11%) were preserved.
Perioperative outcomes
The use of TXA during urological surgery was associated with shorter operative duration (MD −10.88 min, 95% CI −21.37 to −0.38, p<0.00001, I2=98%) (Figure 3A). When assessing surgical procedures individually, this finding remained true for PCNL (MD −14.85 min, 95% CI −21.73 to −7.97, p<0.04, I2=77%) but not for TURP (MD −14.55 min, 95% CI −32.56 to 3.47, p<0.00001, I2=98%). Length of stay following urological surgery was also shorter in the by TXA group (MD −0.73 days, 95% CI −1.15 to −0.31, p<0.00001, I2=94%) (Figure 3B). This finding was again preserved in the PCNL subgroup (MD −1.05 days, 95% CI −1.53 to −0.56, p<0.00001, I2=96%).
Figure 3.
Forest plot comparing (A) operative duration and (B) length of hospital stay between patients undergoing urological surgery who did or did not receive tranexamic acid (TXA). CI: confidence interval; PCNL: percutaneous nephrolithotomy; RP: radical prostatectomy; TURP: transurethral resection of prostate.
Thromboembolic events and other complications
Ten studies described rates of thrombotic adverse events in patients undergoing urological surgery. In seven studies, there were no thrombotic adverse events reported in either group. Meta-analysis of the three studies that reported >0 thrombotic adverse events in either group found the risk of VTE was not significantly different in the TXA group compared to patients receiving placebo (RR 0.86, 95% CI 0.31–2.41, p=0.31, I2=14%) (Figure 4).
Figure 4.
Forest plot comparing the rate of venous thromboembolism between patients undergoing urological surgery who did or did not receive tranexamic acid. CI: confidence interval.
When examining the pooled complication rate, including VTE, we found that TXA use during urological surgery was associated with fewer complications (RR 0.66, 95% CI 0.54–0.79, p=0.34, I2=11%) (Supplementary Figure 3; available at cuaj.ca). When assessing the complication rate by type of procedure, this finding was consistent in PCNL (RR 0.61, 95% CI 0.46–0.82, p=0.10, I2=51%) but not in RP (RR 0.55, 95% CI 0.21–1.48, p=0.62, I2=0%).
DISCUSSION
The use of TXA to limit perioperative bleeding has been explored in numerous surgical specialties. A meta-analysis by Ker et al found that the use of TXA reduced blood loss by an average of 34% among surgeries of all specialties, including urology; in addition, while the method of administration varied between studies, these variations in technique did not significantly impact blood loss.2 Another meta-analysis by the same author found that the use of TXA did not impact the rates of postoperative thrombotic events, including myocardial infarction, stroke, deep vein thrombosis, or pulmonary embolism.48
Our systematic review and meta-analysis found that the use of TXA was associated with reduced EBL, hemoglobin decrease, and transfusion rates. Furthermore, TXA administration was associated with decreased operative times and shorter hospital stay. TXA administration in PCNL was associated with improved EBL, hemoglobin drop, transfusion rate, operative time, and length of stay. In contrast, TXA use in TURP was only associated with improved EBL, with transfusion rates, hemoglobin drop, operative times, and length of stay being similar between the TXA and control groups. This discrepancy is likely due to TURP being associated with a lower baseline risk of bleeding and complications when compared to PCNL.
Excessive bleeding in PCNL most often results from injury to the renal parenchyma or perinephric vessels, though more rarely, it can result from injury to nearby organs, such as the spleen and liver.49,50 Previously published studies assessing the bleeding risk associated with PCNL have reported transfusion rates ranging widely from <1–55%.51,52 Indeed, Rosette et al found that bleeding requiring transfusion was the most common complication of PCNL.53 As a result, the application of TXA specifically in PCNL may improve surgical outcomes, both by reducing blood loss and decreasing operative time, which is a known risk factor for excessive bleeding during PCNL.51
By comparison, in modern transurethral prostatic surgery, the risk of bleeding requiring transfusion is much lower at 0.4–3.8%.54,55 Rates of postoperative bleeding and transfusion associated with TURP have decreased significantly over the last several decades, with more recent studies showing decreased rates of postoperative bleeding and transfusion.54,55 A number of different factors, including preoperative administration of 5-alpha reductase inhibitors, changes to resection technique, and the advent of bipolar cautery, may be contributing to this improvement over time. In addition, the increased adoption of newer transurethral procedures for prostate resection, such as Greenlight photovaporization and holmium laser enucleation, have also improved blood loss associated transurethral prostatic procedures.56–59 Despite this, the utility of TXA in transurethral prostate surgery should not be minimized, particularly in low-resources settings, where the specialized equipment and training required for these newer procedures may not be readily available.60
Our finding suggest TXA can help reduce costs associated with urological surgery, particularly in PCNL. Excessive postoperative bleeding can place heavy financial burdens on healthcare systems due to the increased use of blood products, prolonged hospital stay, need for further procedures, and management of complications associated with hemorrhage and transfusions.61,62 One major advantage of TXA is its relative cost-effectiveness; this has been best demonstrated in orthopedic surgery, where multiple studies have demonstrated that the use of TXA was associated with reduced healthcare costs.63–67 A similar economic benefit has been demonstrated in other surgical specialties, as well as in trauma medicine.68–70 This financial advantage may be more pronounced in low-resource environments, where the costs associated with blood transfusion can be significantly greater than in developed countries, both due to decreased availability of blood products and the higher risk of bloodborne infections.71
We also found that rates of VTE did not increase significantly with TXA administration. This aligns with previous studies, which have demonstrated that TXA administration in prostate surgery did not increase the risk of VTE.72 While this may reflect the relatively benign safety profile of low-dose TXA, considering that six included studies reported that there were no VTE in either group, one potential confounder is the low baseline rate of VTE associated with urological surgery.
Limitations
Our study is not without its limitations. As there was also only a single paper assessing the use of TXA in reducing bleeding in RC, we were unable to compare outcomes of TXA administration in RC, though we were able to identify at least one RCT in progress examining the utility of TXA in preventing blood loss during RC.73 In addition, only three studies examining TXA administration in RP were able to be included in our systematic review. There is a clear lack of studies examining the use of TXA to limit bleeding in high-risk urological surgeries and further prospective and controlled studies are required to better assess the role TXA can play in these types of procedures.
Another limitation was that the route, dosage, and timing of TXA administration differed significantly between papers, making it unclear how these differences might affect surgical outcomes; however, the majority of included studies involved the pre- or intraoperative IV administration of at least 1g of TXA. Given that pharmacokinetic studies suggest that a 1 g dose of IV TXA provides an adequate plasma concentration for inhibition of fibrinolysis for 5–6 hours, the overall effect of reducing bleeding may not be substantially influenced by the differences in administration among our included studies.74 Indeed, our meta-analysis found that the use of TXA improved blood loss in both TURP and PCNL regardless of these variations. Similarly, sensitivity analysis found that the improvement in overall EBL, hemoglobin drop, and transfusion risk associated with TXA was preserved when including only studies using an IV dose of 1 g or more.
Previous studies have demonstrated that TXA use results in reduced blood loss regardless of route in the setting of trauma, postpartum hemorrhage, and orthopedic surgery.75–78 Additionally, multiple studies examining the use of TXA in total hip arthroplasty found no difference in the risk of thrombotic adverse events when comparing oral to IV routes of TXA administration.79,80 This suggests that, while further investigation is needed into the optimal strategy of TXA administration, the use of TXA is relatively safe and effective regardless of dosing strategy or route of administration. Nevertheless, it would be interesting to compare differences in outcomes with differing modalities of TXA administration, such as the oral route or via the irrigation fluid.
In addition, many of the comparisons in our meta-analysis showed high heterogeneity, suggesting a high degree of variability between studies. Of our outcomes, only transfusion rate and VTE incidence demonstrated low heterogeneity. Given the inherent differences between different urological procedures, it is difficult to compare outcomes between them. It is clear that further studies are needed to elucidate the impact of TXA use on bleeding risk, transfusion rates, and perioperative outcomes.
CONCLUSIONS
Our systematic review and meta-analysis found that TXA administration was associated with decreased blood loss, transfusion rate, length of stay, and operative duration. TXA was not associated with an increased risk of VTE, further suggesting that TXA is a cost-effective medication for management of surgical bleeding. Further comparative studies are required to assess the utility of TXA in reducing blood loss and improving perioperative outcomes in urological surgery. Future studies that explore the risk of VTE and economic impact associated with TXA administration will also help further elucidate the role of TXA in urological surgery.
Visit https://www.cua.org/UROpedia to complete the multiplechoice questionnaire associated with this article. This program is an Accredited Self-Assessment Program (Section 3) as defined by the Maintenance of Certification Program of The Royal College of Physicians & Surgeons of Canada, and approved by the Canadian Urological Association. You may claim a maximum of 1 hour of credit.
Supplementary Information
Footnotes
Appendix avaialble at cuaj.ca
COMPETING INTERESTS: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
REFERENCES
- 1.Cai J, Ribkoff J, Olson S, et al. The many roles of tranexamic acid: An overview of the clinical indications for TXA in medical and surgical patients. Eur J Haematol. 2020;104:79–87. doi: 10.1111/ejh.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100:1271–9. doi: 10.1002/bjs.9193. [DOI] [PubMed] [Google Scholar]
- 3.Reed MR, Woolley LT. Uses of tranexamic acid. Contin Educ Anaesth Crit Care Pain. 2015;15:32–7. doi: 10.1093/bjaceaccp/mku009. [DOI] [Google Scholar]
- 4.Turan A, Yang D, Bonilla A, et al. Morbidity and mortality after massive transfusion in patients undergoing non-cardiac surgery. Can J Anesth Can Anesth. 2013;60:761–70. doi: 10.1007/s12630-013-9937-3. [DOI] [PubMed] [Google Scholar]
- 5.Negi G, Gaur DS, Kaur R. Blood transfusion safety: A study of adverse reactions at the blood bank of a tertiary care center. Adv Biomed Res. 2015;4:237. doi: 10.4103/2277-9175.168604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean CL, Wade J, Roback JD. Transfusion-transmitted infections: An update on product screening, diagnostic techniques, and the path ahead. J Clin Microbiol. 2018;56:e00352–18. doi: 10.1128/JCM.00352-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scornik JC, Bromberg JS, Norman DJ, et al. An update on the impact of pre-transplant transfusions and allosensitization on time to renal transplant and on allograft survival. BMC Nephrol. 2013;14:217. doi: 10.1186/1471-2369-14-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng W, Jerath A, Wąsowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intens Ther. 2015;47:339–50. doi: 10.5603/AIT.a2015.0011. [DOI] [PubMed] [Google Scholar]
- 9.Benipal S, Santamarina JL, Vo L, et al. Mortality and thrombosis in injured adults receiving tranexamic acid in the Post-CRASH-2 Era. West J Emerg Med. 2019;20:443–53. doi: 10.5811/westjem.2019.4.41698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers SP, Kutcher ME, Rosengart MR, et al. Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J Trauma Acute Care Surg. 2019;86:20–7. doi: 10.1097/TA.0000000000002061. [DOI] [PubMed] [Google Scholar]
- 11.Anonymous. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet. 2019;394:1713–23. doi: 10.1016/S0140-6736(19)32233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.July J, Pranata R. Tranexamic acid is associated with reduced mortality, hemorrhagic expansion, and vascular occlusive events in traumatic brain injury — meta-analysis of randomized controlled trials. BMC Neurol. 2020;20:119. doi: 10.1186/s12883-020-01694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng S, Wang W, Wei Q, et al. Effect of tranexamic acid in patients with traumatic brain injury: A systematic review and meta-analysis. World Neurosurg. 2019;123:128–35. doi: 10.1016/j.wneu.2018.11.214. [DOI] [PubMed] [Google Scholar]
- 14.Chornenki NLJ, Um KJ, Mendoza PA, et al. Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: A systematic review and meta-analysis. Thromb Res. 2019;179:81–6. doi: 10.1016/j.thromres.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Devereaux PJ, Marcucci M, Painter TW, et al. Tranexamic acid in patients undergoing noncardiac surgery. N Engl J Med. 2022;386:1986–97. doi: 10.1056/NEJMoa2201171. [DOI] [PubMed] [Google Scholar]
- 16.Montroy J, Fergusson NA, Hutton B, et al. The safety and efficacy of lysine analogues in cancer patients: A systematic review and meta-analysis. Transfus Med Rev. 2017;31:141–8. doi: 10.1016/j.tmrv.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Fowler H, Law J, Tham SM, et al. Impact on blood loss and transfusion rates following administration of tranexamic acid in major oncological abdominal and pelvic surgery: A systematic review and meta-analysis. J Surg Oncol. 2022;126:609–21. doi: 10.1002/jso.26900. [DOI] [PubMed] [Google Scholar]
- 18.Nettleton J, Adimonye A, Manley J, et al. The use of peri-operative tranexamic acid and its potential applications to urological surgery. Open Urol Nephrol J. 2018;11 doi: 10.2174/1874303X01811010079. [DOI] [Google Scholar]
- 19.Tikkinen KAO, Craigie S, Agarwal A, et al. Procedure-specific risks of thrombosis and bleeding in urological non-cancer surgery: Systematic review and meta-analysis. Eur Urol. 2018;73:236–41. doi: 10.1016/j.eururo.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLOS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ. 2019. p. 4898. [DOI] [PubMed]
- 22.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 23.Siddiq A, Khalid S, Mithani H, et al. Preventing excessive blood loss during percutaneous nephrolithotomy by using tranexamic acid: A double-blinded, prospective, randomized controlled trial. J Urol Surg. 2017:195–201. doi: 10.4274/jus.1589. [DOI] [Google Scholar]
- 24.Crescenti A, Borghi G, Bignami E, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: Double-blind, randomized, placebo-controlled trial. BMJ. 2011;343:d5701. doi: 10.1136/bmj.d5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng QQ, Pan N, Xiong JY, et al. Tranexamic acid is beneficial for reducing perioperative blood loss in transurethral resection of the prostate. Exp Ther Med. 2019;17:943–7. doi: 10.3892/etm.2018.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammadi Sichani M, Kazemi R, Nouri-Mahdavi K, et al. Re-evaluation of the efficacy of tranexamic acid in reducing blood loss in percutaneous nephrolithotomy: A randomized clinical trial. Minerva Urol E Nefrol Ital J Urol Nephrol. 2019;71:55–62. doi: 10.23736/S0393-2249.18.03151-X. [DOI] [PubMed] [Google Scholar]
- 27.Alfredo CC, Vicentini FC, Monga M, et al. Pd01–04 impact of intraoperative use of tranexamic acid in patients with complex kidney stones undergoing percutaneous nephrolithotomy: Prospective, randomized, double-blind, placebo controlled trial. J Urol. 2020;203:e61–e61. doi: 10.1097/JU.0000000000000821.04. [DOI] [Google Scholar]
- 28.Karkhanei B, Musavi-Bahar SH, Bayat M, et al. Safety and efficacy of intraoperative administration of intravenous tranexamic acid in transurethral resection of prostate: A double-blind, randomized, placebo-controlled trial. J Clin Urol. 2020;13:125–31. doi: 10.1177/2051415819855887. [DOI] [Google Scholar]
- 29.Pourfakhr P, Gatavi E, Gooran S, et al. Local administration of tranexamic acid during prostatectomy surgery: Effects on reducing the amount of bleeding. Nephro Urol Mon. 2016;8:e40409. doi: 10.5812/numonthly.40409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Randhawa MS, Ganesamoni R, et al. Tranexamic acid reduces blood loss during percutaneous nephrolithotomy: A prospective, randomized controlled study. J Urol. 2013;189:1757–61. doi: 10.1016/j.juro.2012.10.115. [DOI] [PubMed] [Google Scholar]
- 31.Rannikko A, Pétas A, Taari K. Tranexamic acid in control of primary hemorrhage during transurethral prostatectomy. Urology. 2004;64:955–8. doi: 10.1016/j.urology.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Bansal A, Arora A. A double-blind, placebo-controlled randomized clinical trial to evaluate the efficacy of tranexamic acid in irrigant solution on blood loss during percutaneous nephrolithotomy: A pilot study from tertiary care center of North India. World J Urol. 2017;35:1233–40. doi: 10.1007/s00345-016-1980-6. [DOI] [PubMed] [Google Scholar]
- 33.Rani R, Mittal S, Choubey SK, et al. Use of tranexemic acid as a topical agent to reduce bleeding during TURP: A novel technique. Int J Sci Res. 2018;7:66–8. [Google Scholar]
- 34.Balík M, Košina J, Hušek P, et al. Safety and efficacy of using tranexamic acid at the beginning of robotic-assisted radical prostatectomy in a double-blind prospective randomized pilot study. Acta Medica (Hradec Kralove) 2020;63:176–82. doi: 10.14712/18059694.2020.60. [DOI] [PubMed] [Google Scholar]
- 35.Cauni V, Mihai V, Barbilian CR, et al. 153 — the use of tranexamic acid for preventing hemorrhagic complications during percutaneous nephrolithotomy. Eur Urol Suppl. 2017;16:e2972. doi: 10.1016/S1569-9056(17)32109-7. [DOI] [Google Scholar]
- 36.Zaid HB, Yang DY, Tollefson MK, et al. Efficacy and safety of intraoperative tranexamic acid infusion for reducing blood transfusion during open radical cystectomy. Urology. 2016;92:57–62. doi: 10.1016/j.urology.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 37.Iskakov Y, Muratov T, Suchshenko A, et al. Percutaneous nephroscopic surgery: Using tranexamic acid to prevent intraoperative bleeding. Res J Pharm Biol Chem Sci. 2016;7 [Google Scholar]
- 38.Rashid AO, Ahmed HK, Ali DMK. The use of tranexamic acid in percutaneous nephrolithotomy. A randomized, controlled study (local experience) Open J Urol. 2018;08:317. doi: 10.4236/oju.2018.812035. [DOI] [Google Scholar]
- 39.Barbilian R, Cauni V, Mihai B, et al. The use of tranexamic acid for preventing bloodloss during percutaneous nephrolithotomy. Mod Med. 2017;24:4. [Google Scholar]
- 40.Jhanwar A, Jain NK, Lashkary N, et al. Does intraoperative administration of tranexamic acid increases the chance of tubeless percutaneous nephrolithotomy. Int J Med Res Prof. 2016;2:109–12. doi: 10.21276/ijmrp.2016.2.6.021. [DOI] [Google Scholar]
- 41.Vezhaventhan G, Soundarya G, Saravanan K, et al. Is tranexamic acid effective in reducing TURP related blood loss? A prospective study. Int J Curr Res Life Sci. 2018;7:2226–8. [Google Scholar]
- 42.Kumsar Ş, Dirim A, Toksöz S, et al. Tranexamic acid decreases blood loss during transurethral resection of the prostate (TURP) Cent Eur J Urol. 2011;64:156–8. doi: 10.5173/ceju.2011.03.art13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan N. Role of intravenous tranexamic acid in decreasing blood loss during transurethral resection of the prostate (TUR-P) Northwest J Med Sci. 2017 https://www.njms.pk/index.php/njms/article/view/163/112[AU: This link does not work and the reference is incomplete. Please verify] [Google Scholar]
- 44.Mirmansouri A, Farzi F, Imantalab V, et al. A survey on the effects of intravenous tranexamic acid on the amount of transfusion in patients undergoing TURP. J Guilan U of Med Serv. 2016;25:110–6. [Google Scholar]
- 45.Abdullah A, Javed A. 2022 does topical tranexamic acid reduce post-TURP hematuria: A double-blind, randomized control trial. J Urol. 2012;187:e816–e816. doi: 10.1016/j.juro.2012.02.2185. [DOI] [Google Scholar]
- 46.Prakash JVS, Balaji AR. Effect of tranexamic acid on blood loss in percutaneous nephrolithotomy. Int J Sci Res. 2017;6:1845–6. https://www.ijsr.net/archive/v6i11/ART20178358.pdf . [Google Scholar]
- 47.Pravin R, Kansal SV, Chaudhary M, et al. Comparative study of role of preoperative injection tranexamic acid in 80 cases of transurethral resection of prostate. Int J Sci Study. 2016;4:167–70. http://galaxyjeevandhara.com/index.php/ijss/article/view/2362 . [Google Scholar]
- 48.Ker K, Edwards P, Perel P, et al. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganpule AP, Vijayakumar M, Malpani A, et al. Percutaneous nephrolithotomy (PCNL) a critical review. Int J Surg. 2016;36:660–4. doi: 10.1016/j.ijsu.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 50.Lee W, Smith A, Cubelli V, et al. Complications of percutaneous nephrolithotomy. Am J Roentgenol. 1987;148:177–80. doi: 10.2214/ajr.148.1.177. [DOI] [PubMed] [Google Scholar]
- 51.Lee JK, Kim BS, Park YK. Predictive factors for bleeding during percutaneous nephrolithotomy. Korean J Urol. 2013;54:448–53. doi: 10.4111/kju.2013.54.7.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keoghane SR, Cetti RJ, Rogers AE, et al. Blood transfusion, embolization and nephrectomy after percutaneous nephrolithotomy (PCNL) BJU Int. 2013;111:628–32. doi: 10.1111/j.1464-410X.2012.11394.x. [DOI] [PubMed] [Google Scholar]
- 53.de la Rosette J, Assimos D, Desai M, et al. The clinical research office of the endourological society percutaneous nephrolithotomy global study: Indications, complications, and outcomes in 5803 patients. J Endourol. 2011;25:11–7. doi: 10.1089/end.2010.0424. [DOI] [PubMed] [Google Scholar]
- 54.Rassweiler J, Teber D, Kuntz R, et al. Complications of transurethral resection of the prostate (TURP) — incidence, management, and prevention. Eur Urol. 2006;50:969–80. doi: 10.1016/j.eururo.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 55.Wendt-Nordahl G, Bucher B, Häcker A, et al. Improvement in mortality and morbidity in transurethral resection of the prostate over 17 years in a single center. J Endourol. 2007;21:1081–8. doi: 10.1089/end.2006.0370. [DOI] [PubMed] [Google Scholar]
- 56.Welliver C, Helo S, McVary KT. Technique considerations and complication management in transurethral resection of the prostate and photoselective vaporization of the prostate. Transl Androl Urol. 2017;6:69503–703. doi: 10.21037/tau.2017.07.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fagerström T, Nyman CR, Hahn RG. Bipolar transurethral resection of the prostate causes less bleeding than the monopolar technique: A single-centre, randomized trial of 202 patients. BJU Int. 2010;105:1560–4. doi: 10.1111/j.1464-410X.2009.09052.x. [DOI] [PubMed] [Google Scholar]
- 58.Westhofen T, Schott M, Keller P, et al. Superiority of holmium laser enucleation of the prostate over transurethral resection of the prostate in a matched-pair analysis of bleeding complications under various antithrombotic regimens. J Endourol. 2021;35:328–34. doi: 10.1089/end.2020.0321. [DOI] [PubMed] [Google Scholar]
- 59.Ahyai SA, Gilling P, Kaplan SA, et al. Meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic enlargement. Eur Urol. 2010;58:384–97. doi: 10.1016/j.eururo.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Gupta NP, Anand A. Lasers are superfluous for the surgical management of benign prostatic hyperplasia in the developing world. Indian J Urol. 2009;25:413–4. doi: 10.4103/0970-1591.56190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shander A. Financial and clinical outcomes associated with surgical bleeding complications. Surgery. 2007;142:S20–5. doi: 10.1016/j.surg.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Vera-Llonch M, Hagiwara M, Oster G. Clinical and economic consequences of bleeding following major orthopedic surgery. Thromb Res. 2006;117:569–77. doi: 10.1016/j.thromres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 63.Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: A cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24:545–51. doi: 10.1007/s00590-013-1225-y. [DOI] [PubMed] [Google Scholar]
- 64.DiBlasi JF, Smith RP, Garavaglia J, et al. Comparing cost, efficacy, and safety of intravenous and topical tranexamic acid in total hip and knee arthroplasty. Am J Orthop Belle Mead NJ. 2016;45:E439–43. [PMC free article] [PubMed] [Google Scholar]
- 65.Gillette BP, Maradit Kremers H, Duncan CM, et al. Economic impact of tranexamic acid in healthy patients undergoing primary total hip and knee arthroplasty. J Arthroplasty. 2013;28:137–9. doi: 10.1016/j.arth.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 66.Demos HA, Lin ZX, Barfield WR, et al. Process improvement project using tranexamic acid is cost-effective in reducing blood loss and transfusions after total hip and total knee arthroplasty. J Arthroplasty. 2017;32:2375–80. doi: 10.1016/j.arth.2017.02.068. [DOI] [PubMed] [Google Scholar]
- 67.Drakos A, Raoulis V, Karatzios K, et al. Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing intertrochanteric fracture surgery. J Orthop Trauma. 2016;30:409–14. doi: 10.1097/BOT.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 68.Williams J, Roberts I, Shakur-Still H, et al. Cost-effectiveness analysis of tranexamic acid for the treatment of traumatic brain injury, based on the results of the CRASH-3 randomized trial: A decision modelling approach. BMJ Glob Health. 2020;5:e002716. doi: 10.1136/bmjgh-2020-002716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wokes JET, Erdmann MWH, McLean NR. The role of tranexamic acid in aesthetic plastic surgery: A survey of the British Association of Aesthetic Plastic Surgeons. Aesthet Surg J. 2021;41:244–9. doi: 10.1093/asj/sjaa149. [DOI] [PubMed] [Google Scholar]
- 70.Ehresman J, Pennington Z, Schilling A, et al. Cost-benefit analysis of tranexamic acid and blood transfusion in elective lumbar spine surgery for degenerative pathologies. J Neurosurg Spine. 2020;33:177–85. doi: 10.3171/2020.1.SPINE191464. [DOI] [PubMed] [Google Scholar]
- 71.Guerriero C, Cairns J, Jayaraman S, et al. Giving tranexamic acid to reduce surgical bleeding in sub-Saharan Africa: An economic evaluation. Cost Eff Resour Alloc. 2010;8:1. doi: 10.1186/1478-7547-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Longo MA, Cavalheiro BT, de Oliveira Filho GR. Systematic review and meta-analyses of tranexamic acid use for bleeding reduction in prostate surgery. J Clin Anesth. 2018;48:32–8. doi: 10.1016/j.jclinane.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Breau RH, Lavallée LT, Cnossen S, et al. Tranexamic acid versus placebo to prevent blood transfusion during radical cystectomy for bladder cancer (TACT): Study protocol for a randomized controlled trial. Trials. 2018;19:261. doi: 10.1186/s13063-018-2626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schutgens REG, Lisman T. Tranexamic acid is not a universal hemostatic agent. HemaSphere. 2021;5:e625. doi: 10.1097/HS9.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan X, Li B, Wang Q, et al. Comparison of 3 routes of administration of tranexamic acid on primary unilateral total knee arthroplasty: A prospective, randomized, controlled study. J Arthroplasty. 2017;32:2738–43. doi: 10.1016/j.arth.2017.03.059. [DOI] [PubMed] [Google Scholar]
- 76.Keyhani S, Esmailiejah AA, Abbasian MR, et al. Which route of tranexamic acid administration is more effective to reduce blood loss following total knee arthroplasty? Arch Bone Jt Surg. 2016;4:65–9. https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/26894222/ [PMC free article] [PubMed] [Google Scholar]
- 77.Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964. doi: 10.1002/14651858.CD012964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stansfield R, Morris D, Jesulola E. The use of tranexamic acid (TXA) for the management of hemorrhage in trauma patients in the prehospital environment: Literature review and descriptive analysis of principal themes. Shock. 2020;53:277–83. doi: 10.1097/SHK.0000000000001389. [DOI] [PubMed] [Google Scholar]
- 79.Wang N, Xiong X, Xu L, et al. Transfusions and cost-benefit of oral versus intravenous tranexamic acid in primary total hip arthroplasty. Medicine. 2019;98:e15279. doi: 10.1097/MD.0000000000015279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao G, Huang Z, Xie J, et al. The effect of oral versus intravenous tranexamic acid in reducing blood loss after primary total hip arthroplasty: A randomized clinical trial. Thromb Res. 2018;164:48–53. doi: 10.1016/j.thromres.2018.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.