Abstract
The plasmid pGT5 from the hyperthermophilic archaeon Pyrococcus abyssi replicates via the rolling circle mechanism. pGT5 encodes the replication initiator protein Rep75 that exhibits a nicking–closing (NC) activity in vitro on single-stranded oligonucleotides containing the pGT5 double-stranded origin (dso) sequence. Some mesophilic Rep proteins present site-specific DNA topoisomerase-like activity on a negatively supercoiled plasmid harbouring the dso. We report here that Rep75 also exhibits topoisomerase activity on a negatively supercoiled DNA substrate. This DNA topoisomerase-like activity is dependent on the amino acids involved in NC activity of Rep75. However, in contrast with mesophilic Rep proteins, Rep75 topoisomerase activity is not dso dependent. Moreover, although pGT5 is known to be relaxed in vivo, Rep75 was not able to act on a relaxed plasmid in vitro, whether or not it contained the dso.
INTRODUCTION
pGT5 is an archaeal plasmid known to replicate via the rolling-circle (RC) mechanism. This mode of DNA replication was first described for single-stranded DNA bacteriophages from Escherichia coli. This mechanism is also widely used by bacterial plasmids and eukaryotic viruses (1–5). The RC replicon encodes a replication initiator protein, usually named Rep, which acts as a site-specific endonuclease-ligase enzyme. The Rep protein initiates replication by nicking the plus strand of the double-stranded origin (dso). Rep protein then remains covalently linked to the 5′ extremity of the nicked strand, as the 3′-OH end is extended by the host DNA replication machinery. At the end of one replication round, Rep introduces a second single-stranded break at the dso and ligates single-stranded extremities, generating one double-stranded plasmid and one circular single-stranded form. Complementary strand synthesis of the single-stranded DNA is then initiated at the single-stranded origin (sso) by the host replication machinery.
Plasmid pGT5 was isolated from the hyperthermophilic archaeon Pyrococcus abyssi and sequenced (6). The pGT5 replication initiator protein Rep75, which has a size of 75 kDa, was identified from the sequence analysis, and the recombinant protein expressed in E.coli was purified to homogeneity (7). We have previously shown that purified Rep75 is able to recognise and cleave the pGT5 dso on single-stranded oligonucleotides. This dso was previously identified tentatively by sequence comparison (6) and is characterised by 11 nucleotides which are conserved between the pGT5 nicking site and the dso of the RC plasmid pC194. The site-specific nicking activity of Rep75 is thermophilic, with an optimum temperature of at least 105°C. After the nicking activity, Rep75, which remains covalently linked to the 5′ DNA end, can religate this extremity to the 3′-OH end of an oligonucleotide containing the dso sequence.
In addition to its nicking–closing (NC) activities, Rep75 exhibits an unusual (d)ATP-dependent nucleotidyl-terminal transferase (NTT) activity, never before described for such a protein. This NTT activity corresponds to the transfer of one adenine or one deoxyadenine nucleotide monophosphate to the 3′-OH end of the nicking site. We have demonstrated by site-directed mutagenesis that the same active site is involved in both the NTT and NC activities of Rep75. However, these two activities can be uncoupled in vitro (8). Analyses of mutated proteins has revealed two essential amino acids: tyrosine 448, which is involved in the NC activity and the covalent fixation to DNA, and arginine 451, which is involved in the closing and NTT activities.
In our previous experiments we used oligonucleotides as in vitro substrates, although they are different to the in vivo substrate. In vivo, the protein should recognise the dso located on a plasmid in order to initiate DNA replication. As a consequence of their NC activities, some Rep proteins can behave as site-specific DNA topoisomerases in vitro (9–12), i.e. they can relax a negatively supercoiled plasmid containing the dso sequence (13–16). Since such activity mimics a priori better the actual role of a Rep protein in vivo than its action on a single-stranded oligonucleotide, it was important to demonstrate such activity for Rep75 as a first step to set up an in vitro replication system for pGT5. We report here that Rep75 exhibits both nicking and topoisomerase activities on a negatively supercoiled plasmid. The topoisomerase activity occurs in a narrow range of conditions, explaining why it was not detected in our earlier studies. Effects of various amino acid substitutions demonstrate that the topoisomerase activity of Rep75 on plasmid DNA is related to its NC activity on single-stranded oligonucleotides. Surprisingly, whereas topoisomerase activities previously associated with RC Rep proteins were dependent on the plasmid dso, the topoisomerase activity of Rep75 does not require pGT5 dso. Furthermore, although plasmids from hyperthermophilic archaea are from relaxed to positively supercoiled in vivo (17,18), a relaxed plasmid is not a substrate for Rep75 in vitro, either in the presence or absence of pGT5 dso.
MATERIALS AND METHODS
Bacterial and archaeal strains
Pyrococcus abyssi strain GE5 (19) was used to isolate pGT5 plasmid. The rep75 gene was cloned in pET3b (Stratagene, La Jolla, CA) using E.coli strain DH5α (7). In vitro mutagenesis was performed in E.coli strain NM522 (Amersham Pharmacia Biotech, Uppsala, Sweden). Escherichia coli strain BL21 (DE3° LysS, Stratagene) was used to overproduce Rep75 and the mutated proteins.
Construction of recombinant plasmids
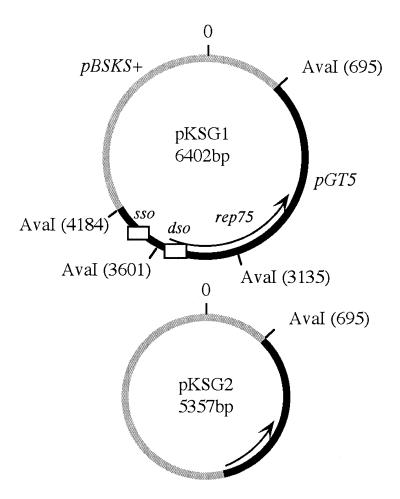
Plasmid pKSG1 (6402 bp) was constructed by ligating HinpI-cleaved plasmid pGT5 with ClaI-cleaved Bluescript pBSKS+ (Stratagene). Plasmid pKSG2 (5357 bp), deleted from a pGT5 fragment containing pGT5 dso, was constructed by AvaI partial cleavage, and the 5357-bp fragment was blunted by Klenow fill-in modification and religated (Fig. 1).
Figure 1.
pKSG1 and pKSG2 plasmidic maps. The two plasmid constructions are presented in Materials and Methods. The pBSKS+ vector is indicated by grey lines and the pGT5 insert by black lines. The rep75 gene is indicated by an arrow. Whites boxes indicate the two pGT5 origins, sso and dso.
Preparation of relaxed plasmids
Plasmids (100 ng) were incubated with 20 U (for pKSG1) and 30 U (for pKSG2) of wheatgerm topoisomerase I (Promega, Madison, WI) for 30 min at 37°C in 50 mM Tris–HCl (pH 7.5), 50 mM NaCl, 0.1 mM EDTA, 1 mM DTT and 20% glycerol.
In vitro mutagenesis
Mutagenesis experiments were performed using the Pharmacia Biotech USE Mutagenesis kit as described previously (8).
Production and purification of recombinant Rep proteins
Rep75 and the mutated proteins were expressed in E.coli and purified as described previously (7,8,20). All the mutated proteins presented the same purification profile. Briefly, the proteins were expressed in an insoluble form and partially purified by isolation of the inclusion bodies. They were then solubilized in a 6 M guanidium solution and renatured by dialysis against a 200 mM NaCl buffer. The dialysates were loaded on a P11 cellulose phosphate column (Whatman, Maidstone, UK) and the Rep proteins were eluted with a buffer containing 400 mM NaCl.
DNA topoisomerase assays
Unless otherwise stated, mixtures of Rep75 (50 ng) and DNA (100 ng), with a protein:DNA molar ratio of 25, were incubated for 10 min at 75°C in 20 µl of R buffer (50 mM HEPES–HCl, 200 mM Na-glutamate, 1 mM DTT, 1 mM EDTA, 5 mM MnCl2, 0.1% Triton X-100, 50 µg ml–1 BSA, pH 8) covered with oil. Reactions were stopped by addition of 25 mM EDTA and incubated with 15 µg of proteinase K for 30 min at 55°C. Proteolysis was stopped on ice by addition of 1% SDS loading buffer. The reaction products were run in 0.7% agarose gel in 45 mM Tris–borate, 1 mM EDTA. The gels were stained with ethidium bromide (EtBr) and photographed under UV illumination.
RESULTS
Rep75 has a topoisomerase activity on a negatively supercoiled plasmid
DNA topoisomerase activities previously detected in vitro with pure preparations of mesophilic Rep proteins from RC replicons were dependent on the presence of the homologous dso on a negatively supercoiled plasmid substrate (9–12). Purified Rep75 was thus first tested for topoisomerase activity using as substrate a hybrid pGT5/pBSK+ plasmid, pKSG1 (Fig. 1), isolated from E.coli and thus negatively supercoiled. As shown in Figure 2A, purified Rep75 was able to both nick and relax pKSG1 at 75°C. Relaxation was indicated by the time-dependent production of multiple pKSG1 topoisomers with a Gaussian distribution when the reaction products were run on agarose gel at room temperature without EtBr. In these conditions, pKSG1 plasmids relaxed at 75°C by Rep topoisomerase activity were separated from nicked plasmids since they became negatively supercoiled at room temperature (17,21). Relaxation was maximal after 30 min incubation. At this stage, and at a Rep75 concentration of 2.5 µg/ml, all pKSG1 plasmids were either relaxed or nicked. Topoisomerase activity was only observed in a narrow range of Rep75 protein concentration (Rep75:DNA molar ratio of 25–50) (Fig. 2B). At higher Rep75 concentrations, all plasmids were cleaved but no more religated, and a linear form appeared. Rep75 relaxation activity was also observed in a narrow range of temperatures around 75°C (Fig. 2C). No activity was observed below 55°C. At 85°C and above, the relaxation activity was not observed and the plasmids were only cleaved with formation of linear forms, indicating that Rep75 was not able to religate DNA. This is in agreement with our previous observation that, in the same conditions, Rep75 nicking activity has a temperature optimum of at least 105°C on dso-containing oligonucleotides, whereas its religation activity on this substrate is optimal at 75°C (7). When the temperature was increased above 85°C, no Rep75 activity could be detected on a plasmid substrate because of DNA aggregation due to the buffer composition (Fig. 2C, control sample) (22).
Figure 2.
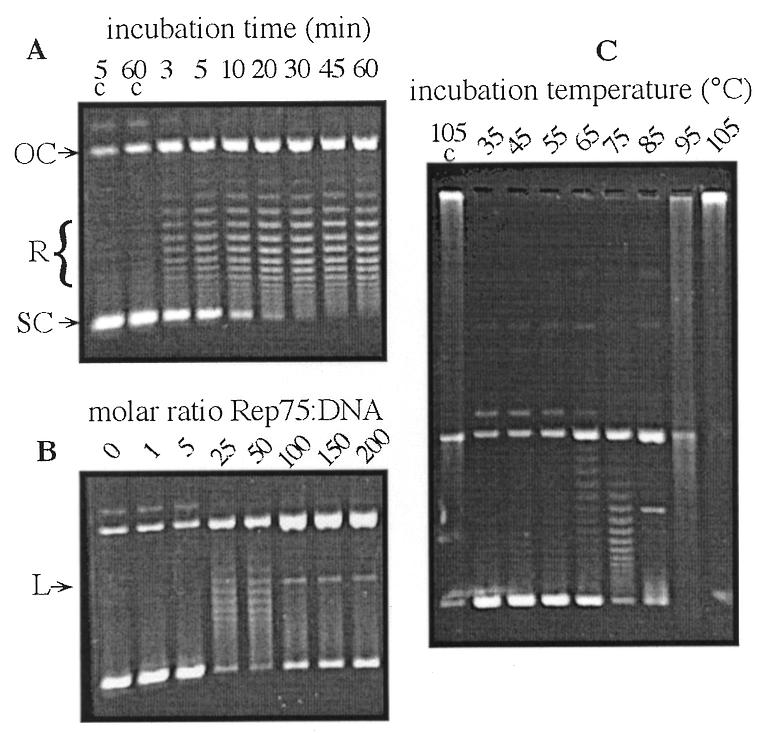
In vitro Rep75 topoisomerase-like activity on the pKSG1 plasmid. The different DNA plasmidic forms are indicated: OC, open circular plasmid; SC, negatively supercoiled plasmid; R, relaxed plasmid at 75°C; L, linear form. c, control samples without Rep75 protein. (A) Topoisomerase reaction kinetics. pKSG1 (100 ng) was incubated with Rep75 at 75°C for the indicated time. (B) Concentration dependence of the topoisomerase reaction on negatively supercoiled plasmid. pKSG1 (100 ng) was incubated with Rep75 at the ratio indicated for 10 min at 75°C. (C) Temperature dependence of Rep75 topoisomerase activity. pKSG1 (100 ng) was incubated with Rep75 for 10 min at the indicated temperature.
Rep75 activity is not dso-specific but requires negatively supercoiled DNA
To test if the topoisomerase activity of the Rep75 protein was dso-dependent, we checked its relaxation activity on the plasmid pKSG2, which has been deleted from a large pGT5 fragment containing both the sso and dso of this plasmid (Fig. 1). Suprisingly, in contrast with the results obtained with mesophilic Rep proteins, Rep75 was able to relax negatively supercoiled (S) pKSG2 with the same efficiency as pKSG1 (compare the reaction products that have been positively supercoiled by EtBr in gel B, Fig. 3). Rep75 was also able to completely relax a plasmid that does not harbour the pGT5 sequence, such as the E.coli plasmid pTZ18, in the same range of molar:DNA ratios and in the same conditions as pKSG1 and pKSG2 (data not shown). Since plasmids from hyperthermophilic archaea, including pGT5, are from relaxed to positively supercoiled at in vivo physiological temperatures (17,18), we thought that Rep75 protein might be site-specific only on a template with such topology. We thus checked the activity of Rep75 on pKSG1 and pGSK2 plasmids that were relaxed at 37°C using a eukaryotic type I DNA topoisomerase. Since nicked DNA was produced during incubation with the topoisomerase, we used preparations also containing nicked DNA for supercoiled plasmids control. As shown in Figure 3, the relaxation of negatively supercoiled plasmids by Rep75 was not inhibited by the presence of nicked DNA. The results in Figure 3B show that plasmids relaxed at 37°C and used as substrate can be discriminated from those relaxed at 75°C by Rep75 since they migrated differently both in gels with or without EtBr at room temperature. Since plasmids relaxed at 37°C become slightly positively supercoiled at 75°C, their relaxation by Rep75 at this temperature would have produced plasmid products with a topology different to those of the plasmid substrates. However, topology of relaxed pKSG1 and pGSK2 was not changed upon incubation at 75°C with Rep75 and there was no increase in the amount of open circular DNA. This indicated that Rep75 has neither relaxation nor nicking activity on slightly positively supercoiled plasmid, even when pGT5 dso is present. Nicking was also not observed at higher protein concentrations (data not shown).
Figure 3.
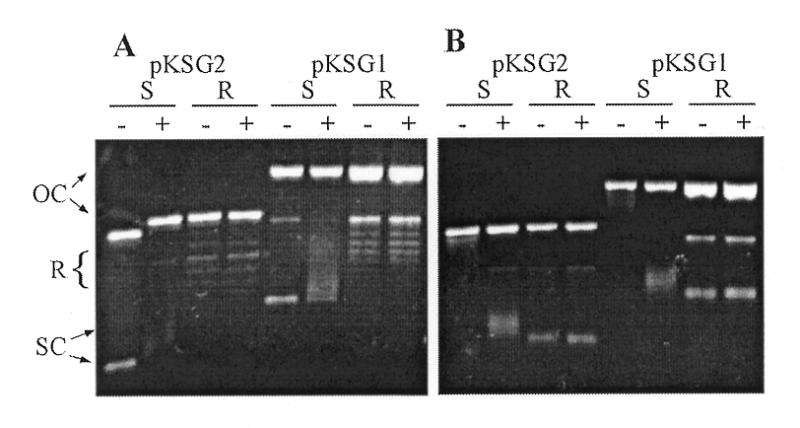
Rep75 topoisomerase activity on different substrates. An aliquot of 100 ng of plasmid pKSG1 or pKSG2, negatively supercoiled (S) or relaxed (R), were incubated with (+) or without (–) 100 ng of Rep75 (protein:DNA ratio of 50:1). The incubations were performed for 15 min at 75°C and the reaction products were loaded on an agarose gel in the absence (A) or in the presence (B) of 10 ng/ml of EtBr. Different DNA plasmidic forms are indicated: OC, open circular plasmid; SC, negatively supercoiled plasmid; R, relaxed plasmid at 75°C.
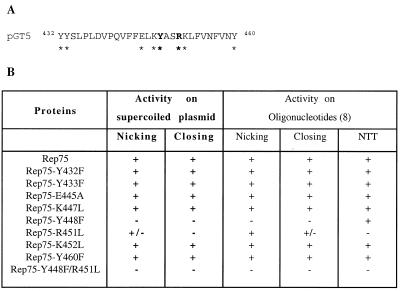
Effect of amino acid substitutions on Rep75 topoisomerase activity
The Rep75 active site was previously identified by site-directed mutagenesis, and the effects of different amino acid substitutions were studied on dso-containing oligonucleotides (8). Here we have tested the effect of the same substitutions (indicated in Fig. 4A) on the topoisomerase activity of Rep75. Only two of the eight substitutions tested, Y448F and R451L, produced a drastic effect on topoisomerase activity (Fig. 4B and C). Identical results were obtained using either pKSG1 or pKSG2 as substrates. The mutated protein Rep75-Y448F, which has no nicking activity on single-stranded DNA (8), also had no topoisomerase or nicking activity on supercoiled plasmids. On the other hand, the mutated protein Rep75-R451L, which has nearly normal nicking activity but strongly reduced closing activity on single-stranded DNA (8), had only weak nicking activity and no topoisomerase activity on supercoiled plasmids (Fig. 4C). These results demonstrate that the topoisomerase activity of Rep75 is related to its NC activity on a single-stranded template.
Figure 4.
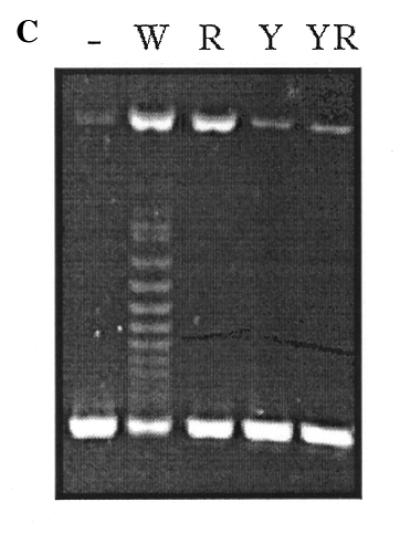
Topoisomerase activities of Rep75 mutated proteins. (A) Sequence of the Rep75 active site. The residues conserved in the active site of different RC replication initiator proteins are indicated in bold (see Fig. 5). The amino acids targeted for substitution are indicated by an asterisk. (B) Activities of Rep75 mutated proteins. The names of the proteins correspond to the mutations they harbour. For the assays performed on supercoiled plasmids, 100 ng of pKSG1 was incubated with 100 ng of the indicated protein (protein:DNA molar ratio of 50:1) for 10 min at 75°C. The oligonucleotide assays have been performed previously (8), and the results are summarised for comparison with topoisomerase activities. The appearance (+) or not (–) of nicking and religation products are indicated. +/– indicates a weak activity. NTT corresponds to the nucleotidyl terminal transferase activity. (C) Activities of three mutated proteins. The experiment was performed as previously described and the BET stained gel is presented. –, control samples without Rep75 protein. W, incubation with Rep75; R, incubation with Rep75-R451L; Y, incubation with Rep75-Y448F; YR, incubation with Rep75-R451L/Y448F.
DISCUSSION
All Rep proteins that have been previously characterised are mesophilic and related to bacteria or eukaryotic viruses. Rep75 protein encoded by the pGT5 plasmid was the first replication initiator protein identified from a hyperthermophilic archaeon and studied in vitro (7,8,20). At high temperatures, Rep75 is able to cleave and religate an oligonucleotide harbouring the dso sequence. These activities testify for the replication initiation activity that consists of opening a circular plasmid and producing a 3′-OH extremity which can be used as a primer by the host replication machinery. At the end of one replication round, Rep should cleave and religate the DNA. This capacity to both cleave and religate DNA substrate is the hallmark of DNA topoisomerases. We report here that Rep75 indeed exhibits a thermophilic topoisomerase activity in vitro when a supercoiled plasmid is used as template. This activity is observed in a narrow range of Rep75 concentrations (Rep75:DNA ratio of 25–50). At higher protein concentrations, the plasmidic products are nicked but not religated. The latter observation suggests that two molecules of Rep75 can bind and nick the two DNA strands close to each other to produce a double-stranded break in the plasmid molecule.
In contrast with previously characterised Rep proteins from mesophiles, Rep75 can nick and relax a negatively supercoiled plasmid without stringent specificity. The dso of some mesophilic Rep proteins contain two sites, explaining their specificity (23,24). These proteins first recognise a binding site (bind) on double-stranded DNA. Rep–DNA interaction at the bind site induces the opening of the double helix, such that Rep proteins cleave single-stranded DNA at the nicking site (nick). The local unwinding of the DNA by these Rep proteins requires negative supercoiling of their substrate. This model probably also generally applies for Rep75. We have shown that Rep75 binds specifically to the right side of its nick site on double-stranded DNA whereas it binds to the left of its nick site without stringent specificity on single-stranded DNA (20). Thus, the non-specific nicking activity of Rep75 reported here possibly occurs on denaturation bubbles that should be present in negatively supercoiled plasmid at high temperatures. One can suppose that the unwinding potential of negatively supercoiled plasmid is not enough at low temperatures to create non-specific cleavage sites for mesophilic Rep proteins.
Analysis of the Rep75 active site by site-directed mutagenesis indicates that its topoisomerase activity is related to the site-specific NC activity previously detected in vitro on single-stranded dso-containing oligonucleotides, and that both activities are performed by the same basic mechanism. We concluded from our experiments with oligonucleotides that tyrosine 448 was implicated in the transesterification reaction and the covalent fixation between Rep75 and the DNA 5′ extremity, and arginine 451 in the stabilisation of the 3′-OH DNA substrate during the religation step (8). The effect of mutations Y448F and R431L on the topoisomerase activity of Rep75 are in agreement with these conclusions. We noticed that a tyrosine homologue of Y448 is conserved in the active sites of all Rep proteins from RC replicons known so far, including the Rep protein of bacteriophage M13 (Fig. 5; 25). Interestingly, we also found that a basic residue corresponding to pGT5 R451 is conserved two positions downstream of this tyrosine in the active sites of many unrelated RC replicons from bacteria, eukaryotes and archaea (Fig. 4). The corresponding lysine in the Rep protein from the Bean Golden Mosaic Geminivirus (BGMG) was shown to be essential for in vivo viral replication (26). We thus propose that a basic residue two positions downstream of the active tyrosine is essential for the activity of all these Rep proteins and for DNA replication of the corresponding replicons.
Figure 5.
Alignment of Rep75 putative active site with those of different RC replication initiator proteins. The residues conserved between all the proteins are indicated in bold characters. The names indicated refer to the plasmid or the phage name: BGMV, Bean Golden Mosaic Geminivirus; MVM, Minute Virus of Mouse.
Topoisomerase activity of mesophilic Rep proteins depends on the plasmid topological state, for example, RepA performs its topoisomerase activity on pMV158 only in a narrow range of supercoiling (13). Thus, since plasmids from hyperthermophilic archaea are not negatively supercoiled but are from relaxed to positively supercoiled (17,18), Rep75 activities might have been dso-specific on a relaxed or positively supercoiled plasmid. However, this is not the case since Rep75 has neither topoisomerase nor nicking activity on relaxed plasmids. This is somewhat paradoxical, since Rep75 activities are thus dependent in vitro on a topological state (negative supercoiling) different to the topological state presumed to be favoured in vivo in hyperthermophilic archaea. However, a similar result was obtained by Hethke et al. (27) when studying in vitro transcription of a specific archaeal promoter at high temperatures using purified RNA polymerase and transcription factors from Pyrococcus furiosus. In that case, negatively supercoiled DNA is the preferred template compared with relaxed DNA. Our result thus suggests that negatively supercoiled DNA could be also the preferred template for DNA replication in vitro in Pyrococcus.
There are two possible non-exclusive explanations for the dependence of Rep75 on negatively supercoiled DNA. First, DNA from hyperthermophilic archaea might actually be negatively supercoiled in vivo by interaction with DNA binding proteins. Such negative supercoiling could be distributed along the whole chromosome or restricted to specific regions and possibly submitted to regulatory mechanisms. Interestingly, plasmid DNA can be either positively or negatively supercoiled around archaeal histones in vitro, depending on the salt concentration (28). It is thus possible that Rep75 interacts in vivo with histone proteins to produce negative supercoiling in the region of pGT5 containing the dso. In the case of the mesophilic RC plasmid pKYM, an interaction of the HU protein with the origin was shown to improve DNA replication (29). Secondly, different protein factors (such as site-specific DNA helicases) might be missing in our topoisomerase assay or in the in vitro transcription system that make them operational on relaxed or positively supercoiled DNA. The nicking activity of Rep75 on plasmid DNA demonstrated here might be helpful to identify such specificity factors by in vitro complementation. Recently, several proteins that could be involved in the elongation step of archaeal DNA replication (e.g. DNA polymerases, PCNA and RFC factors) have been isolated from P.furiosus (30). Combination of these proteins with Rep75 and specificity factors should help to isolate additional archaeal replication proteins and ultimately produce an in vitro hyperthermophilic DNA replication system for pGT5.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Emily Umpleby for technical assistance. This work was supported by grants from the Association pour la Recherche contre le Cancer and the European Community Program Cell Factory (BIO4-CT 96-0488).
REFERENCES
- 1.del Solar G., Giraldo,R., Ruiz-Echevarria,M.J., Espinosa,M. and Diaz Orejas,R. (1998) Microbiol. Mol. Biol. Rev., 62, 434–464. [DOI] [PMC free article] [PubMed]
- 2.Khan S.A. (1997) Microbiol. Mol. Biol. Rev., 61, 442–455. [DOI] [PMC free article] [PubMed]
- 3.Espinosa M., del Solar,G., Rojo,F. and Alonso,J.C. (1995) FEMS Microbiol. Lett., 130, 111–120. [DOI] [PubMed]
- 4.Koonin E.V. and Ilynia,T.V. (1992) J. Gen. Virol., 73, 2763–2766. [DOI] [PubMed]
- 5.Gruss A. and Ehrlich,S.D. (1989) Microbiol. Rev., 53, 231–241. [DOI] [PMC free article] [PubMed]
- 6.Erauso G., Marsin,S., Benbouzid-Rollet,N., Baucher,M.F., Barbeyron,T., Zivanovic,Y., Prieur,D. and Forterre,P. (1996) J. Bacteriol., 178, 3232–3237. [DOI] [PMC free article] [PubMed]
- 7.Marsin S. and Forterre,P. (1998) Mol. Microbiol., 27, 1183–1192. [DOI] [PubMed]
- 8.Marsin S. and Forterre,P. (1999) Mol. Microbiol., 33, 537–546. [DOI] [PubMed]
- 9.van Mansfeld A.D., Langeveld,S.A., Baas,P.D., Jansz,H.S., van der Marel,G.A., Veeneman,G.H. and van Boom,J.H. (1980) Nature, 288, 561–566. [DOI] [PubMed]
- 10.Meyer T.F. and Geider,K. (1979) J. Biol. Chem., 254, 12642–12646. [PubMed]
- 11.Zoch J.M., Patrick,B. and Khan,S.A. (1990) J. Biol. Chem., 265, 3484–3488. [PubMed]
- 12.Moscoso M., Eritja,R. and Espinosa,M. (1995) J. Mol. Biol., 268, 840–856. [DOI] [PubMed]
- 13.Moscoso M., del Solar,G. and Espinosa,M. (1995) J. Bacteriol., 177, 7041–7049. [DOI] [PMC free article] [PubMed]
- 14.Dempsey L.A., Birch,P. and Khan,S.A. (1992) Proc. Natl Acad. Sci. USA, 89, 3083–3087. [DOI] [PMC free article] [PubMed]
- 15.Thomas C.D., Balson,D.F. and Shaw,W.V. (1990) J. Biol. Chem., 265, 5519–5530. [PubMed]
- 16.Koepsel R.R. and Khan,S.A. (1987) Nucleic Acids Res., 15, 4085–4097. [DOI] [PMC free article] [PubMed]
- 17.Charbonnier F., Erauso,G., Barbeyron,T., Prieur,D. and Forterre,P. (1992) J. Bacteriol., 174, 6103–6108. [DOI] [PMC free article] [PubMed]
- 18.Lopez-Garcia P. and Forterre,P. (1997) Mol. Microbiol., 23, 1267–1279. [DOI] [PubMed]
- 19.Erauso G., Reysenbach,A.L., Godfroy,A., Meunier,J.R., Crump,B., Partensky,F., Baross,J.A., Marteinsson,V., Barbier,G., Pace,N.R. and Prieur,D. (1993) Arch. Microbiol., 160, 338–349.
- 20.Marsin S. and Forterre,P. (2000) Methods Enzymol., in press. [DOI] [PubMed]
- 21.Duguet M. (1993) Nucleic Acids Res., 21, 463–468. [DOI] [PMC free article] [PubMed]
- 22.Marguet E. and Forterre,P. (1998) Extremophiles, 2, 115–122. [DOI] [PubMed]
- 23.Baas P.D. and Jansz,H.S. (1988) Curr. Top. Microbiol. Immunol., 136, 31–70. [DOI] [PubMed]
- 24.Khan S.A. (1997) Microbiol. Mol. Biol. Rev., 61, 442–455. [DOI] [PMC free article] [PubMed]
- 25.Asano S., Higashitani,A. and Horiuchi,K. (1999) Nucleic Acids Res., 27, 1882–1889. [DOI] [PMC free article] [PubMed]
- 26.Hoogstraten R.A., Hanson,S.F. and Maxwell,D.P. (1991) Mol. Plant Microbe Interact., 9, 594–599. [DOI] [PubMed]
- 27.Hethke C., Bergerat,A., Hausner,W., Forterre,P. and Thomm,M. (1999) Genetics, 152, 1325–1333. [DOI] [PMC free article] [PubMed]
- 28.Musgrave D., Forterre,P. and Slezarev,A. (2000) Mol. Microbiol., 35, 341–349. [DOI] [PubMed]
- 29.Yasukawa H., Ozaki,E., Nakahama,K. and Masamune,Y. (1997) Mol. Gen. Genet., 254, 548–554. [DOI] [PubMed]
- 30.Cann I.K. and Ishino,Y. (1999) Genetics, 152, 1249–1267. [DOI] [PMC free article] [PubMed]