Abstract
Background and Objectives
Bell palsy is the third most frequent diagnosis in children with sudden-onset neurologic dysfunction. The cost-effectiveness of treating Bell palsy with prednisolone in children is unknown. We aimed to assess the cost-effectiveness of prednisolone in treating Bell palsy in children compared with placebo.
Methods
This economic evaluation was a prospectively planned secondary analysis of a double-blinded, randomized, placebo-controlled superiority trial (Bell Palsy in Children [BellPIC]) conducted from 2015 to 2020. The time horizon was 6 months since randomization. Children aged 6 months to <18 years who presented within 72 hours of onset of clinician-diagnosed Bell palsy and who completed the trial were included (N = 180). Interventions were oral prednisolone or taste-matched placebo administered for 10 days. Incremental cost-effectiveness ratio comparing prednisolone with placebo was estimated. Costs were considered from a health care sector perspective and included Bell palsy–related medication cost, doctor visits, and medical tests. Effectiveness was measured using quality-adjusted life-years (QALYs) based on Child Health Utility 9D. Nonparametric bootstrapping was performed to capture uncertainties. Prespecified subgroup analysis by age 12 to <18 years vs <12 years was conducted.
Results
The mean cost per patient was A$760 in the prednisolone group and A$693 in the placebo group over the 6-month period (difference A$66, 95% CI −A$47 to A$179). QALYs over 6 months were 0.45 in the prednisolone group and 0.44 in the placebo group (difference 0.01, 95% CI −0.01 to 0.03). The incremental cost to achieve 1 additional recovery was estimated to be A$1,577 using prednisolone compared with placebo, and cost per additional QALY gained was A$6,625 using prednisolone compared with placebo. Given a conventional willingness-to-pay threshold of A$50,000 per QALY gained (equivalent to US$35,000 or £28,000), prednisolone is very likely cost-effective (probability is 83%). Subgroup analysis suggests that this was primarily driven by the high probability of prednisolone being cost-effective in children aged 12 to <18 years (probability is 98%) and much less so for those <12 years (probability is 51%).
Discussion
This provides new evidence to stakeholders and policymakers when considering whether to make prednisolone available in treating Bell palsy in children aged 12 to <18 years.
Trial Registration Information
Australian New Zealand Clinical Trials Registry ACTRN12615000563561.
Bell palsy is the third most frequent diagnosis in children with sudden-onset neurologic dysfunction1 and the most common acute facial paralysis for people of all ages.2 In the United Kingdom, the incidence rate is more than 6 per 100,000 person-years in children younger than 14 years and more than 20 per 100,000 person-years in people aged 15–29 years.3 The symptoms of Bell palsy can affect the functioning of the face, mouth, and eyes resulting in impaired verbal communication and social interaction.4 Children with Bell palsy have been reported to be distressed, embarrassed, and treated differently because of the facial condition.4
Steroids are inexpensive and may reduce the inflammatory process, neural edema, and compression of the nerve in the facial canal.5 In adults, there is high-level evidence that steroids improve recovery in Bell palsy,6,7 and the American Academy of Otolaryngology—Head and Neck Surgery Foundation published a clinical practice guideline recommending the use of oral steroids for patients with Bell palsy aged 16 years and older within 72 hours of symptom onset.8 Whether steroids may also be appropriate for treating Bell palsy in children is unknown.9 One systematic review of 6 studies reported that the role of steroid treatment for Bell palsy in children is inconclusive.10
In line with the effectiveness evidence and regarding cost-effectiveness, treating Bell palsy with steroids has been suggested to be cost-effective in adults. One economic evaluation based on a National Institute for Health Research–commissioned trial conducted in 2004–2006 found that compared with no prednisolone, prednisolone was on average less costly and more effective (77% probability of being cost-effective at a £30,000 willingness-to-pay threshold).11 However, there is no evidence regarding the cost-effectiveness of using steroids to treat Bell palsy in children.
Prednisolone is a commonly used steroid. We recently conducted a double-blinded, randomized, placebo-controlled superiority trial (Bell Palsy in Children [BellPIC]) comparing prednisolone with placebo for the treatment of Bell palsy in children.12 At 1 month after randomization, no statistically significant difference was found in complete recovery (49% vs 57% for prednisolone compared with placebo), which is the primary outcome of the trial. Complete recovery rates at 3 and 6 months were not significantly different either (90% vs 85% and 99% vs 93%, respectively). In this study, we focused on comparing the cost and effectiveness of using prednisolone vs placebo to facilitate health care planning decisions and comparison across health conditions, where effectiveness is mainly estimated using a generic health-related quality-of-life instrument collected out to 6 months after randomization.
Methods
An economic evaluation was prospectively planned alongside the randomized clinical trial,9,12 conducted following the Second Panel on Cost-Effectiveness in Health and Medicine guidelines.13 The reporting of the economic evaluation followed the updated Consolidated Health Economic Evaluation Reporting Standards.14 The cost-effectiveness analysis was conducted from a health care sector perspective, and the time horizon is the 6-month period since randomization.
Standard Protocol Approvals, Registrations, and Patient Consents
The trial was approved by the institutional ethics committee at the Royal Children's Hospital (HREC/15/RCHM/V4) and received governance approval by the institutional ethics offices at each participating site. Written informed consent was obtained for each participant from a parent or legal guardian and the child if deemed competent. The study was registered with the Australian New Zealand Clinical Trials Registry ACTRN12615000563561.
Data
The full trial protocol9 and main study findings12 have been published elsewhere. Briefly, the BellPIC trial was a randomized, double-blinded, placebo-controlled trial of the use of prednisolone to improve recovery from Bell palsy. Study sites were 10 hospitals in Australia and 1 site in New Zealand in the Paediatric Research in Emergency Department International Collaborative research network.15 Patients aged 6 months to <18 years, diagnosed by an emergency department (ED) clinician with Bell palsy and onset of symptoms less than 72 hours before randomization were recruited. A 10-day treatment without dosage taper was administered. Participants received either oral prednisolone 1 mg/kg/d (based on weight categories) to a maximum of 50 mg/d or taste-matched placebo for 10 days. The primary outcome of the trial was complete recovery defined by House-Brackmann facial paralysis scales at 1 month, with grades 1, 2, 3, 4, 5 and 6 indicating normal (complete recovery), mild dysfunction, moderate dysfunction, moderately severe dysfunction, severe dysfunction, and total paralysis, respectively.16 Health-related quality of life as a secondary outcome was collected for the economic evaluation as specified in the published protocol at 1, 3, and 6 months after randomization or until the participant fully recovered.
Costs
Prednisolone was supplied as Redipred oral liquid, which contains the active ingredient prednisolone (equivalent to prednisolone 5 mg/mL). Redipred and the taste-matched placebo were supplied by Aspen Pharmacare Pty Ltd. (St Leonards, NSW, Australia). Bell palsy–related health service costs were assessed by a self-reported survey administered at months 1, 3, and 6 after randomization, and unrelated service use was not asked. The health service use categories included general practitioner (GP), hospital inpatient, hospital outpatient, or ED visits and other health services (patients were asked to describe in text) and medical tests including blood tests, neuroimaging (head CT, head MRI), and lumbar puncture. Costs for the initial nonadmitted ED visit that led to the diagnosis of Bell palsy were also included.
Costs were estimated using the physical units of health care items used multiplied by unit costs, expressed in 2020 Australian currency price. Unit cost for GP visits was obtained from the Medical Benefits Schedule.17 Unit cost for ED visits was obtained from the Independent Hospital Pricing Authority.18 There were no hospital inpatient admissions reported by patients during the study period, thus costing for inpatient visits was not relevant. Detailed unit costs including those for other care items are summarized in eTable 1 (links.lww.com/WNL/C752). The total health care cost over a 6-month period was the sum of all medication and health service costs during that time.
Effectiveness
The effectiveness outcome focused on quality-adjusted life-years (QALYs) over 6 months because QALYs are comparable across health conditions and routinely used to facilitate health care planning decisions. Complete recovery (defined as a House-Brackmann facial paralysis scale = 1) at 6 months was also considered. The QALYs were calculated based on health utilities estimated using the Child Health Utility 9D (CHU9D)19 and the Pediatric Quality of Life Inventory (PedsQL).20
The CHU9D is a pediatric generic preference-based measure of health-related quality of life consisting of a descriptive system and a set of preference weights.21 It gives a single value ranging from 0 (equivalent to dead) to 1 (equivalent to perfect health) for a health state defined by its descriptive system.22 CHU9D is available for children aged 5–18 years (5–7 years parent-reported and 8–18 years self-reported). For children aged 2–4 years, utilities were obtained using PedsQL scores mapped to CHU9D using established mapping algorithms.23 PedsQL is a nonpreference-based quality-of-life instrument and is available for children aged 2–18 years (2–4 years parent-reported and 5–18 years self-reported).
Participants were followed until full recovery or 6 months after randomization, whichever occurred first. For those who recovered earlier than 6 months, it is assumed that they remained recovered for the rest of the follow-up period sustaining their last observable quality of life. Utility for patients at the time of recruitment (baseline) was estimated using the mean from unrecovered patients at 1 month, assuming that baseline utilities are the same for prednisolone and placebo groups because of randomization. The QALYs over the 6-month period were estimated by calculating the area under the curve using the trapezium rule (eFigure 1, links.lww.com/WNL/C752).24
Cost-effectiveness
The incremental cost-effectiveness ratio, defined as the difference in cost divided by the difference in effectiveness, was estimated and interpreted as the cost per additional QALY gained at 6 months or cost to achieve 1 additional complete recovery at 6 months.
A prespecified cost-effectiveness subgroup analysis by age (6 months to <12 years vs 12 to <18 years) was also conducted. Age 12 years was chosen as the cut point to be consistent with the preplanned subgroup analysis of the primary outcome.12
Missing Data
Missingness, including CHU9D utilities (15.2% missing) and facial paralysis scales (7.8% missing), was imputed using multiple imputation with predictive mean matching within chained equations, assuming that data were missing at random (missing at random assumption was tested using the Little Missing Completely at Random test25,26). The imputation model included baseline characteristics of age, sex, weight, treatment group, hospital, length of illness, side of palsy, face pain, and facial paralysis severity. Imputations were drawn from a pool of 5 donors (nearest neighbors).27,28 Missing costs were limited (1.5% missing) and were assumed to be zero because it is most common to have no health care use.
Uncertainty and Sensitivity Analysis
To capture sampling uncertainty, probabilistic sensitivity analysis was conducted using bootstrapping with 1,000 replications drawing from cost and effectiveness data observed at the patient level. The bootstrapping results are graphically presented on a cost-effectiveness plane, with each of the 1,000 dots representing 1 mean cost and effectiveness difference between the treatment and placebo groups. We avoided presenting CIs directly for the cost-effectiveness ratios because the CIs may not be interpretable when the lower and upper bounds locate in different quadrants;29 nevertheless, the cost-effectiveness plane can be used to locate the middle 95% of all bootstrapped dots, which correspond to the conventional 95% CI. The acceptability curves were also constructed by counting the proportion of bootstrap replicates that are acceptable under various willingness-to-pay levels, summarized as the probability that the therapy is cost-effective.30 We used A$ 28,000–A$ 76,000 per QALY as the range of willingness-to-pay threshold according to published literature, reporting on an A$50,000/QALY threshold, which corresponds to approximately US$35,000 or £28,000.29,31,32
One-way sensitivity analyses were performed to assess the robustness of the findings. First, cost inputs were varied. This includes varying the unit cost of medication and medical health service use and removing extreme cost outliers (3 patients with costs over A$1,500 and 1 patient with cost over A$2,000, respectively). Second, alternative approaches for dealing with missing data including complete case analysis, mean imputation, median imputation, and regression imputation were used. Third, we considered an alternate mapping approach using PedsQL to estimate CHU9D utilities.23
The Student t test for continuous outcomes and the χ2 test for dichotomous outcomes were used. All analyses were conducted using STATA version 16 (Statacorp, College Station, TX).
Data Availability
The authors support data sharing. The BellPIC trial has used identifiable individual patient data that are subject to restriction because of ethics, consent, and privacy issues. Anonymized participant data and data dictionary will be available on request from the corresponding author where possible within these constraints.
Results
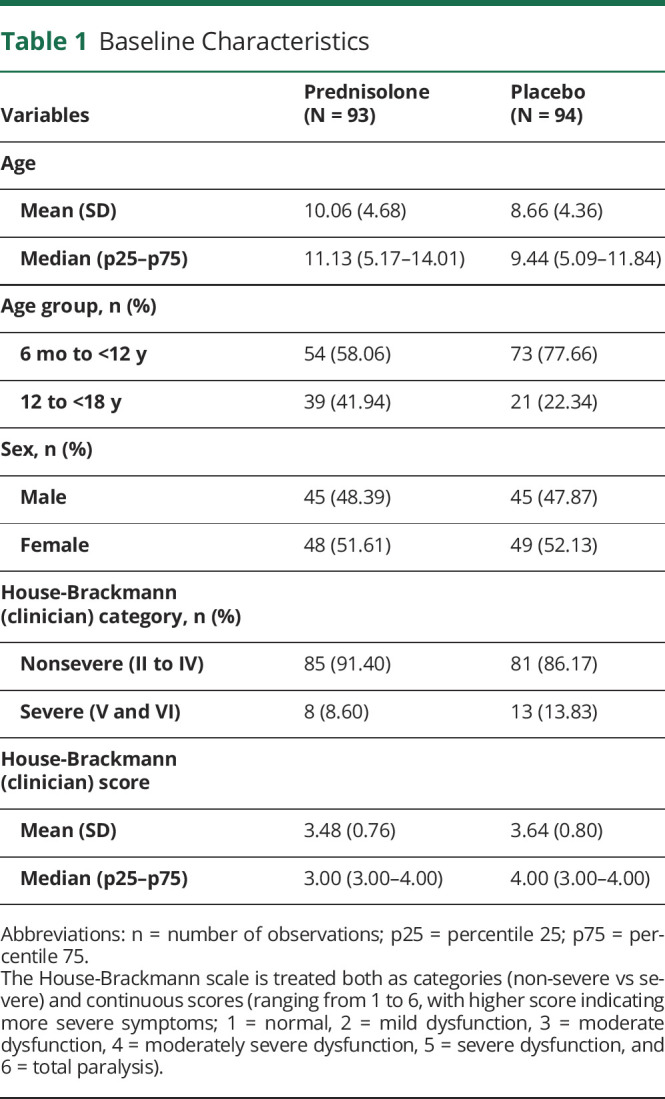
A total of 187 participants aged 6 months to <18 years were recruited, 93 randomized to prednisolone and 94 to placebo. The baseline sample characteristics of each group are presented in Table 1. Seven participants who withdrew or were lost to follow-up and had no quality-of-life or cost data (5 from prednisolone, 2 from placebo) were dropped. A total of 180 participants were included.
Table 1.
Baseline Characteristics
Cost-effectiveness
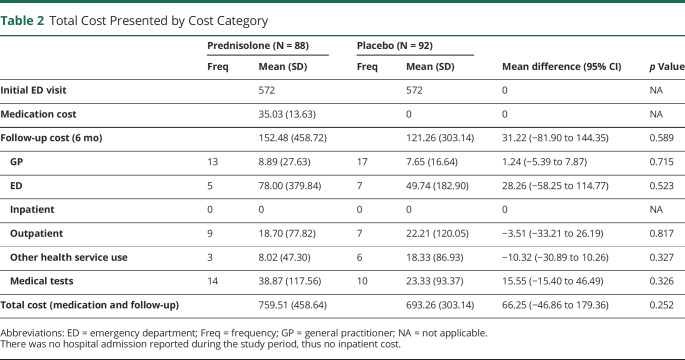
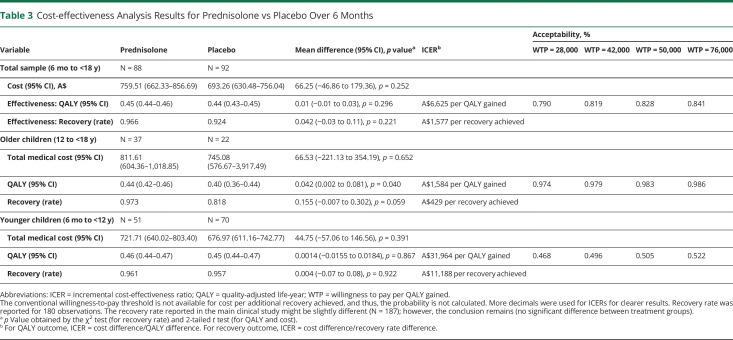
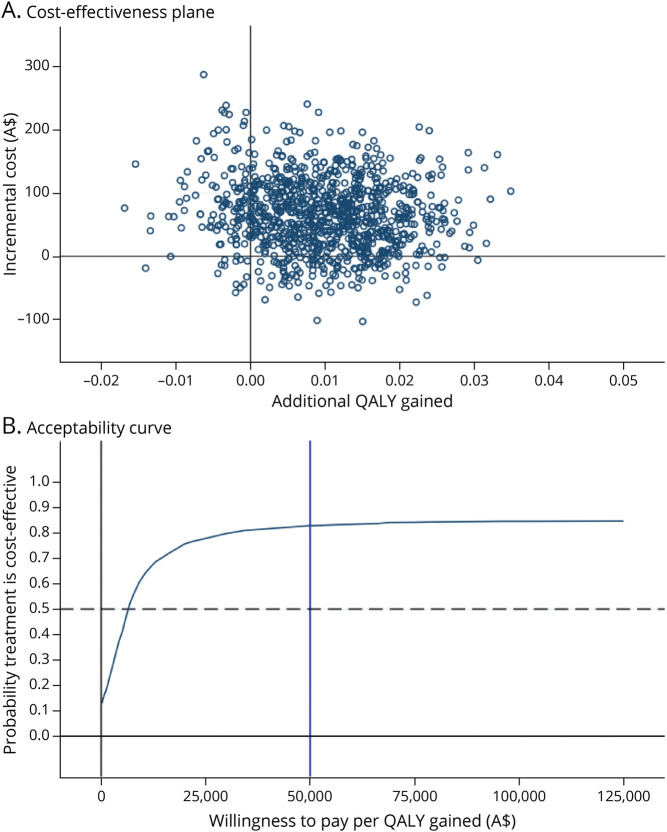
Total costs disaggregated by the type of health care used are presented in Table 2 (costs by different age groups presented in eTable 2, links.lww.com/WNL/C752). The cost-effectiveness outcomes are summarized in Table 3. Participants in the prednisolone group had a mean incremental cost of A$66 (95% CI −A$47 to A$179) compared with patients in the placebo group. Participants in the prednisolone group had a mean incremental QALY gain of 0.01 (95% CI −0.01 to 0.03), resulting in an incremental cost-effectiveness ratio of A$6,625 per QALY gained. This is lower than conventional willingness-to-pay thresholds of A$50,000 per QALY,31,32 suggesting that prednisolone is likely cost-effective relative to placebo. The acceptability curve (Figure 1) indicated that prednisolone was cost-effective compared with placebo in 83.0% of bootstrapped replicates if willingness to pay for a QALY gain is $50,000 (Table 3). The probability that prednisolone is cost-effective rises from 79.0% to 84.1% when willingness to pay increases from A$28,000 to A$76,000, implying that the conclusion remains unchanged given common willingness-to-pay thresholds (Table 3).
Table 2.
Total Cost Presented by Cost Category
Table 3.
Cost-effectiveness Analysis Results for Prednisolone vs Placebo Over 6 Months
Figure 1. Cost-effectiveness Plane and Acceptability Curve Comparing Prednisone With Placebo, Total Sample.
QALY = quality-adjusted life-year.
The recovery rate at 6 months for the prednisolone group was 0.97 and for the placebo group was 0.92 (difference in proportions 0.04, 95% CI −0.03 to 0.11, p = 0.221, Table 3). The incremental cost to achieve 1 additional recovery was estimated to be A$1,577 for prednisolone compared with placebo. The uncertainty when using recovery rate as the effectiveness outcome was also demonstrated with the cost-effectiveness plane and acceptability curve (eFigure 2, links.lww.com/WNL/C752).
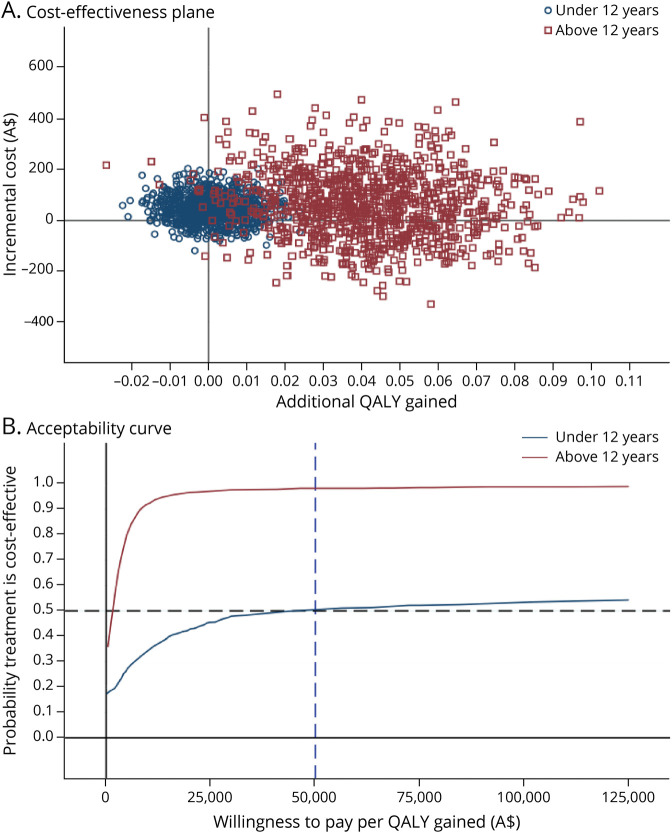
The subgroup analysis by age revealed that prednisolone is more cost-effective in children aged 12 to <18 years than those <12 years (Figure 2). This is primarily driven by the much greater incremental QALY gain of 0.042 (95% CI 0.002–0.081) in children aged 12 to <18 years comparing prednisolone with placebo, whereas the QALY gain was 0.001 (95% CI −0.016 to 0.018) in those <12 years. There was also a larger gain in recovery of 0.16 (95% CI −0.01 to 0.30; p = 0.059) comparing prednisolone with placebo in children aged 12 to <18 years, compared with 0.004 (95% CI −0.07 to 0.08; p = 0.922) in the younger age group at 6 months. The cost difference between prednisolone and placebo was similar in different age groups. The probability of prednisolone being cost-effective is 98% in children aged 12 to <18 years and 51% in those <12 years at a willingness-to-pay threshold of A$50,000 per QALY. One-way sensitivity analyses suggest that the conclusion remains under various scenarios (eFigure 3, links.lww.com/WNL/C752).
Figure 2. Cost-effectiveness Planes and Acceptability Curves Comparing Prednisolone With Placebo, by Age.
QALY = quality-adjusted life-year.
Discussion
We evaluated the cost-effectiveness of prednisolone treatment compared with placebo for Bell palsy in children. Prednisolone was more costly; however, it also led to more QALYs gained over a 6-month period. The additional cost is likely worthwhile given the additional QALYs gained using conventional value judgment thresholds, whereas the gains are primarily driven by children aged 12 to <18 years. Given a willingness to pay of A$50,000 per QALY gained, the probability that prednisolone is of good value is 98% in children aged 12 to <18 years. For children <12 years, on the other hand, the probability that prednisolone is cost-effective is 51%, meaning that it is uncertain whether prednisolone is of good value for this age group. In addition, the average incremental cost to achieve 1 additional recovery seems to be quite reasonable considering the social effect of Bell palsy in children aged 12 to <18 (A$429); for children <12 years, it seems to be much more expensive (A$11,188). This added evidence to the conclusion we made above. The focus of this study is cost-effectiveness, different from past studies on Bell's palsy treatment in children where the focus was on clinical efficacy or effectiveness.
The results that prednisolone is highly likely cost-effective and of good value for children aged 12 to <18 years but may not be for those younger than 12 years are not surprising. In adults, oral steroids are recommended by the American Academy of Otolaryngology—Head and Neck Surgery Foundation to help improve recovery in Bell palsy in patients 16 years and older.8 Our trial population includes children up to <18 years and and there is little biological reason that treatment with prednisolone would suddenly stop being efficacious for children who are slightly younger. It is reasonable to find that steroids have offered good value in those children aged 12 to <18 years. Our results are thus in line with the only previous cost-effectiveness analysis in adults of prednisolone treatment for Bell palsy,11 which reported that prednisolone is likely cost-effective with a 77% probability compared with placebo at a willingness to pay of £30,000 (2006/07 currency price). For children younger than 12 years, on the other hand, our results suggest that evidence supporting the use of steroids is not strong, consistent with the overall clinical findings.
It is worth noticing that no significant difference in the primary outcome (recovery defined by facial palsy score at 1 month) was found between the 2 treatment groups, nor for the prespecified subgroup of children aged 12 to <18 years, in the main clinical study.12 The authors concluded that the study, although underpowered, does not provide evidence that early treatment with prednisolone improves complete recovery.12 The clinical study and the current economic evaluation agree in conclusion for children younger than 12 years and differ for 12 to <18 years. The difference may be because of the following reasons. The economic evaluation is centered around patient quality-of-life outcomes, where a generic instrument instead of a condition-specific measure is used to capture general quality-of-life effect. Furthermore, it is possible that prednisolone improves health-related quality of life independent of its effect on Bell palsy recovery. In this study, we found that prednisolone has resulted significantly better QALYs in older children over the 6-month period. This is possible because Bell palsy had a larger effect on quality of life for adolescents than for younger children, which is supported by our supplementary results that showed a larger quality-of-life difference between recovered and unrecovered patients in the older age group (eTable 3, links.lww.com/WNL/C752). A previous systematic review also reported an increase in social difficulties with age in children with facial palsy.33 QALY reflected the accumulative quality-of-life difference over the whole 6-month period and thus may drive the high probability of prednisolone being cost-effective in 12 to <18 years.
Our study has several strengths including using individual-level data from a gold standard randomized controlled trial, with a wide range of child age (6 months to <18 years). We used quality-of-life observations collected as part of the trial instead of assumed utilities based on published literature. Comprehensive sensitivity analyses were performed to capture uncertainty. A prespecified subgroup analysis was also conducted to capture heterogeneity of patient population.
Several limitations were also identified. The time horizon was 6 months, and longer term effects were not measured. Nevertheless, Bell palsy is an acute disease with 97% and 93% children recovered at 6 months in the treatment and placebo groups in this trial and the literature shows all children recovered within 12 months.34 Recurrent Bell palsy is uncommon;35 hence, long-term evaluation is unlikely to have a meaningful effect on our results and conclusion. In actual clinical practice, a placebo would not be administered in patients not receiving a medication (prednisolone). The use of a placebo, although in line with the guidelines for clinical trials of no known or available alternative therapy, may underestimate the benefit of prednisolone compared with usual practice because of its psychosomatic effects. Another limitation is that CHU9D was not designed for children younger than 4 years, and thus, the CHU9D utilities for children aged 2–4 years in this study were mapped from an established algorithm. Further evidence based on directly collected health utilities for 2–4 years may be valuable. We primarily reported on an A$50,000/QALY threshold corresponding to approximately US$35,000 or £28,000, and appropriate willingness-to-pay levels may vary across countries and over time. Nevertheless, we used A$28,000 to A$76,000 per QALY as the range of willingness to pay and the results suggested that this has little meaningful effect on the probability that prednisolone is cost-effective (probability rises from 79.0% to 84.1%) and thus the conclusion remains. Finally, baseline utility was not collected because of concerns for patients and parents’ stress when at diagnosis and recruitment and was assumed to be the same for both patient groups because of randomization. We used the mean utility from unrecovered patients at 1 month as baseline for both groups. Nevertheless, the QALY difference between the 2 groups would remain unchanged even if other baseline utility values were applied as long as the same level of baseline utility can be expected for both groups given randomization.
Our results suggest that using prednisolone to treat Bell palsy in children is likely cost-effective compared with placebo over a 6-month period in children aged 12 to <18 years. The benefit primarily comes from improvement in children's quality of life and QALYs, which are increasingly valued by clinicians in practice. In children aged <12 years, there is no strong evidence supporting the use of prednisolone to treat Bell palsy. Prednisolone for treating Bell palsy in 12 to <18 years may be considered by stakeholders and policymakers.
Glossary
- BellPIC
Bell Palsy in Children
- CHU9D
Child Health Utility 9D
- ED
emergency department
- GP
general practitioner
- PedsQL
Pediatric Quality of life Inventory
- QALY
quality-adjusted life-year
Appendix. Authors

Footnotes
Editorial, page 1123
Infographic NPub.org/ig10024
Study Funding
X. Xiong was supported by the China Scholarship Council (201906010310). The sponsor was not involved in the study design, data collection, data analyses, results interpretation, and writing of this manuscript. F.E. Babl's time was part funded by a grant from the Royal Children's Hospital Foundation, Melbourne, Victoria, Australia, and an NHMRC Practitioner Fellowship. S.R. Dalziel's time was part funded by the Health Research Council of New Zealand (HRC13/556) and Cure Kids, Auckland, New Zealand. Aspen Australia (St Leonards, NSW, Australia) provided the study drug (prednisolone and taste-matched placebo) as a donation free of charge. Aspen did not sponsor the study and had no influence on study design, execution, analysis, and publication. The clinical trial was funded by a grant from the National Health and Medical Research Council (NHMRC, project grant GNT1078069), Canberra, Australia; the Emergency Medicine Foundation (EMSS-312R26-2016-GEORGE), Brisbane, Australia; and the Perth Children's Hospital Foundation project grant 9670, Perth, Australia.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Mackay MT, Chua ZK, Lee M, et al. Stroke and nonstroke brain attacks in children. Neurology. 2014;82(16):1434-1440. [DOI] [PubMed] [Google Scholar]
- 2.Hato N, Murakami S, Gyo K. Steroid and antiviral treatment for Bell's palsy. Lancet. 2008;371(9627):1818-1820. [DOI] [PubMed] [Google Scholar]
- 3.Rowlands S, Hooper R, Hughes R, Burney P. The epidemiology and treatment of Bell's palsy in the UK. Eur J Neurol. 2002;9(1):63-67. [DOI] [PubMed] [Google Scholar]
- 4.Lee M, Mackay M, Blackbourn L, Babl FE. Emotional impact of Bell's palsy in children. J Paediatr Child Health. 2014;50(3):245-246. [DOI] [PubMed] [Google Scholar]
- 5.Cope D, Bova R. Steroids in otolaryngology. Laryngoscope. 2008;118(9):1556-1560. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan FM, Swan IRC, Donnan PT, et al. Early treatment with prednisolone or acyclovir in Bell's palsy. N Engl J Med. 2007;357(16):1598-1607. [DOI] [PubMed] [Google Scholar]
- 7.Engström M, Berg T, Stjernquist-Desatnik A, et al. Prednisolone and valaciclovir in Bell's palsy: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2008;7(11):993-1000. [DOI] [PubMed] [Google Scholar]
- 8.Baugh RF, Basura GJ, Ishii LE, et al. Clinical practice guideline: Bell's palsy. Otolaryngology. 2013;149(3 suppl):S1-S27. [DOI] [PubMed] [Google Scholar]
- 9.Babl FE, Mackay MT, Borland ML, et al. Bell's Palsy in Children (BellPIC): protocol for a multicentre, placebo-controlled randomized trial. BMC Pediatr. 2017;17(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitaro J, Waissbluth S, Daniel SJ. Do children with Bell's palsy benefit from steroid treatment? A systematic review. Int J Pediatr Otorhinolaryngol. 2012;76(7):921-926. [DOI] [PubMed] [Google Scholar]
- 11.Hernández RA, Sullivan F, Donnan P, Swan I, Vale L. Economic evaluation of early administration of prednisolone and/or aciclovir for the treatment of Bell's palsy. Fam Pract. 2009;26(2):137-144. [DOI] [PubMed] [Google Scholar]
- 12.Babl FE, Herd D, Borland M, et al. Efficacy of prednisolone for Bell palsy in children: a randomized, double-blind, placebo-controlled, multicenter trial. Neurology. 2022. doi: 10.1212/WNL.0000000000201164. [DOI] [PubMed] [Google Scholar]
- 13.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-1103. [DOI] [PubMed] [Google Scholar]
- 14.Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babl F, Borland M, Ngo P, et al. Paediatric Research in Emergency Departments International Collaborative (PREDICT): first steps towards the development of an Australian and New Zealand research network. Emerg Med Australas. 2006;18(2):143-147. [DOI] [PubMed] [Google Scholar]
- 16.House JW, Brackmann DE. Facial nerve grading system. Otolaryngology. 1985;93(2):146-147. [DOI] [PubMed] [Google Scholar]
- 17.MBS. Unit Cost for GP. Accessed December 1, 2021. www9.health.gov.au/mbs/search.cfm?q=23&sopt=I. [Google Scholar]
- 18.The Independent Hospital Pricing Authority. The Round 23 National Hospital Cost Data Collection (NHCDC) Collected Public Hospital Cost Information for the 2018-19 Financial Year. The Independent Hospital Pricing Authority. [Google Scholar]
- 19.Stevens KJ. Working with children to develop dimensions for a preference-based, generic, pediatric, health-related quality-of-life measure. Qual Health Res. 2010;20(3):340-351. [DOI] [PubMed] [Google Scholar]
- 20.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800-812. [DOI] [PubMed] [Google Scholar]
- 21.Measuring & Valuing Health. A brief overview of the Child Health Utility 9D (CHU9D). licensing.sheffield.ac.uk/product/CHU-9D.
- 22.Ratcliffe J, Flynn T, Terlich F, Stevens K, Brazier J, Sawyer M. Developing adolescent-specific health state values for economic evaluation. Pharmacoeconomics. 2012;30(8):713-727. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney R, Chen G, Gold L, Mensah F, Wake M. Mapping PedsQL(TM) scores onto CHU9D utility scores: estimation, validation and a comparison of alternative instrument versions. Qual Life Res. 2020;29(3):639-652. [DOI] [PubMed] [Google Scholar]
- 24.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83(404):1198-1202. [Google Scholar]
- 26.Rhon DI, Kim M, Asche CV, Allison SC, Allen CS, Deyle GD. Cost-effectiveness of physical therapy vs intra-articular glucocorticoid injection for knee osteoarthritis: a secondary analysis from a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2142709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manca A, Palmer S. Handling missing data in patient-level cost-effectiveness analysis alongside randomised clinical trials. Appl Health Econ Health Policy. 2005;4(2):65-75. [DOI] [PubMed] [Google Scholar]
- 28.Franklin M, Hunter RM, Enrique A, Palacios J, Richards D. Estimating cost-effectiveness using alternative preference-based scores and within-trial methods: exploring the dynamics of the quality-adjusted life-year using the EQ-5D 5-level version and recovering quality of life utility index. Value Health. 2022;25(6):1018-1029. [DOI] [PubMed] [Google Scholar]
- 29.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. OUP; 2014. [Google Scholar]
- 30.Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187(2):106-108. [DOI] [PubMed] [Google Scholar]
- 31.George B, Harris A, Mitchell A. Cost-effectiveness analysis and the consistency of decision making: evidence from pharmaceutical reimbursement in Australia (1991 to 1996). Pharmacoeconomics. 2001;19(11):1103-1109. [DOI] [PubMed] [Google Scholar]
- 32.Edney LC, Haji Ali Afzali H, Cheng TC, Karnon J. Estimating the reference incremental cost-effectiveness ratio for the Australian Health System. Pharmacoeconomics. 2018;36(2):239-252. [DOI] [PubMed] [Google Scholar]
- 33.Hotton M, Huggons E, Hamlet C, et al. A systematic review of the psychosocial adjustment of children and adolescents with facial palsy: the impact of Moebius syndrome. Int J Environ Res Public Health. 2020;17(15):5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ünüvar E, Oğuz F, Sıdal M, Kılıç A. Corticosteroid treatment of childhood Bell's palsy. Pediatr Neurol. 1999;21(5):814-816. [DOI] [PubMed] [Google Scholar]
- 35.Pitts DB, Adour KK, Hilsinger RL Jr. Recurrent Bell's palsy: analysis of 140 patients. Laryngoscope. 1988;98(5):535-540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors support data sharing. The BellPIC trial has used identifiable individual patient data that are subject to restriction because of ethics, consent, and privacy issues. Anonymized participant data and data dictionary will be available on request from the corresponding author where possible within these constraints.