Abstract
Background and Objectives
Neurologic outcomes in people with HIV (PWH) on long-duration antiretroviral therapy (ART) are not fully understood, and the underlying pathophysiology is unclear. To address this, we established a cohort of such individuals and compared them with HIV-negative controls using a novel matching technique. Both groups underwent extensive cognitive testing, evaluation for psychiatric measures, and MRI and CSF analyses.
Methods
Participants underwent comprehensive neuropsychological testing and completed standardized questionnaires measuring depressive symptoms, perceptions of own functioning, and activities of daily living as part of an observational study. Brain MRI and lumbar puncture were optional. Coarsened Exact Matching was used to reduce between-group differences in age and sex, and weighted linear/logistic regression models were used to assess the effect of HIV on outcomes.
Results
Data were analyzed from 155 PWH on ART for at least 15 years and 100 HIV-negative controls. Compared with controls, PWH scored lower in the domains of attention/working memory (PWH least square mean [LSM] = 50.4 vs controls LSM = 53.1, p = 0.008) and motor function (44.6 vs 47.7, p = 0.009) and a test of information processing speed (symbol search 30.3 vs 32.2, p = 0.003). They were more likely to self-report a higher number of cognitive difficulties in everyday life (p = 0.011). PWH also reported more depressive symptoms, general anxiety, and use of psychiatric medications (all with p < 0.05). PWH had reduced proportions of subcortical gray matter on MRI (β = −0.001, p < 0.001), and CSF showed elevated levels of neurofilament light chain (664 vs 529 pg/mL, p = 0.01) and tumor necrosis factor α (0.229 vs 0.156 ng/mL, p = 0.0008).
Discussion
PWH, despite effective ART for over a decade, displayed neurocognitive deficits and mood abnormalities. MRI and CSF analyses revealed reduced brain volume and signs of ongoing neuronal injury and neuroinflammation. As the already large proportion of virologically controlled PWH continues to grow, longitudinal studies should be conducted to elucidate the implications of cognitive, psychiatric, MRI, and CSF abnormalities in this group.
Neurologic outcomes for people with HIV (PWH) are of major concern for clinicians and patients alike, even in the era of effective antiretroviral therapy (ART).1-3 Although severe cognitive disorders attributed to HIV in this group are rare, even mild-to-moderate cognitive impairment can affect quality of life, employment, and medication adherence.4,5 Cognitive impairment in PWH is often defined with a spectrum of HIV-associated neurocognitive disorders (HAND). HAND is assessed using Frascati criteria, which relies on normative data to adjust for age, sex, race, and education level. A 2020 meta-analysis of Frascati criteria–based HAND found that the prevalence of neurocognitive impairment was 44.9% across 18 studies.6 Concerns have been raised that these criteria overestimate the prevalence of impairment and are not specific for HIV.7 The criteria have an inherent false-positive rate for ANI of 16%–20%, and in studies with well-matched controls, more than a third of the HIV-negative group met the criteria for cognitive impairment.8 Another confounder that may contribute to high rates of reported impairment is treatment variability in many HIV patient cohorts. In the landmark CNS HIV Antiretroviral Therapy Effects Research study, 59% of participants had detectable HIV RNA in their plasma, 29% were not on any ART, and no control group was used for comparison.2 These factors make it difficult to extrapolate results to individuals on effective treatment for many years.
In practice, clinicians find lower rates of cognitive impairment in PWH. At 1 large specialized center in the United Kingdom, the prevalence of symptomatic cognitive impairment via clinical evaluation was only 7.5%.9 In part, this discrepancy may be due to biases inherent to reliance on participant self-report in classifying HAND in research settings. As the population of PWH on effective ART grows and ages, the long-term effects of HIV, particularly neurocognitive consequences, are of increasing interest. Given the discrepancy in prevalence assigned by research reports vs clinical practice, there is a need to produce more clinically relevant results.
The standard of care for PWH is lifelong ART, so we aimed to directly compare cognitive and psychiatric outcomes, MRI brain volumes, and CSF biomarkers in PWH on very-long-duration ART (>15 years) with an HIV-negative control group recruited from the same sociodemographic communities. Using a technique that reduced reliance on external normative data, we preprocessed our data with Coarsened Exact Matching (CEM) to reduce imbalance between groups on relevant variables.10 Then, we broadly analyzed cognition, MRI brain volumes, CSF biomarkers, and psychiatric measurements to determine how neurologic outcomes are affected in PWH with long-duration, treated infection.
Methods
Participant Selection
Data for this study were collected under longitudinal natural history studies of PWH and uninfected same-community controls conducted in parallel by the NIH and the Uniformed Services University of the Health Sciences (USU) with a common protocol design. Participants completed research evaluations including neurologic and psychiatric evaluations, laboratory studies, and MRI.
The eligibility criteria for the overall study included at least 18 years old at the time of enrollment, no prior neurologic infection, no confounding neurologic or psychiatric disease (e.g., untreated depression or sleep apnea and stroke), no ongoing illicit substance use, ability to complete MRI, and no use of confounding medications. Eligible participants had at least 7 years of education; the ability to speak, read, and understand English; and performance validity scores in the valid range on neuropsychological (NP) testing.11
For the present analysis, all participants with HIV started ART at least 15 years before their NP testing visit per participant interview or medical record review. Note that these criteria do not confirm viral suppression for the entire duration of ART use. Controls were recruited through local bus advertisements, through flyers mailed to the same ZIP codes as PWH, as family members or close friends of PWH, or as patients at the same primary care clinics.
Clinical and Laboratory Parameters
Vital signs, medical history, and medication use history were assessed for all participants at the time of NP testing. Psychiatric disorders and substance use disorders were measured using the Client Diagnostic Questionnaire, a tool that has been validated in PWH.12 For PWH, current CD4 lymphocyte T-cell count, plasma HIV viral load, past and current ART regimens, number of years since HIV diagnosis, number of years since ART initiation, and nadir CD4 T cell count were recorded. If nadir CD4 was recorded as <200 or >200 cells/μL rather than a definite value, 199 and 201 were used, respectively, in the analysis. This occurred for 7 PWH.
NP Testing
All participants underwent NP testing overseen by a board-certified neuropsychologist (J.S.) including a 3- to 4-hour battery of neurocognitive tests, as well as the Beck Depression Inventory (BDI)-II, the Patient's Assessment of Own Functioning Inventory (PAOFI), and the Texas Functional Living Scale (TFLS), as previously described.13 Cognition was measured in 2 ways: The first used T scores generated for each NP domain (verbal fluency, information processing speed, executive functioning, attention/working memory, learning, memory, and psychomotor speed). These T scores were based on each participant's performance on specific NP tests and demographics (Heaton normative values, Calibrated Neuropsychological Normative System norms, or Wechsler Adult Intelligence Scale [WAIS]-III/Wechsler Memory Scale-III demographic norms).14-16 The second method used the raw individual test scores that compose each domain.
MRI
MRI scans were performed on a 3T brain MRI (Philips Medical Systems, the Netherlands) using an 8-channel head coil. The MRI included T1-MPRAGE (176 slices, 1 mm isotropic resolution). The T1-weighted images were segmented with FreeSurfer (version 7.0), and the volumes of 6 prespecified brain regions were calculated as a proportion of the total estimated intracranial volume. Participants who completed an MRI scan within 15 months of their NP testing and were included in the analysis of MRI outcomes.
Lumbar Puncture
An optional lumbar puncture was completed for CSF collection within 12 months of their NP testing. The Quanterix immunoassay was used for CSF measurements of neurofilament light (NfL), total tau, interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF) α. The NfL and total tau assay was performed on all included CSF samples. Cytokine levels were unavailable for 9 (6 PWH and 3 control) CSF samples.
Statistics
Covariates were chosen a priori based on known associations with outcome variables. Age, sex, race, education, and visit number (as a surrogate for NP test practice effect) were included as covariates for cognitive outcomes. Age, sex, and race were included as covariates for MRI and CSF outcomes. CEM was used to retrospectively match the HIV-negative controls to the PWH cases on age and sex.17,18 Matching on age and sex effectively reduced imbalance in other covariates. Visit number was balanced between the groups before matching.
Participants with MRI and CSF outcome data were selected from the larger group to create subsets for analysis of these outcomes. Using the same parameters used to match groups for cognitive analysis, subset groups were rematched using CEM. Weights were applied to the control group based on age and sex, and participants with HIV were each weighted at 1. After matching, balance was verified for age, sex, visit number, and other relevant variables including race and education level using a weighted t test (age), a weighted Wilcoxon rank-sum test (visit), or a weighted χ2 (sex, race, and education level). Preprocessing our data with CEM allowed us to control for the influence of confounding variables by reducing imbalance between groups, without eliminating a large proportion of our participants from analysis.10
Weighted linear regression was used to assess the effect of HIV on continuous outcomes, including cognition, MRI, and CSF outcomes. Least square means (LSMs) and CIs are reported. Logistic regression was used to assess the effect of HIV on binary outcomes (psychiatric and functional variables), with weight included as a covariate. The odds ratio (OR) and 95% CI are reported. Box-Cox transformation was used for skewed continuous outcomes before regression. Regression model residuals were tested for normality (Shapiro-Wilk, skewness, and kurtosis) to confirm whether transformation was appropriate. For transformed variables, back-transformed means and 95% CI are reported in tables. Variables where transformation was ineffective were dichotomized with a cutoff chosen based on clinical or statistical relevance—2 SDs from the control group mean (for PAOFI and TFLS)—or prior studies using the same variable (for BDI-II).19,20 Mean and SD are reported for normally distributed continuous variables, median and interquartile range (IQR) for non-normal continuous variables, and frequency and percentage for categorical variables. Given the exploratory nature of this analysis, p values were not corrected for multiple comparisons. A significance level of 0.05 was used in all analyses. All statistical analyses were conducted using R version 4.0.5.
Standard Protocol Approvals, Registrations, and Patient Consents
The NIH Institutional Review Board (IRB) reviewed and approved the NIH study (NCT01875588), and USU IRB reviewed and approved the Department of Defense study (IDCRP-078). All participants provided written informed consent.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
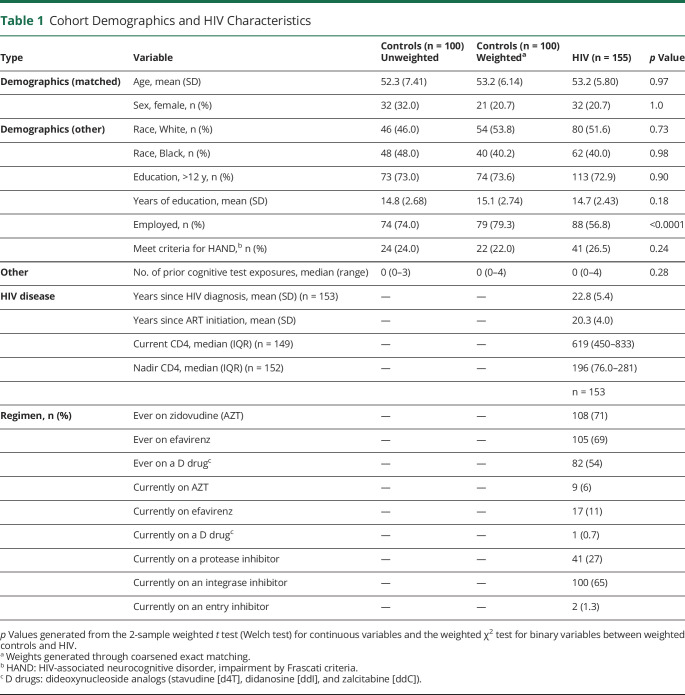
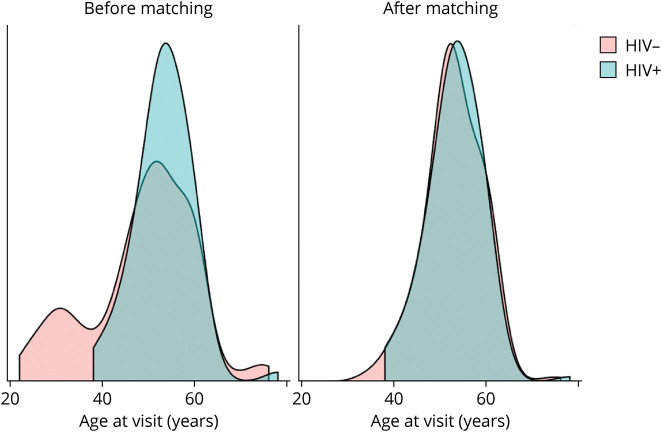
The analysis population included 155 PWH and 100 HIV-negative controls with demographic characteristics reported in Table 1. The HIV-negative control demographics are shown before and after weighting was applied through CEM. After matching on age and sex, the PWH and control groups were similar in age, sex, race, and education level (Figure 1). PWH were an average of 53.2 (SD 5.8) years old, 21% female, 52% White, and 40% Black, with 73% of participants having an education level of a high school degree or greater and a median visit number of 1 (median prior exposures to cognitive testing of 0). There were no significant differences in demographic variables or visit number between PWH and controls in any subset analysis (MRI, CSF).
Table 1.
Cohort Demographics and HIV Characteristics
Figure 1. Age Distributions by Group, Before and After Coarsened Exact Matching.
All PWH were receiving ART for at least 15 years and had plasma HIV RNA levels <50 copies/mL for at least 1 year before enrollment. PWH had a median CD4+ T lymphocyte count of 619 cells/μL (IQR 450–833 cells/μL) at the time of their visit and a median nadir CD4 of 196 cells/μL (IQR 76.0–281.0 cells/μL). The overall mean duration since HIV diagnosis was 22.8 (SD 5.4) years, and the mean duration of ART was 20.3 (SD 4.0) years.
Cognitive, Psychiatric, and Functional Outcomes
For comparisons of cognitive, psychiatric, and functional outcomes, the matching algorithm resulted in a control group (n = 100) with an effective sample size (ESS) of n = 62, meaning that analyses carried the same precision as an unweighted sample of this size. Using Frascati criteria, 22.0% of controls and 26.6% of PWH were categorized as impaired.
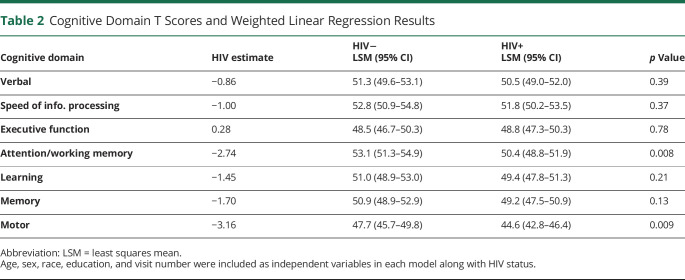
First, using a more traditional method of measuring cognition in PWH, we compared cognitive domain T scores between the 2 groups. HIV infection had a significant effect on the domains of attention/working memory (PWH LSM = 50.4 vs controls LSM = 53.1, p = 0.008) and motor function (44.6 vs 47.7, p = 0.009) (Table 2).
Table 2.
Cognitive Domain T Scores and Weighted Linear Regression Results
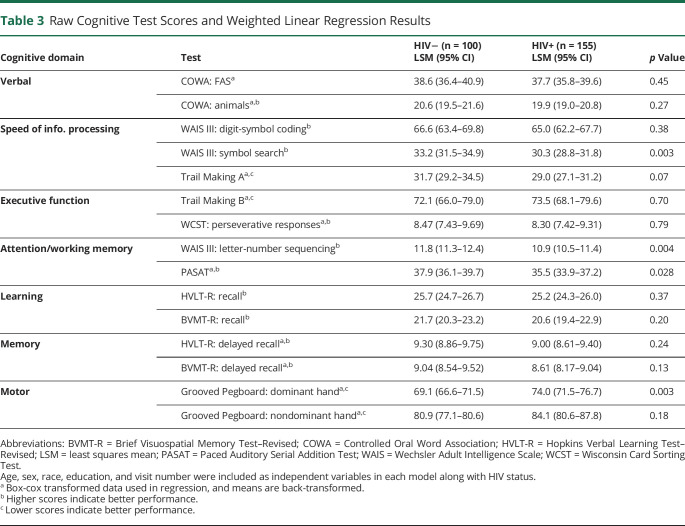
Because groups were well matched on demographic factors, we were also able to directly compare participants' individual test raw scores. PWH performed worse, compared with controls, on both tests composing the attention/working memory domain—WAIS–letter-number sequencing (LSM 10.9 vs 11.8, p = 0.004) and the Paced Auditory Serial Addition Test (35.5 vs 37.9, p = 0.028); 1 speed of information processing test—WAIS–symbol search (30.3 vs 32.2, p = 0.003); and 1 motor function test—Grooved Pegboard–dominant hand (74.0 vs 69.1, p = 0.003) (Table 3). In the motor function test, a higher score indicates longer time taken to complete the task (i.e., worse performance).
Table 3.
Raw Cognitive Test Scores and Weighted Linear Regression Results
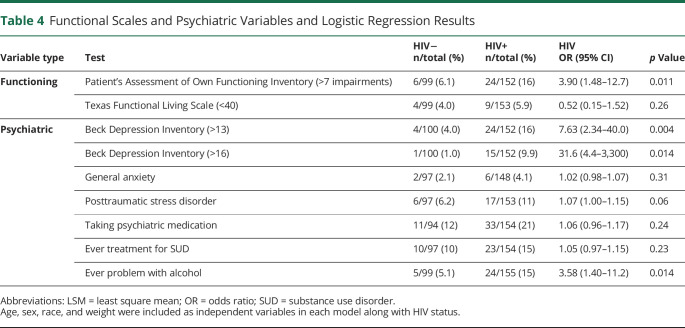
PWH were almost 4 times as likely to perceive a high number of cognitive impairments (>7, 2 SD above the control group mean) on the PAOFI (OR 3.90; 95% CI 1.48–12.7; p = 0.011) (Table 4). On the BDI, PWH were more than 7 times as likely to score ≥14, indicating at least mild depression (OR 7.63; 95% CI 2.34–40.0; p = 0.004), and almost 32 times as likely to score ≥17 (OR 31.6; 95% CI 4.40–3,300; p = 0.01), a threshold previously identified as more relevant for PWH.19 HIV infection also slightly increased the odds of ever having a problem with alcohol (OR 3.58; 95% CI 1.40–11.2; p = 0.014) (Table 4).
Table 4.
Functional Scales and Psychiatric Variables and Logistic Regression Results
MRI
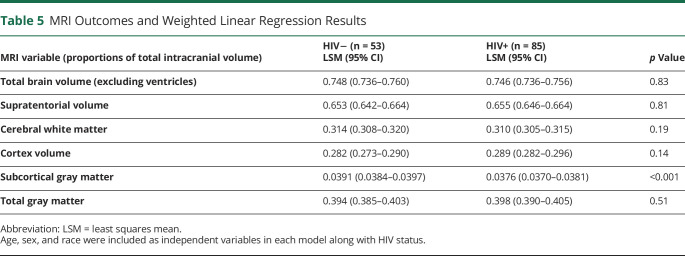
Eighty-five PWH and 53 HIV-negative controls (ESS n = 32) had MRI scans within 15 months of their NP testing. PWH had lower subcortical gray matter volume, measured as a proportion of the total intracranial volume (β = −0.001, p < 0.001) (Table 5). When we examined which regions within the subcortical gray matter were driving this difference, limbic structures (hippocampus, amygdala, and ventral diencephalon) (i.e., hypothalamus, along with the putamen) all had HIV estimates with an uncorrected p value <0.05. No significant differences were found between groups in any other regions of the brain.
Table 5.
MRI Outcomes and Weighted Linear Regression Results
CSF
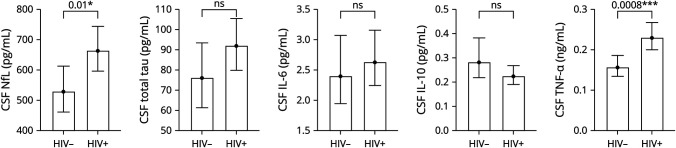
Forty-nine PWH and 23 HIV-negative controls (ESS of n = 20) were included in the analysis of CSF NfL and total tau. Forty-three PWH and 20 HIV-negative controls (ESS of n = 18) were included in the analysis of cytokines (IL-6, IL-10, and TNF-α). Multivariate linear regression found significant effects of HIV infection on NfL chain (PWH LSM 664 vs controls LSM 529 pg/mL, p = 0.01) and TNF-α (0.229 vs 0.156 ng/mL, p = 0.0008) in CSF (Figure 2). No difference between groups was seen in CSF total tau, IL-6, or IL-10 levels.
Figure 2. Cytokine/Biomarker Levels in CSF by Group.
CSF outcomes were box-cox transformed for linear regression analysis. Back-transformed means and 95% CIs are plotted. Age, sex, and race were included as independent variables in each model along with HIV status. NfL = neurofilament light chain; ns = p value not significant; TNF = tumor necrosis factor.
Discussion
We evaluated comprehensive cognitive, psychiatric, MRI, and CSF findings in a group of PWH on ART for at least 15 years. This study characterizes these outcomes with a focus on prospectively defined, long-duration HIV and long-duration ART use with a well-matched group of controls. As PWH are surviving longer, this population continues to grow, and understanding the ongoing effects on the brain is critically important. The largest differences between PWH and HIV-negative controls were found in the cognitive domains of attention/working memory and motor function, depression, subcortical gray matter volume, and CSF biomarkers of neuronal injury and inflammation.
Results from treated HIV cohorts, such as Comorbidity in Relation to AIDS (COBRA) and AGEhIV, have revealed specific effects of HIV on cognition,21,22 but lacked diversity in race and sex, with greater than 90% White participants and very few women. These factors limit the generalizability of results to the population living with HIV in the United States, where 1 in 5 PWH are women, and 44% of new infections occur in Black Americans.23 Our cohort benefits from balanced representation, with demographics similar to national averages. Using a robust matching technique, CEM, we were able to make a series of meaningful comparisons across a broad spectrum of neurologic outcomes with a diverse representation of participants. An important limitation of prior HIV cohort studies is that they have wide heterogeneity in the duration of treatment with ART, with inclusion criteria of more than 12 months on ART. In contrast, we included participants with at least 15 years of treatment with ART to explore the long-term consequences of HIV infection.
Several tests showed that PWH had impairment of attention/working memory and motor function, relative to HIV-negative controls. This pattern was seen when looking at comparisons of the traditional cognitive domain scores and on individual tests. On these individual test comparisons, we also saw a difference in symbol search, a test that measures information processing speed in addition to sustained attention.16 Most other domain scores (5/7) and individual test results (11/15) were not affected by HIV status.
A limitation of composite T scores is that they use external norms, which are not always generalizable to study populations. Another HIV cohort study found that both PWH and controls, when tested at baseline, scored >0.5 SDs above normative data standards in the memory domain,22 highlighting the difficulty of applying these standards to make inferences about research groups. Thus, a strength of our study was the use of a well-matched control group, which allowed for direct comparisons between the 2 groups without the use of external norms or demographic normalization.
Although there is debate over whether demographic norms, specifically race, are inherently harmful,24 existing norms show clear failures. Comparison samples used to develop the Heaton norms (most recently updated in 2004), which are widely used to assess NP test results, were acquired from centers across the country; however, almost all the data from Black participants were acquired in San Diego.14 While analyzing cognitive domains vs individual tests yielded largely similar results, we support a move away from normative data based on race. Demographic covariates did not significantly affect results, indicating that the matching process we used effectively reduced reliance on both normative data and statistical adjustment. This technique is unable to assess individual cognitive test performance but has the potential to be applied in other fields that use NP testing at a group level.
Objective findings of cognitive dysfunction were also corroborated by PWH who subjectively reported more cognitive difficulties compared with controls. In line with prior reports,25 we found a significant increase in the odds of PWH having mild depression. Depression has been associated with cognitive impairment and represents a potentially modifiable factor that clinicians should be aware of in this population. PWH in our cohort were also more likely to self-report ever having a problem with alcohol. To evaluate whether prior problematic alcohol use could explain the group differences in cognitive testing, we performed the domain score analysis with affected individuals removed (5 controls, 24 PWH) and found that attention/working memory and motor function remained significantly different. Because several factors may contribute to cognitive impairment in PWH, we conducted imaging and CSF studies.
Our results show loss of subcortical gray matter in PWH. Deep gray matter structures take up a very small proportion of the intracranial space, averaging 3.7% of the intracranial volume. Thus, even a small reduction in the volume of these regions may result in functional changes.26 On cognitive testing, PWH were slower to complete a motor task measuring manual dexterity with their dominant hand. It is possible that this impairment may be related to reductions in basal ganglia volume, a region important in motor pathways.
Subcortical gray matter, specifically the basal ganglia, has been shown to be affected by HIV infection on imaging, both in severely immunosuppressed individuals with HIV-associated dementia27 and in cohorts with well-treated disease.28-30 Why subcortical gray matter is particularly susceptible to HIV infection is not entirely clear, but in untreated patients, the HIV viral load is also higher in this region.31 Reduced volumes in this region have been associated with deficits in executive function,32 HIV DNA levels in peripheral blood,32 and duration of HIV infection.28 PET studies have also found abnormalities in subcortical gray regions, with significantly reduced relative F-fluorodeoxyglucose uptake in the thalamus in well-treated PWH.33 One study found that well-treated PWH had smaller subcortical brain volumes at baseline. However, when researchers looked at longitudinal scans over a 2-year period, they did not find differences in volume change between PWH and controls.29,34 A meta-analysis showed that there is substantial variation in HIV-associated brain volume changes in the literature.35 An international study on subcortical gray matter volumes in treated demographically diverse cohort of PWH found that HIV factors are consistently associated with reduced volume in limbic areas in the post-ART era.36 Of interest, our well-treated cohort showed similar reductions, including in the hippocampus, amygdala, and hypothalamic region, which may in part explain some the memory deficits and mood changes. Longitudinal follow-up studies are underway to determine whether there are progressive changes in brain volume and if they may correlate with other cognitive and CSF biomarkers.
Autopsy studies show that HIV viral load is maximal in the basal ganglia and the temporal lobe. Our neurocognitive and MRI studies also support the involvement of these areas of the brain. It is therefore likely that the CSF findings of neurodegeneration and neuroinflammation also originate, at least in part, from these same anatomic regions. The reasons for viral tropism in these regions remain unexplained.
CSF studies were performed to determine whether there was any evidence of ongoing neuronal injury or neuroinflammation. NfL levels were measured as an indicator of neuronal or axonal damage. In agreement with prior studies, we found that levels in PWH are higher than matched controls, despite effective ART.37 This suggests that although ART initially reduces NfL levels in treatment-naive PWH,38 ART does not fully suppress NfL and ongoing neuronal injury occurs. Furthermore, CSF NfL is known to increase with age in the general population.39 However, because our HIV and control groups were well matched for age, elevated NfL levels in PWH may be driven by HIV proteins such as Tat. Tat is produced despite adequate use of ART and suppressed viremia40 and is known to cause neurotoxicity.41
TNF-α levels were measured as an indicator of neuroinflammation. TNF-α is a proinflammatory cytokine released in the CNS by activated microglia and macrophages. Overproduction in the brain leads to aberrant immune activation that has been implicated in the pathogenesis of many CNS neuroinflammatory diseases.39 We observed elevated TNF-α in our long-term treated group of PWH. This finding is consistent with persistent glial cell activation and may be driven by the release of HIV proteins.42
Although elevations of NfL and TNF-α are not specific to HIV infection and are also associated with other neurodegenerative and neuroinflammatory disorders, they indicate persistent cerebral injury. An important question that remains unanswered in the field is whether the cognitive dysfunction in treated PWH represents a legacy effect from the initial infection or if there might be concern for ongoing injury despite the use of ART. Legacy effects can result from neuronal damage caused at the time of seroconversion and before initiation of ART. When HIV is untreated, low nadir CD4 counts are associated with poor neurologic outcomes,2,29,43,44 and the use of ART can halt further brain atrophy.45 Although legacy effects may be a reasonable concern, our studies show that current ART use alone is insufficient to control the production of HIV proteins once the viral reservoir is established, and there is ongoing neuroglial injury. Longitudinal studies are needed to further validate these observations.
Based on HAND's high false positivity rate and the variability of other methods used to assess HAND, some researchers have called for new criteria with a lesser focus on ANI.46 Although the classification of ANI has unclear clinical significance, it has been shown to increase the probability of developing further symptomatic cognitive decline.47 We suggest that future studies focus on identifying HAND phenotypes that include biological abnormalities or biotypes. HAND is a heterogeneous group of diseases and may be better identified with markers, including MRI, that reveal pathophysiology. In this way, we may be able to link differences in cognitive function to specific brain regions or biomarker abnormalities.
Our study design has several limitations. First, this is a cross-sectional study; longitudinal data are needed to confirm the progressive effects of HIV on cognition, regional effects on the brain, and neuroimmune dysfunction. Second, our study did not identify distinct biotypes of HAND. This may be related to the stringent inclusion criteria, which may have selected for a relatively homogeneous population, or a much larger sample size may be needed for such analyses. Third, by focusing on participants on ART for ≥15 years, we may have introduced a survivor bias, such that we selected for PWH who are more resilient to the effects of untreated/poorly treated HIV or more highly retained in care. Seventy-one percent of our participants were prescribed zidovudine (AZT), indicating that they were infected before or close to the time that the first effective combination ART became available in the mid-1990s. Patients who were given AZT monotherapy, or other less effective regimens, may be different from those who started on a fully suppressive treatment.48 Fourth, there are potential immunologic differences between patients who started ART during their acute infection and those who started ART later. Future studies may be improved by stratifications based on the duration of untreated HIV infection. Fifth, the group of PWH interested in participating in clinical research may not represent the general population of PWH. For example, in our cohort, both groups had a high level of educational attainment. A research setting allowed us to do a broader array of testing but may limit the generalizability of our results to all PWH in the United States. Last, although we avoided the use of normative data in the primary analysis, we chose to covary for race and other demographic factors within our cohort. Race is an imperfect proxy for factors such as socioeconomic status, access to care, health effects of racism and discrimination, educational attainment, and more. Although it has proven complex and difficult to account for these factors in other ways, we note that the development of better, more standardized measures is paramount to promoting equity in research and health care.
This study provides several lines of evidence that despite adequate control of HIV replication and over a decade of ART treatment, PWH have markers of cerebral injury and inflammation and cognitive dysfunction. These observations have important implications for understanding the neuropathogenesis of HIV infection and for future approaches to treatment. Going forward, it is critical to develop modes of therapy that can either completely eradicate HIV or halt production of viral transcripts/proteins. Alternatively, adjunctive neuroprotective and anti-neuroinflammatory therapies may be developed through clinical trials to support neurologic health in PWH.
Glossary
- ART
antiretroviral therapy
- AZT
zidovudine
- BDI
Beck Depression Inventory
- CEM
Coarsened Exact Matching
- ESS
effective sample size
- HAND
HIV-associated neurocognitive disorders
- IL
interleukin
- IQR
interquartile range
- IRB
Institutional Review Board
- LSM
least square mean
- NfL
neurofilament light
- NP
neuropsychological
- OR
odds ratio
- PAOFI
Patient's Assessment of Own Functioning Inventory
- PWH
people with HIV
- TFLS
Texas Functional Living Scale
- TNF
tumor necrosis factor
- USU
Uniformed Services University of the Health Sciences
- WAIS
Wechsler Adult Intelligence Scale
Appendix. Authors
Study Funding
This research was supported by the Intramural Research Training Award program at the NIH, by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke, and by the Division of Intramural Research Programs of the National Institute of Mental Health. This project has been funded in whole or in part with federal funds from the NIH Office of AIDS Research. Support for this work (IDCRP-078) was also provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been funded by the National Institute of Allergy and Infectious Diseases, NIH, under Inter-Agency Agreement Y1-AI-5072, and the Defense Health Program, US DoD, under award HU0001190002.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol. 2011;17(2):176-183. [DOI] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75(23):2087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winston A, Arenas-Pinto A, Stöhr W, et al. Neurocognitive function in HIV infected patients on antiretroviral therapy. PLoS One. 2013;8(4):e61949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alford K, Daley S, Banerjee S, Vera JH. Quality of life in people living with HIV-associated neurocognitive disorder: a scoping review study. PLoS One. 2021;16(5):e0251944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis RJ, Deutsch R, Heaton RK, et al. Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch Neurol. 1997;54(4):416-424. [DOI] [PubMed] [Google Scholar]
- 6.Wei J, Hou J, Su B, et al. The prevalence of Frascati-criteria-based HIV-associated neurocognitive disorder (HAND) in HIV-infected adults: a systematic review and meta-analysis. Front Neurol. 2020;11(1613):581346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisslén M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis. 2011;11(1):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su T, Schouten J, Geurtsen GJ, et al. Multivariate normative comparison, a novel method for more reliably detecting cognitive impairment in HIV infection. AIDS. 2015;29(5):547-557. [DOI] [PubMed] [Google Scholar]
- 9.Ferretti F, Mora-Peris B, Underwood J, Waldman A, Everitt A, Winston A. Cognitive impairment in a clinical setting. J Acquir Immune Defic Syndr. 2018;77(1):e10-e13. [DOI] [PubMed] [Google Scholar]
- 10.Iacus SM, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal. 2012;20(1):1-24. [Google Scholar]
- 11.Green P. Medical Symptom Validity Test (MSVT) for Microsoft Windows: User's Manual. Paul Green Pub; 2004. [Google Scholar]
- 12.Aidala A, Havens J, Mellins C, et al. Development and validation of the Client Diagnostic Questionnaire (CDQ): a mental health screening tool for use in HIV/AIDS service settings. Psychol Health Med. 2004;9(3):362-380. [Google Scholar]
- 13.Shirazi TN, Summers AC, Smith BR, et al. Concordance between self-report and performance-based measures of everyday functioning in HIV-associated neurocognitive disorders. AIDS Behav. 2017;21(7):2124-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heaton RKM, Walden S, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources; 2004. [Google Scholar]
- 15.Schretlen DJ. CNNS, Calibrated Neuropsychological Normative System: Professional Manual. PAR; 2010. [Google Scholar]
- 16.Tulsky DH, Saklofske DH, Heaton RK, et al. Clinical Interpretation of the WAIS-III and WMS-III. Academic Press; 2003. [Google Scholar]
- 17.Ho D, Imai K, King G, Stuart E. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):28. [Google Scholar]
- 18.Ho D, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15(3):199-236. [Google Scholar]
- 19.Hobkirk AL, Starosta AJ, De Leo JA, Marra CM, Heaton RK, Earleywine M. Psychometric validation of the BDI-II among HIV-positive CHARTER study participants. Psychol Assess. 2015;27(2):457-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; 1996. [Google Scholar]
- 21.Schouten J, Su T, Wit FW, et al. Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy. AIDS. 2016;30(7):1027-1038. [DOI] [PubMed] [Google Scholar]
- 22.Cole JH, Caan MWA, Underwood J, et al. No evidence for accelerated aging-related brain pathology in treated human immunodeficiency virus: longitudinal neuroimaging results from the comorbidity in relation to AIDS (COBRA) project. Clin Infect Dis. 2018;66(12):1899-1909. [DOI] [PubMed] [Google Scholar]
- 23.HIV Surveillance Report 2019. Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 24.Byrd DA, Rivera-Mindt MG. Neuropsychology's race problem does not begin or end with demographically adjusted norms. Nat Rev Neurol. 2022;18(3):125-126. [DOI] [PubMed] [Google Scholar]
- 25.Rezaei S, Ahmadi S, Rahmati J, et al. Global prevalence of depression in HIV/AIDS: a systematic review and meta-analysis. BMJ Support Palliat Care. 2019;9(4):404. [DOI] [PubMed] [Google Scholar]
- 26.Ilves N, Lõo S, Ilves N, et al. Ipsilesional volume loss of basal ganglia and thalamus is associated with poor hand function after ischemic perinatal stroke. BMC Neurol. 2022;22(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40(2-3):122-131. [DOI] [PubMed] [Google Scholar]
- 28.Becker JT, Sanders J, Madsen SK, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011;5(2):77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanford R, Fellows LK, Ances BM, Collins DL. Association of brain structure changes and cognitive function with combination antiretroviral therapy in HIV-positive individuals. JAMA Neurol. 2018;75(1):72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R, Qi Y, Shi L, et al. Brain volumetric alterations in preclinical HIV-associated neurocognitive disorder using automatic brain quantification and segmentation tool. Front Neurosci. 2021;15(991):713760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nath A. Eradication of human immunodeficiency virus from brain reservoirs. J Neurovirol. 2015;21(3):227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallianpur KJ, Shikuma C, Kirk GR, et al. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013;80(19):1792-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammoud DA, Sinharay S, Steinbach S, et al. Global and regional brain hypometabolism on FDG-PET in treated HIV-infected individuals. Neurology. 2018;91(17):e1591-e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corrêa DG, Zimmermann N, Tukamoto G, et al. Longitudinal assessment of subcortical gray matter volume, cortical thickness, and white matter integrity in HIV-positive patients. J Magnet Reson Imaging. 2016;44(5):1262-1269. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor E, Zeffiro TA, Zeffiro TA. Brain structural changes following HIV infection: meta-analysis. Am J Neuroradiol. 2018;39(1):54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nir TM, Fouche JP, Ananworanich J, et al. Association of immunosuppression and viral load with subcortical brain volume in an international sample of people living with HIV. JAMA Netw Open. 2021;4(1):e2031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jessen Krut J, Mellberg T, Price RW, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One. 2014;9(2):e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mellgren Å, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology. 2007;69(15):1536-1541. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz A, Blennow K, Hagberg L, et al. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev Mol Diagn. 2017;17(8):761-770. [DOI] [PubMed] [Google Scholar]
- 40.Henderson LJ, Johnson TP, Smith BR, et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS. 2019;33(suppl 2):S145-S157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37(3):373-380. [DOI] [PubMed] [Google Scholar]
- 42.Chen P, Mayne M, Power C, Nath A. The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases. J Biol Chem. 1997;272(36):22385-22388. [DOI] [PubMed] [Google Scholar]
- 43.Cohen RA, Harezlak J, Schifitto G, et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16(1):25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanford R, Ances BM, Meyerhoff DJ, et al. Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin Infect Dis. 2018;67(11):1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nightingale S, Dreyer AJ, Saylor D, Gisslén M, Winston A, Joska JA. Moving on from HAND: Why we need new criteria for cognitive impairment in persons living with human immunodeficiency virus and a proposed way forward. Clin Infect Dis. 2021;73(6):1113-1118. [DOI] [PubMed] [Google Scholar]
- 47.Grant I, Franklin DR, Deutsch R, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br J Clin Pharmacol. 2015;79(2):182-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.