药食两用真菌红曲霉转运真菌毒素桔霉素的途径目前尚不清楚。本文通过CRISPR/Cas9基因编辑和过表达,分析了紫色红曲霉中一个假定的主要协同转运蛋白超家族(MFS)基因CtnC,证实了CtnC参与桔霉素的外排以及抑制菌丝体中桔霉素的积累,但CtnC蛋白不是唯一通道。同时,本研究结果发现CtnC过表达会负调控桔霉素合成相关基因(CtnD、CtnE、CtnF和pksCT)的表达。综上,本研究发现的红曲霉中桔霉素转运体基因为真菌毒素的防控策略研究提供了新的依据。
Keywords: 桔霉素, CtnC基因, CRISPR/Cas9, 过表达, 转运体, 真菌毒素
Monascus is one of the most essential microbial resources in China, with thousands of years of history. Modern science has proved that Monascus can produce pigment, ergosterol, monacolin K, γ-aminobutyric acid, and other functionally active substances. Currently, Monascus is used to produce a variety of foods, health products, and pharmaceuticals, and its pigments are widely used as food additives. However, Monascus also makes a harmful polyketide component called citrinin in the fermentation process; citrinin has toxic effects on the kidneys such as teratogenicity, carcinogenicity, and mutagenicity (Gong et al., 2019). The presence of citrinin renders Monascus and its products potentially hazardous, which has led many countries to set limits and standards on citrinin content. For example, the citrinin limit is less than 0.04 mg/kg according to the Chinese document National Standard for Food Safety Food Additive Monascus (GB 1886.181-2016) (National Health and Family Planning Commission of the People’s Republic of China, 2016), and the maximum level in food supplements based on rice fermented with Monascus purpureus is 100 µg/kg in the European Union (Commission of the European Union, 2019).
In order to study the metabolic pathway of citrinin and to explore ways to effectively reduce its content, Hajjaj et al. (1999) reported that citrinin biosynthesis begins with the condensation of one acetyl‐coenzyme A (acetyl-CoA) with three malonyl‐CoAs via a fatty acid synthase (FAS) and a polyketide synthase (PKS). Shimizu et al. (2005) cloned a PKS gene pksCT from M. purpureus. Its disruption reduced citrinin production to barely detectable levels. Then, the group cloned a 21-kb region flanking pksCT, in which the Orf2 gene, designated CtnA, was a major activator of citrinin biosynthesis (Shimizu et al., 2007). Li et al. (2012) cloned a 43-kb DNA region from Monascus aurantiacus, containing 16 open reading frames (ORFs) designated CtnD, CtnE, Orf6, Orf1, CtnA, Orf3, Orf4, pksCT, Orf5, CtnF, Orf7, CtnR, Orf8, CtnG, CtnH, and CtnI. Later, Li et al. (2013, 2015, 2020) further analyzed and verified the functions of CtnB (Orf4), CtnG, and CtnF in citrinin biosynthesis. Other researchers have identified the functions of CtnE, Orf1, Orf3, and Orf6 (Balakrishnan et al., 2016; Liang et al., 2017; Ning et al., 2017) in the citrinin biosynthesis gene cluster. Although many genes associated with citrinin production have been studied, the transmembrane transport mechanism of citrinin has yet to be elucidated, and little is known about the genes involved in citrinin tolerance and transport.
There is only one putative membrane transporter gene, CtnC (GenBank: AB243687), in the 43-kb gene cluster region, also named Orf5 in M. purpureus (Shimizu et al., 2007) and mrr1 in Monascus ruber (He and Cox, 2016). The DNA sequence of CtnC in M. purpureus is 1678 bp in length and contains two introns. The sequence structures of its homologs in M. ruber (GenBank: KT781075) and Penicillium camemberti (GenBank: MZ233788) have three introns, while Aspergillus sp.(GenBank: MZ503795) has four introns (Fig. 1a). The protein sequence of CtnC encodes 521 amino acids belonging to the major facilitator superfamily (MFS) transporter family and has a detoxification-related MFS_Tpo1_MDR_like functional domain, similar to that of multidrug resistance transporters. Phylogenetic tree analysis showed that CtnC shared 95.01% identity with the mrr1 of M. ruber and 86.82% with that of Aspergillus sp. (Fig. 1b). There have been reports of fungal transporters that are able to secrete mycotoxins and defend themselves against fungicides, such as the MFS transporter trichothecene-related gene 12 (Tri12), which is involved in the efflux of trichothecene in Fusarium graminearum (Menke et al., 2012). Thus, it has been suggested that it would be possible for CtnC to be involved in the transport of compounds in M. purpureus. CtnC would be a good target for investigating the regulatory mechanism of citrinin accumulation in Monascus. However, whether CtnC has a transport function for citrinin and how it would accomplish this are still unknown.
Fig. 1. CtnC gene structure and cluster analysis. (a) The gene structure of CtnC compared with those of its homologs in other fungi. Exons are represented by black boxes, introns by a line, and UTRs by a white box. (b) A neighbor-joining tree constructed with MEGA 7.0 software based on the full-length amino acid sequences. Bootstrap analysis (1000 replicates) was performed. UTRs: untranslated regions.
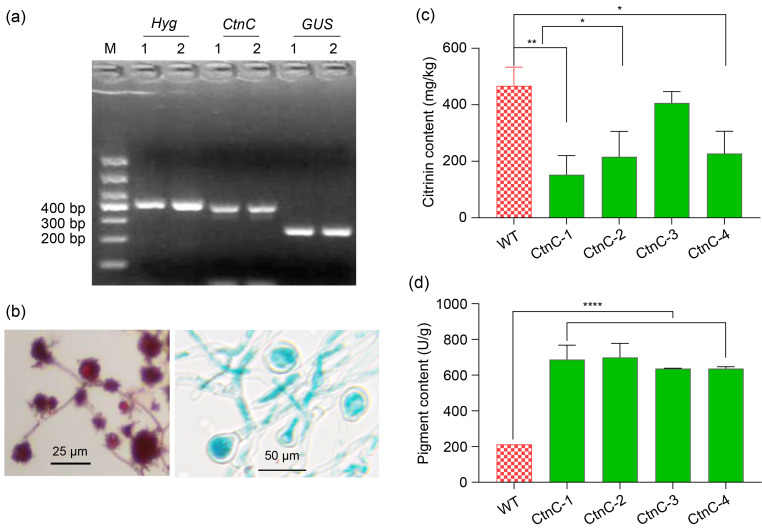
In this study, the expression cassette PgpdA-GUS-CtnC-Tnos was transformed into the M. purpureus strain by the Agrobacterium tumefaciens-mediated transformation (ATMT) method, and the transformation was verified by amplifying the hygromycin resistance gene (Hyg)and β-glucuronidase gene (GUS)(Fig. 2a). GUS staining showed that cleistothecium and hyphal cells were stained blue in the transformed strain, while wild-type cells were red (Fig. 2b). The CtnC-overexpressed strain was genetically stable after five contiguous cultures. Compared to the wild type, the overexpressed strain had a larger colony diameter and fewer aerial hyphae. Citrinin levels were significantly reduced by 12.93%–67.34% (Fig. 2c), but the levels of red pigment increased up to 228.70% (Fig. 2d). The expression level of CtnC is closely related to the content of polyketide components of pigment in mycelium.
Fig. 2. Analysis of CtnC-overexpressed strains. (a) PCR test for the Hyg, CtnC, and GUS. M: DL1000 DNA marker; 1, 2: detection duplication. (b) GUS staining of the mycelium (left: wild type; right: transformant). (c) Detection of citrinin content. (d) Detection of red pigment content. * P<0.05, ** P<0.01, **** P<0.0001. Data are expressed as mean±SD (n=3). PCR: polymerase chain reaction; Hyg: hygromycin resistance gene; GUS: β-glucuronidase gene; WT: wild type; CtnC-1‒CtnC-4: different CtnC-overexpressed strains; SD: standard deviation.
The M. purpureus strains constitutively expressing clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 gene (Cas9) were also constructed via ATMT. Its protoplasts were transformed in vitro with the single-guide RNAs (sgRNAs) of both orotidine-5'-phosphate decarboxylase (pyrG) and CtnC (Fig. 3a) mediated by polyethylene glycol (PEG) for target edition; then the double-site mutant strains were generated. We used the pyrG mutation as a marker for gene-editing strain screening. The wild-type Monascus strain had difficulties in growing on a medium supplemented with 5-fluoroorotic acid (5-FOA) and uridine, while the pyrG mutant strain was able to grow normally. The mutant strains were screened in a medium containing 20 mg/L hygromycin, 20 g/L uridine, and 1.5 g/L 5-FOA. The CtnC sequences of the resistant strains were verified by polymerase chain reaction (PCR) amplification and sequencing. Some sequences had obvious partial nesting peaks, indicating that the base sequence was mutated and resulted in a partial mismatch. The T7 endonuclease 1 (T7E1) endonuclease detection showed that there was only one electrophoresis band of PCR products of the wild type. However, we cut two bands in the mutant (MT) lane and in the mutant and wild-type mixed lane. We assumed that the MT1‒MT6 strains all had mutation loci and were heterozygous genotypes. The fact that no band was cut from MT7 mixed with wild type indicated that there was no mutation site (Fig. 3b). Sequencing revealed that the CtnC gene was successfully edited by the sgRNA and CRISPR/Cas9 systems; a single-base C deletion occurred at position 160 of the CtnC exon in the MT1, MT2, and MT3 strains, resulting in subsequent sequence-translation errors. The double-site mutation of CtnC in the MT4 strain at positions 161 (T>A) and 163 (T>G) resulted in amino acid mutations of 54 (Leu>Gln) and 55 (Trp>Gly) (Fig. 3c), and the mutation site was located inside the conserved functional domain of MFS_Tpo1_MDR_like. The constructed Cas9 chassis strain and in vitro sgRNA co-transformation system facilitated rapid editing of multiple genes in M. purpureus.
Fig. 3. Analysis of the edited loci of CtnC by CRISPR/Cas9. (a) Schematic diagram of sgRNA for pyrG and CtnC. (b) T7E1 cuts the PCR production of CtnC. (c) Mutant loci in CtnC edited by CRISPR/Cas9. CRISPR: clustered regularly interspaced short palindromic repeats; Cas9: CRISPR-associated protein 9; sgRNA: single-guide RNA; pyrG: orotidine-5'-phosphate decarboxylase; T7E1: T7 endonuclease 1; PAM: protospacer adjacent motif; PCR: polymerase chain reaction; WT: wild type; MT1‒MT7: different mutant strains; M: DL1000 DNA marker.
Uridine supplementation in the medium significantly reduced the citrinin content of wild-type strains (by up to 44.49%) while increasing the red pigment by 189%. Compared to the wild-type strains, the gene-editedstrains with CtnC and PyrG double mutations were able to grow on a medium containing 5-FOA and uridine with a faster growth rate and more pigment secretion (Fig. 4a). The CtnC mutation resulted in a significant increase in citrinin content (by 28.13%‒38.96%), while the content of red pigment did not change significantly (Fig. 4b). It was evident that CtnC was involved in citrinin transport, but was not the only channel, and that CtnC may not be involved in the transport of pigments. To detect transcriptional changes in CtnC and other genes involved in citrinin metabolism, we employed reverse transcription-quantitative PCR (RT-qPCR) for analysis. The results showed a significant increase in transcriptional levels of CtnC in the overexpressed strain. The change in expression of CtnA was not obvious. In contrast, transcription levels of CtnD, CtnE, CtnF, and pksCT were significantly reduced. This was closely related to the decrease in citrinin content of overexpressed strains. In the CtnC mutant strain, the mutation had a limited effect on transcription, while the expression levels of CtnA, CtnD, CtnE, CtnF, and pksCT were significantly increased, which was consistent with the increase in citrinin content in the mutant strain. In addition, inactivation of CtnC promoted transcription of the M. purpureus polyketide synthase gene 5 (MpPKS5), a gene involved in the synthesis of the orange pigment (Fig. 4c). So, CtnC activity was closely related to expression levels of CtnD, CtnE, CtnF, and pksCT. Presumably, CtnC is not only a channel protein, but also has a significant regulatory effect on citrinin synthesis.
Fig. 4. Morphology and chemical analyses of the mutant strains and RT-qPCR analyses of the citrinin-related genes. (a) Morphology of different mutant strains. (b) Comparison of the contents of citrinin and pigment in different mutant strains. (c) RT-qPCR analyses of the overexpressed strains and mutant strains. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001. Data are expressed as mean±SD (n=3). RT-qPCR: reverse transcription-quantitative polymerase chain reaction; WT: wild type; WU: wild type cultured with uridine; MT1‒MT4: different mutant strains; CtnC-1‒CtnC-3: different CtnC-overexpressed strains; pksCT: citrinin polyketide synthase; MpPKS5: M. purpureus polyketide synthase gene 5; SD: standard deviation.
Citrinin is a polyketide compound, a secondary metabolite generated during fermentation, which makes Monascus and its products potentially hazardous. Many studies have been done on the metabolism and regulatory mechanisms of citrinin in order to reduce it efficiently, but its transmembrane transport mechanism has yet to be elucidated. As a putative transporter, the specific function of CtnC in the citrinin-metabolism pathway has also not been reported. In this paper, we describe the construction of CtnC-overexpressed and CRISPR/Cas9 gene-edited strains. Compared with the wild‐type strain, citrinin content is significantly reduced in the mycelium of the CtnC-overexpressed strain and increased in the mycelium of the mutant strain. It is clear that CtnC is involved in citrinin transport and has an inhibitory effect on intracellular accumulation of citrinin. Transcription levels of CtnD, CtnE, CtnF, and pksCT were significantly reduced by CtnC overexpression, while they increased dramatically in CtnC-inactivated strains, according to RT-qPCR results. CtnC activity is closely related to expression levels of CtnD, CtnE, CtnF, and pksCT. It seems that an increase in extracellular citrinin concentration due to CtnC overexpression results in feedback inhibition of the expression of genes involved in citrinin synthesis, while a decrease in extracellular citrinin concentration due to CtnC mutation activates the expression of these genes.
Moreover, the addition of uridine in wild-type strains significantly reduces citrinin (by 44.49%) and increases red pigment (by 189%); the mechanisms of these effects remain to be clarified. Zhang et al. (2020) reported that disruption of pyrG in Aspergillus niger results in enhanced glycolysis and produces certain amounts of citrate and its precursors. It has been conjectured that uridine nucleosides are closely related to the metabolism of acetyl-CoA, the precursor to citrinin synthesis. The Cas9 chassis strain and sgRNA co-transformation system constructed in this paper are convenient for rapid editing of multiple genes. The findings of this study provide original insights into the citrinin efflux regulated by an MFS transporter gene, CtnC, which will assist in the exploration of mycotoxin transport and thus provide a valuable theoretical basis for improving the safety of Monascus-related products.
Materials and methods
Detailed methods are provided in the electronic supplementary materials of this paper.
Supplementary information
Acknowledgments
This work was supported by the Science and Technology Planning Project of Guizhou Province (No. 2019-2776) and the National Natural Science Foundation of China (No. 81960692).
Funding Statement
This work was supported by the Science and Technology Planning Project of Guizhou Province (No. 2019-2776) and the National Natural Science Foundation of China (No. 81960692).
Author contributions
Jie HAN and Jiehong ZHAO designed the project and wrote the manuscript. Yanling GUI and Guangfu TANG carried out the transgenic experiment. Yanling GUI, Haiqiao MAN, and Jiao WANG conducted the chemical analysis. Guangfu TANG conducted the RT-qPCR analysis. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Yanling GUI, Guangfu TANG, Haiqiao MAN, Jiao WANG, Jie HAN, and Jiehong ZHAO declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Balakrishnan B, Chandran R, Park SH, et al. , 2016. Delineating citrinin biosynthesis: Ctn-ORF3 dioxygenase-mediated multi-step methyl oxidation precedes a reduction-mediated pyran ring cyclization. Bioorg Med Chem Lett, 26(2): 392-396. 10.1016/j.bmcl.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Commission of the European Union , 2019. Commission Regulation (EU) 2019/1901 of 7 November 2019 amending Regulation (EC) No. 1881/2006 as regards maximum levels of citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. OJ, L 293: 2-4. [Google Scholar]
- Gong L, Zhu H, Li TT, et al. , 2019. Molecular signatures of cytotoxic effects in human embryonic kidney 293 cells treated with single and mixture of ochratoxin A and citrinin. Food Chem Toxicol, 123: 374-384. 10.1016/j.fct.2018.11.015 [DOI] [PubMed] [Google Scholar]
- Hajjaj H, Klaebe A, Loret MO, et al. , 1999. Biosynthetic pathway of citrinin in the filamentous fungus Monascus ruber as revealed by 13C nuclear magnetic resonance. Appl Environ Microbiol, 65(1): 311-314. 10.1128/AEM.65.1.311-314.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Cox RJ, 2016. The molecular steps of citrinin biosynthesis in fungi. Chem Sci, 7(3): 2119-2127. 10.1039/c5sc04027b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Xu Y, Huang ZB, 2012. Isolation and characterization of the citrinin biosynthetic gene cluster from Monascus aurantiacus. Biotechnol Lett, 34(1): 131-136. 10.1007/s10529-011-0745-y [DOI] [PubMed] [Google Scholar]
- Li YP, Pan YF, Zou LH, et al. , 2013. Lower citrinin production by gene disruption of CtnB involved in citrinin biosynthesis in Monascus aurantiacus Li AS3.4384. J Agric Food Chem, 61(30): 7397-7402. 10.1021/jf400879s [DOI] [PubMed] [Google Scholar]
- Li YP, Tang X, Wu W, et al. , 2015. The CtnG gene encodes carbonic anhydrase involved in mycotoxin citrinin biosynthesis from Monascus aurantiacus. Food Addit Contam Part A, 32(4): 577-583. 10.1080/19440049.2014.990993 [DOI] [PubMed] [Google Scholar]
- Li YP, Wang N, Jiao XX, et al. , 2020. The CtnF gene is involved in citrinin and pigment synthesis in Monascus aurantiacus. J Basic Microbiol, 60(10): 873-881. 10.1002/jobm.202000059 [DOI] [PubMed] [Google Scholar]
- Liang B, Du XJ, Li P, et al. , 2017. Orf6 gene encoded glyoxalase involved in mycotoxin citrinin biosynthesis in Monascus purpureus YY-1. Appl Microbiol Biotechnol, 101(19): 7281-7292. 10.1007/s00253-017-8462-7 [DOI] [PubMed] [Google Scholar]
- Menke J, Dong YH, Kistler HC, 2012. Fusarium graminearum Tri12p influences virulence to wheat and trichothecene accumulation. Mol Plant Microbe Interact, 25(11): 1408-1418. 10.1094/MPMI-04-12-0081-R [DOI] [PubMed] [Google Scholar]
- National Health and Family Planning Commission of the People’s Republic of China , 2016. National Standard for Food Safety Food Additive Monascus, GB 1886. 181-2016. National Standards of People’s Republic of China. [Google Scholar]
- Ning ZQ, Cui H, Xu Y, et al. , 2017. Deleting the citrinin biosynthesis-related gene, ctnE, to greatly reduce citrinin production in Monascus aurantiacus Li AS3.4384. Int J Food Microbiol, 241: 325-330. 10.1016/j.ijfoodmicro.2016.11.004 [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kinoshita H, Ishihara S, et al. , 2005. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl Environ Microbiol, 71(7): 3453-3457. 10.1128/AEM.71.7.3453-3457.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Kinoshita H, Nihira T, 2007. Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl Environ Microbiol, 73(16): 5097-5103. 10.1128/AEM.01979-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LH, Zheng XM, Cairns TC, et al. , 2020. Disruption or reduced expression of the orotidine-5'-decarboxylase gene pyrG increases citric acid production: a new discovery during recyclable genome editing in Aspergillus niger. Microb Cell Fact, 19: 76. 10.1186/s12934-020-01334-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.