Graphical abstract
Keywords: Echocardiography, Cardiovascular magnetic resonance, Cancer
Highlights
-
•
Echocardiography is the modality of choice for initial cardiac tumor diagnosis.
-
•
CMR aids in tissue characterization, perfusion assessment, and delineating anatomy.
-
•
Intimal sarcomas are the most common primary cardiac sarcomas.
-
•
All intimal sarcomas exhibit MDM-2 gene overexpression and amplification.
-
•
Overall prognosis of intimal sarcomas is dismal.
Introduction
Primary cardiac tumors overall are extremely rare, with an annual incidence rate of 1.38 cases per 100,000 patients, with only 5% to 6% of primary cardiac tumors being malignant.1,2 As such, diagnosis and management of primary cardiac tumors, particularly malignant ones, can be challenging, and multimodal imaging can play an important role in the process.
Here we report the case of a young man who presented with vague neurologic symptoms and was ultimately found to have a metastatic primary cardiac intimal sarcoma. We describe the clinical presentation, the thought process behind the diagnostic approach, multimodal imaging, and management of the malignancy.
Case Presentation
A 35-year-old man with a history of left skull fracture (age 16 years) and traumatic brain injury (age 33 years) presented to the emergency department with a 1-week history of headache following a motor vehicle accident as a restrained driver. In addition, the patient had been having decreased concentration, blurry vision, short-term memory loss, and headaches for several weeks.
Vital signs in the emergency department were normal, and physical examination was unremarkable, but neurologic examination showed right homonymous hemianopia. A computed tomographic scan of the head without intravenous contrast and showed a large cystic mass located in the left posterior cerebral hemisphere with associated edema, and midline shift (Figure 1). Magnetic resonance imaging of the head confirmed a 7.0-cm peripherally enhancing, partially cystic mass in the left occipital lobe with a 7-mm midline shift, along with several other small foci of enhancement in the cerebral hemispheres bilaterally (Figure 1). These findings were concerning for metastatic disease.
Figure 1.
(A) Initial CT scan of the head showing left occipital brain tumor with a 7-mm rightward shift. (B) T2-weighted brain magnetic resonance imaging showing a 7-cm peripherally enhancing lesion (blue arrow), with associated significant mass effect on the occipital horn of the left lateral ventricle (red arrow). (C) Diffusion-weighted image showing a partially cystic mass (blue arrow).
Investigation
To determine the primary tumor location, a computed tomographic scan of the chest, abdomen, and pelvis with intravenous contrast was done. The results showed a large nonenhancing left atrial mass originating from the left atrial appendage (LAA) with protrusion into the left ventricle (Figure 2). The remainder of the computed tomographic results were negative for other locations of metastases.
Figure 2.
Contrast-enhanced chest computed tomography showing nonenhancing left atrial mass protruding into the left ventricle on coronal (A) and axial (B) views (blue arrows).
Transthoracic echocardiography with an ultrasound enhancing agent was subsequently performed and showed a 7.0-cm mass located in the left atrium that appeared to originate from the left upper pulmonary vein. The mass protruded into the left ventricle through the mitral valve, causing severe functional mitral stenosis (mean mitral gradient, 15 mm Hg). The mass did not enhance after administration of the ultrasound enhancing agent (Figure 3, Videos 1 and 2). The patient otherwise had normal biventricular and valvular structure and function.
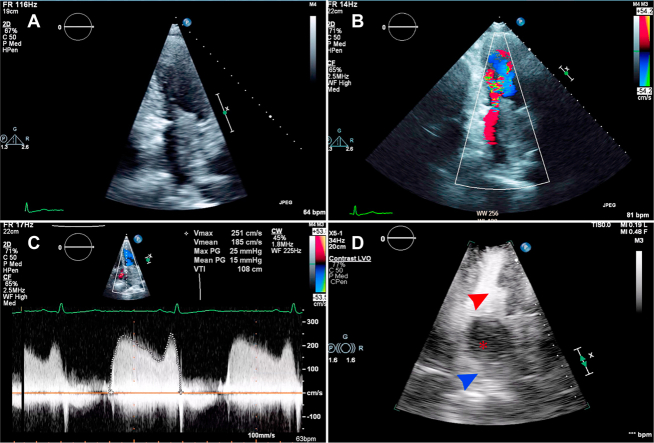
Figure 3.
(A) Two-dimensional transthoracic echocardiography, narrow-sector, left heart–focused apical four-chamber view, diastolic phase, showing left atrial mass originating from the area of the left pulmonary veins. Color Doppler (B) shows significant flow acceleration around the mass, with severe functional mitral stenosis indicated on continuous wave Doppler (C). (D) The tumor does not enhance with ultrasound enhancing agent (asterisk indicates mass, blue arrowhead indicates left atrium, and red arrowhead indicates left ventricle).
At this point, given the metastatic brain lesions, there was a high suspicion that the mass was a primary cardiac malignant tumor with resultant metastases to the brain, but the lack of enhancement of the mass was also concerning for thrombus. In addition, cardiac myxoma was not completely excluded, as there had been case reports of rare metastatic cardiac myxoma,3 but the location of the tumor and presentation were very atypical. Therefore, to further define anatomy, structure, and composition of the tumor, cardiovascular magnetic resonance imaging (CMR) was performed. CMR showed a heterogenous mass in the left atrium arising from the LAA. The more basal portion of the mass arising from the LAA did not show early perfusion but did have late gadolinium enhancement and high T1 and T2 signal. The remainder of the mass did not show any perfusion or late gadolinium enhancement (Figure 4, Videos 3 and 4). These findings were thought more likely to represent a left atrial myxoma arising from the LAA with a layered thrombus at various stages of development as opposed to cardiac sarcoma, given the lack of early perfusion or vascularity in the majority of the mass.
Figure 4.
CMR. (A) T2 image showing heterogenous mass in the left atrium originating from the LAA and extending into the left ventricle through the mitral valve. (B) The tumor does not show any early perfusion after contrast injection. The proximal portion of the tumor near the LAA shows late gadolinium enhancement (C, red arrow).
Management and Outcome
To avoid hemodynamic complications during neurosurgery, and to prevent delaying cardiac tumor resection, which would have to occur several weeks later if the brain mass was resected first, the decision was to proceed with the resection of the cardiac tumor. Cardiac surgery was uncomplicated, and findings were of a large mass originating from the area of the LAA and occupying most of the left atrium. The mass was attached in its anterior portion to the confluence of the left pulmonary veins, without attachment to the mitral valve or annulus (Figure 5).
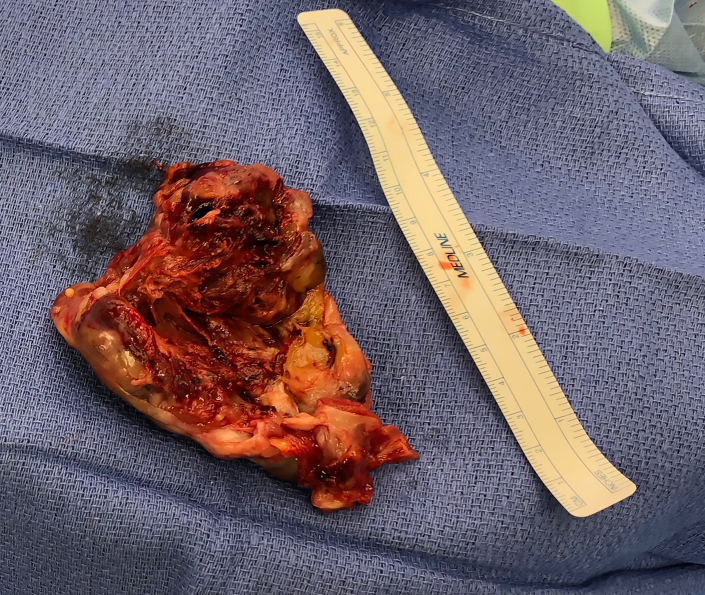
Figure 5.
Large gross resected tumor with a predominantly necrotic core and gross vascular thrombosis.
Intraoperative frozen sections showed a high-grade pleomorphic malignancy consistent with sarcoma. Final surgical pathology showed a pleomorphic spindle cell and epithelioid sarcomatoid high-grade tumor with myxoid matrix showing abundant mitotic activity and extensive necrosis. The tumor was positive for vimentin and smooth muscle actin by immunohistochemistry. Fluorescent in situ hybridization analysis showed MDM-2 gene amplifications. These findings were consistent with intimal sarcoma (Figure 6).
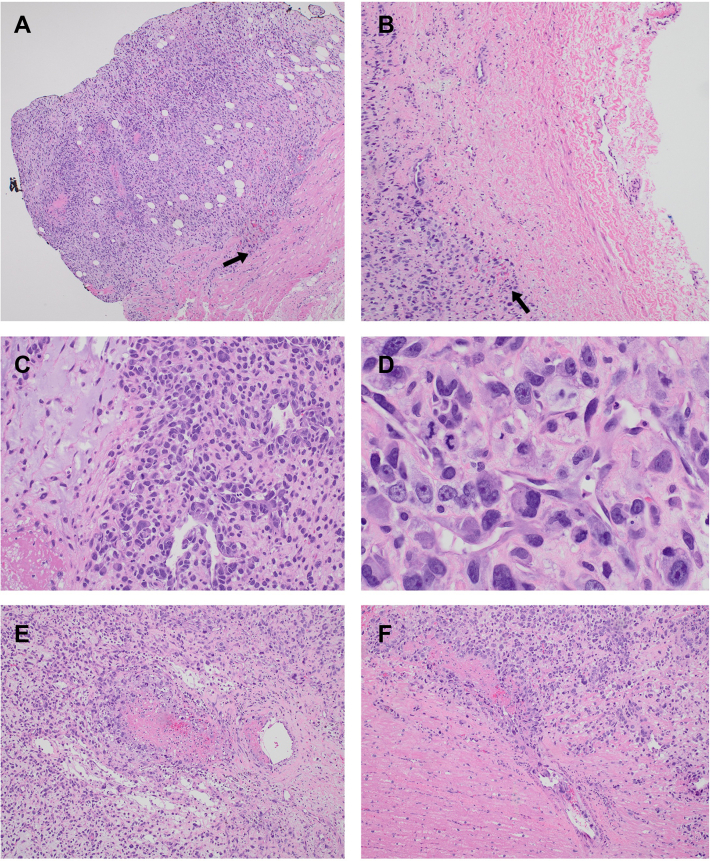
Figure 6.
Hematoxylin and eosin–stained histopathologic sections of the intimal sarcoma. The top row shows the intimal sarcoma invading into the left atrial myocardium (A, black arrow; 4× magnification), and pericardium (B, black arrow; 10× magnification). (C) Focal myxoid stromal production and vasoformative intravascular angiosarcoma pattern (20× magnification). (D) Large, high-grade, pleomorphic epithelioid tumor cells with multinucleation, prominent nucleoli, and mitotic figures (40× magnification). Malignant cells invade vessels causing thrombosis and occlusion: 10× magnification (E) and 20× magnification (F).
The patient subsequently underwent resection of the large brain mass 8 days later without complication, and the histology of the brain mass was identical to that of the primary cardiac malignancy.
The patient was discharged home and is planned to start systemic therapy with ifosfamide and radiation therapy for 6 weeks, followed by ifosfamide and doxorubicin for a total of 6 months of therapy.
Discussion
Our patient presented with nonspecific central nervous system symptoms related to the metastatic brain lesions but did not have any cardiovascular symptoms despite the large size of the primary tumor and the associated severe functional mitral stenosis. Multimodality cardiac imaging, particularly CMR, is fundamental to understand the anatomic location, composition, vascularity, and physiologic impact of cardiac masses and to aid in surgical planning. However, imaging results were not definitive, and surgical pathology was necessary for confirmation of diagnosis. In this case, the mass had typical CMR characteristics of cardiac myxoma, including the tumor’s lack of first-pass perfusion and late gadolinium enhancement.2 However, the size, irregular appearance, metastatic lesions, and atypical location suggested a malignant neoplasm. Gross examination showed that the tumor had a large necrotic core, and histopathologic examination revealed evidence of angiogenesis along with extensive vascular thrombosis explaining the necrotic core and the absence of first-pass perfusion on cardiac imaging. This finding in intimal sarcoma is in contrast to angiosarcomas, which generally have normal to accelerated first-pass perfusion on imaging compared to normal myocardium.2
Finally, the surgical decision was not straightforward in our patient and required discussion between cardiothoracic surgery and neurosurgery. The concern with resection of the cardiac tumor prior to the brain tumor is intracranial bleeding while on cardiopulmonary bypass. However, the concern with resecting the brain tumor first was hemodynamic compromise secondary to functional mitral stenosis and the need to delay cardiac surgery for several weeks after neurosurgery to prevent bleeding into the tumor bed. Ultimately, the cardiac tumor was resected first without any complications.
Cardiac sarcomas have a poor overall prognosis, with an associated 5-year survival rate of 14%.2 Traditionally, angiosarcomas were considered the most common type of primary cardiac sarcoma, but this has changed following the 2015 World Health Organization classification for cardiac tumors. Intimal sarcoma has been proven to be the most common primary cardiac sarcoma in a study by Neuville et al.4 Intimal sarcomas are malignant, poorly differentiated sarcomas made up of pleomorphic/atypical spindle cells and may contain areas with myxoid or epithelioid morphology. They typically arise in the large vessels of the heart or in the left atrium. All intimal sarcomas show overexpression, and amplification of the MDM-2 gene, an E3 ubiquitin protein ligase gene.4,5 Treatment is ideally with complete surgical resection, but usually this is not possible given the location of the tumor and invasion into nearby tissues. Adjuvant anthracycline-based chemotherapy is associated with a 38% response rate, although prognosis continues to be dismal with recurrence-free survival of 14.6 months in patients with localized disease and progression-free survival of 7.7 months in patients with advanced disease.6 Targeted therapy against MDM-2 has shown some promise in small clinical studies and could be a potential therapeutic option in the future.7,8
Conclusion
The diagnosis of primary cardiac tumors can be challenging, and multimodality cardiac imaging, particularly CMR, is indispensable in confirming the diagnosis and identifying the type of tumor with its ability for tissue characterization. However, in many cases of malignant tumors, especially sarcomas, surgical and histopathologic diagnosis remains the gold standard, particularly in cases in which rapid proliferation has led to central core necrosis of the tumor.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
The authors declare that since this was a non-interventional, retrospective, observational study utilizing de-identified data, informed consent was not required from the patient under an IRB exemption status.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Footnotes
Given his role as Editor-in-Chief, Vincent Sorrell, MD, FASE, had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Chittur A. Sivaram, MD, FASE.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2023.01.008.
Supplementary Data
Two-dimensional transthoracic echocardiography, apical four-chamber view with color Doppler, showing the large left atrial mass protruding into the left ventricle with flow acceleration.
Two-dimensional transthoracic echocardiography, apical four-chamber view with ultrasound enhancing agent, showing nonenhancing left atrial tumor.
CMR perfusion imaging showing lack of tumor perfusion (two-chamber view).
CMR perfusion imaging showing lack of tumor perfusion (four-chamber view).
References
- 1.Cresti A., Chiavarelli M., Glauber M., et al. Incidence rate of primary cardiac tumors: a 14-year population study. J Cardiovasc Med (Hagerstown) 2016;17:37–43. doi: 10.2459/JCM.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 2.Tyebally S., Chen D., Bhattacharyya S., et al. Cardiac tumors: JACC CardioOncology state-of-the-art review. JACC CardioOncol. 2020;2:293–311. doi: 10.1016/j.jaccao.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinjikji W., Morris J.M., Brown R.D., et al. Neuroimaging findings in cardiac myxoma patients: a single-center case series of 47 patients. Cerebrovasc Dis. 2015;40:35–44. doi: 10.1159/000381833. [DOI] [PubMed] [Google Scholar]
- 4.Neuville A., Collin F., Bruneval P., et al. Intimal sarcoma is the most frequent primary cardiac sarcoma: clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas. Am J Surg Pathol. 2014;38:461–469. doi: 10.1097/PAS.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 5.Burke A., Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol. 2016;11:441–452. doi: 10.1016/j.jtho.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Frezza A.M., Assi T., Lo Vullo S., et al. Systemic treatments in MDM2 positive intimal sarcoma: a multicentre experience with anthracycline, gemcitabine, and pazopanib within the World Sarcoma Network. Cancer. 2020;126:98–104. doi: 10.1002/cncr.32508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray-Coquard I., Blay J.Y., Italiano A., et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol. 2012;13:1133–1140. doi: 10.1016/S1470-2045(12)70474-6. [DOI] [PubMed] [Google Scholar]
- 8.Tovar C., Graves B., Packman K., et al. MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res. 2013;73:2587–2597. doi: 10.1158/0008-5472.CAN-12-2807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional transthoracic echocardiography, apical four-chamber view with color Doppler, showing the large left atrial mass protruding into the left ventricle with flow acceleration.
Two-dimensional transthoracic echocardiography, apical four-chamber view with ultrasound enhancing agent, showing nonenhancing left atrial tumor.
CMR perfusion imaging showing lack of tumor perfusion (two-chamber view).
CMR perfusion imaging showing lack of tumor perfusion (four-chamber view).