Graphical abstract
Keywords: Mitral stenosis, Mitral annular calcification, Degenerative mitral disease, Echocardiography, Multimodality imaging
Highlights
-
•
DMS has a high morbidity and a 5-year mortality rate of >50%.
-
•
DMS frequently occurs as mixed mitral disease as well as multivalvular disease.
-
•
Assessment of severity requires use of TTE, TEE, and stress echocardiography.
-
•
CT is used for periprocedural planning.
-
•
Treatment can be surgical or transcatheter.
Introduction
Due to improvements in living conditions, greater access to health care, and the widespread availability of antimicrobials, the prevalence of rheumatic heart disease has declined significantly in the developed countries.1 Additionally, with increasing life expectancy, age-related or degenerative forms of valvular disease have become increasingly prevalent. This includes mitral annular calcification (MAC) and degenerative mitral stenosis (DMS). Mitral annular calcification results from progressive calcification of the fibrous mitral annulus (particularly the posterior annulus). The prevalence of MAC in the general population is estimated to be around 10% based on data from the Multi-Ethnic study of Atherosclerosis but has been found to be closer to 22% in patients referred for indicated echocardiography.2, 3, 4 Prevalence increases with age, with one study reporting that 42% of its cohort with a mean age of 76 years had MAC.5 Degenerative mitral stenosis, also known as calcific mitral stenosis (MS), occurs via progression of this calcification along the mitral annulus, causing degeneration of the fibrous mitral support.6 Six to eight percent of patients with MAC are found to have MS.7,8 In this case series, we aim to provide an overview of the epidemiology of DMS and highlight the challenges of echocardiographic evaluation and management.
Case Presentation 1: Multimodality Imaging for DMS
A 70-year-old woman with a history of type 1 diabetes mellitus (DM), hypertension, dyslipidemia, stage 4 chronic kidney disease (CKD), and breast cancer (treated 5 years ago with left breast lumpectomy and radiation therapy) was admitted with an episode of acute decompensated heart failure and was found on transthoracic echocardiography (TTE) to have a normal ejection fraction. The patient was found to have hyperdynamic left ventricular (LV) function with a mean transmitral gradient (TMG) of 10 mm Hg at a heart rate (HR) of 74 bpm. There was severe MAC with leaflet calcification. There was mild mitral regurgitation by color Doppler and no other significant valvular disease. The patient was treated with intravenous diuretics with improvement in symptoms. A month after discharge a repeat TTE revealed a mean TMG of 6.3 (HR = 62 bpm). These findings raised the following questions: (1) What is the etiology of MS and (2) how severe is the valvular stenosis?
A review of the patient’s TTE reveals severe MAC (on visual assessment of two-dimensional [2D] images) with calcification of the mitral valve (MV) leaflets and with restricted leaflet motion (Figure 1, Videos 1 and 2). The patient had multiple risk factors for DMS including CKD, hypertension, and left-sided radiation therapy. However, a TTE 6 years prior to the diagnosis of breast cancer demonstrated moderate MAC with a mean TMG of 6.6 mm Hg (HR not available). Therefore, the valve morphology and disease progression were consistent with DMS likely secondary to CKD and hypertension. On the index TTE (during inpatient hospitalization), the pressure half time–derived valve area was 1.7 cm2 and using an MV velocity-time integral (VTI) of 74 cm and an LV VTI of 31 cm, the MV doppler velocity index (DVI) was 0.42 (Figure 1). On the subsequent TTE, the mean TMG was lower (6.3 mm Hg with an HR of 62 bpm) with an MV DVI of 0.44. These DVI values are suggestive of a valve area <1.5 cm2. Given significant mitral leaflet and LV outflow tract (LVOT) calcification, an accurate LVOT measurement could not be obtained to allow for continuity-derived MV area (MVA) calculation; therefore, a transesophageal echocardiogram (TEE) was performed (Video 3). The LVOT was measured on the TEE as 1.8 cm (Figure 2). Of note, this measurement was obtained by carefully excluding the calcification on the anterior MV leaflet. To obtain accurate LVOT velocities, supplementary TTE images were performed. Using an LVOT diameter of 1.8 cm, LV VTI of 34, and MV VTI of 55, the following calculations were performed:
Figure 1.
Two-dimensional TTE zoomed apical 4-chamber view, without (left) and with (right) color flow Doppler of the mitral inflow, demonstrates severe MAC (∗) and leaflet calcification (#) with associated proximal diastolic flow acceleration (A). Continuous-wave Doppler spectrum demonstrates an increased mean TMG of 10 mm Hg (B).
Figure 2.
Two-dimensional TEE midesophageal apical 4-chamber view (0°), diastolic phase, demonstrates severe MAC (∗) with calcification of the mitral leaflets (#) and restricted leaflet opening (A). Continuous-wave Doppler spectrum across the mitral inflow demonstrates a normal TMG of 3 mm Hg (B). Spectral ghosting (mirroring artifact) is seen and should be avoided when tracing. The LVOT was measured in the apical 3-chamber, midsystolic view (120°) inner edge to inner edge at the hinge points (blue double arrow) while avoiding the calcification (∗) on the anterior mitral leaflet (C).
DVI = LV VTI/MV VTI = 0.6.
MVA = LVOT VTI ∗ LVOT area/MV VTI = 34 ∗ 2.5/55 = 1.54.
We also performed three-dimensional (3D) planimetry to derive an MVA by obtaining a 3D MV data set in the 3-chamber midesophageal view (Figure 3, Video 4). Using multiplanar reconstruction, the cross-sectional planimetered MVA was 1.6 cm2. We further conducted a recumbent bike stress test, where this patient was able to exercise for a total of 2 minutes 49 seconds (reaching 44% of the maximum predicted HR). The test was terminated secondary to limiting symptoms (dyspnea). The mean TMG increased from 5 mm Hg at rest (HR = 58 bpm) to 7 mm Hg (HR = 66 bpm) with a peak estimated right ventricular systolic pressure of 33 mm Hg. These findings suggest nonsevere MS based on criteria by the American Society of Echocardiography.9 Together, these findings are suggestive of significant but nonsevere MS (moderate MS), with the patient’s symptoms of exertional dyspnea unlikely to be entirely related to MS.
Figure 3.
Three-dimensional TEE acquisition and multiplanar, diastolic reconstruction demonstrates the MV leaflets in 2 orthogonal planes (A, B), short-axis leaflet tip display (C), and volume-rendered reconstruction in the surgeon's view orientation from the perspective of the LA (D).
Case Presentation 2: Assessment of Severity of DMS With Concomitant Severe Aortic Stenosis
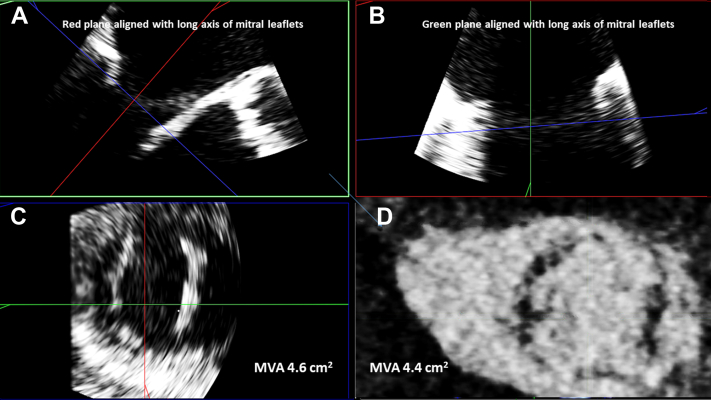
A 74-year-old man with a history of coronary artery disease (CAD), hypertension, dyslipidemia, stage 4 CKD, type 2 DM, and peripheral arterial disease with multiple recent hospitalizations for decompensated heart failure with preserved ejection fraction presented to the valve clinic for evaluation of known severe aortic stenosis (AS). An outpatient TTE was performed that revealed severe AS (valve area = 0.8 cm2, mean gradient = 55 mm Hg, and maximum velocity = 4.7 m/sec). There was mild MAC (on visual assessment of 2D images) without any leaflet calcification and a mean TMG of 9 mm Hg (118 bpm; Figure 4, Videos 5 and 6). The patient was a high-risk surgical candidate, and therefore the severity of MS needed to be more clearly delineated to determine the necessity of MV intervention. If only an aortic valve replacement (AVR) was needed, transcatheter aortic valve implantation (TAVI) would be appropriate for this patient. However, if the MS was significant, a surgical approach would have been favored. A more in-depth evaluation of the index TTE showed a continuity equation-derived MVA of 3.0 cm2 (LVOT diameter of 2.2 cm, LVOT VTI of 74, and MV VTI of 27). A TEE revealed normal biventricular size and function. The MV was noted to have mild MAC and a mean TMG of 3 mm Hg (HR = 73 bpm) with mild mitral regurgitation. Three-dimensional planimetry revealed an MVA of 4.6 cm2 (Figure 5, Videos 7 and 8). Additionally, a contrast-enhanced cardiac computed tomography (CCT) TAVI study revealed a planimetered MVA of 4.5 (60% RR interval). Based on these additional data, MS was not deemed to be severe. This patient subsequently underwent successful TAVI and was noted to have a mean TMG of 4 mm Hg (HR = 74 bpm) 1 month later.
Figure 4.
Two-dimensional TTE parasternal long-axis view (A) and apical 4-chamber (B) diastolic images demonstrate moderate posterior MAC (∗). The continuous-wave Doppler spectral display of the mitral inflow obtained from the apical 4-chamber view demonstrates an increased TMG of 9 mm Hg (C).
Figure 5.
Three-dimensional TEE acquisition and multiplanar, diastolic reconstruction demonstrates the MV leaflets in 2 orthogonal planes (A, B) and short-axis leaflet tip display (C), which allows for direct planimetry (MVA = 4.4 cm2). A corresponding CCT-derived short-axis slice of the MV leaflet tips (D) during diastole demonstrates a similar MVA by planimetry (MVA = 4.4 cm2).
Case Presentation 3: Assessment and Management of Concomitant Severe AS and Severe DMS
A 68-year-old man with a multivessel CAD, type 2 DM, chronic obstructive pulmonary disease, and gastroesophageal adenocarcinoma with recent radiation and chemotherapy was referred to the valve clinic for combined MS and AS. An outpatient TTE revealed normal biventricular function with a low stroke volume (SVindexed = 34.8 mL/m2) with severe low-flow low-gradient AS (valve area 0.8 cm2). There was severe MAC and calcification of the anterior mitral leaflet with restriction in motion of both leaflets. The mean TMG was 11 mm Hg (HR = 84 bpm), and the continuity-derived MVA was 1.0 cm2 (Video 9). Further evaluation was done with a retrospective gated CCT, revealing a planimetered MVA of 1.1 cm2, further suggesting severe DMS. Given the high surgical risk for dual-valve surgery, this case was discussed in a multidisciplinary valve conference that included cardiologists and cardiothoracic surgeons. A percutaneous transcatheter mitral valve replacement (TMVR) in MAC was considered, but they were not a good candidate due to the lack of a circumferential ring of calcium. The patient is being considered for a hybrid procedure with TAVI followed by staged surgical valve-in-MAC. This case highlights the recognition of true severe MS and the need for a multidisciplinary approach while dealing with mixed and multiple valvular disease.
Case Presentation 4: Guiding Decisions for Combined Valve Surgery
An 84-year-old man with a history of hypertension, end-stage renal disease with subsequent renal transplant, and peripheral arterial disease presented to our clinic with known mixed mitral and aortic disease. The outpatient TTE revealed normal biventricular size and function. There was noted to be severe AS by Doppler interrogation (valve area = 0.7 cm2, aortic Vmax = 4 m/sec, and mean gradient = 35 mm Hg), severe MAC (on visual assessment of 2D images) with leaflet calcification, and restricted leaflet motion. The mean mitral gradient was 11 mm Hg (HR = 86 bpm) with moderate mitral regurgitation. Given the presence of moderate to severe mitral regurgitation by Doppler interrogation, a continuity-derived MVA was not possible. The DVI using an LV VTI of 15 cm and MV VTI of 56 cm was 0.26, suggestive of very severe DMS (with a correlated valve area of <1 cm2). After a multidisciplinary discussion, a decision was made to proceed with open surgical AVR and transatrial TMVR. After placing a 21 mm St. Jude Trifecta valve in the aortic position, the MV was inspected and the anterior mitral leaflet was resected. Thereafter, a stented 28 mm Edwards Sapien valve was inflated and deployed in the mitral position. Surgical pathology of the resected leaflet revealed nodular dystrophic calcification. The patient had significant improvement in symptoms.
Discussion
Epidemiology/Pathophysiology
In the European Heart Survey, 13% of MS was from a degenerative etiology.10 It is also present in ∼25% patients with degenerative AS.11 Mitral annular calcification is more commonly found in elderly patients, patients with CKD, patients who received radiation therapy, and patients with increased LV pressures (including AS, hypertrophic obstructive cardiomyopathy, and hypertension).12 Mitral annular calcification also appears to be more common among women and Caucasians.
Pathophysiology and Natural History
The pathophysiology of DMS is thought to be similar to that of atherosclerosis. First, chronic valvular stress causes disruption of the endothelium, allowing for invasion of inflammatory cells and inflammatory mediators.12,13 This process subsequently leads to the differentiation and activation of myofibroblasts into osteoblast-type cells, which ultimately results in calcification of the annulus and valve.12,14 This explains why patients with elevated LV pressures and LV hypertrophy, including those with AS, MV prolapse, and hypertrophic obstructive cardiomyopathy, have a propensity for MAC, as their additional hemodynamic stress exerted upon valvular tissue causes inflammatory changes and expedites this process of dystrophic calcification.14
Early development of MAC typically involves the posterior annulus of the MV.15 Initially, MV leaflet motion is preserved with little impact on LV pressures.15 As the process progresses, calcification spreads inward, typically sparing the leaflet tips and commissures until late in the disease progression. Once this occurs, leaflet mobility becomes restricted.15,16 In contrast, rheumatic MS typically involves early fusion of the commissures, particularly the leaflet tips, resulting in the valve having a funnel shape.16 Patients with severe MAC appear to be susceptible to rapid progression as restricted motion of the mitral annulus impairs the folding motion of the valve, increasing the hemodynamic stress on the valve, leading to progression of calcification.12,14 Calcification is unpredictable and has the potential to expand in any direction. If calcification occurs inferiorly and medially, it can invade the myocardium and involve the atrioventricular node, causing conduction delays.14 Calcium can also expand superiorly into the atrium or inferiorly into the ventricle causing mitral regurgitation or even chordal rupture via increased tension, ultimately leading to MV prolapse.14 Rarely, calcium can dislodge, leading to systemic embolization.14
Based on current data limited to small single-center retrospective studies, DMS carries a poor prognosis, with a 5-year mortality rate of >50%.1,6 In another study, ∼75% of patients were symptomatic at the time of the index echocardiogram. An additional 10% of patients developed symptoms over a mean follow-up of 2.8 years. Event-free survival in these patients was as low as 53% at 1 year and 34% at 3 years, with 54% mortality over a mean follow-up of 2.8 years.6 was Figure 6 provides an overview of risk factors and epidemiology of DMS.
Figure 6.
Etiology, outcomes, and associations of DMS. ESRD, End-stage renal disease; LV, left ventricle; HOCM, hypertrophic cardiomyopathy; MR, mitral regurgitation; TR, tricuspid regurgitation.
Evaluation of DMS
Evaluation of DMS is challenging. Echocardiography, in particular TTE, is usually the first imaging modality to be obtained. Table 1 provides an overview of the echocardiographic parameters utilized for assessment of DMS.
Table 1.
| Imaging modality | Traditional strengths | Pitfalls in MAC |
|---|---|---|
| 2D planimetry | Ability to directly measure MVA Historically accurate correlations with Gorlin's hydraulic formula and with directly measured anatomic orifices in explanted valves |
Acoustic shadowing from calcifications may hinder measurement Optimal timing and optimal positioning of the MV orifice requires operator experience. |
| 3D planimetry from multiplanar reconstruction | Good correlation with continuity-derived valve area Can be used in mixed regurgitant and stenotic disease Avoids assumptions of continuity equation |
Acoustic shadowing from calcifications may hinder measurement Generally, requires TEE, which is semi-invasive Technical expertise and operator dependent |
| Pressure half time | Easy to perform | May overestimate valve area secondary to diastolic dysfunction |
| Continuity equation | Fundamentally accurate and favored method in absence of aortic or mitral regurgitation Transmitral flow-independent modality |
Inaccuracy in setting of arrhythmias (especially atrial fibrillation) Limitations with concomitant aortic or mitral regurgitation Accuracy and reproducibility are hampered by number of measurements and increasing impact of measurement error Calcification of the aortomitral curtain hinders accurate assessment of the LVOT diameter |
| Transmitral gradient | Easy to perform | Flow dependent and varies with HR Tends to overestimate severity of stenosis |
| Dimensionless index | Less prone to measurement error than the continuity equation | Further studies and validation of cutoffs are needed |
The TMG, while commonly used, can be misleading in DMS. There are many factors that adversely affect the ability to accurately assess MS severity. First, unlike in rheumatic MS where there is greatest narrowing at the leaflet tips (resulting in a funnel-shaped or cone-shaped narrowing), the valve in DMS is primarily restricted at the annulus and the base of the leaflets, leading to relatively unrestricted motion of the leaflet tip.16 Therefore, the “y descent” (early ventricular filling) is blunted in rheumatic MS, whereas in DMS, there is usually a tall V wave (passive venous filling of the left atrium [LA]) with a sharp y descent. A tall V wave may result in a very high TMG, which is not truly representative of the degree of valve stenosis. The echocardiographic analogues of these catheterization findings are a tall mitral E-wave with rapid E-wave deceleration. Additionally, patients with DMS often have multiple comorbidities such as diabetes, hypertension, and CAD, all of which are associated with LV diastolic dysfunction as well as poor left atrial (LA) compliance.16,21 Together, LV diastolic dysfunction and poor LA compliance can result in an elevated V wave and therefore TMG, which may be increased further in the presence of MAC and DMS. Therefore, even a nonsignificant stenosis can result in a significant TMG. On the other hand, it is possible that a reduced LV stroke volume, such as in elderly individuals with small end-diastolic volumes and concentric hypertrophy, may result in a lower TMG. Reduced mitral annular excursion may also play a role in decreasing LV stroke volume.9,14 Cases 1 and 2 highlight the variability of TMG with hemodynamic conditions and therefore its fallacy as the sole criterion for the assessment of severity of DMS.
Pressure half time for the estimation of MVA has a level 1 recommendation for use in rheumatic MS. However, the guidelines note that deceleration time (and in turn pressure half time) will be prolonged with impaired LV relaxation and shortened when ventricular compliance is poor. Therefore, this method is not reliable in patients with impaired diastolic function such as DMS.19 Pressure half time is usually shortened in these patients, resulting in overestimation of the MVA.6,14
Direct 2D planimetry measurement of MVA is challenging given acoustic shadowing from annular and leaflet calcification.6,22 However, we have found 3D planimetry to be helpful and feasible in a majority of the patients. It is important to acquire high temporal resolution (multiple beats if necessary) images, carefully creating a data set that includes the entirety of the MV apparatus (from just above the annulus to just below the leaflet tips on the ventricular side). Thereafter, the mid-diastolic phase with the largest MV opening is carefully selected. Using multiplanar reconstruction, the cross-sectional plane is aligned to the leaflet tips (narrowest portion of the annular leaflet complex) in the 2 orthogonal views. Gain settings are adjusted to visualize the leaflet tips without overgaining. In our experience, optimizing the gain settings during postprocessing is especially helpful. Additionally, obtaining higher gain images during 3D acquisition is helpful as it allows for better gain adjustment during postprocessing. This method may be technically challenging due to acoustic shadowing in some patients. Therefore, in the absence of significant (greater than mild) mitral or aortic regurgitation, the continuity equation is used for assessment of MVA. The accuracy and reproducibility of the continuity equation are attenuated by the number of measurements and resulting combined impact of individual measurement errors.19 Arrythmias such as atrial fibrillation also decrease the accuracy of measurement significantly. Lastly, measurement of the LVOT diameter can be inaccurate in the presence of calcification of the aortomitral curtain, which may also make the LVOT noncircular and therefore make the continuity equation invalid. Some of these errors can be addressed by using an MV DVI (analogous to the aortic valve dimensionless index). This is derived by dividing the LVOT VTI by the MV inflow VTI. In a study of 64 patients with DMS, a DVI of 0.31 ± 0.04 was correlated with a valve area ≤ 1 cm2, and a 0.43 ± 0.07 was correlated with a valve area between 1 and 1.5 cm2.23 Further validation of cutoff is needed before routine use of this metric.
Grading MAC severity is critical in understanding the pathophysiology of DMS and in determining treatment options. While there are several approaches for grading the severity of MAC, we rely on the classification proposed by Eleid et al.14 (Table 2). Of note, MAC is easily visible on both contrast and noncontrast computed tomography (CT) scans. However, contrast allows for delineation of leaflets and myocardium and differentiates from blood.15 Electrocardiogram gating is preferred to reduce motion artifact, allowing for multiphasic images and highlighting the motion of leaflets and annulus.15 Figure 7 provides an algorithmic approach for imaging evaluation of DMS.
Table 2.
Proposed system for grading the severity of degenerative MV stenosis using echo and CT (adapted from Eleid et al.15)
| MAC grading | Annular calcification | Extra-annular calcification |
|---|---|---|
| Mild | Focal noncontiguous calcification limited to <180° total annular circumference. | None |
| Moderate | Dense continuous calcification limited to <270° total annular circumference. | Posterior and/or anterior leaflet calcification may be present. |
| Severe | Dense continuous calcification extending past the commissures or complete circumferential annular calcification (≥270° calcification arc) | Posterior and/or anterior leaflet calcification may be present. Papillary muscle or ventricular myocardial calcification may be present. |
Figure 7.
Proposed algorithm featuring summary of steps for echocardiographic evaluation of DMS. CW, Continuous wave.
Treatment
Treatment of DMS remains controversial, and current guidelines provide limited recommendations on the assessment and management of patients with DMS.6 Most of the data currently applied to these patients come from rheumatic MS.24 Strategies in medical management of symptomatic DMS are aimed at volume optimization with diuretics, HR control with β-blockers or calcium channel blockers, risk factor modification, prevention of thromboembolism, and control of atrial arrhythmias.14,25 While medical therapy may transiently improve symptoms, it neither slows nor reverses the progression of calcification.8 Surgical interventions are typically offered only to patients with severe, symptomatic MS who are not adequately controlled with medical management.14 This is due to the high morbidity and mortality associated with surgical interventions, largely a result of an elderly patient population with high prevalence of comorbidities such as CAD, hypertension, and CKD as well as surgical considerations related to the difficulty of annular debridement.
Determining who will benefit from intervention for DMS remains challenging. Determining the degree of true inflow obstruction (and differentiating it from a high TMG secondary to poor LA compliance and diastolic dysfunction) is essential.16 Preoperative pulsed-wave Doppler MV inflow showing E < A with prolonged deceleration of the E wave may be reassuring as it suggests that gradient is not secondary to poor LA compliance.14 Prior retrospective data show that a TMG ≥ 8 mm Hg and right ventricular systolic pressure ≥ 50 mm Hg were associated with higher all-cause mortality independent of comorbidities, signifying that patients with these parameters may benefit from intervention.6 In cases of diagnostic uncertainty, an invasive hemodynamic assessment with measurement of accurate LA and LV pressures along with evaluation of the contours of the v wave and y descent may be helpful. Additionally, provocative maneuvers such as exercise and nitroprusside can bring out the relative contribution of the MV to the elevation in LA pressure.16
Surgical mitral valvular replacement (SMVR), with or without calcium resection, has traditionally been the method of choice for treating patients with symptomatic DMS. The gold standard surgical technique for SMVR is standard annular debridement with reconstruction.15 In cases of MAC with concurrent severe mitral regurgitation (MR), intra-atrial placement of a mitral prosthesis has been investigated.15 There is also growing use of experimental therapy with LA-to-LV apical conduits by means of MV bypass.15 Although SMVR is the mainstay of MVR treatment, there have been significant advancements in TMVR with use of aortic transcatheter heart valves in the mitral position. In a retrospective registry of 521 patients undergoing TMVR, those undergoing valve-in-valve TMVR had procedural success in three-fourths of the cases, with a 30-day mortality of 6%. However, the mortality was significantly higher for valve-in-ring (10%) and even higher for valve-in-MAC (35%). The 1-year mortality was similarly higher for valve-in-MAC (63%) as compared with valve-in-ring (31%) and valve-in-valve (14%). Twenty-two percent of the patients with valve-in-MAC required reintervention.26 In the prospective MITRAL (Mitral Implantation of Transcatheter Valves) trial, 92 patients were presented for case review, of which only 31 were enrolled for valve-in-MAC intervention.27 The exclusions were mainly secondary to the risk of LVOT obstruction as well as risk for embolization. Most of these patients either had DMS or combined DMS and mitral regurgitation. The in-hospital mortality was 16% with a technical success rate of 74%. The 30-day mortality was 17%, and the 1-year mortality was 35%. At 30 days, the mean mitral gradient was 6 mm Hg, and only 1 patient had 3+ mitral regurgitation. The authors attribute the improved outcomes to improved patient selection as well as use of strategies such as preemptive alcohol septal ablation for reduction in risk of LVOT obstruction. The scope of transcatheter interventions is evolving. The MITRAL II trial will study the safety and effectiveness of transseptal valve-in-MAC with the SAPIEN 3 Ultra THC in patients at high surgical risk.
Conclusion
The prevalence of DMS is increasing in the developed world due to increased life expectancy and risk factors for cardiac structural calcification. Transmitral mean gradient is dependent on hemodynamic conditions and is unreliable as the sole criterion for assessment of severity. A multiparametric algorithmic approach with reliance on the continuity equation and direct planimetry (2D or 3D) utilizing a combination of TTE, TEE, and stress echocardiography along with CT imaging is essential for assessment of severity and determining need and candidacy for valve intervention.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
Patients were included in an existing IRB approval number PRO00043184.
Funding Statement
The authors declare that this report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2022.12.014.
Supplementary Data
Two-dimensional TTE, apical 2-chamber view without (left) and with (right) color flow Doppler, demonstrates mitral annular and leaflet calcification as well as restricted leaflet motion with diastolic flow acceleration across the MV exemplified by color flow aliasing and the formation of a proximal isovelocity surface area at the valve proximal to flow.
Two-dimensional TTE, apical 4-chamber view without (left) and with (right) color flow Doppler, demonstrates mitral annular and leaflet calcification as well as restricted leaflet motion with diastolic flow acceleration across the MV. In addition to aliasing of color flow, the inflow jet is visibly narrower than what would be expected for normal MV inflow. Left atrial enlargement is noted.
Two-dimensional TEE, midesophageal 4-chamber view (0°), demonstrates severe MAC that extends into the posterior MV leaflet involving ∼50% of the length and causing restricted leaflet motion. A mild degree of calcification of the mitral subvalvular chord is also noted.
Three-dimensional TEE view of the MV from the surgeon’s view looking down on the valve from the LA demonstrates bulky MAC extending into the posterior MV leaflet with commissural fusion. Leaflet motion is restricted, and the diastolic valve area is reduced.
Two-dimensional TTE, apical 4-chamber view demonstrating MAC without extension into the leaflets and normal leaflet motion. Normal right ventricular and LV systolic function is also seen.
Two-dimensional TTE, zoomed apical 4-chamber view without (left) and with (right) color flow Doppler, demonstrates MAC without leaflet involvement and normal leaflet motion. There is minimal proximal flow acceleration and a mild degree of mitral regurgitation.
Third-dimensional TEE, volume-rendered image in the surgeon’s view demonstrates posterior MAC with normal unrestricted leaflet mobility and normal commissures.
Two-dimensional TEE midesophageal, zoomed 4-chamber view (0°) without (left) and with (right) color flow Doppler, demonstrates unrestricted leaflet motion and normal color flow Doppler without flow acceleration and mild mitral regurgitation.
Two-dimensional TTE, zoomed 4-chamber view without (left) and with (right) color flow Doppler, demonstrates severe MAC involving the MV leaflets leading to significantly restricted mobility, notable aliasing and narrowing of the mitral inflow jet indicative of elevated inflow velocities, and a small diastolic valve area. There is also mild mitral regurgitation and LA dilation noted.
References
- 1.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., III, Gentile F., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2021;77:450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 2.Kato N., Guerrero M., Padang R., Amadio J.M., Eleid M.F., Scott C.G., et al. Prevalence and natural history of mitral annulus calcification and related valve dysfunction. Mayo Clin Proc. 2022;97:1094–1107. doi: 10.1016/j.mayocp.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura R.A., Vahanian A., Eleid M.F., Mack M.J. MV disease–current management and future challenges. Lancet. 2016;387:1324–1334. doi: 10.1016/S0140-6736(16)00558-4. [DOI] [PubMed] [Google Scholar]
- 4.Kanjanauthai S., Nasir K., Katz R., Rivera J.J., Takasu J., Blumenthal R.S., et al. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2010;213:558–562. doi: 10.1016/j.atherosclerosis.2010.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barasch E., Gottdiener J.S., Larsen E.K., Chaves P.H., Newman A.B., Manolio T.A. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS) Am Heart J. 2006;151:39–47. doi: 10.1016/j.ahj.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 6.Kato N., Padang R., Scott C.G., Guerrero M., Pislaru S.V., Pellikka P.A. The Natural history of severe calcific mitral stenosis. J Am Coll Cardiol. 2020;75:3048–3057. doi: 10.1016/j.jacc.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 7.Aronow W.S., Kronzon I. Correlation of prevalence and severity of mitral regurgitation and mitral stenosis determined by Doppler echocardiography with physical signs of mitral regurgitation and mitral stenosis in 100 patients aged 62 to 100 years with mitral anular calcium. Am J Cardiol. 1987;60:1189–1190. doi: 10.1016/0002-9149(87)90423-1. [DOI] [PubMed] [Google Scholar]
- 8.Labovitz A.J., Nelson J.G., Windhorst D.M., Kennedy H.L., Williams G.A. Frequency of MV dysfunction from mitral anular calcium as detected by Doppler echocardiography. Am J Cardiol. 1985;55:133–137. doi: 10.1016/0002-9149(85)90314-5. [DOI] [PubMed] [Google Scholar]
- 9.Carlhäll C., Wigström L., Heiberg E., Karlsson M., Bolger A.F., Nylander E. Contribution of mitral annular excursion and shape dynamics to total left ventricular volume change. Am J Physiol Heart Circ Physiol. 2004;287:H1836–H1841. doi: 10.1152/ajpheart.00103.2004. [DOI] [PubMed] [Google Scholar]
- 10.Iung B., Baron G., Butchart E.G., Delahaye F., Gohlke-Bärwolf C., Levang O.W., et al. A prospective survey of patients with valvular heart disease in Europe: the Euro heart Survey on valvular heart disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 11.Iwataki M., Takeuchi M., Otani K., Kuwaki H., Yoshitani H., Abe H., et al. Calcific extension towards the MV causes non-rheumatic mitral stenosis in degenerative aortic stenosis: real-time 3D transoesophageal echocardiography study. Open Heart. 2014;1:e000136. doi: 10.1136/openhrt-2014-000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmariah S., Budoff M.J., Delaney J.A., Hamirani Y., Eng J., Fuster V., et al. Risk factors associated with the incidence and progression of mitral annulus calcification: the multi-ethnic study of atherosclerosis. Am Heart J. 2013;166:904–912. doi: 10.1016/j.ahj.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sud K., Agarwal S., Parashar A., Raza M.Q., Patel K., Min D., et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation. 2016;133:1594–1604. doi: 10.1161/CIRCULATIONAHA.115.020185. [DOI] [PubMed] [Google Scholar]
- 14.Silbiger J.J. Mitral annular calcification and calcific mitral stenosis: role of echocardiography in hemodynamic assessment and management. J Am Soc Echocardiogr. 2021;34:923–931. doi: 10.1016/j.echo.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Eleid M.F., Foley T.A., Said S.M., Pislaru S.V., Rihal C.S. Severe mitral annular calcification: multimodality imaging for Therapeutic strategies and interventions. JACC Cardiovasc Imaging. 2016;9:1318–1337. doi: 10.1016/j.jcmg.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Reddy Y.N.V., Murgo J.P., Nishimura R.A. Complexity of defining severe "stenosis" from mitral annular calcification. Circulation. 2019;140:523–525. doi: 10.1161/CIRCULATIONAHA.119.040095. [DOI] [PubMed] [Google Scholar]
- 17.Nichol P.M., Gilbert B.W., Kisslo J.A. Two-dimensional echocardiographic assessment of mitral stenosis. Circulation. 1977;55:120–128. doi: 10.1161/01.cir.55.1.120. [DOI] [PubMed] [Google Scholar]
- 18.Faletra F., Pezzano A., Jr., Fusco R., Mantero A., Corno R., Crivellaro W., et al. Measurement of MV area in mitral stenosis: four echocardiographic methods compared with direct measurement of anatomic orifices. J Am Coll Cardiol. 1996;28:1190–1197. doi: 10.1016/S0735-1097(96)00326-9. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner H., Hung J., Bermejo J., Chambers J.B., Evangelista A., Griffin B.P., et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101-2. [DOI] [PubMed] [Google Scholar]
- 20.Chu J.W., Levine R.A., Chua S., Poh K.K., Morris E., Hua L., et al. Assessing MV area and orifice geometry in calcific mitral stenosis: a new solution by real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2008;21:1006–1009. doi: 10.1016/j.echo.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris D.A., Gailani M., Vaz Perez A., Blaschke F., Dietz R., Haverkamp W., et al. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:651–662. doi: 10.1016/j.echo.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Silbiger J.J. Pitfalls in the echocardiographic evaluation of mitral annular size, shape, and dynamics in patients with mitral annular calcification. J Am Soc Echocardiogr. 2015;28:1255–1256. doi: 10.1016/j.echo.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Oktay A.A., Riehl R., Kachur S., Khan Z., Tutor A., Chainani V., et al. Dimensionless index of the MV for evaluation of degenerative mitral stenosis. Echocardiography. 2020;37:1533–1542. doi: 10.1111/echo.14847. [DOI] [PubMed] [Google Scholar]
- 24.Bertrand P.B., Mihos C.G., Yucel E. Mitral annular calcification and calcific mitral stenosis: therapeutic challenges and considerations. Curr Treat Options Cardiovasc Med. 2019;214:19. doi: 10.1007/s11936-019-0723-6. [DOI] [PubMed] [Google Scholar]
- 25.Al-Taweel A., Almahmoud M.F., Khairandish Y., Ahmad M. Degenerative MV stenosis: diagnosis and management. Echocardiography. 2019;36:1901–1909. doi: 10.1111/echo.14495. [DOI] [PubMed] [Google Scholar]
- 26.Yoon S.-H., Whisenant B.K., Bleiziffer S., Delgado V., Dhoble A., Schofer N., et al. Outcomes of transcatheter MV replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2018;40:441–451. doi: 10.1093/eurheartj/ehy590. [DOI] [PubMed] [Google Scholar]
- 27.Guerrero M., Wang D.D., Eleid M.F., Pursnani A., Salinger M., Russell H.M., et al. Prospective study of TMVR using balloon-expandable aortic transcatheter valves in MAC: MITRAL trial 1-year outcomes. JACC Cardiovasc Interv. 2021;14:830–845. doi: 10.1016/j.jcin.2021.01.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE, apical 2-chamber view without (left) and with (right) color flow Doppler, demonstrates mitral annular and leaflet calcification as well as restricted leaflet motion with diastolic flow acceleration across the MV exemplified by color flow aliasing and the formation of a proximal isovelocity surface area at the valve proximal to flow.
Two-dimensional TTE, apical 4-chamber view without (left) and with (right) color flow Doppler, demonstrates mitral annular and leaflet calcification as well as restricted leaflet motion with diastolic flow acceleration across the MV. In addition to aliasing of color flow, the inflow jet is visibly narrower than what would be expected for normal MV inflow. Left atrial enlargement is noted.
Two-dimensional TEE, midesophageal 4-chamber view (0°), demonstrates severe MAC that extends into the posterior MV leaflet involving ∼50% of the length and causing restricted leaflet motion. A mild degree of calcification of the mitral subvalvular chord is also noted.
Three-dimensional TEE view of the MV from the surgeon’s view looking down on the valve from the LA demonstrates bulky MAC extending into the posterior MV leaflet with commissural fusion. Leaflet motion is restricted, and the diastolic valve area is reduced.
Two-dimensional TTE, apical 4-chamber view demonstrating MAC without extension into the leaflets and normal leaflet motion. Normal right ventricular and LV systolic function is also seen.
Two-dimensional TTE, zoomed apical 4-chamber view without (left) and with (right) color flow Doppler, demonstrates MAC without leaflet involvement and normal leaflet motion. There is minimal proximal flow acceleration and a mild degree of mitral regurgitation.
Third-dimensional TEE, volume-rendered image in the surgeon’s view demonstrates posterior MAC with normal unrestricted leaflet mobility and normal commissures.
Two-dimensional TEE midesophageal, zoomed 4-chamber view (0°) without (left) and with (right) color flow Doppler, demonstrates unrestricted leaflet motion and normal color flow Doppler without flow acceleration and mild mitral regurgitation.
Two-dimensional TTE, zoomed 4-chamber view without (left) and with (right) color flow Doppler, demonstrates severe MAC involving the MV leaflets leading to significantly restricted mobility, notable aliasing and narrowing of the mitral inflow jet indicative of elevated inflow velocities, and a small diastolic valve area. There is also mild mitral regurgitation and LA dilation noted.