Abstract
Transition metal catalyzed intermolecular hydroamination of the alkynes with aliphatic amine is generally problematic due to the good coordination between amine and metal cation. With the combination of 1,2,3-triazole coordinated gold(I) catalyst (TA-Au) and Zn(OTf)2 co-catalyst, this challenging transformation was achieved with good to excellent yields and regioselectivity. Compared to previously reported methods, this approach offered an alternative catalyst system to achieve this fundamental chemical transformation with high efficiency and practical conditions.
Graphical Abstract

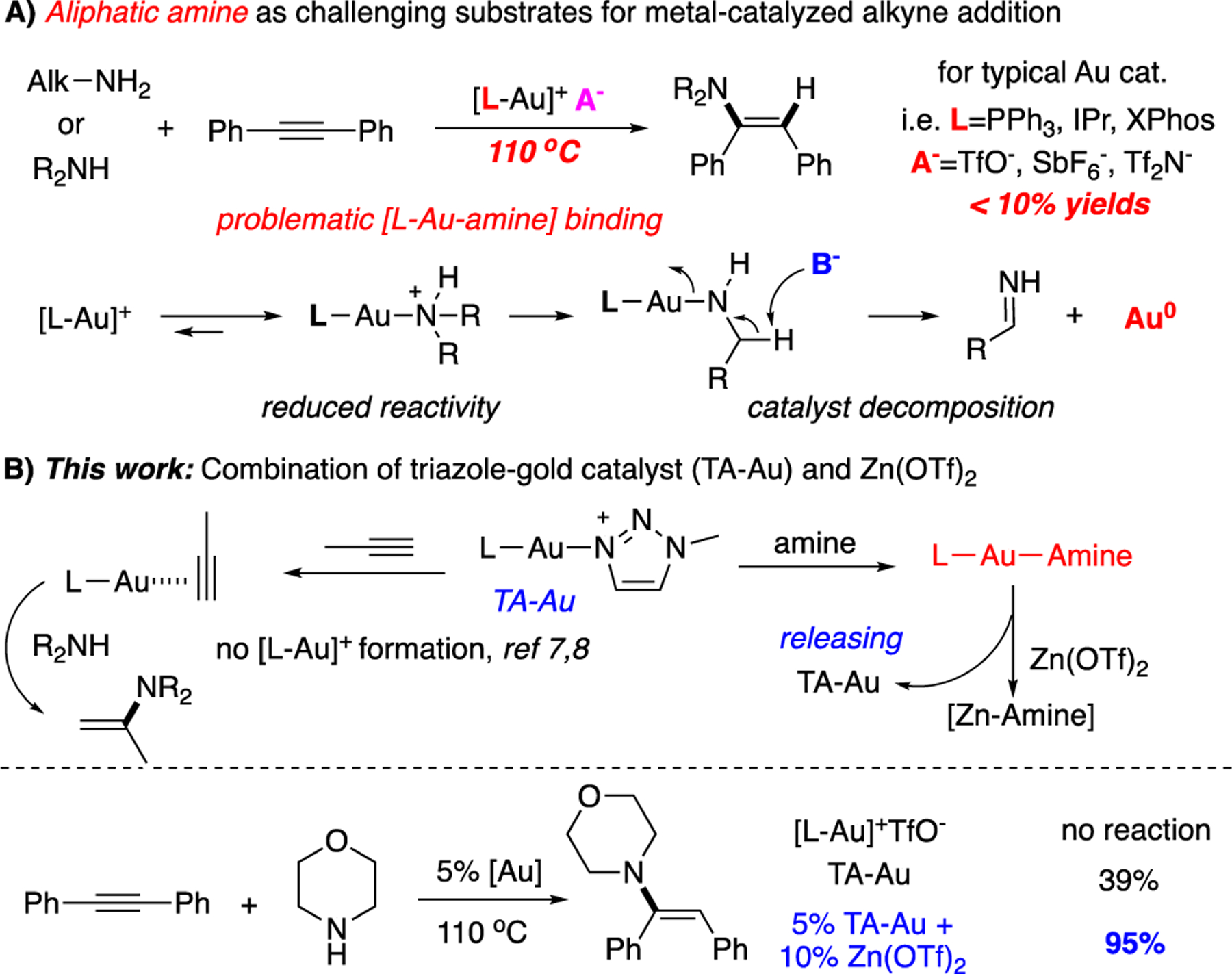
Hydroamination of alkene and alkyne is one of the critical transformations in chemical synthesis since it offers the atom economic approach through direct C-N bond construction.1 Comparing with the alkene, alkyne activation is more challenging due to the high reaction kinetic barrier, especially for non-activated (by EWG or EDG) internal alkynes.2 With the combined efforts from researchers worldwide, various catalytic systems have been developed to achieve effective alkyne activation under mild conditions.3 The choice of amine is also one important concern, which has been often overlooked. Compared to commonly used stabilized amine (such as aniline and amide), aliphatic amines are more basic with a better coordination ability towards metal cations. Therefore, aliphatic amines are usually problematic substrates in hydroamination reaction as the N-metal coordination significantly reduced both amine nucleophilicity and metal catalyst π-activation capability.4 As a result, alkyne hydroamination with aliphatic amine is one extremely challenging task, and new methods to achieve this transformation under mild conditions are highly desirable.
Over the past two decades, homogeneous gold catalysis has been considered one of the most effective strategies for alkyne activation due to its superior π-acid reactivity.5 Both Au(I) and Au(III) catalytic systems have been applied for alkyne hydroamination.6 However, while reactions with stabilized amine work well, most reported systems gave a poor performance with the aliphatic amine. This is mainly due to the amine coordination, which quenched gold catalyst reactivity (Scheme 1A). A high reaction temperature is generally demanded, facilitating the needed alkyne activation by releasing gold cation from dynamic Au-N binding. However, gold cation decomposition at an elevated temperature significantly reduced the overall efficiency of the transformation with a low gold catalyst turnover number (TON). To overcome the stability and reactivity dilemma, some special ligands have been developed and applied in this challenging aliphatic amine addition to the alkyne. Two representative recent examples are the cyclic(alkyl)(amino)carbene (CAAC) ligand7 and P, N-ligand Mor-DalPhos ligand8. In both cases, special bulky counter ion such as B(C6F5)4− was required to achieve satisfying results by minimizing potential base-mediated elimination. The requirement of special primary ligand and counter anion not only reduced the practicality of these gold catalytic systems but also highlighted the challenges associated with this transformation.
Scheme 1.
TA-Au catalyzed hydroamination of alkynes
To overcome gold cation stability issue, our group developed the 1,2,3-triazole gold complexes (TA-Au) as effective catalyst with enhanced stability, especially at elevated temperatures.9 The general idea of TA-Au catalysis was to have 1,2,3-triazole serving as the dynamic L-ligand (DLL), forming stable [L-Au-(DLL)]+ precatalyst as the off-cycle resting state. Our initial assumption was that triazole could dissociate from [L-Au-(DLL)]+, giving the active [L-Au]+ catalysts to react with alkyne substrates. Based on this assumption, several new transformations with the TA-Au system have been discovered. However, the different reactivity of TA-Au catalyst (even with the same primary ligand) raised our concern on whether this simple TA-releasing hypothesis was accurate as different reactivity was observed between TA-Au and [L-Au]+.10 Through detailed mechanistic investigation using react IR and in-suit NMR, we successfully confirmed the dissociation mechanism with alkyne direct addition to TA-Au, forming alkyne-TA-Au π-complex without going through the formation of [L-Au]+.11 This study not only explains the significantly improved gold catalyst stability observed with TA-Au, but also offers new mechanistic insight to achieve new reactivity with TA-Au over the [L-Au]+ even bearing same primary ligand. Taking advantage of TA-Au catalytic system, we reported the alkyne hydroamination with stabilized amine (aniline) at a high temperature. However, the reaction did work well with aliphatic amines using only TA-Au, giving poor yields (< 20%).9 Herein, we reported a combination of TA-Au and Zn(OTf)2 as a practical system for aliphatic amine addition to both internal and terminal alkynes, giving corresponding hydroamination products in good to excellent yields (up to 95%) with a large scope of amine choices (>50 examples).
Our interest in tackling this challenging problem was initiated by the counter anion effect associated with [(CAAC)-Au]+ system for the aliphatic amine addition to the alkyne, where B(C6F5)4− produced the optimal result while other anions gave significantly worse results. Although it makes sense that the electron-rich CAAC ligand helps to stable [L-Au]+ from decomposition, it is not apparent that why non-coordinated anion B(C6F5)4− is also critical for improved performance. After carefully considering the plausible reaction pathway, we postulated that Lewis base-assisted gold reduction might cause trouble in gold catalyzed reaction involving aliphatic amines. As shown in Scheme 1A, aliphatic amine could easily coordinate with [L-Au]+, forming [L-Au-NHR2]+ as the dominated gold species in the reaction mixture. Therefore, a high temperature is required to break this coordination (releasing [L-Au]+ catalyst). Aliphatic amine usually contains α-proton, which might cause gold reduction (decomposition) through a potential elimination pathway, especially in the presence of coordination anions at a high temperature. Therefore, a Lewis base mediated β-deprotonation could potentially occur, causing gold cation decomposition (formation of Au°). Therefore, the key to facilitating effective aliphatic amine hydroamination is to avoid the formation of this gold-amine complexes.
Based on this analysis and previously reported TA-Au catalytic reaction mechanism, one solution to prevent the intrinsic aliphatic amine reduction pathway is to develop a practical system adopting TA-Au as the resting state instead of the [L-Au-amine]+. With this proposed solution in mind, we first monitored the DLL ligand exchange between TA-Au and morpholine 2a using 31P NMR. As shown in Figure 1A, mixing 2a with TA-Au Cat-1 gives the formation of [L-Au-2a]+ instantly even at room temperature, suggesting the good binding ability of aliphatic amine toward gold cations even over 1,2,3-triazole. After screening various amine-scavengers,12 Zn(OTf)2 was identified as the optimal choice, recovering the formation of TA-Au in almost 80% with the addition of 1eq of Zn2+ salt. This result suggested that the addition of Zn(OTf)2 will help to break the equilibrium between TA-Au and [L-Au-amine]+, avoiding the formation of the undesired [L-Au-amine]+ intermediates. As the result, the combination of TA-Au and Zn(OTf)2 could be the potential solution for the challenging alkyne hydroamination with the aliphatic amine. To testify this hypothesis, reactions between internal alkyne 1a and amine 2a were performed under various gold-catalyzed conditions. The results are summarized in Figure 1B. First, simple [L-Au]+ gave almost no conversion due to a rapid gold decomposition at high temperatures. In addition, only a trace amount of hydroamination product 3a was observed while using TA-Au alone, indicating the stabilization effect of triazole at a high temperature. Notably, the low yield with TA-Au suggested that [L-Au-amine]+ is the dominant gold-complexes in the catalytic cycle which is subjected to decomposition. Finally, the combination of TA-Au (5 mol%) and Zn(OTf)2 (10 mol%) gave the desired product 3 in 20% yield. This result was promising and proved the feasibility of Lewis Acid as an amine-scavenger to promote the TA-Au alkyne activation. Further condition optimization was performed. 5 mol% JohnsPhosAu(TA-Me)OTf, 10 mol% Zn(OTf)2 in Toluene(1 M) at 110 °C was identified as the optimal conditions, giving 3a in 95% yield. Reaction results with some representative alternative conditions are summarized in Table 1.
Figure 1.

Prevent gold decomposition with TA-Au.
Table 1.

| ||
|---|---|---|
| Entry | Reaction conditions varies from standard | Yield of 3 |
| 1 | None | 95% |
| 2 | PPh3Au(TA-Me)OTf | 6% |
| 3 | (ArO)3PAu(TA-Me)OTf | n.r. |
| 4 | XPhosAu(TA-Me)OTf | 85% |
| 5 | JohnPhosAuNTf2, no Zn(OTf)2 | 30% |
| 6 | JohnPhosAuNTf2 | 60% |
| 7 | JohnPhosAuCl | 40% |
| 8 | No Zn(OTf)2 | 39% |
| 9 | Cu(OTf)2 as Lewis acid | 60% |
| 10 | Ga(OTf)3, Yb(OTf)3, or In(OTf)3 as Lewis acid | 40–42% |
| 11 | Zn(NTf2)2 instead of Zn(OTf)2 | 70% |
| 12 | Zn(OAc)2 instead of Zn(OTf)2 | 45% |
| 13 | Dioxane as solvent | 85% |
| 14 | 0.5 M | 85% |
| 15 | at 100 °C | 87% |
| 16 | No gold | n.r |
General reaction conditions: 1 (0.2 mmol, 1.0 equiv.), 2 (0.4 mmol, 2.0 equiv.), [Au] catal. (0.01 mmol, 5 mol%), Lewis Acid (0.02 mmol, 10 mol%) in dry solvent (0.2 mL) under Ar at 110 °C.
Yield was determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
Similar to other literature reported hydroamination of internal alkynes, cis-addition was observed as the only product due to the enamine equilibrium to the formation of more stable isomers under this condition.7,8 The primary ligands were crucial for good reaction performance. In general, electron-deficient phosphine ligands, such as PPh3 and (ArO)3P, gave poor results due to the quick catalyst decomposition, even with triazole as a stabilization factor (entry 2 and 3). Electron-rich phosphine ligands, such as XPhos, can promote this reaction due to a more stabilized gold cation (entry 4). JohnsPhosAuNTf2 was tested as an alternative silver-free catalyst. As expected, a lower yield was observed (30%, entry 5). Screening of Lewis acids (Ga2+, Yb3+, In3+, Cu2+ etc.) revealed Zn(OTf)2 as the optimal choice. A similar LA-assisted effect was also observed with Cu(OTf)2 as co-catalyst, though less effective than Zn(OTf)2 (entry 9). Other commonly used Lewis acid, such as Ga2+, Yb3+, and In3+, could not increase the yield of desired hydroamination product 3a (entry 10) compared to the reaction without Lewis acid (entry 8). This result suggested that these Lewis acids were not valid amine-scavengers. The coordination counter ions, NTf2 and OAc, led to decreased yields (entry11 and 12), which further supported our hypothesis on the Lewis base induced gold decomposition pathway. Dioxane was also suitable solvent with a slightly decreased yield (entry 13). This reaction required a relatively harsh condition (high temperature at 110 °C and concertation 1 M) to reach the complete conversion, in which the most gold catalyst suffered from decomposition. No gold decomposition (by forming the gold mirror) was observed using our TAAu/LA system, highlighting the advantage of enhancing thermal stability while remaining high catalyst efficiency (entry 14 and 15). A control experiment showed that Zn(OTf)2 alone could not catalyze the reaction, ruling out the possible early-transition metal alkyne activation pathway13 (entry 16).
With the optimal condition in hand, the reaction scope was explored.14 The results are summarized in Scheme 2. We first evaluated the scope of alkynes. For diaryl alkyne(4a-4c), the reaction works well for both electro-deficient and electron-rich aromatic rings. Alkynes with electron-rich aromatic ring afforded desired hydroamination product with good to excellent yields. In comparison, electron-deficient aromatic alkynes gave moderate yields (i.e. 6k). Unsymmetric alkynes (4e-4g) were also evaluated, affording good to excellent regioselectivity. Interestingly, the regioselectivity could be improved by employing the electron-rich aromatic rings, leading to 4g as the only product. This result is consistent with previously reported examples using Mor-DalPhos-Au. Besides internal alkynes, the scope of terminal alkynes (4i-4r) was also explored. Similarly, both electron-deficient and electron-rich aromatic alkynes worked well, giving the desired products in good to excellent yields. Aromatic rings with electron-donation groups gave high yields due to the better coordination capability. Notably, electron-rich heterocyclic thiophene alkyne (4r) was suitable substrate for this transformation, though with modest yields. No reaction was observed with 1-Hexyne and 3-Hexyne, indicating a high activation barrier for aliphatic alkynes.
Scheme 2.

Reaction scope for alkyne and aliphatic aminea,b
aConditions: For internal alkyne: 1 (0.2 mmol, 1.0 eq), [Au] cat. (5.0 mol%), Zn(OTf)2 (10.0 mol%), and 2 (0.4 mmol, 2.0eq) was added into dry toluene (1M) under Ar. The reaction mixture was stirred at 110 °C for 12 h. For terminal alkyne: 1 (0.2 mmol, 1.0 eq), [Au] cat. (5.0 mol%), Zn(OTf)2 (10.0 mol%), and 2 (0.3 mmol, 1.5 eq) was added into dry toluene (0.05 M) under Ar. The reaction mixture was stirred at 110 °C for 6 h. bIsolated yield. cThe ratio of 4 and 5 was determined by crude 1H NMR spectra of reaction mixture. d 80 °C
Various aliphatic amines were applied to this transformation. To our great satisfaction, many of these substrates are suitable for this transformation. Both 1-phenylpiperazine (6a, 6f-6k) and 1-pyridylpiperaziene (6l-6s) were applied to react with either internal or terminal alkynes, giving the desired products in good to excellent yields. Interestingly, the 2-pyridine group on nitrogen (6v, 6w) were tolerated under this condition, suggesting good coordination capability of triazole over pyridine and highlighting the good reactivity of TA-Au. Though under strong Lewis-acid conditions, the N-Boc carbamate (6b) was compatible, giving the desired product in excellent yield. This result highlighted the relative mild condition of gold catalyzed hydroamination, allowing further N-modification through simple Boc-deprotection and NH coupling. Both Acyclic secondary amines (6c, 6e, 6z-6ah) and benzyl amine (6d) worked well in this transformation, giving the desired products in good yields in general.
Primary aliphatic amine, 1-butylamine, did not give any conversion, likely due to a stronger coordination of primary aliphatic amine towards gold cation. Nevertheless, this result revealed a new facile route to achieve 2-benzyl amine derivatives with high efficiency.
In summary, we disclosed a new protocol to achieve aliphatic amine addition to terminal and internal alkynes using the combination of TA-Au and Zn(OTf)2. The amine binding with gold cation was the main challenge for the observed poor performance of aliphatic amine hydroamination. The key discovery here is the application of Zn(OTf)2 as amine-scavengers to release TA-Au and re-activate the reaction path for the formation of gold-alkyne π-complexes. TA-Au was crucial with enhanced stability, especially at elevated temperatures. The process was proved by NMR study and challenging aliphatic amine hydroamination was achieved with broad substrate scope and good overall yields. This new catalytic system not only revealed an effective and practical strategy to construction synthetic challenge aliphatic amine derivatives, but also suggested the great advantage of triazole as dynamic-L-Ligand in tuning metal center reactivity. The appreciation of TA-Au and Lewis acid catalytic systems in other challenging transformations is expected and under investigation.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the NSF (CHE-1665122), the NIH (1R01GM120240-01), and Jilin Province (20170307024YY, 20190201080JC) for financial support.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experiment procedures, conditions optimization, and characterization for all products (PDF)
The authors declare no competing financial interest.
REFERENCE
- 1.(a) Muller TE; Hultzsch KC; Yus M; Foubelo F; Tada M Hydroamination: Direct addition of amines to alkenes and alkynes. Chem. Rev 2008, 108, 3795–3892. [DOI] [PubMed] [Google Scholar]; (b) Hesp KD; Stradiotto M Rhodium- and Iridium-Catalyzed Hydroamination of Alkenes. Chemcatchem 2010, 2, 1192–1207. [Google Scholar]; (c) Huang LB; Arndt M; Goossen K; Heydt H; Goossen LJ Late Transition Metal-Catalyzed Hydroamination and Hydroamidation. Chem. Rev 2015, 115, 2596–2697. [DOI] [PubMed] [Google Scholar]; (d) Pirnot MT; Wang YM; Buchwald SL Copper Hydride Catalyzed Hydroamination of Alkenes and Alkynes. Angew. Chem. Int. Ed 2016, 55, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Colonna P; Bezzenine S; Gil R; Hannedouche J Alkene Hydroamination via Earth-Abundant Transition Metal (Iron, Cobalt, Copper and Zinc) Catalysis: A Mechanistic Overview. Adv. Synth. Catal 2020, 362, 1550–1563. [Google Scholar]; (f) Sengupta M; Das S; Islam SM; Bordoloi A Heterogeneously Catalysed Hydroamination. Chemcatchem 2021, 13, 1089–1104. [Google Scholar]; (g) Streiff S; Jerome F Hydroamination of non-activated alkenes with ammonia: a holy grail in catalysis. Chem. Soc. Rev 2021, 50, 1512–1521. [DOI] [PubMed] [Google Scholar]
- 2.Gorin DJ; Toste FD, Relativistic effects in homogeneous gold catalysis. Nature 2007, 446, 395–403. [DOI] [PubMed] [Google Scholar]
- 3.(a) Yim JCH; Schafer LL Efficient Anti-Markovnikov-Selective Catalysts for Intermolecular Alkyne Hydroamination: Recent Advances and Synthetic Applications. Eur. J. Org. Chem 2014, 6825–6840. [Google Scholar]; (b) Patel M; Saunthwal RK; Verma AK Base-Mediated Hydroamination of Alkynes. Acc. Chem. Res 2017, 50, 240–254. [DOI] [PubMed] [Google Scholar]; (c) Tashrifi Z; Khanaposhtani MM; Biglar M; Larijani B; Mahdavi M Recent Advances in Alkyne Hydroamination as a Powerful Tool for the Construction of C-N Bonds. Asian J. Org. Chem 2020, 9, 969–991. [Google Scholar]
- 4.Yang Y; Shi SL; Niu DW; Liu P; Buchwald SL Catalytic asymmetric hydroamination of unactivated internal olefins to aliphatic amines. Science 2015, 349, 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Hashmi ASK; Hutchings GJ Gold catalysis. Angew. Chem. Int. Ed 2006, 45, 7896–7936. [DOI] [PubMed] [Google Scholar]; (b) Gorin DJ; Sherry BD; Toste FD Ligand effects in homogeneous Au catalysis. Chem. Rev 2008, 108, 3351–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Furstner A Gold and platinum catalysis-a convenient tool for generating molecular complexity. Chem. Soc. Rev 2009, 38, 3208–3221. [DOI] [PubMed] [Google Scholar]; (d) Fensterbank L; Malacria M Molecular Complexity from Polyunsaturated Substrates: The Gold Catalysis Approach. Acc. Chem. Res 2014, 47, 953–965. [DOI] [PubMed] [Google Scholar]; (e) Hashmi ASK Dual Gold Catalysis. Acc. Chem. Res 2014, 47, 864–876. [DOI] [PubMed] [Google Scholar]; (f) Wang YM; Lackner AD; Toste FD Development of Catalysts and Ligands for Enantioselective Gold Catalysis. Acc. Chem. Res 2014, 47, 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Dorel R; Echavarren AM Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev 2015, 115, 9028–9072. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Asiri AM; Hashmi ASK Gold-catalysed reactions of diynes. Chem. Soc. Rev 2016, 45, 4471–4503. [DOI] [PubMed] [Google Scholar]; (i) Hopkinson MN; Tlahuext-Aca A; Glorius F Merging Visible Light Photoredox and Gold Catalysis. Acc. Chem. Res 2016, 49, 2261–2272. [DOI] [PubMed] [Google Scholar]; (j) Zi WW; Toste FD Recent advances in enantioselective gold catalysis. Chem. Soc. Rev 2016, 45, 4567–4589. [DOI] [PubMed] [Google Scholar]; (k) Liu L; Zhang JL, Gold-catalyzed transformations of alpha-diazocarbonyl compounds: selectivity and diversity. Chem. Soc. Rev 2016, 45 (3), 506–516. [DOI] [PubMed] [Google Scholar]; (l) Zheng Z; Ma X; Cheng X; Zhao K; Gutman K; Li T; Zhang L, Homogeneous Gold-Catalyzed Oxidation Reactions. Chem. Rev 2021. 10.1021/acs.chemrev.0c00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Mizushima E; Hayashi T; Tanaka M Au(I)-catalyzed highly efficient intermolecular hydroamination of alkynes. Org. Lett 2003, 5, 3349–3352. [DOI] [PubMed] [Google Scholar]; (b) Brouwer C; He C Efficient gold-catalyzed hydroamination of 1,3-dienes. Angew. Chem. Int. Ed 2006, 45, 1744–1747. [DOI] [PubMed] [Google Scholar]; (c) Han XQ; Widenhoefer RA Gold(I)-catalyzed intramolecular hydroamination of alkenyl carbamates. Angew. Chem. Int. Ed 2006, 45, 1747–1749. [DOI] [PubMed] [Google Scholar]; (d) Liu XY; Li CH; Che CM Phosphine gold(I)-catalyzed hydroamination of alkenes under thermal and microwave-assisted conditions. Org. Lett 2006, 8, 2707–2710. [DOI] [PubMed] [Google Scholar]; (e) Nishina N; Yamamoto Y Gold-catalyzed intermolecular hydroamination of allenes with arylamines and resulting high chirality transfer. Angew. Chem. Int. Ed 2006, 45, 3314–3317. [DOI] [PubMed] [Google Scholar]; (f) Widenhoefer RA; Han XQ Gold-catalyzed hydroamination of C-C multiple bonds. Eur. J. Org. Chem 2006, 2006, 4555–4563. [Google Scholar]; (g) Zhang JL; Yang CG; He C Gold(I)-catalyzed intra- and intermolecular hydroamination of unactivated olefins. J. Am. Chem. Soc 2006, 128, 1798–1799. [DOI] [PubMed] [Google Scholar]; (h) LaLonde RL; Sherry BD; Kang EJ; Toste FD Gold(I)-catalyzed enantioselective intramolecular hydroamination of allenes. J. Am. Chem. Soc 2007, 129, 2452. [DOI] [PubMed] [Google Scholar]; (i) Zhang YH; Donahue JP; Li CJ Gold(III)-catalyzed double hydroamination of o-alkynylaniline with terminal alkynes leading to N-vinylindoles. Org. Lett 2007, 9, 627–630. [DOI] [PubMed] [Google Scholar]; (j) Enomoto T; Girard AL; Yasui Y; Takemoto Y Gold(I)-Catalyzed Tandem Reactions Initiated by Hydroamination of Alkynyl Carbamates: Application to the Synthesis of Nitidine. J. Org. Chem 2009, 74, 9158–9164. [DOI] [PubMed] [Google Scholar]; (k) Kramer S; Dooleweerdt K; Lindhardt AT; Rottlander M; Skrydstrup T Highly Regioselective Au(I)-Catalyzed Hydroamination of Ynamides and Propiolic Acid Derivatives with Anilines. Org. Lett 2009, 11, 4208–4211. [DOI] [PubMed] [Google Scholar]; (l) Zhang ZB; Lee SD; Widenhoefer RA Intermolecular Hydroamination of Ethylene and 1-Alkenes with Cyclic Ureas Catalyzed by Achiral and Chiral Gold(I) Complexes. J. Am. Chem. Soc 2009, 131, 5372. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Patil NT; Lakshmi P; Singh V Au-I Catalyzed Direct Hydroamination/Hydroarylation and Double Hydroamination of Terminal Alkynes. Eur. J. Org. Chem 2010, 2010, 4719–4731. [Google Scholar]; (n) Wang ZJ; Benitez D; Tkatchouk E; Goddard WA; Toste FD Mechanistic Study of Gold(I)-Catalyzed Intermolecular Hydroamination of Allenes. J. Am. Chem. Soc 2010, 132, 13064–13071. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Kinjo R; Donnadieu B; Bertrand G Gold-Catalyzed Hydroamination of Alkynes and Allenes with Parent Hydrazine. Angew. Chem. Int. Ed 2011, 50, 5560–5563. [DOI] [PubMed] [Google Scholar]; (p) Alvarado E; Badaj AC; Larocque TG; Lavoie GG N-Heterocyclic Carbenes and Imidazole-2-thiones as Ligands for the Gold(I)-Catalysed Hydroamination of Phenylacetylene. Chem. Eur. J 2012, 18, 12112–12121. [DOI] [PubMed] [Google Scholar]; (q) He YP; Wu H; Chen DF; Yu J; Gong LZ Cascade Hydroamination/Redox Reaction for the Synthesis of Cyclic Aminals Catalyzed by a Combined Gold Complex and BrOnsted Acid. Chem. Eur. J 2013, 19, 5232–5237 [DOI] [PubMed] [Google Scholar]
- 7.(a) Zeng XM; Frey GD; Kinjo R; Donnadieu B; Bertrand G Synthesis of a Simplified Version of Stable Bulky and Rigid Cyclic (Alkyl)(amino)carbenes, and Catalytic Activity of the Ensuing Gold(I) Complex in the Three-Component Preparation of 1,2-Dihydroquinoline Derivatives. J. Am. Chem. Soc 2009, 131, 8690–8696. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Melaimi M; Jazzar R; Soleilhavoup M; Bertrand G Cyclic (Alkyl)(amino)carbenes (CAACs): Recent Developments. Angew. Chem. Int. Ed 2017, 56, 10046–10068. [DOI] [PubMed] [Google Scholar]
- 8.Hesp KD; Stradiotto M Stereo- and Regioselective Gold-Catalyzed Hydroamination of Internal Alkynes with Dialkylamines. J. Am. Chem. Soc 2010, 132, 18026–18029. [DOI] [PubMed] [Google Scholar]
- 9.Duan HF; Sengupta S; Petersen JL; Akhmedov NG; Shi XD, Triazole-Au(I) Complexes: A New Class of Catalysts with Improved Thermal Stability and Reactivity for Intermolecular Alkyne Hydroamination. J. Am. Chem. Soc 2009, 131 (34), 12100. [DOI] [PubMed] [Google Scholar]
- 10.(a) Chen YF; Yan WM; Akhmedov NG; Shi XD 1,2,3-Triazole as a Special “X-Factor” in Promoting Hashmi Phenol Synthesis. Org. Lett 2010, 12, 344–347. [DOI] [PubMed] [Google Scholar]; (b) Wang DW; Ye XH; Shi XD Efficient Synthesis of E-alpha-Haloenones Through Chemoselective Alkyne Activation Over Allene with Triazole-Au Catalysts. Org. Lett 2010, 12, 2088–2091. [DOI] [PubMed] [Google Scholar]; (c) Wang QY; Aparaj S; Akhmedov NG; Petersen JL; Shi XD Ambient Schmittel Cyclization Promoted by Chemoselective Triazole-Gold Catalyst. Org. Lett 2012, 14, 1334–1337. [DOI] [PubMed] [Google Scholar]; (d) Wang QY; Motika SE; Akhmedov NG; Petersen JL; Shi XD Synthesis of Cyclic Amine Boranes through Triazole-Gold(I)Catalyzed Alkyne Hydroboration. Angew. Chem. Int. Ed 2014, 53, 5418–5422. [DOI] [PubMed] [Google Scholar]
- 11.Xi YM; Wang QY; Su YJ; Li MY; Shi XD, Quantitative kinetic investigation of triazole-gold(I) complex catalyzed 3,3 -rearrangement of propargyl ester. Chem. Commun 2014, 50 (17), 2158–2160. [DOI] [PubMed] [Google Scholar]
- 12.For selected example of TA-Au and Lewis acid catalysis system, see:; (a) a) Xi YM; Dong BL; McClain EJ; Wang QY; Gregg TL; Akhmedov NG; Petersen JL; Shi XD Gold-Catalyzed Intermolecular C -S Bond Formation: Efficient Synthesis of a- Substituted Vinyl Sulfones**. Angew. Chem. Int. Ed 2014, 53, 4657–4661. [DOI] [PubMed] [Google Scholar]; (b) Xi YM; Wang DW; Ye XH; Akhmedov NG; Petersen JL; Shi XD Synergistic Au/Ga Catalysis in Ambient Nakamura Reaction. Org. Lett 2014, 16, 306–309. [DOI] [PubMed] [Google Scholar]; (c) Dong BL; Xi YM; Su YJ; Akhmedov NG; Petersen JL; Shi XD Gold/gallium-catalyzed annulation of 1,3-dicarbonyl compounds and cyclopropylacetylenes for synthesis of substituted cyclopentenes. Rsc Advances 2016, 6, 17386–17389. [Google Scholar]; (d) Hosseyni S; Wojtas L; Li MY; Shi XD Intermolecular Homopropargyl Alcohol Addition to Alkyne and a Sequential 1,6-Enyne Cycloisomerization with Triazole-Gold Catalyst. J. Am. Chem. Soc 2016, 138, 3994–3997. [DOI] [PubMed] [Google Scholar]; (e) Motika SE; Wang QY; Akhmedov NG; Wojtas L; Shi XD Regioselective Amine-Borane Cyclization: Towards the Synthesis of 1,2-BN-3-Cyclohexene by Copper-Assisted Triazole/Gold Catalysis. Angew. Chem. Int. Ed 2016, 55, 11582–11586. [DOI] [PubMed] [Google Scholar]; (f) Yuan T; Ye XH; Zhao PY; Teng S; Yi YP; Wang J; Shan C; Wojtas L; Jean J; Chen H; Shi X Regioselective Crossed Aldol Reactions under Mild Conditions via Synergistic Gold-Iron Catalysis. Chem 2020, 6, 1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For example, see; (a) Al-huniti MH; Lepore SD, Zinc(II) Catalyzed Conversion of Alkynes to Vinyl Triflates in the Presence of Silyl Triflates. Org. Lett 2014, 16, 4154–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Werkmeister S; Fleischer S; Zhou S; Junge K; Beller M, Development of New Hydrogenations of Imines and Benign Reductive Hydroaminations: Zinc Triflate as a Catalyst. ChemSusChem 2012, 5, 777–782. [DOI] [PubMed] [Google Scholar]
- 14.The enamine product 3 was intended to hydrolysis during the separation using silica column chromatography. Therefore, the reduction is performed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


