Abstract
Hexafluoroisopropanol (HFIP) promoted disulfidation and diselenation of C-C unsaturated bonds is reported. Reactions of un-activated alkyne, alkene, and allene, respectively, with disulfides or diselenides in HFIP led to desired products in good to excellent yields (up to 96%). In contrast, other solvents, such as isopropanol and dichloroethane, could not promote the same reaction. This method revealed an example of HFIP promoted transformations under the mild conditions, which greatly highlighted the unique reactivity of this special solvent.
Graphical Abstract

One basic mission of synthetic chemistry is to develop new methods for effective transformations, achieving complex molecular scaffold from simple starting materials under mild conditions.1 As one of the crucial reaction tuning factors, solvent could dramatically impact the reaction performance. With the discovery of ionic liquid and fluorinated solvents, unexpected results were obtained, greatly enriching the synthetic toolbox.2 Fluorinated compounds have received tremendous attention in chemical, medicinal, and material research.3 The high electronegativity and good hydrophobicity of fluorine atom give fluorinated compounds unique chemical properties. Recently, HFIP has emerged as a unique polar organic solvent in promoting chemical reactions.4 With two acidic protons, low nucleophilicity, and high ionizing power, HFIP has been used as an alternative solvent in substituting Lewis acid or Brønsted acid in promoting chemical transformations through H-bond networks under very mild conditions. In addition, HFIP is a promising solvent in industrial processing, owing to its recyclability and low cost.5 Some representative HFIP promoted transformations, including Friedel-Crafts reaction,6 polyene cyclization,7 and, Schmidt reaction,8 are shown in Scheme 1A. In comparison with other solvents which failed to produce the desired products, HFIP significantly promoted the reaction to obtain good to excellent yields. These results greatly highlighted the unique reactivity of HFIP in facilitating organic transformations under mild conditions.
Scheme 1.
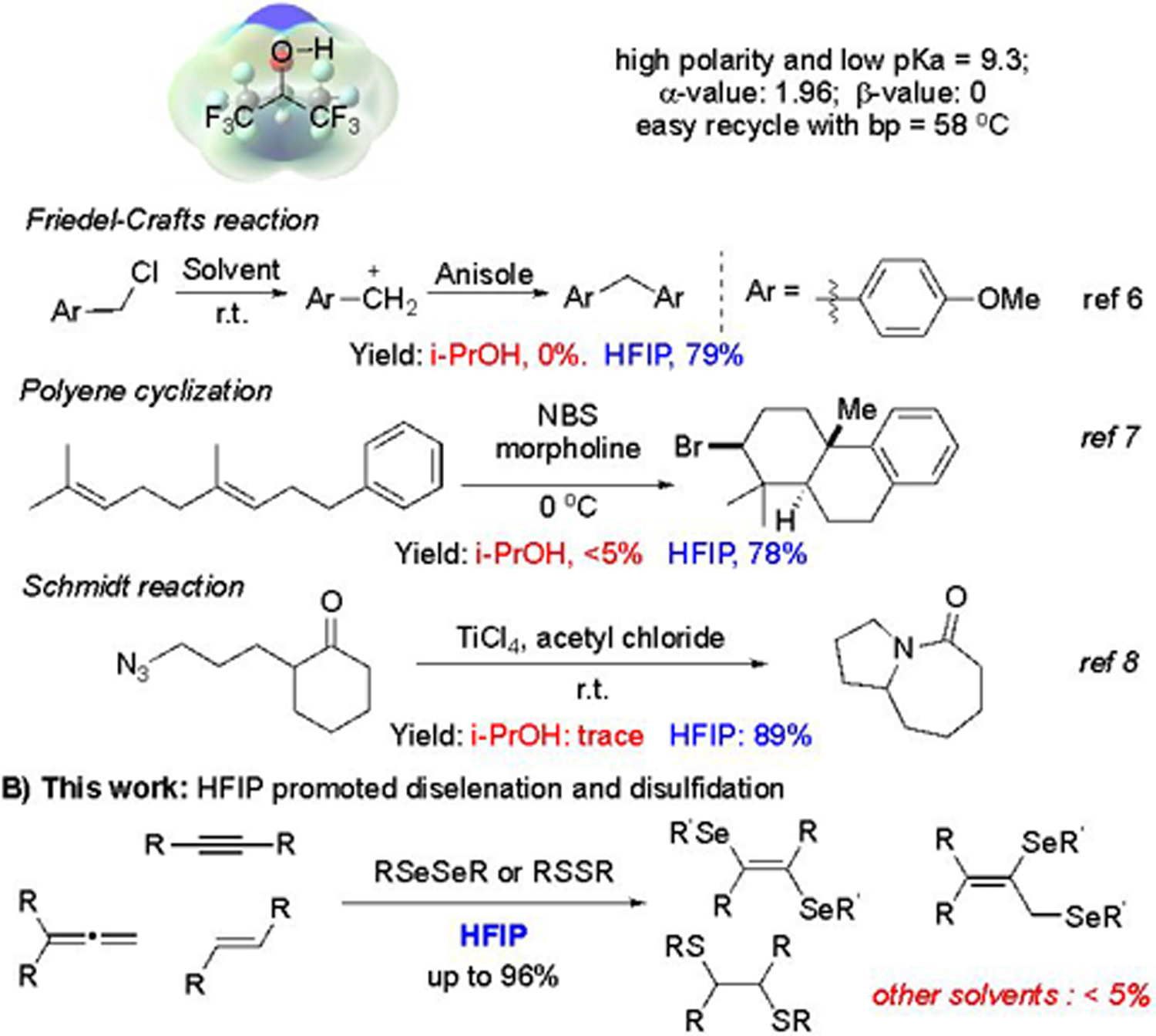
HFIP promoted challenging transformations
Recently, we reported thioallylation and diselenation of alkynes under gold redox catalysis.9 Based on the solvent screening, we observed that HFIP could greatly promote the reaction of even more challenging unactivated internal alkyne without gold catalysts. Herein, we report this interesting solvent effect of HFIP on diselenation and disulfidation of the C-C unsaturated bond. Good to excellent yields were achieved with broad reaction substrate scope under metal-free condition (Scheme 1B), which demonstrated the unique reactivity of the HFIP solvent in promoting challenging organic transformations.
Disulfidation10 and diselenation11 of C-C unsaturated bonds are the basic organic transformations with many chemical and medicinal applications. Great progress has been made regarding these transformations by using transition metal promoters/catalysts, Lewis acid, and photocatalysts to achieve the desired products. Our interest in this reaction was originated from our continuous efforts in gold-catalyzed alkyne activation.12 As shown in Figure 1A, we recently reported the diselenation of alkynes through a gold(I)-gold(III) redox catalytic cycle, giving diselenation products in excellent yields with high stereoselectivity (trans-addition only). However, this transformation did not work well on simple internal alkynes, even with an increased catalyst loading (10% [Au]) at 60 °C.
Figure 1.
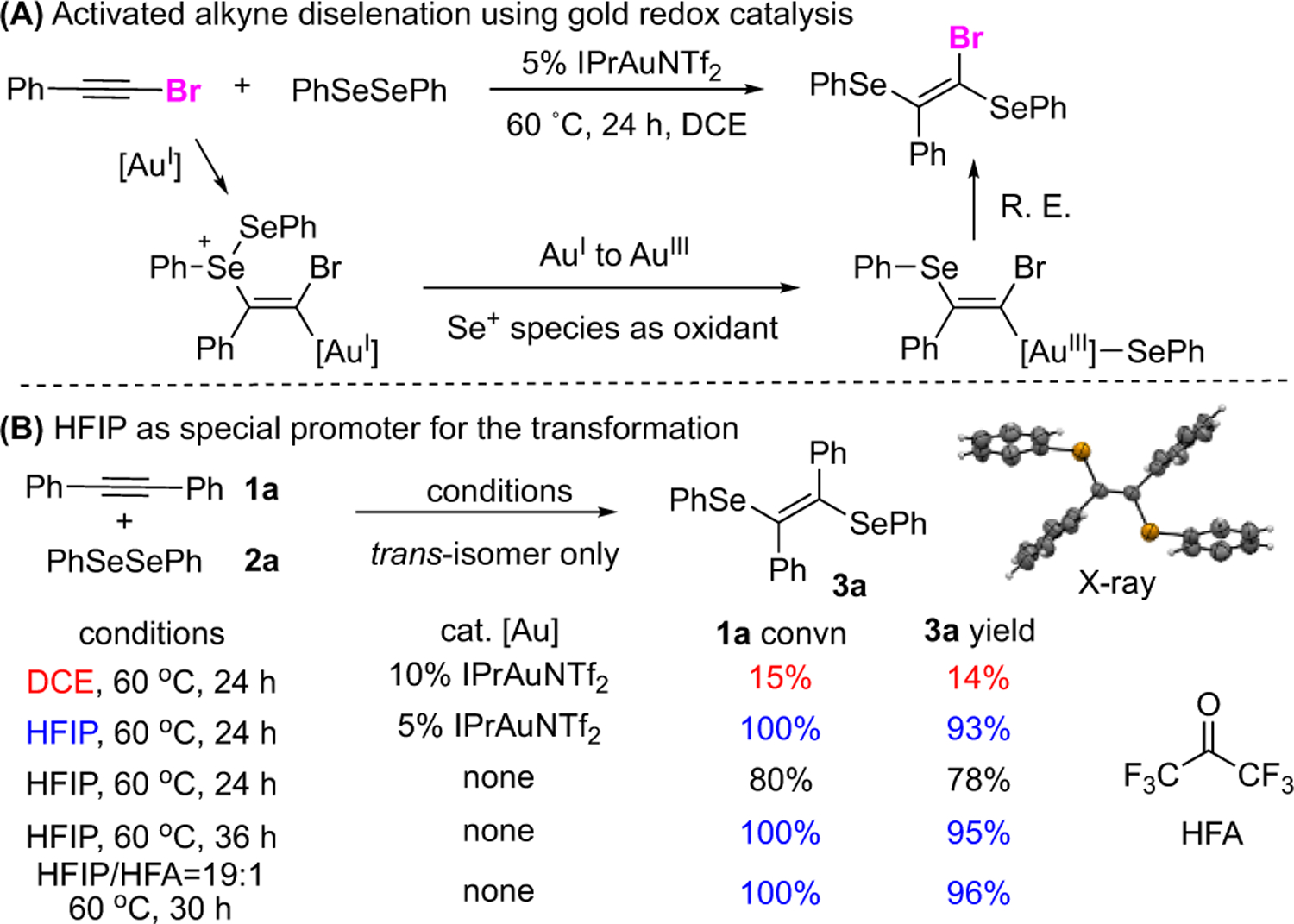
HFIP promoted internal alkyne diselenation
As shown in Figure 1B, treating internal alkyne 1a with diselenide 2a under previous gold catalytic conditions (DCE, 60 °C, 24 h) gave diselenation product 3a in poor conversion (15%) and low yield (14%) associated with completed gold catalyst decomposition. Interestingly, switching solvent to HFIP greatly enhanced the reaction performance, giving 3a in nearly quantitative yield (93%). Control reaction using HFIP as solvent without gold catalyst was then carried out, achieving 3a in 78% yield with 80% conversion of 1a in 24 h. Extending the reaction time to 36 h gave a full conversion of 1a, and the desired product 3a was obtained in excellent yield (95%). HFIP is necessary to be a solvent (0.2 M). When applying a mixture of DCE/HFIP (9:1), only trace amount of product was obtained. In addition, applying hexafluoroacetone (HFA) as additive could accelerate the reaction with comparable yield in 30 hours(For the detailed screening on optimal ratio of HFIP/HFA, see Table S1 in SI) Other solvents, including iPrOH, DMSO, DMF, CH3CN, acetone, chlorobenzene, and nitromethane, could not promote this reaction, resulting in less than 5% yield in all cases. Lowering temperature to 40 °C led to decreasing of conversion due to slower reaction kinetic. The detailed screening conditions are summarized in the Supporting Information. Notably, only trans-isomer 3a was observed under this metal-free condition, suggesting a concerted bi-molecular trans-addition of diselenides to the plausible selenium cation intermediate formed from alkyne addition toward HFIP activated diselenides. Encouraged by these results, we evaluated the reaction scope of this metal-free HFIP promoted transformations. The results are summarized in Scheme 2.
Scheme 2.
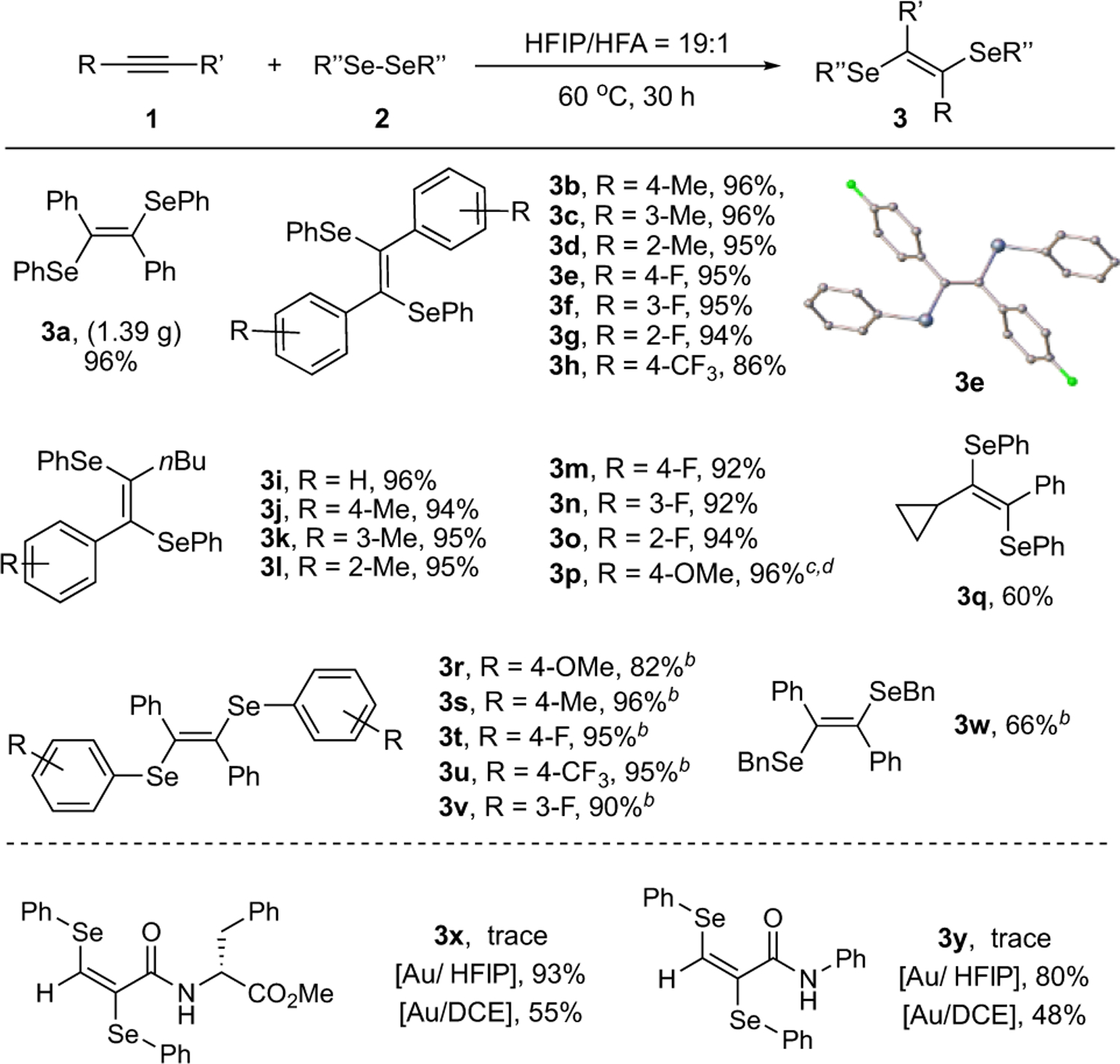
HFIP Promoted Alkyne Diselenationa
aReaction conditions: alkyne (0.4 mmol) and diselenide (0.2 mmol) were added into HFIP:HFA = 19:1 (1 mL), and the mixture was stirred at 60 °C for 24 h. Isolated yield. b3 days. cUnder room temperature. d12 h.
Various substituted alkynes were evaluated in this HFIP “boosted” transformation. First, several diaryl acetylenes with different substituents on the aromatic rings were tested. Both electron-donating group (EDG) and electron-withdrawing group (EWG) substituted aromatic rings worked well in this reaction, giving the desired products (3a-3h) in excellent yields. Second, aliphatic alkynes (3i-3q) gave similar results with desired products obtained mostly in excellent yields. The cyclopropyl substituted alkyne (3q) gave no ring-opening product, ruling out the potential radical mechanism. Third, a series of diselenides were also employed for this transformation (3r-3v), giving the expected products in good to excellent yields. A lower yield was observed with dibenzylselenide 3w due to the slow reaction rate. The terminal alkynes (3x, 3y) did not work under this HFIP condition. After switching the conditions to Au catalysis, significantly improved yields were obtained. Notably, employing HFIP as solvent instead of DCE, the reaction performance could be improved to 93% and 80%, respectively. No racemization of the chiral center on the amino acid after reaction highlighted the mild condition, as well as the great promoting effects of HFIP solvent in gold redox catalysis (For chiral HPLC characterization, see Section VI in SI).
Although the detailed mechanism of this HFIP promoting effect remains to be explored, one plausible reason behind this “boosting effect” is that HFIP activates RSeSeR to form a selenium species as a good leaving group through H-bond network.4j Therefore, under gold catalytic pathway, this HFIP boosting effect could help the oxidation of gold(I) to gold(III)with the activated selenium, which is likely the turnover limiting step. Similarly, in the non-metal catalytic conditions, diselenides are good electrophiles, which form an equilibrium with alkyne addition. This hypothesis also explained the reason why carbonyl-conjugated alkynes (3x, 3y), as bad nucleophiles, did not work under non-metal catalytic condition. The cation stabilizing effect of HFIP would also help the activation of unsaturated substrates under HFIP/Au condition.5 Overall, through the formation of H-bond, HFIP could greatly assist diselenides activation and increase the reactivity of this diselenation. Based on this hypothesis, we wondered if this activation mode could be applied to similar transformations using alkene and allene as the substrate. To confirm this idea, reactions of diselenides with alkenes and allenes were performed.
As shown in Figure 2A, styrene failed to give diselenation product with the formation of complex reaction mixtures without diselenation product. With allene substrates, lower yields were observed. Monitoring the reactions with NMR revealed the decompositions of products overtime under the reaction conditions. The addition of gold catalyst in the allene case could give a faster reaction. As a result, improved yields were observed (Figure 2A).
Figure 2.
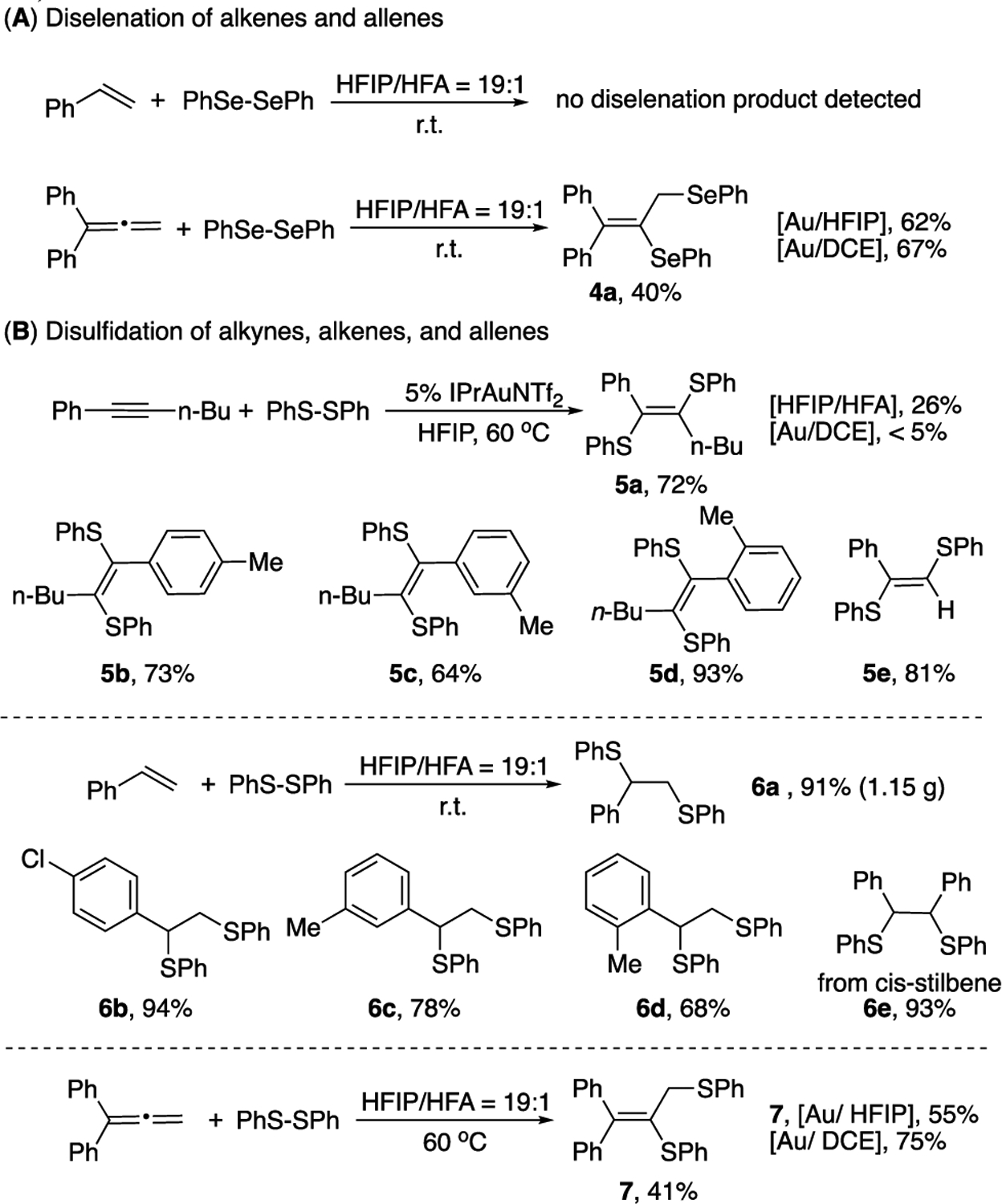
Disulfidation and diselenation reactions
Considering that HFIP might provide a similar H-bond activation effect toward disulfide (RSSR), we started out our investigations on disulfidation with various unsaturated C-C bonds under Au/HFIP condition. For alkyne substrates (Figure 2B), HFIP could not promote this transformation, giving desired disulfide products in low yield. In comparison, the combination of gold and HFIP afforded the desired products in good yield (72%), while DCE solvent gave only a trace amount of product. Different internal and terminal alkynes were tested with desired products observed in good to excellent yields (5a-5e). For aliphatic substituted alkynes, both internal and terminal, give almost no products under either HFIP or HFIP/Au catalysis conditions (for the detailed scope for aliphatic alkynes, see Table S3 in SI). Overall, this result greatly highlighted the boosting effect of HFIP solvent applied in gold catalysis, which was critical for alkyne activations.
Furthermore, reactions of disulfide with alkene under the HFIP condition gave the desired product 6a in 91% yield with gram-scale synthesis. Some representative alkenes were tested with the desired disulfidation products obtained in good to excellent yields (6b-6e). Notably, 1,2-disubstituted alkene, cis-stilbene, accomplished the reaction in excellent yield (6e, 93%), while the trans-stilbene did not work under this condition. Notably, other solvents, such isopropanol and DCE, gave almost no reaction (<5%). Allene was also tested under the HFIP conditions, with or without gold catalyst presented. Moderate yield was observed using non-metal condition. With gold catalyst presented, no significantly improved yield was observed in HFIP comparing with DCE solvents due to the decomposition of product in HFIP. Nevertheless, HFIP presented a solvent promoting effect over other solvents, suggesting this boosting effect could also be applied in C-C unsaturated bonds disulfidation reaction under mild conditions.
In conclusion, the diselenation and disulfidation of C-C unsaturated bonds were reported using HFIP as the promoting solvent. Compared with other common organic solvents, HFIP greatly enhanced the reaction performance by activating the diselenide and disulfide through H-bond networks. The desired products were achieved under mild conditions with good to excellent yield and high stereoselectivity. These results offered another prospective of using HFIP as the solvent to boost reactivity of substrates and achieve challenging transformations.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to the NSF (CHE-1665122) and NIH (1R01GM120240-01) for financial support.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details, NMR spectra, and details of the experiments
The authors claim no competing financial interest.
REFERENCES
- (1).(a) Corey EJ Angew. Chem. Int. Ed 1991, 30, 455–465. [Google Scholar]; (b) Nicolaou KC; Bulger PG; Sarlah D Angew. Chem. Int. Ed 2005, 44, 4442–4489. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC; Bulger PG; Sarlah D Angew. Chem. Int. Ed 2005, 44, 4490–4527. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC; Chen JS Chem. Soc. Rev 2009, 38, 2993–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Nicolaou KC; Edmonds DJ; Bulger PG Angew. Chem. Int. Ed 2006, 45, 7134–7186. [DOI] [PubMed] [Google Scholar]; (f) Nicolaou KC; Snyder SA; Montagnon T; Vassilikogiannakis G Angew. Chem. Int. Ed 2002, 41, 1668–1698. [DOI] [PubMed] [Google Scholar]; (g) Nicolaou KC; Vourloumis D; Winssinger N; Baran PS Angew. Chem. Int. Ed 2000, 39, 44–122. [PubMed] [Google Scholar]; (h) Tasker SZ; Standley EA; Jamison TF Nature 2014, 509, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Parmar D; Sugiono E; Raja S; Rueping M Chem. Rev 2014, 114, 9047–9153. [DOI] [PubMed] [Google Scholar]; (j) Akiyama T; Itoh J; Fuchibe K Adv. Synth. Catal 2006, 348, 999–1010. [Google Scholar]; (k) Hashmi ASK; Hutchings GJ Angew. Chem. Int. Ed 2006, 45, 7896–7936. [DOI] [PubMed] [Google Scholar]; (l) Noyori R; Hashiguchi S Acc. Chem. Res 1997, 30, 97–102. [Google Scholar]; (m) Colby DA; Bergman RG; Ellman JA Chem. Rev 2010, 110, 624–655. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Ley SV; Thomas AW Angew. Chem. Int. Ed 2003, 42, 5400–5449. [DOI] [PubMed] [Google Scholar]; (o) Herrmann WA Angew. Chem. Int. Ed 2002, 41, 1290–1309. [DOI] [PubMed] [Google Scholar]; (p) Prier CK; Rankic DA; MacMillan DW C. Chem. Rev 2013, 113, 5322–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Furstner A Angew. Chem. Int. Ed 2000, 39, 3012–3043. [PubMed] [Google Scholar]; (r) Yan M; Kawamata Y; Baran PS Chem. Rev 2017, 117, 13230–13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Shuklov IA; Dubrovina NV; Boerner A Synthesis 2007, 2925–2943. [Google Scholar]; (b) de Visser SP; Kaneti J; Neumann R; Shaik SJ Org. Chem 2003, 68, 2903–2912. [DOI] [PubMed] [Google Scholar]; (c) Kirste A; Elsler B; Schnakenburg G; Waldvogel SR J. Am. Chem. Soc 2012, 134, 3571–3576. [DOI] [PubMed] [Google Scholar]; (d) De K; Legros J; Crousse B; Bonnet-Delpon DJ Org. Chem 2009, 74, 6260–6265. [DOI] [PubMed] [Google Scholar]; (e) Arai T; Yokoyama N Angew. Chem. Int. Ed 2008, 47, 4989–4992. [DOI] [PubMed] [Google Scholar]; (f) Marques A-S; Duhail T; Marrot J; Chataigner I; Coeffard V; Vincent G; Moreau X Angew. Chem. Int. Ed 2019, 58, 9969–9973. [DOI] [PubMed] [Google Scholar]; (g) Wasserscheid P; Keim W Angew. Chem. Int. Ed 2000, 39, 3772–3789. [DOI] [PubMed] [Google Scholar]
- (3).(a) Purser S; Moore PR; Swallow S; Gouverneur V Chem. Soc. Rev 2008, 37, 320–330. [DOI] [PubMed] [Google Scholar]; (b) O’Hagan D Chem. Soc. Rev 2008, 37, 308–319. [DOI] [PubMed] [Google Scholar]; (c) Furuya T; Kamlet AS; Ritter T Nature 2011, 473, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shimizu M; Hiyama T Angew. Chem. Int. Ed 2005, 44, 214–231. [DOI] [PubMed] [Google Scholar]; (e) Berger R; Resnati G; Metrangolo P; Weber E; Hulliger J Chem. Soc. Rev 2011, 40, 3496–3508. [DOI] [PubMed] [Google Scholar]; (f) Begue JP; Bonnet-Delpon DJ Fluor. Chem 2006, 127, 992–1012. [Google Scholar]
- (4).(a) Dherbassy Q; Schwertz G; Chessé M; Hazra CK; Wencel-Delord J; Colobert F Chem. Eur. J 2016, 22, 1735–1743. [DOI] [PubMed] [Google Scholar]; (b) Wencel-Delord J; Colobert F Org. Chem. Front 2016, 3, 394–400. [Google Scholar]; (c) Colomer I; Chamberlain AER; Haughey MB; Donohoe TJ Nat. Rev. Chem 2017, 1, 12. [Google Scholar]; (d) Elsler B; Wiebe A; Schollmeyer D; Dyballa KM; Franke R; Waldvogel SR Chem. Eur. J 2015, 21, 12321–12325. [DOI] [PubMed] [Google Scholar]; (e) Wiebe A; Schollmeyer D; Dyballa KM; Franke R; Waldvogel SR Angew. Chem. Int. Ed 2016, 55, 11801–11805. [DOI] [PubMed] [Google Scholar]; (f) Wang H; Moselage M; Gonzalez MJ; Ackermann L ACS Catal. 2016, 6, 2705–2709. [Google Scholar]; (g) Trillo P; Baeza A; Najera CJ Org. Chem 2012, 77, 7344–7354. [DOI] [PubMed] [Google Scholar]; (h) Gaster E; Vainer Y; Regev A; Narute S; Sudheendran K; Werbeloff A; Shalit H; Pappo D Angew. Chem. Int. Ed 2015, 54, 4198–4202. [DOI] [PubMed] [Google Scholar]; (i) Tao Z; Robb KA; Zhao K; Denmark SE J. Am. Chem. Soc 2018, 140, 3569–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Berkessel A; Adrio JA; Huettenhain D; Neudorfl JM J. Am. Chem. Soc 2006, 128, 8421–8426. [DOI] [PubMed] [Google Scholar]
- (5).Takahashi I; Fujita T; Shoji N; Ichikawa J Chem. Commun 2019, 55, 9267–9270. [DOI] [PubMed] [Google Scholar]
- (6).Weisner N; Khaledi MG Green Chem. 2016, 18, 681–685. [Google Scholar]
- (7).Arnold AM; Pothig A; Drees M; Gulder TJ Am. Chem. Soc 2018, 140, 4344–4353. [DOI] [PubMed] [Google Scholar]
- (8).Motiwala HF; Fehl C; Li SW; Hirt E; Porubsky P; Aube JJ Am. Chem. Soc 2013, 135, 9000–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Wang J; Zhang S; Xu C; Wojtas L,; Akhmedov NG; Chen H; Shi X Angew. Chem. Int. Ed 2018, 57, 6915–6920. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang J; Wei C; Li X; Zhao P; Shan C; Wojtas L; Chen H; Shi X Chem. Eur. J 2020, 26, 5946–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Kondo T; Uenoyama S.-y.; Fujita K.-i.; Mitsudo T.-a. J. Am. Chem. Soc 1999, 121, 482–483. [Google Scholar]; (b) Kondo T; Mitsudo T.-a. Chem. Rev 2000, 100, 3205–3220. [DOI] [PubMed] [Google Scholar]; (c) Beletskaya I; Moberg C Chem. Rev 2006, 106, 2320–2354. [DOI] [PubMed] [Google Scholar]; (d) Yamagiwa N; Suto Y; Torisawa Y Bioorg. Med. Chem. Lett 2007, 17, 6197–6201. [DOI] [PubMed] [Google Scholar]; (e) Caserio MC; Fisher CL; Kim JK J. Org. Chem 1985, 50, 4390–4393. [Google Scholar]; (f) Matsumoto K; Fujie S; Suga S; Nokami T; Yoshida J.-i. Chem. Commun 2009, 5448–5450. [DOI] [PubMed] [Google Scholar]; (g) Wang X-R; Chen F Tetrahedron 2011, 67, 4547–4551. [Google Scholar]; (h) Usugi S.-i.; Yorimitsu H; Shinokubo H; Oshima K Org. Lett 2004, 6, 601–603. [DOI] [PubMed] [Google Scholar]; (i) Ananikov VP; Gayduk KA; Orlov NV; Beletskaya IP; Khrustalev VN; Antipin MY Chem. Eur. J 2010, 16, 2063–2071. [DOI] [PubMed] [Google Scholar]; (j) Song T; Li H; Wei F; Tung C-H; Xu Z Tetrahedron Lett. 2019, 60, 916–919. [Google Scholar]
- (11).(a) Ogawa A; Yokoyama H; Yokoyama K; Masawaki T; Kambe N; Sonoda NJ Org. Chem 1991, 56, 5721–5723. [Google Scholar]; (b) Kawaguchi S.-i.; Shirai T; Ohe T; Nomoto A; Sonoda M; Ogawa A J. Org. Chem 2009, 74, 1751–1754. [DOI] [PubMed] [Google Scholar]; (c) Shi M; Lu J-MJ Org. Chem 2006, 71, 1920–1923. [DOI] [PubMed] [Google Scholar]; (d) Kamiya I; Nishinaka E; Ogawa A Tetrahedron Lett. 2005, 46, 3649–3652. [Google Scholar]; (e) Wang M; Cheng L; Hong B; Wu Z Organometallics 2009, 28. [Google Scholar]; (f) Prochnow T; Back DF; Zeni G Adv. Synth. Catal 2016, 358, 1119–1129. [Google Scholar]; (g) Ananikov VP; Beletskaya IP; Aleksandrov GG; Eremenko IL Organometallics 2003, 22, 1414–1421. [Google Scholar]; (h) Ananikov VP; Kabeshov MA; Beletskaya IP; Khrustalev VN; Antipin MY Organometallics 2005, 24, 1275–1283. [Google Scholar]; (i) Ogawa A; Doi M; Ogawa I; Hirao T Angew. Chem. Int. Ed 1999, 38, 2027–2029. [DOI] [PubMed] [Google Scholar]; (j) Sartori G; Neto JSS; Pesarico AP; Back DF; Nogueira CW; Zeni G Org. Biomol. Chem 2013, 11, 1199–1208. [DOI] [PubMed] [Google Scholar]; (k) Moro AV; Nogueira CW; Barbosa NBV; Menezes PH; da Rocha JBT; Zeni GJ Org. Chem 2005, 70, 5257–5268. [DOI] [PubMed] [Google Scholar]; (l) Kuniyasu H; Ogawa A; Miyazaki S; Ryu I; Kambe N; Sonoda NJ Am. Chem. Soc 1991, 113, 9796–9803. [Google Scholar]; (m) Cai M; Wang Y; Hao W Green Chem. 2007, 9, 1180–1184. [Google Scholar]; (n) Casola KK; Back DF; Zeni GJ Org. Chem 2015, 80, 7702–7712. [DOI] [PubMed] [Google Scholar]
- (12).(a) Yuan T; Ye X; Zhao P; Teng S; Yi Y; Wang J; Shan C; Wojtas L; Jean J; Chen H; Shi X Chem 2020, 6, 1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang J; Wei C; Li X; Zhao P; Shan C; Wojtas L; Chen H; Shi X Chem. Eur. J 2020, 26, 5946–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ye X; Zhao P; Zhang S; Zhang Y; Wang Q; Shan C; Wojtas L; Guo H; Chen H; Shi X Angew. Chem. Int. Ed 2019, 58, 17226–17230. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jimoh AA; Hosseyni S; Ye X; Wojtas L; Hu Y; Shi X Chem. Commun 2019, 55, 8150–8153. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wei C; Ye X; Xing Q; Hu Y; Xie Y; Shi X Org. Biomol. Chem 2019, 17, 6607–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Li P; Zhao J; Shi L; Wang J; Shi X; Li F Nat. Commun 2018, 9, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ye X; Peng H; Wei C; Yuan T; Wojtas L; Shi X Chem 2018, 4, 1983–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Wang J; Zhang S; Xu C; Wojtas L; Akhmedov NG; Chen H; Shi X Angew. Chem. Int. Ed 2018, 57, 6915–6920. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Ye X; Wang J; Ding S; Hosseyni S; Wojtas L; Akhmedov NG; Shi X Chem. Eur. J 2017, 23, 10506–10510. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Dong B; Peng H; Motika SE; Shi X Chem. Eur. J 2017, 23, 11093–11099. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Smith CA; Motika SE; Wojtas L; Shi X Chem. Commun 2017, 53, 2315–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Cai R; Ye X; Sun Q; He Q; He Y; Ma S; Shi X ACS Catal. 2017, 7, 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


