Abstract
Insulin-producing β cells created from human pluripotent stem cells have potential as a therapy for insulin-dependent diabetes, but human pluripotent stem cell-derived islets (SC-islets) still differ from their in vivo counterparts. To better understand the state of cell types within SC-islets and identify lineage specification deficiencies, we used single-nucleus multi-omic sequencing to analyse chromatin accessibility and transcriptional profiles of SC-islets and primary human islets. Here we provide an analysis that enabled the derivation of gene lists and activity for identifying each SC-islet cell type compared with primary islets. Within SC-islets, we found that the difference between β cells and awry enterochromaffin-like cells is a gradient of cell states rather than a stark difference in identity. Furthermore, transplantation of SC-islets in vivo improved cellular identities overtime, while long-term in vitro culture did not. Collectively, our results highlight the importance of chromatin and transcriptional landscapes during islet cell specification and maturation.
Subject terms: Embryonic germ cells, Type 1 diabetes, Stem-cell biotechnology
Augsornworawat et al. perform single-nucleus multi-omics and integrated transcriptional and chromatin analysis to identify differences between human stem cell-derived and primary islets.
Main
The development of methods to differentiate hPSCs into islet-like clusters has the potential to generate an unlimited number of insulin-producing stem cell-derived β (SC-β) cells for the treatment of insulin-dependent diabetes1–3. This process utilizes temporal combinations of small molecules and growth factors4–6, microenvironmental cues7 and other sorting or aggregation4,8–14 approaches to drive cells through several intermediate progenitor cell types. The resulting SC-β cells possess many features of primary human β cells, including the expression of β-cell-specific markers, glucose-responsive insulin secretion and the ability to reverse severe diabetes in animal models5,6,15–18. As a result, these cells have the potential to provide a functional cure for human patients with type 1 diabetes. However, these stem cell-derived islets (SC-islets) are a heterogeneous tissue that also contains stem cell-derived α (SC-α) and δ (SC-δ) cells, as well as an endocrine cell type of intestinal identity denoted here as stem cell-derived enterochromaffin (SC-EC) cells19. The presence of this awry population suggests that there are inefficiencies in lineage specification during these directed differentiation protocols9. Correcting this aberrant signalling could enhance specification to a β-cell identity and further improve the function of SC-β cells.
The specific pattern of gene expression and chromatin state within a cell directs differentiation and maintains its final identity13,14,20–23. Thus, characterizing both the transcriptional and chromatin landscape of cells during this differentiation process can provide insight into the degree that a particular cell type resembles its in vivo analogue. Single-cell RNA sequencing has been used to characterize the transcriptional environment of SC-islets, demonstrating that SC-β cells express many, but not all, of the important genes found in human primary β cells9,16,17,24,25. Studies have also begun to investigate the chromatin state within pancreatic cell differentiations using bulk approaches26–29, and recent work has demonstrated the advantages of studying primary human islets using single-cell assay for transposase-accessible chromatin (ATAC) sequencing30–32. ATAC sequencing can describe whether particular chromatin regions are in an open, accessible state that is ready to be transcribed or interacted with, providing valuable information about cell identity that is missing when only transcriptional data are investigated. This data can define, for instance, the chromatin accessibility of promoters for specific genes that are capable of being transcribed. Furthermore, it can describe the chromatin accessibility of the DNA sequence motifs where specific transcription factors bind to help activate the transcription of different genes. Importantly, modulating the chromatin states of particular genes and motifs that are mis-expressed in in vitro-derived cell types are likely to drive cells closer to the identity of their in vivo counterparts. However, a comprehensive index of the combined chromatin accessibility and transcriptional signatures of each in vitro-differentiated SC-islet cell type is currently lacking.
In this Resource, we have provided and analysed the transcriptional and chromatin landscapes of SC-islets at the single-cell level, identifying important genes and motifs for each cell type. Interestingly, SC-EC and SC-β cells formed a gradient of cell identities, with subpopulations exhibiting characteristics of the other. Primary human islets had a more defined chromatin state than SC-islets, the latter of which had open chromatin regions associated with other lineages. Transplantation into mice for 6 months closed many of these accessible chromatin regions and improved lineage-specific gene expression, while extended in vitro culture did not have the same effect. We identified and modulated chromatin regulators important for SC-β cell identity, highlighting the importance of the chromatin landscape. Our findings improve the characterization of cellular identities within SC-islets and provide a resource to guide the development of strategies to improve SC-β cell differentiation.
Results
Multi-omic SC-islet analysis improves cell identity resolution
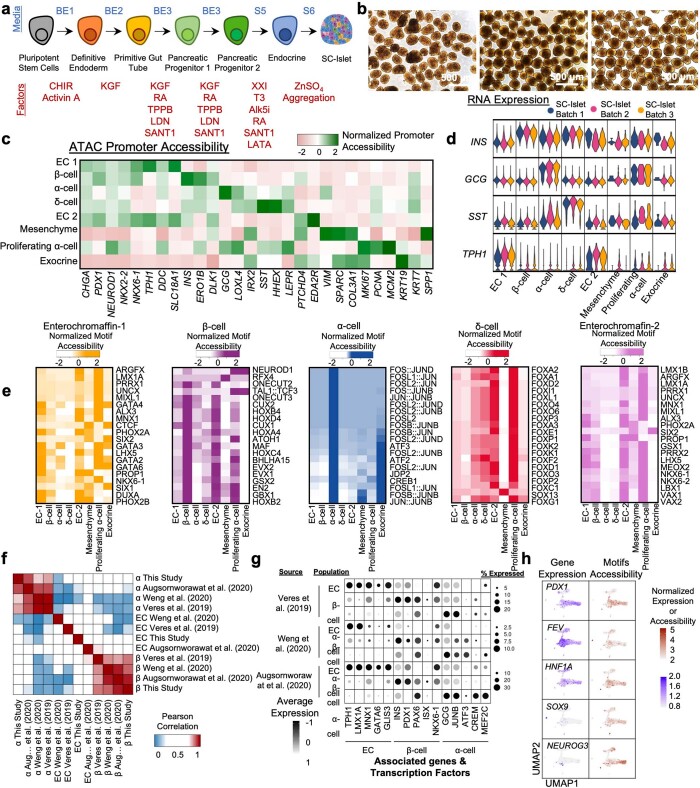
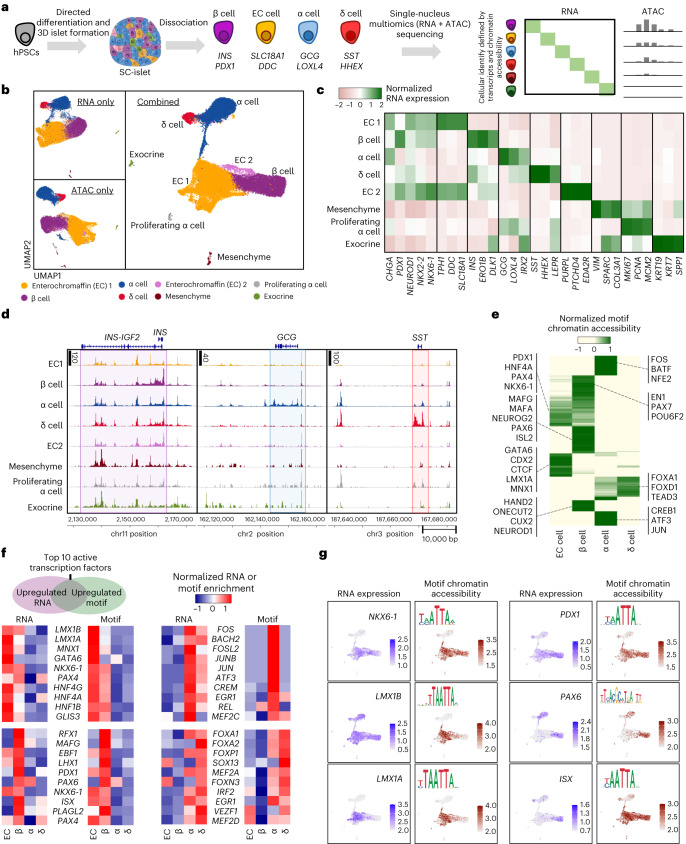
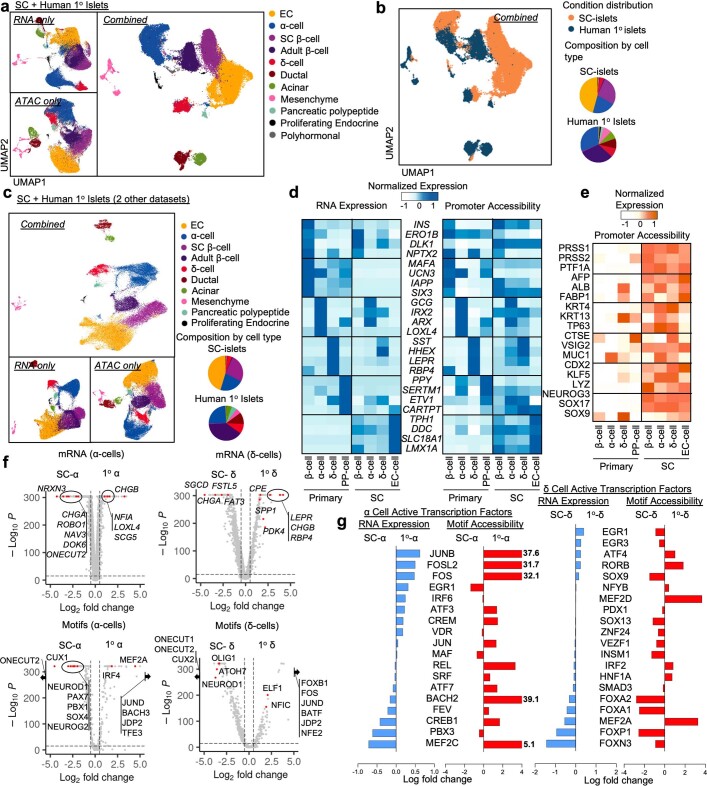
We analysed transcriptional and chromatin features of cells produced through directed differentiation of hPSCs to pancreatic islets to define cell identity and investigate lineage specification deficiencies rigorously7,33 (Extended Data Fig. 1a,b and Supplementary Table 1). These SC-islets were processed and sequenced using single-nucleus multi-omics, obtaining both gene expression (messenger RNA) and chromatin accessibility (ATAC) information for each cell (Fig. 1a and Supplementary Table 2). Analysis of gene expression and chromatin accessibility both individually and in combination enabled us to identify specific islet cell types, including SC-β cells (Fig. 1b,c, Extended Data Fig. 1c,d and Supplementary Tables 3 and 4; 29,526 cells from 3 independent differentiations). We were able to identify two subpopulations of SC-EC cells (denoted as SC-EC1 and SC-EC2) only when using the integrated analysis of both mRNA and ATAC data (Fig. 1b). SC-β, SC-α and SC-δ populations had strong chromatin peaks represented by their respective INS, GCG and SST gene regions, as expected (Fig. 1d). Unexpectedly, however, the INS gene region had open accessibility across all detected cell types, including non-endocrine cell types to some extent. In contrast, GCG and SST gene regions had relatively few chromatin peaks outside of SC-α and SC-δ cells, respectively.
Extended Data Fig. 1. Single-cell Multiomic ATAC and gene expression characterization of SC-islets.
a, Schematic diagram outlining the differentiation protocol for generating SC-islets. b, Brightfield images of differentiated SC-islets from the three batches used for multiomic sequencing. Individual image representative of 1 sample. c, Heatmap showing ATAC promoter accessibility of markers associated with each cell type (29526 cells from 3 independent differentiations; integration of all samples). d, Violin plots for INS, GCG, SST, and TPH1 gene expression in SC-islet cell types from different datasets of different differentiation batches. e, Heatmaps showing the top 20 enriched motifs in SC-EC1, SC-β, SC-α, SC-δ, and SC-EC2 cells. f, Pearson correlation analysis using the top 2000 variable genes comparing correlation of SC-β, SC-α, and SC-EC in SC-islets from this study and other literatures using other differentiation protocols. SC-β, and SC-α have similar gene expression profiles across multiple datasets. g, Dot plots showing the upregulation, by gene expression, of identified active transcription factors in SC-β, SC-α, and SC-EC cells of datasets from other studies. h, Feature plot showing gene expression of progenitor transcription factors and chromatin accessibility of their binding motifs. EC, enterochromaffin cells.
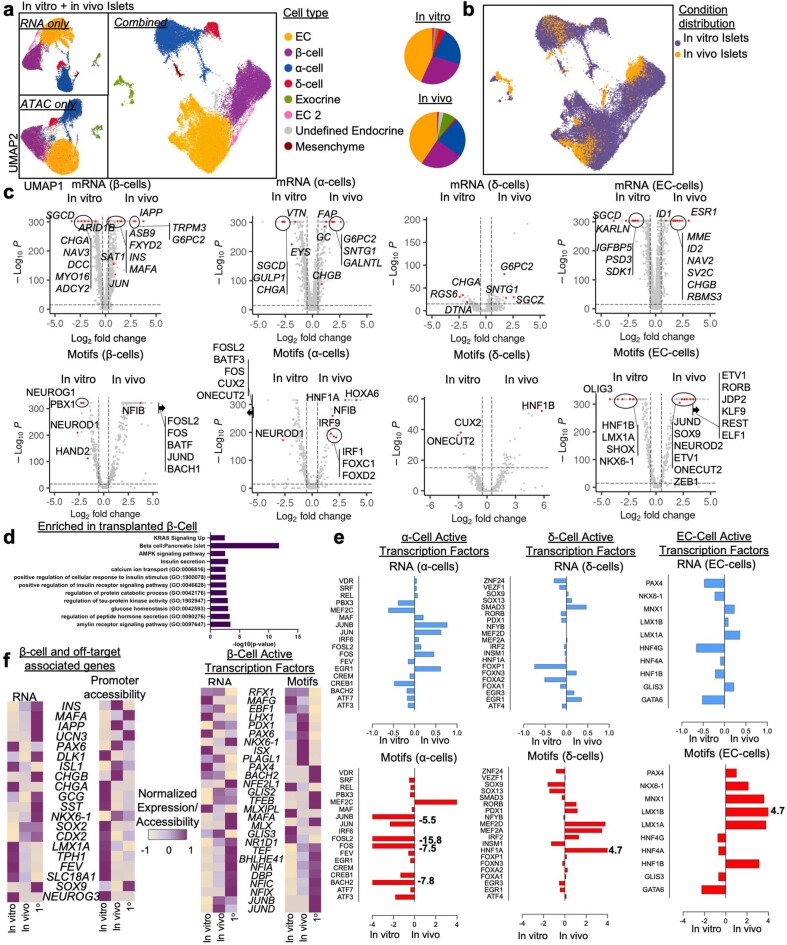
Fig. 1. Multi-omic profiling of SC-islets shows unique chromatin accessibility signatures in endocrine cell types.
a, Schematic of SC-islet differentiation and multi-omic sequencing. b, UMAPs showing identified cell types in SC-islets 2 weeks into stage 6 using both and either chromatin accessibility (ATAC) or gene (mRNA) information (29,526 cells from 3 independent differentiations; integration of all samples). c, Heat map showing gene expression of markers associated with each cell type. d, ATAC plots showing chromatin accessibility of SC-islet cell types around the INS, GCG and SST genomic regions. e, Heat map showing the top 200 variable DNA-binding motif accessibility within endocrine cell populations and highlighting markers for each cell type. f, Heat maps highlighting gene expression and ATAC motif accessibility of top ten active transcription factors co-enriched with both features in SC-β, SC-α, SC-δ and SC-EC cells. g, UMAP showing gene expression and motif accessibility of selected transcription factors associated with SC-β cells or SC-EC cells. SC, stem cell derived; EC, enterochromaffin.
We found distinct transcription factor-binding motif groups enriched in each specific cell type (Fig. 1e, Extended Data Fig. 1e and Supplementary Table 5). Within the endocrine population, SC-β cells had enrichment of motifs that correspond to known β-cell-associated transcription factors9,25,34,35. Interestingly, this included enrichment of the MAFA binding motif, but the expression of the transcription factor itself remained at very low levels in SC-β cells, as previously described19,36. Motifs that were specifically enriched in SC-EC cells include GATA6, CDX2 and LMX1A, which are known intestinal markers37,38. Interestingly, SC-β and SC-EC cells possessed shared enrichment of certain motifs, such as PDX1, PAX4 and NKX6-1, that are important in human primary β cells31 (Fig. 1e).
In an effort to better characterize these cell populations within SC-islets, we cross-referenced transcription factor DNA-binding motif chromatin accessibility and gene expression information to identify active transcription factors that have both high expression and that can access their target binding motifs to promote transcription of other genes (Supplementary Table 5). Here we highlight the top ten identified active transcription factors enriched in specific endocrine populations compared with the average of the other endocrine populations (Fig. 1f). Highly active transcription factors in SC-β cells include RFX1, PDX1 and PAX6, which are important for β-cell identity31,39. Other highly active transcription factors, such as MAFG, EBF1, ISX and PLAGL2, have not been highlighted previously, demonstrating the utility of using both mRNA and chromatin accessibility data for identifying cell types. In contrast, SC-EC cells have co-enrichment of LMX1B, LMX1A, MNX1 and GATA6. Notably, the SC-β and SC-EC populations both have high activities of NKX6-1 and PAX4, suggesting that they share common features essential for their identities. This similarity is further demonstrated with motif chromatin accessibility plots, where NKX6-1 and PDX1 were enriched in both SC-β and SC-EC populations (Fig. 1e,g). In contrast, binding motifs for PAX6 were enriched in SC-β cells compared with SC-EC cells while LMX1A was enriched in SC-EC cells compared with SC-β cells. Unexpectedly, we found a mismatch in RNA expression and motif accessibility across cell types for certain transcription factors, such as LMX1B and ISX (Fig. 1f,g). We also investigated transcription factors specifically implicated during endocrine cell development by RNA expression and motif accessibility (Extended Data Fig. 1h). Of note, we observed FEV being upregulated in the SC-EC population, HNF1A in all endocrine populations, and PDX1 expressed predominantly in SC-EC and SC-β cells by both RNA and chromatin motif accessibility. We also observed a mismatch of NEUROG3 in the final SC-β cell population, where there was no detectable RNA but enriched motif accessibility. Collectively, this multi-omic analysis has generated important lists of genes to better classify the cell types found within SC-islets, providing better resolution of cell identity and insights into the relative importance of different transcription factor activity within each cell type.
SC-EC and SC-β cells show gradient, not distinct, identities
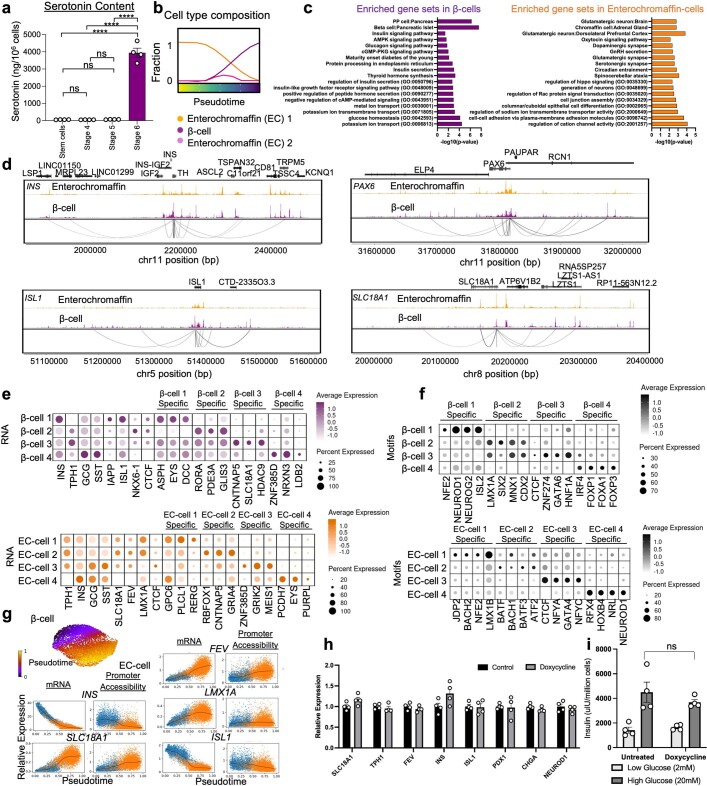
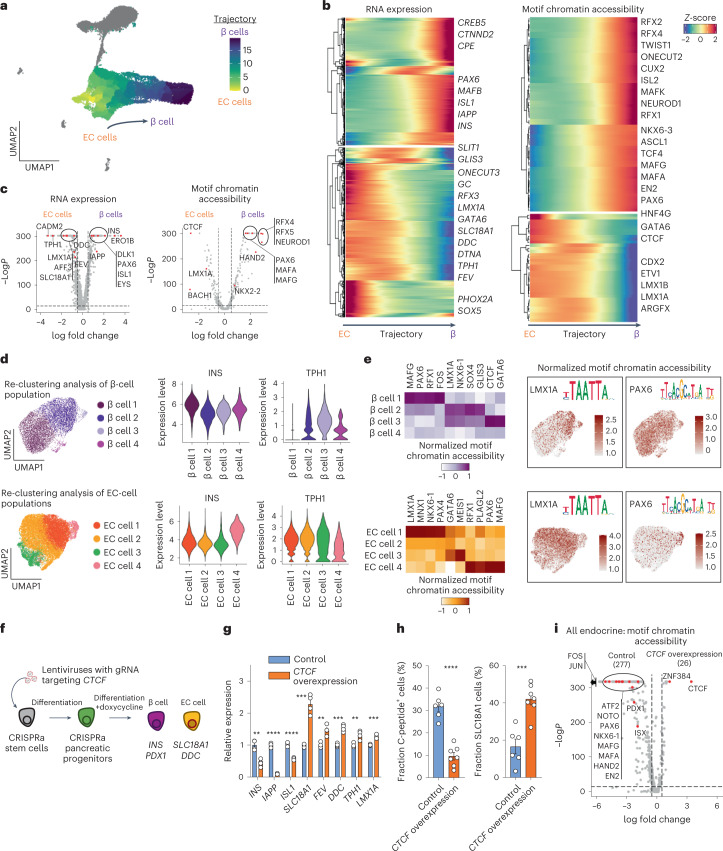
The serotonin-producing SC-EC cells comprise an awry cell population that arises during in vitro SC-islet differentiation protocols but that does not positively contribute to tissue function9,40,41 (Fig. 1b). Detection of substantial amounts of serotonin occurred only at the end of this differentiation protocol (Extended Data Fig. 2a). While it is not known how serotonin affects in vitro differentiation to SC-β cells, a study has reported its involvement in regulating β-cell mass during pregnancy42,43. Although both enterochromaffin and β cells arise from a definitive endoderm precursor during in vivo development, enterochromaffin cells originate from an intestinal lineage, while islet cells differentiate from a distinct pancreatic origin. During in vitro differentiation of SC-islets, however, they appear to share a common progenitor lineage, and how this enterochromaffin cell population emerges is not well understood9. Interestingly, the combined mRNA/ATAC clustering analysis allowed us to resolve two distinct SC-EC populations that were adjacent to the SC-β cell population (Fig. 1a), suggesting that there may be multiple or a gradient of cell states between SC-EC and SC-β cells. Given the apparent similarity of SC-β and the two SC-EC cell populations in our multi-omic clustering (Fig. 1b), we performed a trajectory analysis to detect differences in both gene expression and chromatin accessibility between SC-β and SC-EC cells (Fig. 2a, Extended Data Fig. 2b and Supplementary Table 6). As expected, enterochromaffin cell identity genes were most highly expressed in the SC-EC side of the trajectory map and β-cell identity genes were most highly expressed towards the SC-β cell side of the trajectory map (Fig. 2b,c and Extended Data Fig. 2c). ATAC peaks around SC-β cell and SC-EC cell marker genes also highlight differences in chromatin accessibility patterns, some of which were predicted to be cis-regulatory elements (Extended Data Fig. 2d). Differential motif chromatin accessibility analysis identified LMX1A, GATA6 and CTCF motifs to be enriched in SC-EC cells, while NEUROD1, HAND2, MAFA, PAX6, ONECUT2 and RFX2 motifs were enriched in SC-β cells (Fig. 2b,c).
Extended Data Fig. 2. Comparing SC-β and SC-EC cells.
a, Protein quantification plot showing mean ± s.e.m. (by ELISA, n = 4 biologically independent samples) of serotonin content in stem cells and stages 4–6 of differentiation (Stem cells vs Stage 4, P = 1.00, Stem cells vs Stage 5, P = 1.00, Stem Cells vs Stage 6, P = 6.17 × 10−10, Stage 4 vs Stage 5, P = 1.00, Stage 4 vs Stage 6, P = 6.06 × 10−10, Stage 5 vs Stage 6, P = 6.42 × 10−10). Statistical significances were assessed by one-way ANOVA with Tukey’s multiple comparison testing, reporting adjusted P-value. b, Composition of cell types represented on the trajectory analysis. c, Gene set enrichment of analysis showing enrichment of gene sets in segments of trajectory representing SC-β and SC-EC cells. Non-adjusted p-values were computed using two-sided Fisher exact test. d, ATAC peaks show chromatin accessibility pattern differences and highlight cis-regulatory elements around the INS, ISL1, PAX6, and SLC18A1 gene regions. e, Dot plots showing marker genes and unique genes associated with subpopulations identified from the reclustering of SC-β and SC-EC cell populations. f, Dot plots showing unique motif chromatin accessibility in subpopulations of SC-β and SC-EC cells. g. Trajectory analysis of the EC cell and β cell population using ‘MultiVelo’ package showing gene expression and promoter accessibility along. The trajectory analysis validates expression features of EC cell and β cell genes identified in the previous analysis with ‘Monocle’ package (Subset of 21317 cells from 3 independent differentiations; integration of all samples). h, qPCR analysis, plotting mean ± s.e.m. (n = 4 biologically independent samples), of non-transduced control cells comparing doxycycline effects on the expression of EC cell and β cell associated genes. All comparisons show no statistical significance. (SLC18A1, P = 0.095; TPH1, P = 0.45; FEV, P = 0.31; INS, P = 0.090; ISL1, P = 0.86; PDX1, P = 0.86; CHGA, P = 0.13; NEUROD1, P = 0.37). Statistical significance was assessed by unpaired two-sided t-test. i, Glucose stimulated insulin secretion assay, plotting mean ± s.e.m. (by ELISA, n = 4 biologically independent samples), of differentiated non-transduced control SC-islets. Treatment of doxycycline on non-transduced control shows no significant changes is insulin secretion (P = 0.38). Statistical significances were assessed using unpaired two-sided t-test. EC, enterochromaffin cells.
Fig. 2. SC-EC and SC-β cells have unique and common transcriptional and chromatin accessibility signatures.
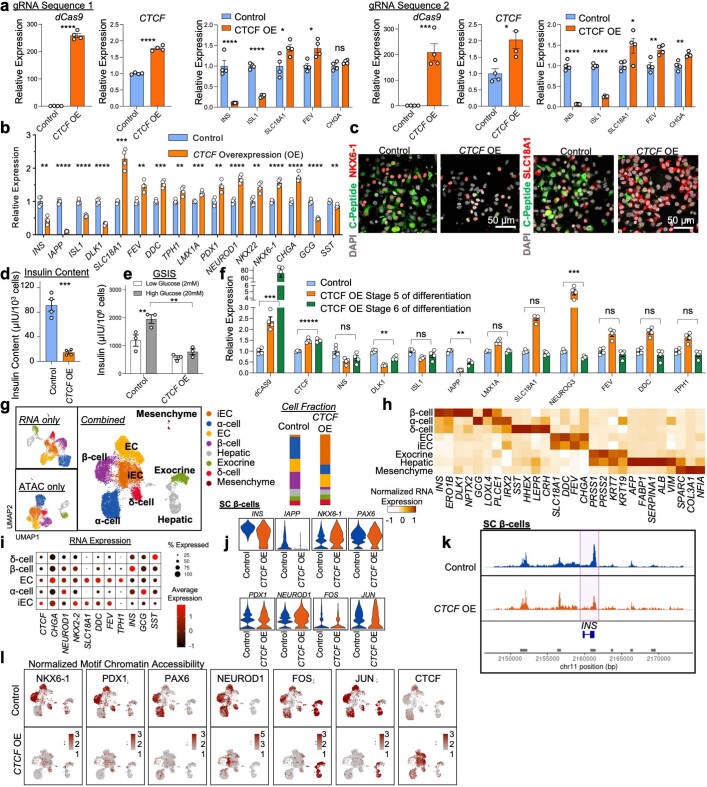
a, UMAP showing the trajectory of cells from the SC-β, SC-EC1 and SC-EC2 populations (subset of 21,317 cells from 3 independent differentiations; integration of all samples). b, Trajectory heat map showing dynamic changes of gene expression and motif accessibility enriched in β and EC groups. c, Volcano plots showing differential gene expression analysis (left) and differential motif accessibility analysis (right), highlighting relevant genes associated with SC-β and SC-EC cell populations. Statistical significance assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility. d, UMAP showing subpopulations by reclustering SC-β or SC-EC cell populations. Violin plots show gene marker expressions of INS and TPH1, highlighting off-target genes. e, Heat map showing DNA-binding motif accessibility associated with SC-β and SC-EC cells in subpopulations. Selected transcription factors plotted to show distribution of cells with target or off-target motif accessibility. f, Schematic of CRISPRa experiment for overexpression of CTCF in differentiating pancreatic progenitor cells. g, qPCR analysis of differentiated SC-islets with CTCF overexpression during endocrine induction, plotting mean ± s.e.m. (n = 4 biologically independent samples), showing expression differences in doxycycline treated compared with untreated control (INS, P = 0.0010; IAPP, P = 1.5 × 10−7; ISL1, P = 3.0 × 10−5) and EC cells (SLC18A1, P = 1.6 × 10−4; FEV, P = 0.0019; DDC, P = 1.2 × 10−4; TPH1, P = 0.0047; LMX1A, P = 4.6 × 10−4). Control represents cells without doxycycline treatment. Statistical significance was assessed by unpaired two-sided t-test. h, ICC quantification of cells expressing C-peptide protein (P = 5.3 × 10−6) and SLC18A1 protein (P = 3.1 × 10−4) with or without CTCF overexpression, plotting mean ± s.e.m. (control; n = 6 biologically independent samples, doxycycline; n = 7 biologically independent samples). Control represents cells without doxycycline treatment. Statistical significance was assessed by unpaired two-sided t-test. i, Volcano plots showing differential motif chromatin accessibility analysis of SC-endocrine population comparing control and CTCF overexpression (12,467 cells from 2 independent biological samples, 1 of each condition from the same differentiation batch; integration of all samples). Statistical significance was assessed by logistic regression. Control represents cells without doxycycline treatment. SC, stem cell derived; EC, enterochromaffin cells; CRISPRa, CRISPR activation.
While key identity genes and motifs were enriched on their respective sides of the trajectory map, their expression was a mixture of SC-β and SC-EC cell identities across the trajectory (Fig. 1b). To further probe the transcriptional and chromatin landscape of cells along this trajectory map, we performed subclustering analyses separately on the SC-β cell population and the SC-EC cell populations (Fig. 2d). Differential expression analyses revealed that each of the SC-β cell subpopulations had distinguishing gene expression and motif accessibility features (Fig. 2d,e, Extended Data Fig. 2e,f and Supplementary Table 7), including SC-β cells with TPH1 expression and more open chromatin accessibility of the SC-EC cell-associated DNA-binding motifs for LMX1A and CTCF. The high INS-expressing SC-β cell population displayed greater motif accessibility to β-cell-associated transcription factors, such as PAX6, and less motif accessibility to EC-cell transcription factors, such as LMX1A. Similarly, differential expression analyses demonstrated that the four SC-EC cell subpopulations also had distinguishing gene expression and motif accessibility features, including SC-EC cells with elevated INS expression and more open chromatin accessibility of the SC-β cell-associated DNA-binding motifs for PAX6 and MAFG. Additionally, we performed trajectory analysis using another tool44 that produced consistent results (Extended Data Fig. 2g). Collectively, these data suggest that the SC-EC and SC-β cell populations produced from in vitro differentiation form a continuum of cell states rather than exhibiting clear exclusivity of gene expression and chromatin accessibility, suggesting deficiencies in fully specifying each endocrine cell population in vitro.
Because changes in chromatin accessibility influence which genes can be expressed in a cell and consequently its identity, chromatin regulators could influence where a cell lies on this gradient between SC-β and SC-EC cells during in vitro differentiation. To this end, our integrated multi-omic analysis identified the chromatin remodeller CCCTC-binding factor (CTCF) as the transcription factor binding motif having the greatest increase in accessibility in SC-EC cells compared with SC-β cells (Fig. 2c). Because CTCF is a chromatin remodeller that is involved in other developmental and differentiation processes45, we sought to further examine its impact on pancreatic differentiation. Unfortunately, we were unsuccessful in also knocking down its expression despite trying several approaches, which we would hypothesize could improve differentiation to SC-β cells. Instead, we utilized a doxycycline-inducible VP64-p65-Rta CRISPR activation (CRISPRa) stem cell line46 to increase transcription of CTCF during differentiation (Fig. 2f and Extended Data Figs. 2h,i and 3a). Upregulation of CTCF during stage 5 endocrine cell induction7,33 resulted in drastic reductions in the expression of β-cell identity markers, insulin content and glucose-stimulated insulin secretion along with notable upregulation of intestinal lineage/EC-cell-associated genes (Fig. 2g,h and Extended Data Fig. 3a–e). CTCF overexpression had the most pronounced impact during the endocrine induction stage, indicating its specific role in selecting between SC-EC and SC-β cell fates (Extended Data Fig. 3f). Furthermore, single-nucleus multi-omic sequencing demonstrated that CTCF overexpression caused increased accessibility of the CTCF binding motif and decreased accessibility of β-cell-associated transcription factor binding motifs (Fig. 2i, Extended Data Fig. 3g–l and Supplementary Table 8; 12,467 cells from 2 datasets, 1 of each condition from the same differentiation batch). These results demonstrate that elevated CTCF expression disrupts the development of SC-β cells and redirects pancreatic progenitors towards an intestinal EC-like cell fate.
Extended Data Fig. 3. CTCF induction during differentiation using doxycycline inducible CRISPRa stem cells. Control represents cells without doxycycline treatment.
a, qPCR, plotting mean ± s.e.m. (n = 4 biologically independent samples), showing upregulation of dCas9 (gRNA sequence 1, P = 4.2 × 10−8; gRNA sequence 2, P = 7.8 × 10−4), CTCF overexpression (gRNA sequence 1, P = 1.4 × 10−6; gRNA sequence 2, P = 0.014), and changes in expression (gRNA sequence 1, INS (P = 5.6 × 10−4) ISL1 (P = 1.1 × 10−6), SLC18A1 (P = 0.034), FEV (P = 0.012), CHGA (ns, P = 0.12); gRNA sequence 2, INS (P = 4.4 × 10−6), ISL1 (P = 7.8 × 10−8), SLC18A1 (P = 0.042), FEV (P = 0.0069), CHGA (P = 0.0050), upon doxycycline. Statistical significance assessed by unpaired two-sided t-test. b, qPCR analysis, plotting mean ± s.e.m. (n = 4 biologically independent samples), of CTCF overexpressed (INS, P = 0.0010; IAPP, P = 1.5 × 10−7; ISL1, P = 3.0 × 10−5; DLK1, P = 3.7 × 10−6), EC cells (SLC18A1, P = 1.6 × 10−4; FEV, P = 0.0019; DDC, P = 1.2 × 10−4; TPH1, P = 0.0047; LMX1A, P = 4.6 × 10−4; PDX1, P = 0.0031; NEUROD1, P = 6.2 × 10−5; NKX2-2, P = 0.0047; NKX6-1, P = 7.5 × 10−5; CHGA, P = 6.8 × 10−5; GCG, P = 5.9 × 10−6; SST, P = 0.0046). Statistical significance assessed by unpaired two-sided t-test. c, Immunocytochemistry of SC-islets with CTCF overexpression showing C-peptide (green), NKX6-1 (red), and SLC18A1 (red). Individual image representative of 6 biologically independent samples. d, Protein quantification plot showing mean ± s.e.m. (by ELISA, n = 4 biologically independent samples) after CTCF overexpression (P = 2.2 × 10−4). Statistical significances assessed by unpaired two-sided t-test. e, Insulin secretion, plotting mean ± s.e.m. (by ELISA, n = 3 biologically independent samples), of control (P = 0.0095) and CTCF overexpression (P = 0.0032). Statistical significance assessed using paired two-sided t-test and unpaired two-sided t-test respectively. f, qPCR analysis, plotting mean ± s.e.m. (n = 4 biologically independent samples), comparing CTCF overexpression during Stage 5 of protocol or at Stage 6 of protocol (dCAS9, P = 6.13 × 10−4; CTCF, P = 2.0 × 10−5; INS, P = 0.10; DLK1, P = 0.0023; ISL1, P = 0.18; IAPP, P = 3.3 × 10−4; LMX1A, P = 0.97; SLC18A1, P = 0.12; NEUROG3, P = 9.3 × 10−4; FEV, P = 0.30; DDC, P = 0.27; TPH1, P = 0.21) Statistical significance assessed by unpaired two-sided t-test. g, UMAPs (12467 cells from 2 independent biological samples; integration of all samples) showing cell types in SC-islets induced with CTCF overexpression. h, Heatmap showing normalized gene expression associated with CTCF experiment. i, Dot plots highlighting expression of CTCF and endocrine-associated genes. j, Violin plot of β-cell associated gene expressions of CTCF overexpression in the SC-β cell population. k, Chromatin accessibility around INS genomic region of SC-β cells. l, Feature plots showing decreased accessibility of β-cell motifs and increased accessibility to CTCF motifs in endocrine populations with CTCF overexpression. EC, enterochromaffin cells; ns, not significant.
Multi-omic analysis defines distinct primary islet cell types
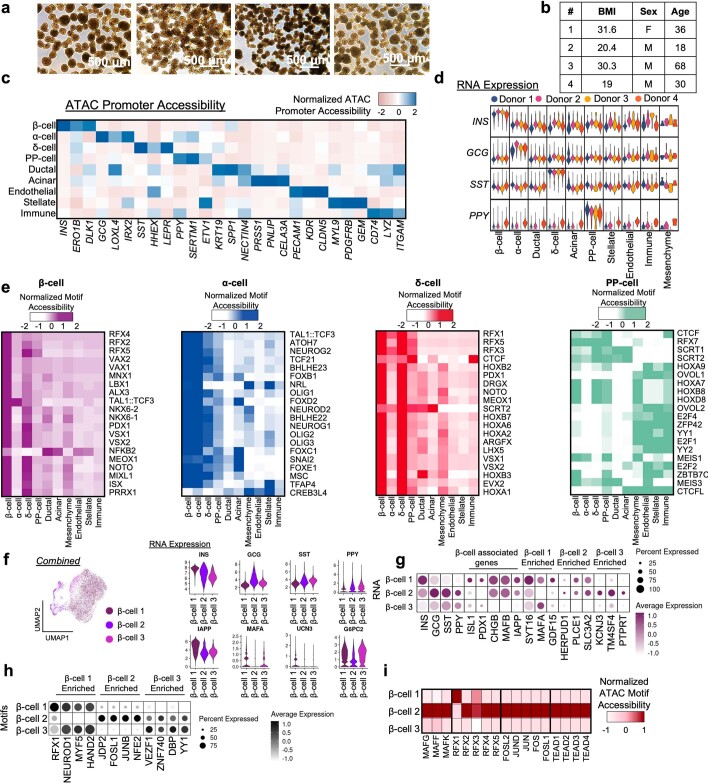
To compare the transcriptional and chromatin signatures identified in our SC-islets to their in vivo counterparts, we sequenced and characterized primary human islets (Fig. 3a, Extended Data Fig. 4a,b and Supplementary Table 2; 30,202 cells from 4 separate donors). We identified ten distinct populations that included both pancreatic endocrine, exocrine and other minority cell populations (Fig. 3b,c, Extended Data Fig. 4c,d and Supplementary Tables 3 and 4). Unlike SC-islets (Fig. 1b,c and Extended Data Fig. 1c), clusters representing all cell types appeared very distinct and exhibited robust expression of their respective gene markers by gene expression and chromatin accessibility. In addition, the chromatin accessibility profiles demonstrated that primary islet endocrine cells contained distinct peak signals around the INS, GCG, SST and PPY genomic regions of β cells, α cells, δ cells and PP cells, respectively (Fig. 3d). We also performed chromatin motif accessibility analyses in primary islets and identified distinguishing features across these endocrine types (Fig. 3e, Extended Data Fig. 4e and Supplementary Table 5). Notably, primary β cells had accessible binding sites for PDX1, NKX6-1, MAFA and ISX, similar to SC-β cells. Surprisingly, motifs identified as enriched in SC-EC cells, such as LMX1A and MNX1, were enriched in β cells when compared with other endocrine populations. Furthermore, re-clustering analysis of the primary β cells identified three subpopulations that displayed unique gene expression and accessible motif signatures (Extended Data Fig. 4f–i and Supplementary Table 7), consistent with previous studies25,31,47,48.
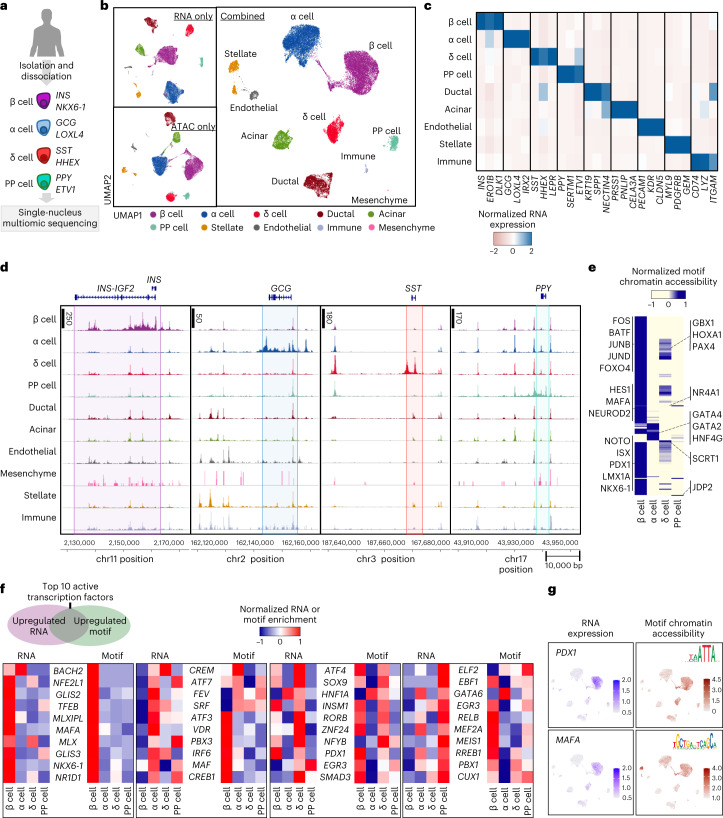
Fig. 3. Multi-omic profiling of human adult primary islets shows unique chromatin accessibility signatures in endocrine cell types.
a, Schematic of human adult primary islets for mutiomic sequencing. b, UMAPs showing identified cell types in primary human islets using both and either chromatin accessibility (ATAC) or gene (mRNA) information (30,202 cells from 4 independent biological donors; integration of all samples). c, Heat map showing gene expression of markers associated with each cell type. d, ATAC plots showing chromatin accessibility primary islet cell types around the INS, GCG, SST and PPY genomic regions. e, Heat map showing the top 200 variable DNA-binding motif accessibility within endocrine cell populations and highlighting motif markers for each cell type. f, Heat maps highlighting gene expression and ATAC motif accessibility of top ten active transcription factors co-enriched with both features in primary β, α, δ and PP cells. g, UMAP showing gene expression and motif accessibility of selected transcription factors associated with primary β cells. PP, pancreatic polypeptide.
Extended Data Fig. 4. Single-cell Multiomic ATAC and gene expression characterization of primary huma islets.
a, Brightfield images of primary islets used for multiomic sequencing. Individual image representative of 1 sample. b, Brief primary islet donor information. c, Heatmap showing ATAC promoter accessibility of markers associated with each cell type (30202 cells from 4 independent biological samples; integration of all samples). d, Violin plots for INS, GCG, SST, and PPY gene expression in primary islet cell types from different donors. e, Heatmaps showing the top 20 enriched motifs in primary β, α, δ, and PP cells. f, Re-clustering analysis of primary β cells highlighting heterogeneity by variations in mature and polyhormonal gene expressions. g, Dotplots showing marker genes and unique genes associated with subpopulations identified from the re-clustering of primary β cells. h, Dotplots showing unique motif chromatin accessibility in in subpopulations primary β cells. i, Chromatin accessibility of transcription factor binding sites of MAF, RFX, FOS/JUN, and TEAD motif family in primary β cell subpopulations. PP, Pancreatic progenitors.
Similar to our multi-omic analysis of SC-islets, we also examined transcription factor activity in primary islet endocrine cells as assessed by both relative increases in mRNA transcripts and chromatin accessibility of the corresponding DNA-binding motif (Fig. 3f,g). For β cells, NKX6-1 was the only transcription factor that was shared in the top ten active factor list with SC-β cells (Figs. 1f and 3f). While some of the active transcription factors were shared with the list from SC-islets, this analysis illustrates that primary islet cells have a unique transcriptional and chromatin landscape when compared with their stem cell-derived counterparts, highlighting specific deficiencies in lineage specification during directed differentiation protocols.
Primary islet chromatin is more restricted than in SC-islets
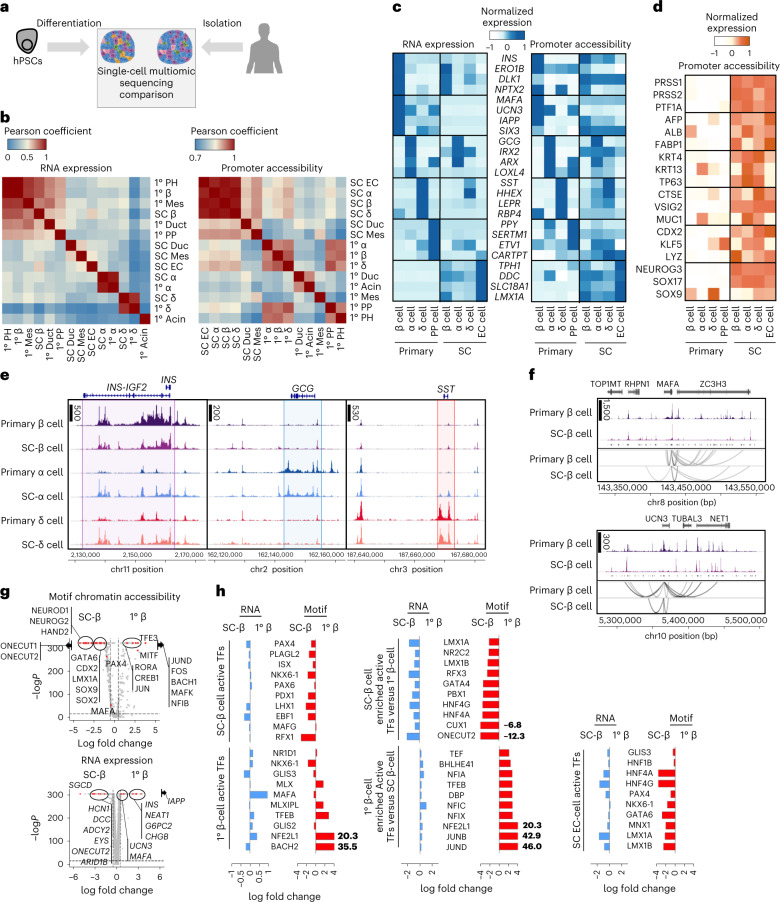
Previous studies have demonstrated that β cells derived from in vitro differentiation of hPSCs are functionally and transcriptionally different than their in vivo counterparts5,6,9,16,17,19. We compared the transcriptional and chromatin landscapes of SC-islets and primary islets using single-nucleus multi-omic sequencing to better understand these differences and similarities (Fig. 4a; 47,566 cells from 5 samples; 3 SC-islets, 2 primary islets). Both SC-islets and primary islets contained β, α and δ cells (Extended Data Fig. 5a,b). However, only SC-islets contained EC-cells, while only primary islets contained PP cells, consistent with prior analysis9. In general, SC-islet cell types were transcriptionally most similar to their primary cell counterparts (Fig. 4b). Surprisingly, promoter chromatin accessibility did not show this trend, as cell origin (in vitro derived versus primary) was a greater factor in determining similarity rather than cell type. In general, promoter accessibility of SC-islet cells was open across cell types for endocrine and non-endocrine identity genes and lacked cell type distinctiveness (Fig. 4c,d), even when compared with additional primary islets (Extended Data Fig. 5c–e; 52,317 cells from 5 samples; 3 SC-islets, 2 primary islets). Primary β cells had greater chromatin accessibility of the INS gene compared with SC-β cells (Fig. 4e). While SC-α and SC-δ cells also shared chromatin accessibility peaks around the INS gene, this was not observed in primary α and δ cells. Chromatin accessibility analysis of MAFA and UCN3 revealed predicted cis-regulatory elements that differed considerably between SC-β and primary β cells (Fig. 4f).
Fig. 4. Comparative analysis of SC-islets and primary human islets shows differences in chromatin accessibility signatures associated with islet identity.
a, Schematic showing comparisons of SC-islet and primary human islets (47,566 cells from 5 independent biological samples; all 3 SC-islets, 2 representative primary islets). b, Pearson correlation analysis comparing cell types in SC-islets and primary islets using gene expression and ATAC promoter accessibility. C, Heat map highlighting and comparing identity (β, adult β, α, δ, PP and EC) associated gene expression and ATAC promoter accessibility of endocrine cells in SC-islets and primary islets. d, Heat map highlighting and comparing off-target identity (exocrine, hepatic, oesophagus, stomach, intestinal and pancreatic progenitor) associated gene expression and ATAC promoter accessibility in SC-islet cells and primary islet cells. e, ATAC plots comparing chromatin accessibility around the INS, GCG and SST genomic regions in β, α and δ cells from SC-islets and primary islets. f, ATAC peaks from SC-β cells and 1° β cells showing chromatin accessibility around β-cell identity marker, MAFA (top) and UCN3 (bottom). Peaks were linked and analysed to depict differences in the number of cis-regulatory elements. g, Volcano plots showing differential motif accessibility analysis (top) and differential gene expression analysis (bottom) comparing SC-β cells and primary β cells. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility. h, Bar graphs showing fold change differences between SC-β cells and primary β cells, showing gene expression and motif accessibility of identified transcription factors (TFs) associated with SC-β cells, primary β cells and SC-EC cells. SC, stem cell derived; EC, enterochromaffin cells; PH, polyhormonal cells; Mes, mesenchyme; Duc, ductal; PP, pancreatic polypeptide cells; Acin, acinar cells.
Extended Data Fig. 5. Multiomic sequencing comparison of SC-islets and primary human islets.
a, Integrative UMAP showing cells from SC-islets and primary islets (47566 cells from 5 independent biological samples; 3 SC-islets, 2 representative primary islets- donor #1 and #2). b, Integrative UMAP plotted by SC-islet or primary islet condition. Pie charts show composition information of cell types identified. c, Validation analysis showing integrative UMAP of cells from SC-islets and other primary islet datasets (41688 cells from 5 independent biological samples; 3 SC-islets, 2 representative primary islets- donor #3 and #4). Pie charts show composition information of cell types identified. d, Validation analysis showing heatmap highlighting and comparing identity (β, adult β, α, δ, PP and EC) associated gene expression and ATAC promoter accessibility of endocrine cells in SC-islets and primary islets (Human islet donors #3 and #4). e, Validation analysis showing heatmap highlighting and comparing off-target identity (Exocrine, hepatic, esophagus, stomach, intestinal, pancreatic progenitor) associated gene expression and ATAC promoter accessibility in SC-islet cells and primary islet cells (Human islet donors #3 and #4). f, Differential gene expression (top) and motif chromatin accessibility analysis (right) for α-cells (left) and δ-cells (right). Statistical significance was assessed by Wilcoxon rank sum test for RNA expression and logistic regression for motif chromatin accessibility. g, Bar graphs showing fold change comparing SC and primary α and δ cell populations, showing gene expression and motif chromatin accessibility of identified transcription factors associated with the respective cell types. EC, enterochromaffin cells.
To explore similarities and differences in endocrine cell identity between SC-islets and primary islets, we analysed differential gene expression and motif chromatin accessibility in β-, α- and δ-cell subpopulations (Fig. 4g, Extended Data Fig. 5f and Supplementary Table 9). The mRNA data from the multi-omic analysis yielded results consistent with previous RNA-only studies, demonstrating SC-β cells had lower expression of IAPP and other maturation genes9,16,25,49,50. ARID1B, a chromatin regulator51, had higher expression in SC-β cells. Surprisingly, ONECUT2 had increased mRNA expression and DNA-binding motif chromatin accessibility in SC-β cells compared with primary β cells, despite this being a gene whose increased expression is associated with adult human β cells compared with juvenile β cells34. The chromatin accessibility data demonstrated that SC-β cells had more enriched motifs that are associated with off-target or progenitor cell states compared with primary β cells. Primary β cells showed enriched motifs linked to FOS/JUN family genes, expression of which is related to better function following transplantation16. MAFA surprisingly had similar chromatin accessibility of the associated DNA-binding motif between SC-β and primary β cells despite low mRNA expression and chromatin accessibility of the gene in SC-β cells (Fig. 4f,g). Thus, the role of MAFA in β-cell identity may be regulated by the chromatin state of the associated gene and not accessibility of its binding motif. These findings reveal substantial differences in mRNA expression and chromatin accessibility between SC-β cells and primary β cells. SC-β cells display more off-target cell type features and lack key adult primary β-cell identity signatures, whereas primary β cells have a more restricted chromatin state.
We compared β, α and δ cells from SC-islets and primary islets by analysing the activity of transcription factors in each cell type from both origins (Fig. 4h and Extended Data Fig. 5g). For SC-β and primary β cells, several transcription factors had comparable mRNA expression and motif accessibility, while others exhibited differences, such as enriched RFX1 motif accessibility, reduced MAFA RNA expression and reduced NFE2L1 and BACH2 motif accessibility in SC-β cells. We identified the top ten transcription factors with the greatest differential activity in SC-β cells compared with primary β cells and found that many of these factors were enriched in SC-β cells, indicating ambiguity in their cell identity due to incorrect or incomplete cell fate specification. Our analyses reveal fundamental differences in mRNA expression, chromatin accessibility and transcription factor DNA-binding motifs between cell types in SC-islets and their in vivo counterparts.
In vivo, not in vitro, time enhances SC-β cell signatures
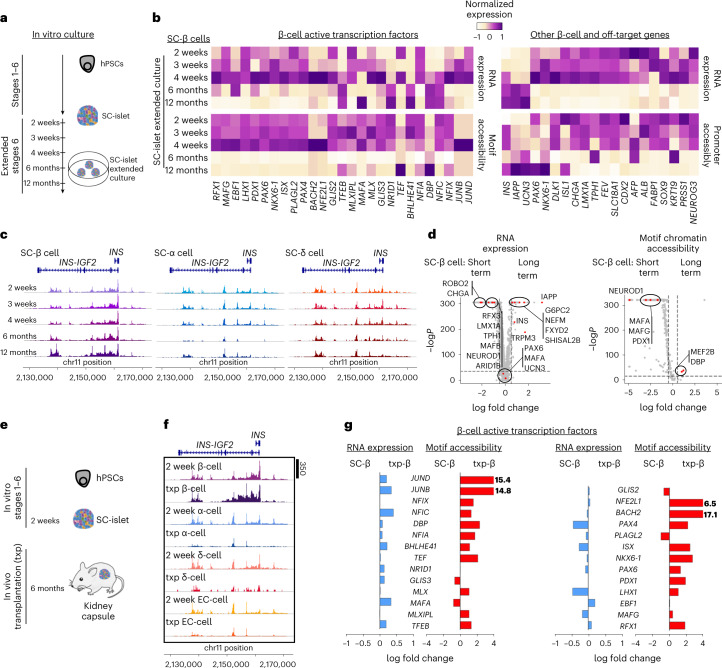
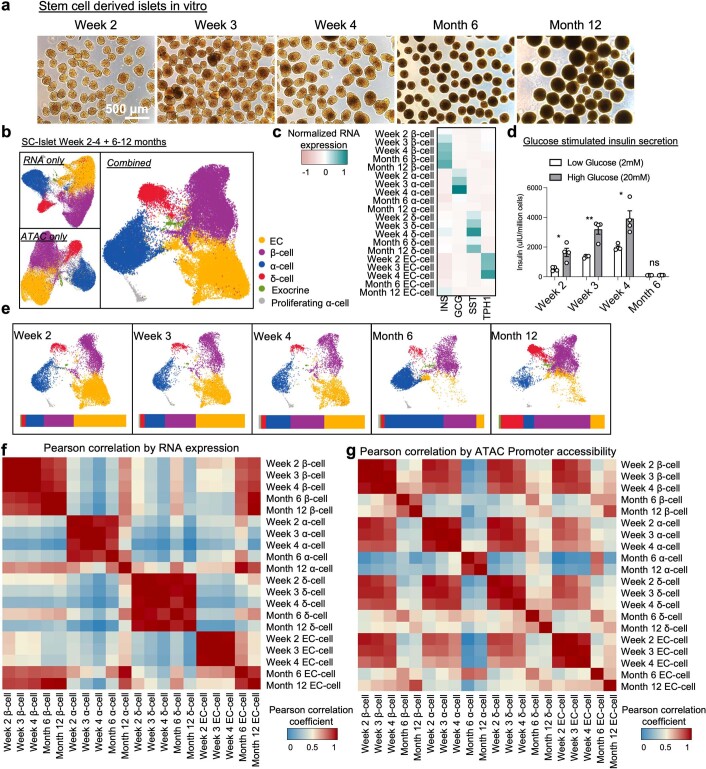
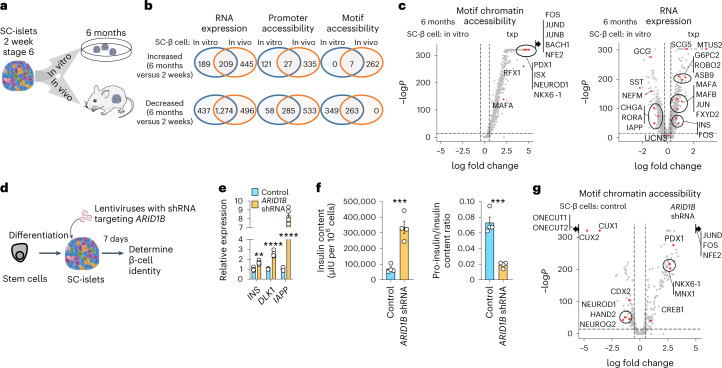
SC-β cells can acquire improved phenotypes with weeks in vitro or months after transplantation4,9,17,18,27,52. We performed multi-omic sequencing on SC-islets that underwent extended in vitro culture or transplantation into mice to characterize the chromatin and transcriptional changes that occur over time under these conditions. SC-islets cultured in vitro were sequenced periodically for up to 12 months (Fig. 5a and Extended Data Fig. 6a,b; 40,332 cells from 5 datasets, 1 from each timepoint). While SC-β cells displayed increased INS transcript with time, insulin secretion improved only until week 4 in vitro (Extended Data Fig. 6c,d). SC-α, SC-δ and SC-EC cells increased GCG, SST and TPH1 expression, respectively, until week 4 but declined by month 6 in vitro (Extended Data Fig. 6c). We found reductions in both the number and identity features for SC-EC cells starting at month 6 and SC-α cell by month 12 (Extended Data Fig. 6e,f). Drastic shifts in chromatin accessibility were observed broadly across all endocrine cell types at months 6 and 12 (Extended Data Fig. 6g).
Fig. 5. Interrogating SC-islet identity with time in vitro and after transplantation.
a, Schematic of SC-islet in vitro culture with extended time course. b, Heat map showing gene expression, motif chromatin accessibility, or ATAC promoter accessibility of gene markers and transcription factors of SC-β cells cultured in vitro with extended time (2, 3 and 4 weeks and 6 and 12 months; 40,332 cells from 5 independent biological samples, 1 from each timepoint). c, Graphs comparing time course of chromatin accessibility around the INS genomic region in SC-β, α and δ cells. Peak signals around the INS gene in SC-β cell increases over time, but decreases in SC-α and δ cells. d, Volcano plots showing differential gene expression analysis (left) and differential motif accessibility analysis (right) comparing SC-β cells cultured short term (weeks 2, 3 and 4) and SC-β cells cultured long term (months 6 and 12). Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility. e, Schematic of differentiated SC-islets maintained long-term by in vivo transplantation. f, ATAC chromatin accessibility around the INS genomic region showing increase of peak signals in SC-β cells after transplantation and decrease in SC-α and δ cells (36,688 cells from 6 independent biological samples; 3 in vitro SC-islets, 3 in vivo SC-islets; integration of all samples). g, Bar graphs showing fold change differences of β-cell-associated active transcription factors in SC-β cells and transplanted SC-β cells. SC, stem cell derived; Txp, transplant; EC, enterochromaffin cells.
Extended Data Fig. 6. Time course characterization and analysis of long-term in vitro SC-islets.
a, Bright field images of SC-islets from week 2, week 3, week 4, month 6, and month 12 of in vitro culture. Individual image representative of 1 sample. b, Integrative UMAP of in vitro SC-islet cells including all time points. c, Heatmap showing increase, or decrease, of marker gene expression in SC-islet cells throughout time (40332 cells from 5 independent biological samples, 1 from each timepoint). d, Glucose stimulated insulin secretion assay, plotting mean ± s.e.m. (by ELISA, n = 4 biologically independent samples), of SC-islets cultured in vitro at week 2 (P = 0.020), week 3 (P = 0.0080), week 4 (P = 0.046), and month 6 (P = 0.27) time point. Statistical significances were assessed using paired two-sided t-test. e, UMAP and bar plots showing composition information of cell types from integrated time course datasets. f and g, Pearson correlation to compare all timepoints, of cell types, (f) using top 2000 most variably expressed genes (mRNA), and (g) using top 2000 most variably accessible promoters from ATAC. EC, enterochromaffin; ns, not significant.
In SC-β cells, expression of many β-cell transcription factors and the accessibility of their associated motifs were upregulated at week 4 of in vitro culture but decreased in the long term (months 6 and 12) (Fig. 5b–d). Expression and promoter accessibility of other β-cell genes, including UCN3, increased, while those associated with off-target identities, such as SC-EC cell, diminished with long-term culture (Fig. 1b,d and Supplementary Table 10). Similarly, ATAC peaks demonstrated accessibility around the INS genomic region increased in SC-β cells but diminished in SC-α and SC-δ cells (Fig. 5c). Collectively, multi-omic analysis of in vitro cultured SC-islets revealed that, while the transcription of some important β-cell genes increased over time, the chromatin state of these cells became much more restricted in long-term culture. While this restriction helped to decrease off-target populations, many crucial β-cell transcription factors were also downregulated, possibly leading to the observed decrease in function.
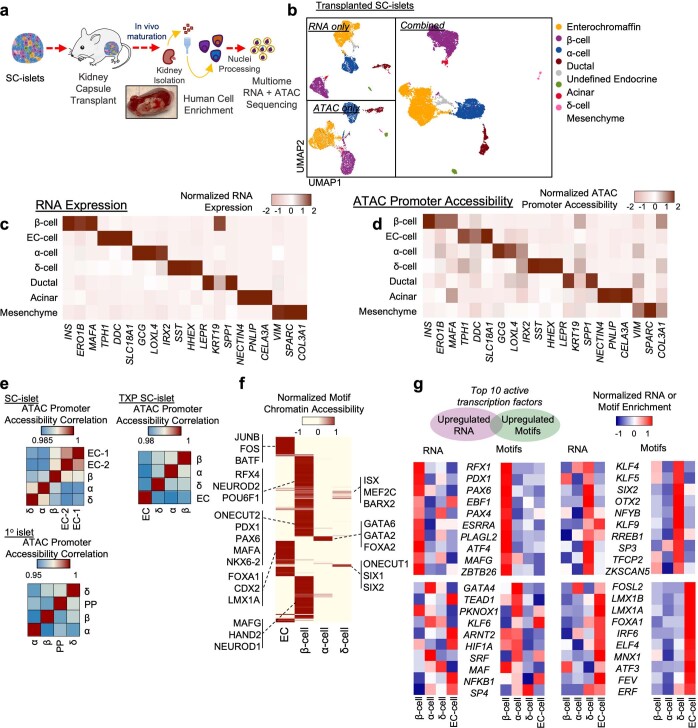
In parallel, we also performed multi-omic sequencing of SC-islets transplanted for 6 months in mice (Fig. 5e and Extended Data Fig. 7a,b; 7,162 cells from 3 datasets, each dataset used 3 mice). Gene expression and promoter accessibility information demonstrated that endocrine identities became even more distinct than before transplantation (Extended Data Fig. 7c–e). Motif accessibility was also well defined for each cell type, with transplanted SC-β and SC-EC cells no longer displaying shared open motifs, such as for PDX1 (Extended Data Fig. 7f,g). Comparison of these 6 month transplanted SC-islets with those before transplantation revealed that transplanted SC-islets acquired gene expression and chromatin accessibility signatures that were more similar to their primary cell counterparts (Extended Data Fig. 8a–c and Supplementary Table 11). These improvements in cell identity were also reflected in the chromatin accessibility around the INS gene, which exhibited diminished peaks in non-β cell populations (Fig. 5f). No major shifts in composition were seen, suggesting this was set by the end of the in vitro differentiation process and that transdifferentiation was likely not occuring (Extended Data Fig. 8a). Gene sets and motif accessibility for many β-cell markers were upregulated in transplanted SC-β cells, whereas immature and off-target populations markers decreased (Fig. 5g and Extended Data Fig. 8d–f). Collectively, transplanted SC-β cells resembled primary β cells more in gene expression, promoter accessibility and motif accessibility, indicating that transplantation enhances both transcriptional and chromatin landscapes.
Extended Data Fig. 7. Single-cell Multiomic ATAC and gene expression characterization of transplanted SC-islets.
a, Schematics of SC-islet transplantation and retrieval of graft after 6 months in vivo. b, UMAP and identification of transplanted SC-islet cells using gene expression and chromatin information (7162 cells from 3 independent biological samples; integration of all samples). c and d, Heatmap showing gene expression (c) and ATAC promoter accessibility (d) of markers associated with each cell type. e, Pearson correlation analysis using top 2000 most variable ATAC promoter accessibility of key endocrine populations from SC-islets, transplanted SC-islets, and primary human islets. This analysis highlights the distinctiveness of chromatin identity acquired in cell types from SC-islets after transplantation. f, Heatmap showing the top 200 variable motifs within endocrine cell populations and highlighting motif markers for each in vivo cell type. g, Heatmaps highlighting gene expression and ATAC motif accessibility of top 10 active transcription factors co-enriched with both features in transplanted SC-β, SC-α, SC-δ, and SC-EC cells.
Extended Data Fig. 8. Multiomic sequencing comparison of in vitro SC-islets and transplanted in vivo SC-islets.
a, Integrative UMAP clustering showing cells from in vitro SC-islets and in vivo SC-islets using gene expression and chromatin information (36688 cells from 6 independent biological samples; 3 in vitro SC-islets, 3 in vivo SC-islets; integration of all samples). The pie chart shows composition of cell types in each condition. b, UMAP showing distribution of SC-islet cells from in vitro or after transplanted in vivo condition. This plot highlights the separation of clusters from transplanted in vivo SC-β and SC-EC cells. c, Differential gene expression analysis (top) and motif chromatin accessibility analysis (bottom) of SC-β, SC-α, SC-δ and SC-EC cell populations from in vitro SC-islets and in vivo SC-islets. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility. d, Gene set enrichment analysis showing enrichment of gene sets comparing in vitro SC-β cells and vivo SC-β cells. Non-adjusted p-values were computed using two-sided Fisher exact test. e, Bar graphs showing fold change differences of cell type associated active transcription factors in SC-α, SC-δ and SC-EC cells from in vitro and in vivo conditions. f, Heatmap comparing gene expression, promoter accessibility, or motif chromatin accessibility in β-cells from in vitro SC-islets, in vivo islets, and primary islets (26697 cells from 5 independent biological samples; 1 representative in vitro SC-islets, 1 representative primary islets, 3 in vivo SC-islets). EC, enterochromaffin.
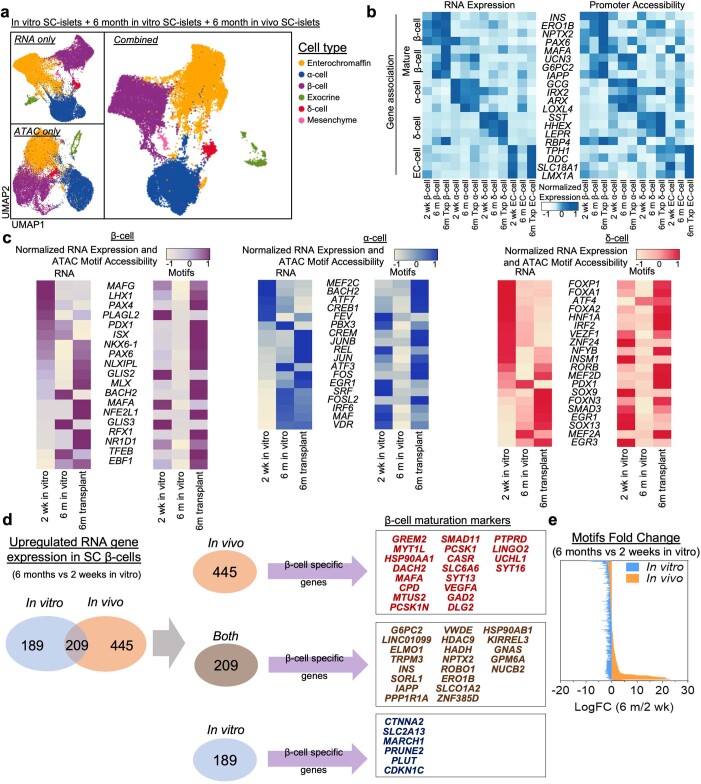
To compare 6 month in vitro and 6 month transplanted SC-islets, we integrated these datasets with the 2 week in vitro data (Fig. 6a and Extended Data Fig. 9a,b). Overall, transplanted SC-islet cells displayed greater enrichment of genes and motifs for transcription factors associated with their cell types, demonstrating that both time and environment are necessary for maturation (Extended Data Fig. 9c). In particular, transplanted SC-β cells exhibited several upregulated and downregulated genes and motifs compared with their 6 month in vitro counterparts, allowing us to compile a list of markers relevant for β-cell identity from both environments (Fig. 6b,c, Extended Data Fig. 9d and Supplementary Table 12). In vitro culture closed many chromatin regions, including some associated with β-cell identity. In vivo time opened chromatin regions linked with cell identity while restricting others, enabling transplanted SC-β cells to acquire a chromatin and transcriptional landscape closer to primary β cells. Furthermore, SC-EC cells also retained expression of identity genes when transplanted, in contrast to during 6 month in vitro culture.
Fig. 6. Comparative analysis of 6 months in vitro and in vivo SC-β cells and improving in vitro maturation by ARID1B gene knockdown.
a, Schematic showing comparison of changes associated with 6 month in vitro culture and 6 month in vivo transplants. b, Number of genes, by gene expression or ATAC promoter accessibility, or motif chromatin accessibility, downregulated or upregulated in 6 months in vitro and in vivo SC-β cells. Number of features were determined by differential gene, promoter accessibility or motif accessibility analysis comparing 2 week SC-β cells with 6 month SC-β cells in vitro or 6 month SC-β cells in vivo (24,491 cells from 5 independent biological samples; week 2 representative SC-islets, month 6 SC-islets, and 3 samples of month 6 in vivo SC-islets). c, Volcano plots showing differential motif accessibility analysis (left) and differential gene expression analysis (right) comparing 6 months SC-β cells from in vitro and in vivo SC-islets. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility. d, Schematic of SC-islets transfected with shRNA lentivirus for ARID1B gene knockdown. e, qPCR plots of SC-islets with ARID1B shRNA showing mean ± s.e.m. (n = 4 biologically independent samples) of expression of β-cell-associated genes, INS (P = 0.0014), DLK1 (P = 4.3 × 10−5) and IAPP (P = 3.0 × 10−6). Statistical significance was assessed by unpaired two-sided t-test. f, Protein quantification plot showing mean ± s.e.m. (n = 4 biologically independent samples) of human insulin content (P = 7.6 × 10−4) by ELISA (left), and pro-insulin/insulin ratio (P = 3.6 × 10−4) by ELISA (right). Statistical significance was assessed by unpaired two-sided t-test. g, Volcano plot from single-nucleus multi-omics comparing motif chromatin accessibility of SC-β cells from control and ARID1B shRNA condition (21,969 cells from 2 independent biological samples, 1 of each condition from the same differentiation batch; integration of all samples). Statistical significance was assessed by two-sided logistic regression. SC, stem cell derived; Txp, transplant; EC, enterochromaffin cells.
Extended Data Fig. 9. Multiomic sequencing comparison of week 2 in vitro SC-islets with 6 months in vitro SC-islets and 6 months in vivo SC-islets.
a, Integrative UMAP clustering showing cells from 2 weeks in vitro SC-islets, 6 months in vitro SC-islets, and 6 months in vivo SC-islets using gene expression and chromatin information (24491 cells from 5 independent biological samples; 1 representative week 2 SC-islets, month 6 SC-islets, 3 month 6 in vivo SC-islets). b, Heatmap showing gene expression and promoter accessibility of gene markers for SC-β, SC-α, SC-δ, and SC-EC cells. c, Gene expression and motifs accessibility of active transcription factors associated with SC-β, SC-α, and SC-δ identity. d, Gene list showing markers for β cell identity 6 months in vitro, in vivo or both. Initial number of genes represent upregulated genes in the 6 month conditions (vitro and vivo) when compared to 2 week SC-islets. List of genes was cross-referenced with β cell upregulated genes from primary human islets. e, Plot highlighting greater increase of motifs accessibility from 6 months in vivo SC-β cells when compared in 6 months in vitro.
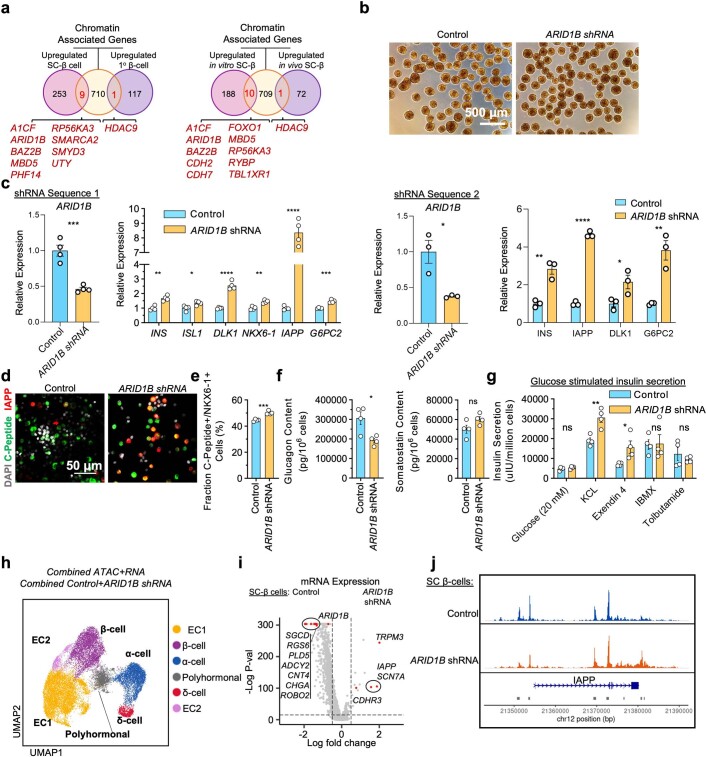
Throughout our analysis, the chromatin regulator ARID1B emerged several times as being differentially expressed in β cells (Figs. 4g and 5d and Extended Data Figs. 8c and 10a). Knockdown of ARID1B during the in vitro culture of SC-islets increased β-cell identity markers, including gene expression and motif accessibility (Fig. 6d–g, Extended Data Fig. 10b–j and Supplementary Table 13; 21,969 cells from 2 datasets, 1 of each condition from the same differentiation batch). These findings demonstrate that the chromatin landscape is important for SC-β cell identity and that targeting chromatin regulators can further improve SC-β cell maturation in vitro.
Extended Data Fig. 10. ARID1B knockdown increases expression of identity genes and chromatin features in SC-β cells.
a, Cross reference map of upregulated SC-β cell and primary β-cell genes (left) or transplanted SC-β cell genes (right) with chromatin associated genes to highlight regulators associated with each cell states. Individual image representative of 1 sample. b, Brightfield images of SC-islets transfected with lentivirus carrying ARID1B shRNA. c, qPCR analysis, plotting mean ± s.e.m. (n = 4 biologically independent samples for shRNA sequence 1; n = 3 biologically independent samples for shRNA sequence 2), showing reduced expression of ARID1B using lentiviruses resulting in increased β cell identity gene expressions. (shRNA sequence 1: ARID1B, P = 5.3 × 10−4; INS, P = 0.0014; ISL1, P = 0.022; DLK1, P = 4.3 × 10−5; NKX6-1, P = 0.0015; IAPP, P = 3.0 × 10−6; G6PC2, P = 2.1 × 10−4) Two shRNA sequences were tested for validation of results. (shRNA sequence 2: ARID1B, P = 0.018; INS, P = 0.0035; IAPP, P = 5.9 × 10−6; DLK1, P = 0.045, G6PC2, P = 0.0054) Statistical significance was assessed by unpaired two-sided t-test. d, Confocal fluorescent images showing increased expression of amylin in SC-islets with ARID1B knockdown. Individual image representative of 5 biologically independent samples. e, Flow cytometry analysis, plotting mean ± s.e.m. (n = 4 biologically independent samples), of SC-islets with ARID1B shRNA showing increased fraction of cells with C-peptide expression (P = 2.2 × 10−5) Statistical significance was assessed by unpaired two-sided t-test. f, ELISA quantification, plotting mean ± s.e.m. (n = 4 biologically independent samples) of glucagon (P = 0.017), and somatostatin (ns, P = 0.087) content. Statistical significance was assessed by unpaired two-sided t-test. g, Glucose stimulated insulin secretion assay, plotting mean ± s.e.m. (by ELISA, n = 4 biologically independent samples), comparing insulin secretion at high glucose (20 mM) stimulation from control (GFP shRNA) and ARID1B shRNA SC-islets in presence of various secretagogues. (Glucose (20 mM), P = 0.27; KCL, P = 0.049; Exendin 4, P = 0.035; IBMX, P = 0.94; Tolbutamide, P = 0.43) Statistical significances were assessed using paired two-sided t-test. h, Single-cell multiomic sequencing UMAPs of ARID1B knockdown SC-Islets, showing identified cell types, and cell knockdown condition using both gene expression and ATAC information (21969 cells from 2 independent biological samples; integration of all samples). i, Differential gene expression analysis of SC-β cells comparing control and ARID1B shRNA. Statistical significance was assessed by Wilcoxon rank sum test. j, Chromatin accessibility around the IAPP genomic region of SC-β cells, showing increased peak signals with ARID1B shRNA. EC, enterochromaffin; ns, not significant.
Discussion
SC-β cells have the potential to functionally cure type 1 diabetes53 but do not perfectly match the transcriptional and functional features of primary β cells. An increased understanding of the deficiencies in lineage specification could improve SC-islet differentiation to prevent non-endocrine cell generation and increase SC-β cell function. Our single-nucleus multi-omic approach provided a more robust definition of cell types than using either data type in isolation and identified important genes and chromatin signatures for SC-islet cell type specification. Comparison with primary human islets allowed for identification of deficiencies in the chromatin and transcriptional landscape of SC-islet cell types. Although the analysis of only four islet donors may not encompass all possible variability across the general population, these data still offer important insights into the chromatin and transcriptional landscape of SC-islet cell types and helped identify differences from their primary counterparts. These differentially expressed genes and chromatin accessibility signatures identified here can be targeted to improve SC-islet cell differentiation.
Our multi-omic analysis revealed important insights about the gene expression and chromatin state dynamics of SC-islet cell types compared with primary human islets. While SC-islet cell types were less distinct from each other by chromatin accessibility compared with primary cells, this difference was mainly driven by continued open chromatin accessibility of genes expressed by progenitor cell types, such as NEUROG3 (ref. 54) and GP2 (ref. 14), and alternative cell fates that were closed in primary cells. After transplantation into mice, SC-islet cell types developed more distinguished transcriptional and chromatin accessibility signatures that matched their respective cell identities16,17. However, extended in vitro culture broadly restricted access to chromatin regions, including those associated with a β-cell identity, and the underlying mechanism responsible for this difference in cellular identity remains unclear. These findings may have implications for other in vitro differentiation systems where immaturity is commonly observed55,56.
Previous research suggested that SC-EC and SC-β cells are distinct populations that arise from the same pancreatic progenitor population during SC-islet differentiation9. However, our multi-omic study revealed that these cells form a continuum of cell types with varying degrees of both enterochromaffin and β-cell features, rather than distinct cell populations. This suggests that current directed differentiation methodologies are insufficient for fully specifying each endocrine cell type. Furthermore, our data support the idea that chromatin accessibility is a major regulator of fate decisions between SC-EC and SC-β cells, particularly by CTCF. Interestingly, a recent publication57 using a different single-cell approach suggested that SC-EC cells resemble a pre-β cell population in the pancreas. Transplanted SC-islets retained SC-EC cells after 6 months in vivo, but this population became less similar to β cells over time. As SC-EC cells may be detrimental9 to SC-β cell function, understanding how to reduce or eliminate them is desired. Analysis tools that take account of both chromatin and mRNA44 could lead to insights on cell fate decisions using our datasets.
SC-islets have been studied due to the success of primary islet transplantation in patients with type 1 diabetes58–60. Several areas of investigation are being pursued for which multi-omic assessment and the dataset presented here could be beneficial. Since the early SC-islet reports5,6,61, groups have focused on changing medium composition or culture methods to improve SC-islet function4,7,8,17,62,63. Controlled assembly of three-dimensional SC-islet aggregates and circadian entrapment have also shown improvements4,8,12,27. Proliferation, purification and increased generation efficacy of progenitors have been pursued to improve final differentiation to SC-islets10,14,64–69. Genetic engineering to lessen immune recognition70 and materials for immune protection have also been reported63,71–80. SC-islets have been generated from patients with diabetes via induced pluripotent stem cells (iPSCs) for diabetes pathology study and as an autologous cell source for β-cell replacement therapy15,81–87. A multi-omic approach could help determine how these modulations affect cell identity within SC-islets with greater resolution, facilitating finer control of differentiation parameters. Further multi-omic sequencing of iPSC-derived SC-islets can reveal the impact of iPSC derivation methods and starting somatic cell types on differentiation strategies.
Our study highlights the important role of chromatin regulators in the generation and final identity of islet cell types, as evidenced by the drastic differences in chromatin accessibility between cell types and within a given cell type from different origins. By modulating the chromatin regulators CTCF and ARID1B, we were able to alter SC-β cell identity, demonstrating the potential for better control of the chromatin landscape to improve SC-islet differentiation protocols. Our comprehensive indexing of single-cell transcriptional and chromatin accessibility states in SC-islets provides a valuable resource for further development of these protocols, as both aspects of islet cell identity will probably need to be targeted for enhanced differentiation strategies. Further comparisons with other multi-omic approaches57 may yield additional insights into SC-islet identity and biology.
Methods
Stem cell culture and differentiation
The HUES8 human embryonic stem cell (hESC) line was provided by Douglas Melton (Harvard University)5. The H1 hESC line was provided by Lindy Barrett (Broad Institute) with permission from WiCell containing doxycycline-inducible dCas9-VPR transgene in the AAVS1 locus (CRISPRa system)46. All hESC work was approved by the Washington University Embryonic Stem Cell Research Oversight Committee (approval no. 15-002) with appropriate conditions and consent. mTeSR1 (StemCell Technologies; 05850) was used for the culture of undifferentiated stem cells. All cell culture was maintained in a humidified incubator at 5% CO2 and 37 °C. Cells were passaged every 4 days by washing cell with phosphate-buffered saline (PBS) and incubating with TrypLE at 0.2 ml cm−2 (Gibco; 12-604-013) for 10 min or less at 37 °C. Dispersed cells were then mixed with an equal volume of mTeSR1 supplemented with 10 µM Y-27632 (Pepro Tech; 129382310MG). Cells were counted on Vi-Cell XR (Beckman Coulter) and spun at 300g for 3 min at room temperature (RT). The supernatant was aspirated, and cells were seeded at a density of 0.8 × 105 cm−2 for propagation onto Matrigel (Corning; 356230)-coated plates in mTeSR1 supplemented with 10 µM Y-27632. After 24 h, medium was replaced daily with mTeSR1 without Y-27632. SC-islet differentiation was performed as described previously7,33. Briefly, hESCs were seeded at a density of 6.3 × 105 cells cm−2. Twenty-four hours later, the mTeSR1 was replaced with differentiation medium supplemented with small molecules and growth factors as outlined in Supplementary Table 1.
SC-islet and primary islet cell culture
After 7 days in stage 6 of the differentiation protocol, cells were dispersed from the culture plate with TrypLE at 0.2 ml cm−2 (Gibco; 12-604-013) for 10 min or less at 37 °C. The cells were mixed with an equal volume of stage 6 enriched serum-free medium (ESFM), centrifuged at 300g, and resuspended in ESFM at a concentration of 1 million cells ml−1. Five millilitres of this solution were pipetted in each well of a six-well plate and placed on an orbital shaker (Orbi-Shaker CO2, Benchmark Scientific) at 100 r.p.m. to form SC-islet clusters. These clusters were maintained by aspirating and replacing 3 ml of ESFM every 2–3 days. SC-islets in long-term culture were similarly maintained with ESFM for up to 1 year without passaging. Primary human islets were acquired as clusters and shipped from Prodo Laboratories, which required consent from the donor relatives for use in research. Consent information can be found on their website (https://prodolabs.com/human-islets-for-research). These islets have been refused for human islet transplants and meet specific criteria for research use. Donor details can be found in Supplementary Table 2. Our study consists of four donors. Upon arrival, islets were transferred into a six-well plate on an orbital shaker at 100 r.p.m. and maintained with 4 ml per well of CMRL1066 Supplemented medium (Corning; 99-603-CV) with 10% heat-inactivated foetal bovine serum (Gibco; 26140-079). Primary human islets were submitted for sequencing within 2 days after arrival.
Mouse transplantations and SC-islet cell retrieval
Mice that were 7 weeks old, male, and with the NOD.Cg-Prkdcscid Il2rgtm1wjl/SzJ (NSG) background (Jackson Laboratories; 005557) were randomly assigned to experimental groups. Mice were housed in an ambient facility with 30–70% humidity and a 12 h light/dark cycle and were fed a chow diet. Animal studies were performed by unblinded individuals in accordance with the Washington University International Care and Use Committee guidelines (approval 21-0240). Mice were anaesthetized using isoflurane and injected with ~5 × 106 SC-islet cells under the kidney capsule. At 6 months post-transplantation, mice were killed, and the kidney transplanted with SC-islets was removed. Sliced kidney samples were placed into a solution of 2 mg ml−1 collagenase D (Sigma; 11088858001) in RPMI (Gibco; 1187-085). A total of nine mice were used to produce three samples. Each sample consisted of pooling three transplanted kidneys (one kidney from each mouse) to achieve sufficient cell numbers required for sequencing. The tissue was incubated for 40 min at 37 °C before diluting with PBS, mechanically disrupting with a pipette, and filtered through a 70 µm strainer (Corning; 431751). The flowthrough was centrifuged, and the remaining cell pellet was resuspended in MACS buffer (0.05% bovine serum albumin (BSA) in PBS). The Miltenyi mouse cell isolation kit (Miltenyi; 130-104-694; LS column, 130-042-401) was used to remove excess mouse cells. The flowthrough was centrifuged, and resuspended in PBS with 0.04% BSA for nuclei processing and sequencing. Data collection and analysis were not performed blind to the conditions of the experiments. No animals or data points associated with transplantation were excluded.
Single-nucleus sample preparation and sequencing
Cells were processed and delivered to the McDonnell Genome Institute at Washington University for library preparation and sequencing. Samples were processed by dispersing cells into a single-cell suspension using TrypLE for 10 min at 37 °C and quantified for viability using the Vi-Cell XR (Beckman Coulter). Before proceeding, all samples were ensured to have >90% viability to minimize dead cell carry over in sequencing. Single-cell suspension samples were processed into nuclei according to the 10X Multiome ATAC + Gene Expression (GEX) protocol (CGOOO338). Cell samples were collected and washed with PBS (with 0.04% BSA), lysed with chilled Lysis Buffer for 4 min, washed three times with wash buffer and resuspended with 10x nuclei buffer at 3,000–5,000 nuclei μl−1. Nucleus samples were processed using the Chromium 10x genomics instrument, with a target cell number of 7,000–1,0000. The 10x Single Cell Multiome ATAC + Gene Expression v1 kit was used according to the manufacturer’s instructions for library preparations. Tape station figures for single-nucleus ATAC libraries can be found in Supplementary Fig. 1. Sequencing of the library was performed using the NovaSeq 6000 System (Illumina).
Processing and filtering of multi-omic sequencing data
Multi-omic sequenced files were processed for demultiplexing and analysed using Cell Ranger ARC v2.0. Genes were mapped and referenced using human reference genome GRCh38. RStudio 1.3.1093 (R version 4.0.3) was used to perform analyses. Datasets were analysed using Seurat 4.01 (ref. 88) and Signac 1.3.0 (ref. 89). For ATAC data, peaks were called using MACS2 and the genomic positions were mapped and annotated with reference human genome EnsDb.Hsapeins.v86 and hg38. Dead cells were removed by excluding cells with very low RNA yields. (Nuclei samples do not contain valid cytoplasmatic mitochondrial content.) Low-quality cells including doublets, dead cells and poor sequencing depth cells were removed by filtering out cells with low RNA counts (nCount_RNA <1,000) and low ATAC counts (nCount_ATAC <1,000); high RNA counts (>40,000–50,000) and high ATAC counts (>40,000–50,000); nucleosome signal >1.25 and transcription start site (TSS) enrichment <2. For transplanted samples, excess mice cells from host mice were removed by filtering out cells expressing kidney marker TTC36 (ref. 16), which is shared in both mouse and human. Highly expressed TTC36 cells (>0.0005 normalized counts) were excluded. Details on filtering can be found in Supplementary Table 2 and Supplementary Fig. 2.
Dataset normalization, integration and assay build
Gene expression data were processed with SCT transform. ATAC data were processed with ‘RunTFIDF’ and ‘RunSVD’. Integration of datasets were performed by anchoring using ‘FindIntegrationAchors’. ‘rpca’ was used as a reduction method, with ‘SCT’ normalization to correct for batch differences for RNA expression. For integrated ATAC data, ‘lsi’ reduction was used along with ‘RunTFIDF’ and ‘RunSVD’ for batch correction. ‘FindMultimodalNeighbors’ with gene expression based ‘pca’ and ATAC based ‘lsi’ reductions were used to generate a joint neighbour graph. ‘FindClusters’ with SLM algorithm was used to identify clusters. Promoter accessibilities were determined from ATAC data using ‘GeneActivity’ (2,000 base pairs upstream of the transcription start sites89,90). ATAC peaks were called using MACS2 and linked using LinkPeaks and Cicero to determine cis-regulatory elements89,91. ‘RunChromVAR’ of chromVAR 1.12.0 package92 and JASPAR version 2020 database93 were used to compute motif enrichment. Motif sequence IDs were converted to transcription factor motifs using ‘ConvertMotifID’. Promoter accessibility and motif enrichment information were incorporated as assay data in the integrated Seurat object files.
Analysis of multi-omic datasets
Cell types in the integrated datasets were identified by performing differential expression analysis. ‘FindMarkers’ using the ‘wilcox’ test method was used to determine highly expressed feartures. ‘LR’ test method in ‘FindMarkers’ was used to determine upregulated motif accessibility. ‘ConveragePlot’ of the Signac package (for example, Figs. 1d and 4f) was used to generate ATAC peaks with cis-regulatory element information. Uniform manifold approximation and projection (UMAP) and violin plots were generated using ‘FeaturePlot’ and ‘VlnPlot’ (for example, Figs. 1g and 2d). Heat maps were generated using average values using ‘AverageExpression’ and ‘heatmap.2’ (for example, Fig. 1c). Top active transcription factors were assessed for top upregulated transcription factor gene expression and motif enrichment by computing the average fold change of selected populations or conditions compared to other cell populations. Shared upregulated transcription factors by both gene expression and motif enrichment assays were determined as active transcription factors (for example, Fig. 1f). Volcano plots were generated using ‘EnhanvedVolcano’ (for example, Fig. 2c). Gene set enrichment analysis was performed using ‘DEenrichRPlot’ of enrichR package with databases from Gene Ontology, KEGG_2021_Human and MSigDB_Hallmark_2020.
Trajectory analysis
Trajectory analysis was performed using SeuratWrappers and the Monocle3 1.0 package94. ‘Subset’ was used to isolate specific populations (enterochromaffin cells and β cells) from the integrated dataset. A monocle compatible cds file was generated using ‘as.cell_data_set’. Pseudotime was computed and determined using the ‘cluster_cells’ and ‘learn_graph’ functions. Trajectories were re-established by selecting the initial node pseudo-timepoint using ‘order_cells’. Dynamic analysis of gene expression and motif enrichment were obtained by conducting differential expression feature analyses along the pseudotime trajectory. ‘graph_test’ was used with ‘neighbor_graph’ parameter set to ‘principal_graph’. Genes or motifs of high differential expression along pseudotime were determined by excluding features with low Moran’s I scores (morans_I >0.05). Expression values were Z-scored and plotted using ‘Heatmap’ with K means parameter (km = 2). Pseudotime information from this analysis was incorporated into the Seurat object using ‘AddMetaData’.
MultiVelo44 package running on Jupyter Notebook (Python) was used to perform alternative trajectory analysis. ATAC and RNA files were imported using ‘sc.read_10x_mtx’ and ‘scv.read’. Aggregated peaks around gene regions were computed using ‘mv.aggregate_peaks_10x’, and mapped with RNA and ATAC information using ‘pd.Index’. RNA counts and ATAC peaks were normalized using ‘scv.pp.filter_and_normalize’ and ‘mv.tfidf_norm’ respectively. Smoothing of gene peak aggregates was performed using ‘mv.knn_smooth_chrom’, followed with ‘mv.recover_dynamics_chrom’ to execute the multi-omic dynamic model to predict cell state trajectories. Pseudotime was computed using ‘mv.latent_time’. Genes of interest were plotted along pseudotime using ‘mv.dynamic_plot’.
Transduction of CTCF gRNA in CRISPRa line
CRISPRa genetic engineering of the H1 dCas9-VPR line46 was performed using custom guide RNAs (gRNAs, MilliporeSigma, Supplementary Table 1). gRNAs were resuspended to a final concentration of 100 µM in water. Primers were phosphorylated and ligated together by adding T4 ligation buffer and T4 Polynucleotide kinase enzyme (NEB; B0202A and M0201S) and running on a thermocycler under the following conditions: 37 °C for 30 min; 95 °C for 5 min; and ramp down to 25 °C at 5 °C min−1. Oligos were then diluted with 90 µl of ultrapure water. These oligos were then inserted into the single guide RNA (sgRNA) library backbone (Lenti sgRNA(MS2)_puro) using the Golden Gate reaction. This was achieved by adding a 25 ng µl−1 plasmid backbone to a master mix of Rapid Ligase Buffer 2X (Enzymatics: B1010L), Fast Digest Esp31 (Thermo: FD0454), dithiothreitol (Promega: PRP1171), BSA (NEB: B9000S), T7 DNA ligase, (Enzymatics: L6020L) and the diluted gRNA oligos in a total reaction volume of 25 µl. The Golden Gate assembly reaction was then performed in a thermocycler under the following conditions: 15 cycles of 37 °C for 5 min, 20 °C for 5 min with final hold at 4 °C. Lenti sgRNA (MS2) puromycin optimized backbone was a generous gift from Feng Zhang (Addgene plasmid no. 73797). This final plasmid was then transfected into STBL3 following the same methods as described below in the ‘Lentiviral design, preparation and transduction’ section.
To transfect the CRISPRa H1 dCas9-VPR stem cell line, lentiviral particles containing gRNA was added at a multiplicity of infection of 5 with polybrene (5 µg ml−1) in culture for 24 h. At confluency, transfected stem cells were passaged and cultured with medium containing puromycin (1 µg ml−1) for selection. To induce CRISPRa expression, doxycycline (MilliporeSigma) was added at 1 µg ml−1 for 7 days during stage 5 of the differentiation protocol.
Real-time PCR
Cells were lysed directly with RLT buffer from the RNeasy Mini Kit (74016; Qiagen) followed by RNA extraction following the manufacturer’s instructions. Complementary DNA was synthesized from the RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems; 129382310MG) on a T100 thermocycler (BioRad). PowerUp SYBR Green Master Mix (Applied Biosystems; A257411) was used to run samples on the Quant Studio 6 Pro (Applied Biosystems), and results were analysed using ΔΔCt methodology. The housekeeping genes TBP and GUSB were both used for normalization. Primer sequences used in this paper are listed in Supplementary Table 1. qPCR data were collected from the QuantStudio6 Pro using Design & Analysis 2.6.0.
ICC
Fluorescence images were taken on a Zeiss Cell Discoverer confocal 7 microscope. For immunocytochemistry (ICC), cells were fixed in 4% paraformaldehyde (Electron Microscopy Science; 15714) for 30 min at RT. For staining, fixed cells were incubated in ICC solution (PBS (Fisher; MT21040CV), 0.1% Triton X (Acros Organics; 327371000) and 5% donkey serum (Jackson Immunoresearch; 01700-121)) for 30 min at RT. Samples were subsequently treated with primary and secondary antibodies in ICC solution overnight at 4 °C and 2 h at RT, respectively. DAPI (Invitrogen; D1306) was used for nuclear staining. Samples were incubated in DAPI for 12 min at RT, washed with ICC solution and stored in PBS until imaging. Antibody details and dilutions can be found in Supplementary Table 1. ImageJ was used for analysis. Quantification was performed by manual counting of cells from analysed fluorescence images and can be found in Supplementary Fig. 3.
Flow cytometry
Cells were single-cell dispersed by washing with PBS and adding 0.2 ml TrypLE cm−2 for 10 min at 37 °C. Cells were washed with PBS, centrifuged and fixed by resuspending the cells in 4% paraformaldehyde at 4 °C for 30 min. After another PBS wash, samples were treated with ICC solution for 45 min at RT. Primary antibodies were prepared in ICC solution and incubated on cells overnight at 4 °C. Samples were washed with PBS and incubated for 2 h with secondary antibodies in ICC at 4 °C. Antibody details and dilutions can be found in Supplementary Table 1. Cells were washed twice with PBS and filtered before running on the LSR Fortessa flow cytometer (BD Bioscience) using BD FACSDiva. FlowJo v10.8.0 (Becton, Dickinson, and Company) was used for analysis. The used gating strategy can be found in Supplementary Fig. 4.
Hormone content
Hormone content was measured by collecting SC-islets, rinsing with PBS and incubating in an acid–ethanol solution for 48 h at −20 °C. Samples were neutralized with 1 M Tris buffer (Millipore Sigma; T6066). Hormone measurements were made using enzyme-linked immunosorbent assay (ELISA) kits: human insulin ELISA (ALPCO; 80-INSHU-E01.1), somatostatin EIA (Phoenix Pharmaceuticals; EK-060-03), glucagon ELISA (Crystal Chem; 81520), serotonin ELISA (ALPCO; 17-SERHU-E01-FST) and human pro-insulin ELISA (Mercodia; 10-1118-01). ELISAs were performed according to the manufacturer instructions. Results were normalized to cell counts performed on Vi-Cell XR (Beckman Coulter).
Lentiviral design, preparation and transduction
Gene knockdown of ARID1B was performed using pLKO.1 TRC plasmids containing short hairpin RNA (shRNA) sequences targeting ARID1B and GFP (Control) (Supplementary Table 1). Glycerol stocks were grown, and plasmid DNA was isolated using Qiagen Mini-prep kit (Qiagen; 27115). Plasmid DNA was transfected into One Shot Stbl3 Chemically Competent Escherichia coli (Invitrogen; C737303) and spread on an agar plate. After 18 h, one colony was selected, cultured and DNA extracted using the Qiagen Maxi-prep-plus kit (Qiagen; 12981). Viral particles were generated using Lenti-X 293T cells (Takara; 632180) cultured in Dulbecco’s modified Eagle medium with 10% heat-inactivated foetal bovine serum (MilliporeSigma; F4135) and 0.01 mM sodium pyruvate (Corning; 25-000-CL) in 10 cm plates (Falcon; 353003). Confluent cells were transfected with 6 μg of shRNA plasmid, 4.5 μg of psPAX2 (Addgene; 12260; gift from Didier Trono) and 1.5 μg pMD2.G (Addgene; 12259; gift from Didier Trono) packaging plasmids in 600 μl of Opti-MEM (Life Technologies; 31985-070) and 48 μl of Polyethylenimine ‘Max’ MW 40,000 Da (Polysciences; 24765-2) per plate. Medium was refreshed after 16 h. Virus-containing supernatant was collected at 96 h post transfection and concentrated using Lenti-X concentrator (Takara; 631232). Collected lentivirus was titred using Lenti-X GoStix Plus (Takara; 631280). Lentiviral transduction was initiated at the beginning of stage 6 with a multiplicity of infection of 5 for 24 h.
Statistics and reproducibility
No statistical methods were used to pre-determine sample sizes, but our sample sizes are similar to those reported in previous publications5,9,16,31,33. This study contains multi-omic sequencing datasets of SC-islets from three independent differentiation batches (three datasets), human islets from four donors (four datasets), SC-islets after 3 weeks, 4 weeks, 6 months and 12 months into stage 6 with one replicate each (five datasets), CTCF CRISPRa differentiations of one replicate from each condition (control and doxycycline-induced CTCF overexpression; two datasets), and ARID1BshRNA SC-islets of one replicate from each condition (control and shARID1B knockdown; two datasets). Information on datasets used and sample details can be found in Supplementary Table 2. Cell selection criteria or exclusion methods for single-cell analysis can be found in Supplementary Fig. 2. Statistical significance from the multi-omic analyses was calculated using the Wilcoxon rank sum test for RNA expression, logistic regression for motif chromatin accessibility and Bonferroni correction to account for multiple testing. For in vitro experiments, we performed unpaired or paired parametric t-tests (two-sided) and one-way ANOVA with Tukey’s multiple comparison testing to determine significance. Data distribution was assumed to be normal, but this was not formally tested. All in vitro experiment data points presented are biological replicates and can be found in Source Data. Significant values are marked on the basis of P values using non-significant (NS) >0.05, *<0.05, **<0.01, ***<0.001 and ****<0.0001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41556-023-01150-8.
Supplementary information
Supplementary Figs. 1–5.
Supplementary Table 1. Materials and methods. This table lists the reagents, cell lines, medium formulations and sequences used in this study. (1.1) Human stem cell lines used. (1.2) Details of the differentiation protocol to generate SC-islets. (1.3) Base medium formulations. (1.4) Factors and compounds used. (1.5) gRNA sequences. (1.6) shRNA sequences. (1.7) Real-time PCR primer sequences. (1.8) Antibodies used. Supplementary Table 2. Multiome sequencing details. This set of tables provides multi-omic sequencing details such as individual data set details, cell filtering parameters and total analysed cell numbers. (2.1) Dataset information used in this study. (2.2) Donor information details for adult human primary islet data sets. (2.3) Cell filtering paraments used to exclude low-quality cells and mouse cells from each dataset. Cells with exceedingly low and high RNA and ATAC fragment counts, and high nucleosome signal were excluded to minimize potential doublets and dead cells. For transplanted samples, we have excluded host cells by filtering out cells with high expression of kidney marker TTC36. (2.4) Number of filtered cells from each dataset used for analysis in this study. SC, stem cell derived; KD, knockdown. Supplementary Table 3. Differential gene expression analysis for cluster identification. This set of tables show gene expression upregulated in each cluster for cell type identification. These include datasets for: (3.1) SC-islets, (3.2) primary human islets, (3.3) transplanted SC-islets and (3.4) CTCF CRISPRa SC-islets. Statistical significance was assessed by two-sided Wilcoxon rank sum test, reporting adjusted P values. Supplementary Table 4. Differential promoter accessibility analysis for cluster identification. This set of tables shows differential promoter accessibilities in each cluster. These include datasets for: (4.1) SC-islets, (4.2) primary human islets, (4.3) transplanted SC-islets and (4.4) CTCF CRISPRa SC-islets. Statistical significance was assessed by two-sided Wilcoxon rank sum test, reporting adjusted P values. Supplementary Table 5. Differential chromatin accessibility analysis of transcription factor (TF) binding motifs for cluster identification. This set of tables show motifs chromatin accessibility analysis for cluster identification for: (5.1) SC-islets, (5.2) primary human islets, (5.3) transplanted SC-islets and (5.4) CTCF CRISPRa SC-islets. Fold change of the chromatin accessibility of transcription factor binding motifs as well as RNA expression of each cell type over other endocrine populations to determine transcriptional activity: (5.5) SC-islets TF activity, (5.6) primary islets TF activity and (5.7) transplanted SC-islets TF activity. Statistical significance was assessed by two-sided logistic regression, reporting adjusted P values. Avg, average; SC, stem cell derived; EC, enterochromaffin; iEC, induced enterochromaffin. Supplementary Table 6. Trajectory analysis for delineation of the SC-β cell and SC-EC cell populations. This set of tables provides the source data for the multi-omic sequencing analysis comparing the SC-β cell and SC-EC cell populations. (6.1) Genes (mRNA) and values from trajectory analysis using Monocle. (6.2) Motif accessibility and values from trajectory analysis using Monocle. (6.3) Differential gene (mRNA) expression analysis comparing SC-β cell and SC-EC cell. (6.4) Differential motifs chromatin accessibility analysis comparing SC-β cell and SC-EC cell. Statistical significance was assessed by two-sided Moran’s test, reporting non-adjusted P values and q values (adjusted P values). Avg, average; SC, stem cell derived; EC, enterochromaffin. Supplementary Table 7. Cluster identification and characterization of subpopulations. This set of tables show differential gene expression and motifs accessibility analysis of a selected subpopulation compared with the rest of the other subpopulations in SC-β cells, SC-EC cells and primary β cells. (7.1) SC-β subpopulation 1, (7.2) SC-β subpopulation 2, (7.3) SC-β subpopulation 3, (7.4) SC-β subpopulation 4, (7.5) SC-EC subpopulation 1, (7.6) SC-EC subpopulation 2, (7.7) SC-EC subpopulation 3, (7.8) SC-EC subpopulation 4, (7.9) primary β subpopulation 1, (7.10) primary β subpopulation 2, (7.11) primary β subpopulation 3. (7.12) Composition information showing the number of cells in each subpopulation for SC-islets and primary islets. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived; EC, enterochromaffin; HI, human islets. Supplementary Table 8. Differential analysis of CTCF CRISPRa experiments. This set of tables provides the source data for the multi-omic sequencing analysis of CTCF overexpression in SC-islets. (8.1) Differential gene expression and motifs chromatin accessibility analysis comparing SC-EC cells and SC-iEC cells. (8.2) Differential gene expression and motifs chromatin accessibility analysis comparing the SC-endocrine population from control and CTCF overexpression. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; EC, enterochromaffin; iEC, induced enterochromaffin; OE, overexpression. Supplementary Table 9. Differential gene expression, motif accessibility and promoter accessibility analysis of SC-islets and primary islets. This set of tables provides differential analysis comparing SC-islet cells with primary islet cells (9.1) SC-β cells versus primary β cells. (9.2) SC-α cells versus primary α cells. (9.3) SC-δ cells versus primary δ cells. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived. Supplementary Table 10. Differential gene expression, and motif accessibility analysis of short-term and long-term in vitro SC-β cells. This set of tables provides differential analysis comparing short-term in vitro (weeks 2–4) with long-term (months 6–12) SC-β cells. (10.1) Differential gene expression. (10.2) Differential motifs accessibility. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived. Supplementary Table 11. Differential gene expression, motif accessibility and promoter accessibility analysis of SC-islets and primary islets. This set of tables provides differential analysis comparing SC-islet cells with transplanted SC-islet cells. (11.1) SC-β cells versus transplanted SC-β cells. (11.2) SC-α cells versus transplanted SC-α cells. (11.3) SC-δ cells versus transplanted SC-δ cells. (11.4) SC-EC cells versus transplanted SC-EC cells. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived. Supplementary Table 12. Differential gene expression and motifs accessibility analysis comparing 6 months in vitro SC-islets with 6 months in vivo SC-β cells. This set of tables provides differential analysis comparing 6 months in vitro SC-β cells with transplanted SC-β cells. (12.1) 6 months in vitro SC-β cells versus 6 months in vivo (transplanted) SC-β cells. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived. Supplementary Table 13. Differential gene expression and motif accessibility analysis comparing SC-β cells from control and ARID1B shRNA condition. This set of tables provides differential analyses comparing control SC-β cells with ARID1B shRNA SC-β cells. (13.1) Control (GFP shRNA) SC-β cells versus ARID1B shRNA SC-β cells. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; GFP, green fluorescent protein; SC, stem cell derived.
Acknowledgements
This work was funded by NIH (R01DK114233 and R01DK127497 to J. R. Millman), JDRF (5-CDA-2017-391-A-N to J. R. Millman) and startup funds from the Washington University School of Medicine Department of Medicine to J. R. Millman. N.J.H. was supported by a JDRF Advanced Postdoctoral Fellowship (3-APF-2020-930-A-N). M.I. was supported by Rita Levi-Montalcini Postdoctoral Fellowship in Regenerative Medicine and the NIH (T32DK007120). M.M.M. was supported by the NIH (T32GM139774). J. R. Miller was supported by a Washington University BioSURF award. D.A.V.-P. was supported by the NSF Graduate Research Fellowship Program (DGE-2139839 and DGE-1745038). L.V.-C. was supported by the NIH (F31DK125068). Microscopy was performed through the Washington University Center for Cellular Imaging, which is supported by the Washington University School of Medicine, the Children’s Discovery Institute (CDI-CORE-2015-505) and the Foundation for Barnes-Jewish Hospital (3770). Microscopy analysis was supported by the Washington University Diabetes Research Center (P30DK020579) and Center of Regenerative Medicine. We thank the Genome Technology Access Center at the McDonnell Genome Institute at Washington University School of Medicine for help with genomic analysis, which is supported by NIH (P30CA91842) support to the Siteman Cancer Center and by ICTS/CTSA (UL1TR002345) from the National Center for Research Resources (NCRR). We thank N. Udomkittivorakul for the graphics design, D. Melton (Harvard University) for the HUES8 cell line and L. Barret (Broad Institute of MIT and Harvard University) for the CRISPRa VPR cell line.
Extended data
Source data
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Author contributions
P.A. and J. R. Millman designed all experiments and performed all in vivo experiments. P.A. and M.I. performed all computational analysis and associated cell culture. P.A., E.M., M.M.M., M.D.S., M.I., D.A.V.-P., J. R. Miller, S.E.G. and L.V.-C. performed in vitro experiments. P.A., N.J.H., M.I. and J. R. Millman wrote the manuscript. All authors revised and approved the manuscript.
Peer review
Peer review information
Nature Cell Biology thanks Heiko Lickert, Yi Zeng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under accession code GSE199636. Source data are provided with this paper. GRCh38 human genome95, MSigDB96 and JASPAR202093 databases were used. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
Codes used for analysing single-nucleus multi-omic sequencing are available on https://github.com/punnaug. No custom codes or mathematical algorithms were developed or used in this study.
Competing interests
P.A., N.J.H., L.V.-C. and J. R. Millman are inventors on related patents and patent applications. J. R. Millman is/has served as a consultant/employee for Sana Biotechnology. L.V.-C. is currently employed by Sana Biotechnology. J. R. Millman and L.V.-C. have stock or stock options in Sana Biotechnology. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/9/2023
A Correction to this paper has been published: 10.1038/s41556-023-01219-4
Extended data
is available for this paper at 10.1038/s41556-023-01150-8.
Supplementary information
The online version contains supplementary material available at 10.1038/s41556-023-01150-8.
References
- 1.Lanzoni G, Ricordi C. Transplantation of stem cell-derived pancreatic islet cells. Nat. Rev. Endocrinol. 2021;17:7–8. doi: 10.1038/s41574-020-00430-9. [DOI] [PubMed] [Google Scholar]
- 2.Millman JR, Pagliuca FW. Autologous pluripotent stem cell-derived β-like cells for diabetes cellular therapy. Diabetes. 2017;66:1111–1120. doi: 10.2337/db16-1406. [DOI] [PubMed] [Google Scholar]
- 3.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2010;6:139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 4.Velazco-Cruz L, et al. Acquisition of dynamic function in human stem cell-derived β cells. Stem Cell Rep. 2019;12:351–365. doi: 10.1016/j.stemcr.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagliuca FW, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezania A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 7.Hogrebe NJ, Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 2020;38:460–470. doi: 10.1038/s41587-020-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair GG, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 2019;21:263–274. doi: 10.1038/s41556-018-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veres A, et al. Charting cellular identity during human in vitro β-cell differentiation. Nature. 2019;569:368–373. doi: 10.1038/s41586-019-1168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahaddalkar PU, et al. Generation of pancreatic beta cells from CD177+ anterior definitive endoderm. Nat. Biotechnol. 2020;38:1061–1072. doi: 10.1038/s41587-020-0492-5. [DOI] [PubMed] [Google Scholar]
- 11.Song J, Millman JR. Economic 3D-printing approach for transplantation of human stem cell-derived β-like cells. Biofabrication. 2016;9:015002. doi: 10.1088/1758-5090/9/1/015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parent AV, et al. Development of a scalable method to isolate subsets of stem cell-derived pancreatic islet cells. Stem Cell Rep. 2022;17:979–992. doi: 10.1016/j.stemcr.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ameri J, et al. Efficient generation of glucose-responsive β cells from isolated GP2+ human pancreatic progenitors. Cell Rep. 2017;19:36–49. doi: 10.1016/j.celrep.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Cogger KF, et al. Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors. Nat. Commun. 2017;8:331. doi: 10.1038/s41467-017-00561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maxwell KG, et al. Gene-edited human stem cell-derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med. 2020;12:eaax9106. doi: 10.1126/scitranslmed.aax9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Single-cell transcriptome profiling reveals β cell maturation in stem cell-derived islets after transplantation. Cell Rep. 2020;32:108067. doi: 10.1016/j.celrep.2020.108067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balboa D, et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat. Biotechnol. 2022;40:1042–1055. doi: 10.1038/s41587-022-01219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maxwell KG, Kim MH, Gale SE, Millman JR. Differential function and maturation of human stem cell-derived islets after transplantation. Stem Cells Transl. Med. 2022;11:322–331. doi: 10.1093/stcltm/szab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuesta-Gomez N, et al. Characterization of stem-cell-derived islets during differentiation and after implantation. Cell Rep. 2022;40:111238. doi: 10.1016/j.celrep.2022.111238. [DOI] [PubMed] [Google Scholar]
- 20.Chen T, Dent SY. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat. Rev. Genet. 2014;15:93–106. doi: 10.1038/nrg3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon JR, et al. Chromatin architecture reorganization during stem cell differentiation. Nature. 2015;518:331–336. doi: 10.1038/nature14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat. Rev. Mol. Cell Biol. 2006;7:540–546. doi: 10.1038/nrm1938. [DOI] [PubMed] [Google Scholar]
- 23.Lund RJ, Närvä E, Lahesmaa R. Genetic and epigenetic stability of human pluripotent stem cells. Nat. Rev. Genet. 2012;13:732–744. doi: 10.1038/nrg3271. [DOI] [PubMed] [Google Scholar]
- 24.Weng C, et al. Single-cell lineage analysis reveals extensive multimodal transcriptional control during directed β-cell differentiation. Nat. Metab. 2020;2:1443–1458. doi: 10.1038/s42255-020-00314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Augsornworawat P, Millman JR. Single-cell RNA sequencing for engineering and studying human islets. Curr. Opin. Biomed. Eng. 2020;16:27–33. doi: 10.1016/j.cobme.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie R, et al. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12:224–237. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez-Dominguez JR, et al. Circadian entrainment triggers maturation of human in vitro islets. Cell Stem Cell. 2020;26:108–122. doi: 10.1016/j.stem.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Hu M, et al. Chromatin 3D interaction analysis of the STARD10 locus unveils FCHSD2 as a regulator of insulin secretion. Cell Rep. 2021;34:108881. doi: 10.1016/j.celrep.2021.108881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackermann AM, Wang Z, Schug J, Naji A, Kaestner KH. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol. Metab. 2016;5:233–244. doi: 10.1016/j.molmet.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiou J, et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature. 2021;594:398–402. doi: 10.1038/s41586-021-03552-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiou J, et al. Single-cell chromatin accessibility identifies pancreatic islet cell type- and state-specific regulatory programs of diabetes risk. Nat. Genet. 2021;53:455–466. doi: 10.1038/s41588-021-00823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su C, et al. 3D chromatin maps of the human pancreas reveal lineage-specific regulatory architecture of T2D risk. Cell Metab. 2022;34:1394–1409. doi: 10.1016/j.cmet.2022.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogrebe NJ, Maxwell KG, Augsornworawat P, Millman JR. Generation of insulin-producing pancreatic beta cells from multiple human stem cell lines. Nat. Protoc. 2021;16:4109–4143. doi: 10.1038/s41596-021-00560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arda HE, et al. Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab. 2016;23:909–920. doi: 10.1016/j.cmet.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hrvatin S, et al. Differentiated human stem cells resemble fetal, not adult, beta cells. Proc. Natl Acad. Sci. USA. 2014;111:3038–3043. doi: 10.1073/pnas.1400709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velazco-Cruz L, Goedegebuure MM, Millman JR. Advances toward engineering functionally mature human pluripotent stem cell-derived β cells. Front. Bioeng. Biotechnol. 2020;8:786. doi: 10.3389/fbioe.2020.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross S, et al. The novel enterochromaffin marker Lmx1a regulates serotonin biosynthesis in enteroendocrine cell lineages downstream of Nkx2.2. Development. 2016;143:2616–2628. doi: 10.1242/dev.130682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egozi A, et al. Insulin is expressed by enteroendocrine cells during human fetal development. Nat. Med. 2021;27:2104–2107. doi: 10.1038/s41591-021-01586-1. [DOI] [PubMed] [Google Scholar]
- 39.Van Gurp L, et al. Generation of human islet cell type-specific identity genesets. Nat. Commun. 2022;13:1–14. doi: 10.1038/s41467-022-29588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haber AL, et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grün D, et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature. 2015;525:251–255. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- 42.Kim H, et al. Serotonin regulates pancreatic β cell mass during pregnancy. Nat. Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon JH, et al. Serotonin regulates adult β-cell mass by stimulating perinatal β-cell proliferation. Diabetes. 2020;69:205–214. doi: 10.2337/db19-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, Virgilio MC, Collins KL, Welch JD. Multi-omic single-cell velocity models epigenome-transcriptome interactions and improves cell fate prediction. Nat. Biotechnol. 2022;41:387–398. doi: 10.1038/s41587-022-01476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hazelbaker DZ, et al. A multiplexed gRNA piggyBac transposon system facilitates efficient induction of CRISPRi and CRISPRa in human pluripotent stem cells. Sci. Rep. 2020;10:635. doi: 10.1038/s41598-020-57500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segerstolpe Å, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24:593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baron M, et al. A single-cell transcriptomic map of the human and mouse pancreas reveals inter-and intra-cell population structure. Cell Syst. 2016;3:346–360. doi: 10.1016/j.cels.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Artner I, et al. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blum B, et al. Functional β-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat. Biotechnol. 2012;30:261. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santen GW, et al. Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin–Siris syndrome. Nat. Genet. 2012;44:379–380. doi: 10.1038/ng.2217. [DOI] [PubMed] [Google Scholar]
- 52.Du Y, et al. Human pluripotent stem-cell-derived islets ameliorate diabetes in non-human primates. Nat. Med. 2022;28:272–282. doi: 10.1038/s41591-021-01645-7. [DOI] [PubMed] [Google Scholar]
- 53.Ramzy A, et al. A century long journey from the discovery of insulin to the implantation of stem cell derived islets. Endocr. Rev. 2022;44:222–253. doi: 10.1210/endrev/bnac021. [DOI] [PubMed] [Google Scholar]
- 54.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 55.Patterson M, et al. Defining the nature of human pluripotent stem cell progeny. Cell Res. 2012;22:178–193. doi: 10.1038/cr.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Zhu, H. et al. Understanding cell fate acquisition in stem-cell-derived pancreatic islets using single-cell multiome-inferred regulomes. Dev. Cell10.1016/j.devcel.2023.03.011(2023). [DOI] [PMC free article] [PubMed]
- 58.Markmann JF, et al. Insulin independence following isolated islet transplantation and single islet infusions. Ann. Surg. 2003;237:741. doi: 10.1097/01.SLA.0000072110.93780.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 60.Barton FB, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35:1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russ HA, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759–1772. doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghazizadeh Z, et al. ROCKII inhibition promotes the maturation of human pancreatic β-like cells. Nat. Commun. 2017;8:298. doi: 10.1038/s41467-017-00129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshihara E, et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature. 2020;586:606–611. doi: 10.1038/s41586-020-2631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng X, et al. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell. 2012;10:371–384. doi: 10.1016/j.stem.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sneddon JB, Borowiak M, Melton DA. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature. 2012;491:765–768. doi: 10.1038/nature11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamura A, et al. Long-term feeder-free culture of human pancreatic progenitors on fibronectin or matrix-free polymer potentiates β cell differentiation. Stem Cell Rep. 2022;17:1215–1228. doi: 10.1016/j.stemcr.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma X, et al. Human expandable pancreatic progenitor-derived β cells ameliorate diabetes. Sci. Adv. 2022;8:eabk1826. doi: 10.1126/sciadv.abk1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nostro MC, et al. Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Rep. 2015;4:591–604. doi: 10.1016/j.stemcr.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukherjee S, French DL, Gadue P. Loss of TBX3 enhances pancreatic progenitor generation from human pluripotent stem cells. Stem Cell Rep. 2021;16:2617–2627. doi: 10.1016/j.stemcr.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deuse T, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019;37:252–258. doi: 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sintov E, et al. Whole-genome CRISPR screening identifies genetic manipulations to reduce immune rejection of stem cell-derived islets. Stem Cell Rep. 2022;17:1976–1990. doi: 10.1016/j.stemcr.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parent AV, et al. Selective deletion of human leukocyte antigens protects stem cell-derived islets from immune rejection. Cell Rep. 2021;36:109538. doi: 10.1016/j.celrep.2021.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vegas AJ, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei J, et al. FasL microgels induce immune acceptance of islet allografts in nonhuman primates. Sci. Adv. 2022;8:eabm9881. doi: 10.1126/sciadv.abm9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bochenek MA, et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2018;2:810–821. doi: 10.1038/s41551-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X, et al. A nanofibrous encapsulation device for safe delivery of insulin-producing cells to treat type 1 diabetes. Sci. Transl. Med. 2021;13:eabb4601. doi: 10.1126/scitranslmed.abb4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stock AA, et al. Conformal coating of stem cell-derived islets for β cell replacement in type 1 diabetes. Stem Cell Rep. 2020;14:91–104. doi: 10.1016/j.stemcr.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang K, et al. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials. 2019;198:37–48. doi: 10.1016/j.biomaterials.2018.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brusko TM, Russ HA, Stabler CL. Strategies for durable β cell replacement in type 1 diabetes. Science. 2021;373:516–522. doi: 10.1126/science.abh1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang K, et al. A therapeutic convection-enhanced macroencapsulation device for enhancing β cell viability and insulin secretion. Proc. Natl Acad. Sci. USA. 2021;118:e2101258118. doi: 10.1073/pnas.2101258118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maehr R, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl Acad. Sci. USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sui L, et al. β-Cell replacement in mice using human type 1 diabetes nuclear transfer embryonic stem cells. Diabetes. 2018;67:26–35. doi: 10.2337/db17-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kishore S, et al. A non-coding disease modifier of pancreatic agenesis identified by genetic correction in a patient-derived iPSC line. Cell Stem Cell. 2020;27:137–146. doi: 10.1016/j.stem.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ng NHJ, et al. HNF4A haploinsufficiency in MODY1 abrogates liver and pancreas differentiation from patient-derived induced pluripotent stem cells. iScience. 2019;16:192–205. doi: 10.1016/j.isci.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kahraman S, et al. Abnormal exocrine–endocrine cell cross-talk promotes β-cell dysfunction and loss in MODY8. Nat. Metab. 2022;4:76–89. doi: 10.1038/s42255-021-00516-2. [DOI] [PubMed] [Google Scholar]
- 86.Leite NC, et al. Modeling type 1 diabetes in vitro using human pluripotent stem cells. Cell Rep. 2020;32:107894. doi: 10.1016/j.celrep.2020.107894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deng H, et al. Bioengineered omental transplant site promotes pancreatic islet allografts survival in non-human primates. Cell Rep. Med. 2023;4:100959. doi: 10.1016/j.xcrm.2023.100959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hao Y, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stuart T, Srivastava A, Madad S, Lareau CA, Satija R. Single-cell chromatin state analysis with Signac. Nat. Methods. 2021;18:1333–1341. doi: 10.1038/s41592-021-01282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma S, et al. Chromatin potential identified by shared single-cell profiling of RNA and chromatin. Cell. 2020;183:1103–1116. doi: 10.1016/j.cell.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pliner HA, et al. Cicero predicts cis-regulatory DNA interactions from single-cell chromatin accessibility data. Mol. Cell. 2018;71:858–871. doi: 10.1016/j.molcel.2018.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schep AN, Wu B, Buenrostro JD, Greenleaf WJ. chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods. 2017;14:975–978. doi: 10.1038/nmeth.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fornes O, et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020;48:D87–D92. doi: 10.1093/nar/gkz1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao J, et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566:496–502. doi: 10.1038/s41586-019-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schneider VA, et al. Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome Res. 2017;27:849–864. doi: 10.1101/gr.213611.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–5.
Supplementary Table 1. Materials and methods. This table lists the reagents, cell lines, medium formulations and sequences used in this study. (1.1) Human stem cell lines used. (1.2) Details of the differentiation protocol to generate SC-islets. (1.3) Base medium formulations. (1.4) Factors and compounds used. (1.5) gRNA sequences. (1.6) shRNA sequences. (1.7) Real-time PCR primer sequences. (1.8) Antibodies used. Supplementary Table 2. Multiome sequencing details. This set of tables provides multi-omic sequencing details such as individual data set details, cell filtering parameters and total analysed cell numbers. (2.1) Dataset information used in this study. (2.2) Donor information details for adult human primary islet data sets. (2.3) Cell filtering paraments used to exclude low-quality cells and mouse cells from each dataset. Cells with exceedingly low and high RNA and ATAC fragment counts, and high nucleosome signal were excluded to minimize potential doublets and dead cells. For transplanted samples, we have excluded host cells by filtering out cells with high expression of kidney marker TTC36. (2.4) Number of filtered cells from each dataset used for analysis in this study. SC, stem cell derived; KD, knockdown. Supplementary Table 3. Differential gene expression analysis for cluster identification. This set of tables show gene expression upregulated in each cluster for cell type identification. These include datasets for: (3.1) SC-islets, (3.2) primary human islets, (3.3) transplanted SC-islets and (3.4) CTCF CRISPRa SC-islets. Statistical significance was assessed by two-sided Wilcoxon rank sum test, reporting adjusted P values. Supplementary Table 4. Differential promoter accessibility analysis for cluster identification. This set of tables shows differential promoter accessibilities in each cluster. These include datasets for: (4.1) SC-islets, (4.2) primary human islets, (4.3) transplanted SC-islets and (4.4) CTCF CRISPRa SC-islets. Statistical significance was assessed by two-sided Wilcoxon rank sum test, reporting adjusted P values. Supplementary Table 5. Differential chromatin accessibility analysis of transcription factor (TF) binding motifs for cluster identification. This set of tables show motifs chromatin accessibility analysis for cluster identification for: (5.1) SC-islets, (5.2) primary human islets, (5.3) transplanted SC-islets and (5.4) CTCF CRISPRa SC-islets. Fold change of the chromatin accessibility of transcription factor binding motifs as well as RNA expression of each cell type over other endocrine populations to determine transcriptional activity: (5.5) SC-islets TF activity, (5.6) primary islets TF activity and (5.7) transplanted SC-islets TF activity. Statistical significance was assessed by two-sided logistic regression, reporting adjusted P values. Avg, average; SC, stem cell derived; EC, enterochromaffin; iEC, induced enterochromaffin. Supplementary Table 6. Trajectory analysis for delineation of the SC-β cell and SC-EC cell populations. This set of tables provides the source data for the multi-omic sequencing analysis comparing the SC-β cell and SC-EC cell populations. (6.1) Genes (mRNA) and values from trajectory analysis using Monocle. (6.2) Motif accessibility and values from trajectory analysis using Monocle. (6.3) Differential gene (mRNA) expression analysis comparing SC-β cell and SC-EC cell. (6.4) Differential motifs chromatin accessibility analysis comparing SC-β cell and SC-EC cell. Statistical significance was assessed by two-sided Moran’s test, reporting non-adjusted P values and q values (adjusted P values). Avg, average; SC, stem cell derived; EC, enterochromaffin. Supplementary Table 7. Cluster identification and characterization of subpopulations. This set of tables show differential gene expression and motifs accessibility analysis of a selected subpopulation compared with the rest of the other subpopulations in SC-β cells, SC-EC cells and primary β cells. (7.1) SC-β subpopulation 1, (7.2) SC-β subpopulation 2, (7.3) SC-β subpopulation 3, (7.4) SC-β subpopulation 4, (7.5) SC-EC subpopulation 1, (7.6) SC-EC subpopulation 2, (7.7) SC-EC subpopulation 3, (7.8) SC-EC subpopulation 4, (7.9) primary β subpopulation 1, (7.10) primary β subpopulation 2, (7.11) primary β subpopulation 3. (7.12) Composition information showing the number of cells in each subpopulation for SC-islets and primary islets. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived; EC, enterochromaffin; HI, human islets. Supplementary Table 8. Differential analysis of CTCF CRISPRa experiments. This set of tables provides the source data for the multi-omic sequencing analysis of CTCF overexpression in SC-islets. (8.1) Differential gene expression and motifs chromatin accessibility analysis comparing SC-EC cells and SC-iEC cells. (8.2) Differential gene expression and motifs chromatin accessibility analysis comparing the SC-endocrine population from control and CTCF overexpression. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; EC, enterochromaffin; iEC, induced enterochromaffin; OE, overexpression. Supplementary Table 9. Differential gene expression, motif accessibility and promoter accessibility analysis of SC-islets and primary islets. This set of tables provides differential analysis comparing SC-islet cells with primary islet cells (9.1) SC-β cells versus primary β cells. (9.2) SC-α cells versus primary α cells. (9.3) SC-δ cells versus primary δ cells. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived. Supplementary Table 10. Differential gene expression, and motif accessibility analysis of short-term and long-term in vitro SC-β cells. This set of tables provides differential analysis comparing short-term in vitro (weeks 2–4) with long-term (months 6–12) SC-β cells. (10.1) Differential gene expression. (10.2) Differential motifs accessibility. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived. Supplementary Table 11. Differential gene expression, motif accessibility and promoter accessibility analysis of SC-islets and primary islets. This set of tables provides differential analysis comparing SC-islet cells with transplanted SC-islet cells. (11.1) SC-β cells versus transplanted SC-β cells. (11.2) SC-α cells versus transplanted SC-α cells. (11.3) SC-δ cells versus transplanted SC-δ cells. (11.4) SC-EC cells versus transplanted SC-EC cells. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived. Supplementary Table 12. Differential gene expression and motifs accessibility analysis comparing 6 months in vitro SC-islets with 6 months in vivo SC-β cells. This set of tables provides differential analysis comparing 6 months in vitro SC-β cells with transplanted SC-β cells. (12.1) 6 months in vitro SC-β cells versus 6 months in vivo (transplanted) SC-β cells. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; SC, stem cell derived. Supplementary Table 13. Differential gene expression and motif accessibility analysis comparing SC-β cells from control and ARID1B shRNA condition. This set of tables provides differential analyses comparing control SC-β cells with ARID1B shRNA SC-β cells. (13.1) Control (GFP shRNA) SC-β cells versus ARID1B shRNA SC-β cells. Statistical significance was assessed by two-sided Wilcoxon rank sum test for RNA expression and two-sided logistic regression for motif chromatin accessibility, reporting adjusted P values. Avg, average; GFP, green fluorescent protein; SC, stem cell derived.
Data Availability Statement
Sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under accession code GSE199636. Source data are provided with this paper. GRCh38 human genome95, MSigDB96 and JASPAR202093 databases were used. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Codes used for analysing single-nucleus multi-omic sequencing are available on https://github.com/punnaug. No custom codes or mathematical algorithms were developed or used in this study.