Abstract
Background
Pulmonary vein isolation (PVI) is an effective treatment for paroxysmal atrial fibrillation (PAF). However, potential complications can occur due to the propagation of thermal energy into nontarget tissues adjacent to the targeted myocardium. Pulsed field ablation (PFA) is a novel ablation modality with the potential for preferential myocardial tissue ablation to minimize damage to collateral cardiac structures. A multielectrode pentaspline catheter has demonstrated safety and efficacy in treating PAF in single-arm, first-in-human studies.
Objective
The study sought to perform a randomized clinical trial to directly compare this PFA catheter with conventional ablation—either radiofrequency or cryoballoon ablation.
Methods
The ADVENT (Randomized Controlled Trial for Pulsed Field Ablation versus Standard of Care Ablation for Paroxysmal Atrial Fibrillation) trial is a multicenter, prospective, single-blind, randomized controlled trial to compare PVI using PFA vs conventional thermal ablation for drug-resistant PAF—with each site employing either (but not both) cryoballoon or radiofrequency ablation as a control condition. The sample size is adaptively determined based on Bayesian statistical methods. All patients will undergo PVI, and be followed for 12 months.
Results
The primary effectiveness endpoint is a composite of acute procedural success and freedom from any documented atrial arrhythmia recurrence, repeat ablation, or use of antiarrhythmic drugs after a 3-month postablation blanking period. The primary safety endpoint is a composite of defined acute and chronic device- and procedure-related serious adverse events. Both primary endpoints will be evaluated for noninferiority of the novel PFA system compared with standard-of-care thermal ablation.
Conclusions
By providing objective, comparative data, this study aims to scientifically determine whether the pentaspline PFA catheter is safe and effective for PVI ablation to treat drug-resistant PAF.
Keywords: Atrial fibrillation, Catheter ablation, Pulsed field ablation, Pulmonary vein isolation, Randomized controlled trial
Key Findings.
-
▪
Pulsed field ablation (PFA) is a novel, nonthermal ablation modality for performing pulmonary vein isolation in paroxysmal atrial fibrillation.
-
▪
Well-established ablation modalities—radiofrequency ablation (RFA) and cryoballoon ablation (CBA)—rely on the propagation of ablative thermal waves that can inadvertently impact structures adjacent to the myocardium.
-
▪
There are limited data in which PFA is prospectively compared with RFA or CBA, and to date, there have been no randomized clinical trials comparing the safety and efficacy of PFA against these well-established ablation modalities.
-
▪
The ADVENT (Randomized Controlled Trial for Pulsed Field Ablation versus Standard of Care Ablation for Paroxysmal Atrial Fibrillation) trial is a multicenter, prospective, randomized controlled trial to evaluate the novel ablation modality, PFA, compared with standard of care thermal ablation—RFA or CBA—for pulmonary vein isolation in patients with paroxysmal atrial fibrillation.
Introduction
Pulmonary vein isolation (PVI) has proven to be safe and effective for treating patients with paroxysmal atrial fibrillation (PAF). However, potential complications are known to occur with thermal-based ablation modalities such as radiofrequency ablation (RFA), cryoballoon ablation (CBA), and laser ablation include stroke, pulmonary vein (PV) stenosis, phrenic nerve palsy (PNP), pericardial tamponade, and atrioesophageal fistula (AEF). Many of these complications result from the propagation of thermal effect into nontarget tissues adjacent to the target myocardium.
Pulsed field ablation (PFA) is a novel ablation modality that, in preclinical and clinical studies, has displayed evidence of an important degree of preferential myocardial tissue ablation.1, 2, 3, 4 This modality involves the application of ultra-rapid, high-voltage microsecond electrical pulses to the endocardium, which generates strong electrical fields in the targeted myocardial tissue. This results in irreversible nanoscale pore formation, dielectric breakdown of the cell membranes, and cellular death.5 Preclinical and clinical studies have demonstrated that by optimizing voltage amplitude, phasic waveforms, and pulse sequences, one can avoid damage to pericardiac structures such as the esophagus and phrenic nerve.2, 3, 4,6, 7, 8
The investigational device used in the ADVENT (Randomized Controlled Trial for Pulsed Field Ablation versus Standard of Care Ablation for Paroxysmal Atrial Fibrillation) trial is a specialized multielectrode pentaspline catheter optimized for PFA energy delivery. While AF ablation with this PFA catheter has been demonstrated to be safe and effective in thousands of patients,3,8, 10 there has not yet been a randomized clinical trial to directly compare its effectiveness with RFA or CBA. The ADVENT trial, a randomized controlled trial with a novel adaptive design, will examine the safety and effectiveness of PFA using the pentaspline ablation catheter compared with conventional thermal ablation modalities in patients with drug-resistant symptomatic PAF.
Methods
Trial design
The ADVENT trial is a multicenter, prospective, single-blind, randomized controlled clinical trial, with blinded endpoint adjudication, that compares the safety and effectiveness of a novel multielectrode pentaspline PFA catheter (Farawave; Farapulse-Boston Scientific Inc, Menlo Park, CA) compared with standard-of-care ablation using either force-sensing RFA or CBA. The trial uses Bayesian statistical methods to adaptively determine sample size, which will range from 350 to 750 randomized subjects. This study will be performed in accordance with the U.S. Code of Federal Regulations, Good Clinical Practice, and ethical principles consistent with the Declaration of Helsinki. The ADVENT trial was registered with the National Library of Medicine’s Clinical Trials site (ClinicalTrials.gov; NCT04612244) on November 2, 2020 and was approved by the Food and Drug Administration (FDA) under the Investigational Device Exemption regulation (G200259) on December 14, 2020. Institutional Review Board approval was obtained at all investigational sites. Informed written consent will be obtained from all trial participants before enrollment and randomization.
Study population
Study subjects will be carefully screened prior to enrollment to meet study eligibility criteria. The inclusion criteria require that the subjects have drug-resistant (therapeutic failure of at least one class I to IV antiarrhythmic drug [AAD] for efficacy and/or intolerance), have documented PAF, are between the ages of 18 and 75 years, and are willing and capable study participants. The full list of inclusion criteria is shown in Table 1. The exclusion criteria prohibit persistent or secondary AF, abnormally enlarged atria or prior atrial procedures, prior ventricular tachycardia or fibrillation, valvular disease, hypertrophic cardiomyopathy, prosthetic valves or implantable devices, or a history of rheumatic fever or congenital heart disease. Baseline heart failure, hypertension, and bradycardia are excluded, as are recent evidence of ischemic heart disease or coronary intervention, stroke or transient ischemic attack (TIA), thromboembolism, or carotid intervention. Cardiac implanted devices, including implantable loop recorders, are not allowed. The full list of exclusion criteria is shown in Table 2.
Table 1.
Inclusion criteria
| Inclusion category | Inclusion definition |
|---|---|
| 1. Drug-resistant symptomatic PAF | |
| a. Paroxysmal | AF that terminates spontaneously or with intervention within 7 d of onset. |
| b. Frequency | i. Physician documentation of recurrent PAF (2 or more episodes) within 6 mo, AND |
| ii. At least 1 documented episode by a recording such as ECG, EM, Holter monitor or telemetry strip within 12 mo of enrollment. | |
| c. Drug-resistant | Failed AAD treatment, meaning therapeutic failure of at least 1 AAD (class I to IV) for efficacy and/or intolerance |
| 2. Age | Patients who are ≥18 and ≤75 years of age on the day of enrollment. |
| 3. Participation | i. Providing informed consent to undergo study procedures, AND |
| ii. Participating in all examinations and follow-up visits and tests associated with this clinical study. | |
AAD = antiarrhythmic drug; AF = atrial fibrillation; ECG = electrocardiography; EM = event monitor; PAF = paroxysmal atrial fibrillation.
Table 2.
Exclusion criteria
| Exclusion criteria |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AHI = apnea-hypopnea index; BP = blood pressure; CPAP = continuous positive airway pressure; CT = computed tomography; DBP = diastolic blood pressure; HgbA1c = hemoglobin A1c; IVC = inferior vena cava; LA = left atrial; LV = left ventricular; LVEF = left ventricular ejection fraction; MRI = magnetic resonance imaging; PV = pulmonary vein; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SBP = systolic blood pressure; SVT = supraventricular tachycardia; TTE = transthoracic echocardiography; TIA = transient ischemic attack.
Randomization and roll-in subjects
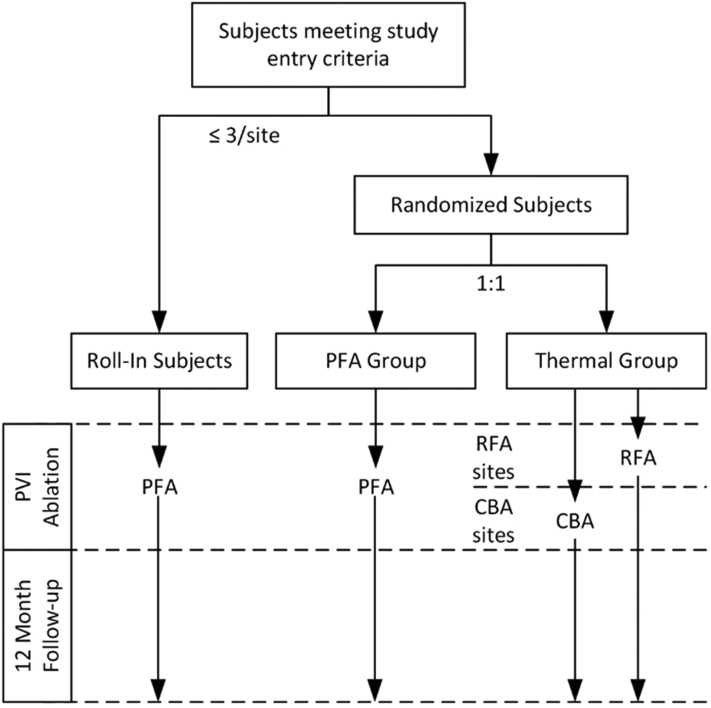
The first 1 to 3 sequentially enrolled subjects at each site are to be nonrandomized roll-in subjects treated with the PFA system to confirm each investigator has experience using the pentaspline PFA ablation system. Thereafter, subjects will be randomized 1:1 either to a treatment group to undergo PVI with the pentaspline PFA catheter (PFA group) or a control group to undergo PVI using thermal ablation with either RFA or CBA catheters (thermal group). Each investigational site is allowed only- one of the 2 thermal modalities, and sites are to be selected to yield approximately equal numbers of RFA and CBA subjects. Randomization is stratified by site and uses a permuted block design with randomly varying block sizes. All patients, roll-in and randomized, will be followed to 12 months (Figure 1).
Figure 1.
Study flow diagram. CBA = cryoballoon ablation; PFA = pulsed field ablation; PVI = pulmonary vein isolation; RFA = radiofrequency ablation.
Preablation protocol
Anticoagulation is as recommended by the 2017 Heart Rhythm Society Expert Consensus Statement11 and the 2019 American Heart Association/American College of Cardiology/Heart Rhythm Society Focused Update.12 Nonanticoagulated subjects will receive therapeutic anticoagulation for at least 3 weeks prior to the index procedure regardless of CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age 65-74 years, sex category) score, and subjects with a CHA2DS2-VASc score ≥2 (men) or ≥3 (women) will receive oral anticoagulants throughout follow-up. Transesophageal echocardiography or intracardiac echocardiography (ICE) will be performed within 48 hours prior to the index procedure to exclude left atrial thrombus. All subjects without contraindications will be maintained on suitable anticoagulation for at least 2 months following the index procedure.
Ablation
Sedation or general anesthesia will be determined according to institutional protocol. A bolus of heparin will be delivered prior to or immediately following transseptal puncture. Procedural activated clotting times will be maintained at a minimum of 300 seconds using intravenous heparin bolus or continuous infusion. The randomized ablation modality will be used to isolate the PVs and entry block will be confirmed after a minimum 20-minute waiting period after the last delivered lesion. Under defined circumstances, subjects may undergo cavotricuspid isthmus (CTI) ablation and a limited range of other ablations if the investigator determines that subject welfare requires ablation for an accessory pathway, atrioventricular nodal re-entrant tachycardia, treatment-emergent left atrial flutter (AFL), or incessant atrial tachycardia (AT), using any approved ablation catheter. Electroanatomical mapping may be performed at the discretion of the operator. Postablation, fluoroscopic examination of diaphragm motion will be performed to assess phrenic nerve integrity.
Pulsed field ablation
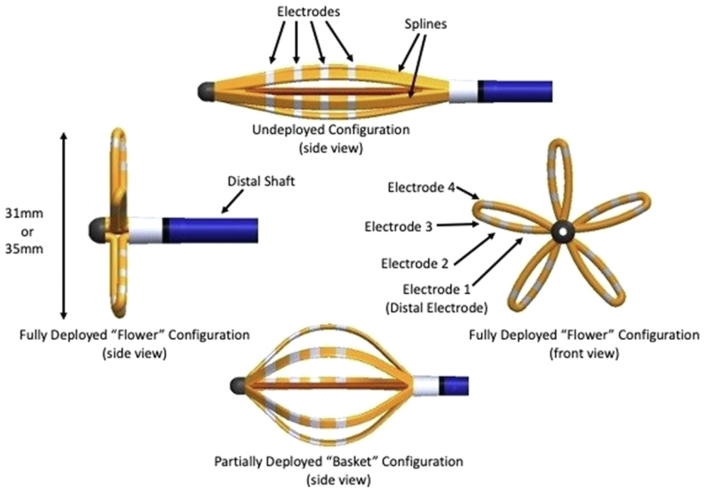
The PFA group in the ADVENT trial will be randomized to undergo PVI using the pentaspline PFA catheter (Figure 2), deflectable sheath (Faradrive; Farapulse-Boston Scientific Inc), and PFA generator (Farastar; Farapulse-Boston Scientific Inc) optimized for left atrial ablation. The 12.8F over-the-wire ablation catheter, described previously,3 has 5 electrode-bearing splines that can be variably deployed after insertion to allow a flexible adaptation to left atrial anatomy, with 1 electrode on each spline separately wired to allow connection to a mapping or recording system. After the PFA catheter has been positioned, the generator will deliver ultra-rapid, high-voltage electrical pulses to the endocardial surface, leading to selective electroporation of targeted cardiac tissue. Each PV will receive 2 applications in a partially open “basket” configuration, followed by rotation and 2 more applications, followed then by a second set of 2 + 2 applications in a fully deployed “flower” configuration (again with rotation between each pair of lesions) for a total of 8 PFA applications per PV. Additional applications may be delivered per operator preference if the PV is not isolated. Per protocol, ICE will be used during PFA to monitor catheter positioning. No esophageal temperature monitoring will be performed.
Figure 2.
Pentaspline pulsed field ablation catheter.
Thermal ablation
The thermal group will consist of subjects undergoing PVI using either of the dominant commercially available modalities (irrigated, force-sensing RFA or CBA), thus allowing comparison of PFA with the current standard-of-care treatment. Esophageal protection (temperature monitoring, cooling, or deviation) will be based on institutional protocols.
Neurologic assessment subgroup
A planned subgroup of up to 80 sequentially enrolled, randomized subjects at selected sites will undergo postprocedural brain magnetic resonance imaging (MRI) for the assessment of silent cerebral events (SCEs) and silent cerebral lesions (SCLs). Standard assessment of the MRI scans will be performed at a Brain Imaging Core Laboratory. Subjects with documented SCEs or SCLs will undergo prespecified neurologic assessment prior to discharge and a follow-up MRI at 3 months.
Follow-up
Subjects will be followed for 12 months with assessments for arrhythmia recurrence, changes in PV morphology, adverse events (AEs), use of AADs, and interventions such as reablation and cardioversion. Table 3 shows the follow-up timing and data collected at discharge, day 7, day 30, day 90, day 180, and day 360.
Table 3.
Schedule of events, randomized subjects
| Assessment | Baseline∗ | Index procedure | Predischarge | Day 7 (7–11 d) | Day 30 (±7 d) | Day 60 (±10 d) | Day 90 (±14 d) | Month 6 (±30 d) | Month 12 (±30 d) | Unscheduled |
|---|---|---|---|---|---|---|---|---|---|---|
| Informed consent | X | |||||||||
| Baseline assessment | X | |||||||||
| TEE/ICE to exclude left atrial thrombus | X | |||||||||
| AAD and anticoagulant medications | X | X | X | X | Discontinue AADs |
X | X | X | X | |
| Recurrent arrhythmia, cardioversions, ablations, hospital admissions | X | X | X | X | X | X | X | |||
| Adverse events | X | X | X | X | X | X | X | X | ||
| 12-lead ECG | X | X | X | X | X | |||||
| Event monitors | Training | Training | Training | Weekly + symptomatic transmissions | X | |||||
| 72-h Holter | Training | Training | X | X | ||||||
| Cardiac CT/MRI | X | X | X† | |||||||
| NIHSS | X | X | ||||||||
| Radiological examination of diaphragm | X | X‡ | X‡ | |||||||
| AF symptom assessment | X | X | ||||||||
| QoL questionnaires | X | X | ||||||||
| Neurological assessment | X§ | X§ | ||||||||
| Assessment of subject blinding | X | X | ||||||||
AAD = antiarrhythmic drug; CT = computed tomography; ICE = intracardiac echocardiography; MRI = magnetic resonance imaging; NIHSS = National Institutes of Health Stroke Scale/Score; PV= pulmonary vein; QoL = quality of life; TEE = transesophageal echocardiography; TIA = transient ischemic attack; TTE = transthoracic echocardiography.
All baseline assessments must be generated in the window beginning 30 days (90 days for TTE and cardiac CT/MRI) prior to the consent date and ending on the date of the index procedure.
Month 12 cardiac MRI/CT only if 3-month imaging revealed any PV with ≥70% reduction in PV measured diameter.
Only if resolution of phrenic nerve palsy has not yet been demonstrated. May be performed either by fluoroscopic sniff test or inspiration/expiration chest radiography.
Non-Neurological Assessment Subgroupsubjects: If NIHSS score has increased by 1 or more points or if there is a clinical suspicion of stroke/TIA, then a consulting neurologist will perform a stroke assessment and include the results of a concurrent brain MRI. If stroke is diagnosed, a modified Rankin Scale assessment will be performed prior to discharge and again at 3 months.
The first 90 days following the index procedure are a blanking period during which recurrent atrial arrhythmia does not constitute a treatment failure. AADs, except amiodarone, may be used during the blanking period at the investigator’s discretion. Per protocol, AADs will be stopped before the end of the blanking period. Following the blanking period, any use of a class I or III AAD constitutes a treatment failure.
Electrocardiograms (ECGs) will be recorded during in-person visits at baseline, predischarge, 90-day follow-up, and 360-day follow-up. Additional ECGs will be performed at unscheduled follow-up visits. Patients will be trained on how to use the Holter monitors and event monitors during their baseline and predischarge appointments. Holter monitors will be used to capture 72-hour continuous ECG at 180- and 360-day follow-up. Additional training for the use of event monitors is given around day 60, and required weekly transmissions supplemented by additional transmissions for any arrhythmic symptoms begin immediately after the blanking period and continue through the remaining 9 months of study follow-up. A qualified Arrhythmia Core Laboratory will receive, review, and assess all protocol-stipulated ECGs, event monitors, and Holter monitors.
Repeat cardiac imaging to assess PV dimensions will be performed in all randomized subjects as part of the Day 90 assessment and will be reviewed by a qualified Cardiac Imaging Core Laboratory. The measured PV diameter is defined as the geometric mean of the 2 orthogonal diameters approximating the longest and shortest diameters at the plane of measurement. If any calculated PV diameter has ≥70% reduction, a 12-month computed tomography (CT)/MRI scan will be performed. Brain imaging will be performed for any subjects with clinical suspicion of stroke.
Redo procedures
Redo ablation procedures require documentation of AF, AFL or AT that persists after the blanking period. When performing a reablation, a commercially available, FDA–approved ablation catheter will be used. If the left atrium is accessed during the procedure, electroanatomical mapping will be performed to assess PVI durability. Reablation does not reset the blanking period.
COVID-19 mitigation
Several mitigations for potential COVID-19 disruptions are included in the study plan, including allowing telemedicine technology for follow-up assessments and the performance of critical testing at nonstudy sites. When these occur or when COVID-related disruptions result in missing data, information will be captured that explains the reasons for the disruption. In addition, severe COVID-19 infection is formally defined and, in sensitivity analyses, subjects who are adjudicated to meet this definition will be censored as of the onset date of infection.
Study endpoints
Primary safety endpoint
The primary safety endpoint, the composite safety endpoint (CSE), is a composite of specifically defined serious adverse events (SAEs) related to either the use of an ablation catheter or the ablation procedure itself with onset within 7 days of the primary procedure. These events include death, myocardial infarction, persistent PNP, stroke, TIA, peripheral or organ thromboembolism, cardiac tamponade or perforation, pericarditis, pulmonary edema, vascular access complications requiring invasive interventions, heart block, or gastric motility or pyloric spasm disorders. In addition, the CSE includes the occurrence of PV stenosis (>70% diameter reduction) or AEF at any time during the 12-month follow-up.
Primary effectiveness endpoint
The primary effectiveness endpoint, treatment success, requires both acute procedural success and chronic success through 12 months. Acute procedural success is defined as the demonstration in each attempted PV of entrance block assessed ≥20 minutes after the last PVI lesion is made with or without adenosine testing, using only the randomized treatment modality. Chronic success is defined as freedom from any reablation for AF, AFL, or AT (excluding CTI-dependent flutter) or any use of amiodarone. Chronic success also requires, after a 90-day blanking period, freedom from any recurrent AF, AFL, or AT (excluding CTI-dependent flutter), any cardioversion for these same arrhythmias, and freedom from use of class I or III AAD. This primary effectiveness endpoint will be compared between the PFA group and the thermal group (combining the RFA and CBA cohorts). The cohorts using the 2 thermal modalities will be aggregated because multiple randomized clinical trials have revealed similar outcomes between RFA and CBA.13
Secondary safety endpoint
The secondary safety endpoint measures the baseline to day-90 changes in each subject’s aggregate PV cross-sectional area, which is then compared between the PFA and thermal groups. PV cross-sectional areas are computed using the formula for area of an ellipse, with half the longest and shortest axis measurements of the PV diameter serving as the radii for calculation. A subject’s aggregate PV cross-sectional area is the sum of the calculated area of each ablated PV.
Secondary effectiveness endpoint
The secondary effectiveness endpoint is the same as the primary effectiveness endpoint but will be tested for treatment superiority rather than noninferiority.
Additional endpoints
Additional safety assessments comparing PFA and thermal subjects include (1) severe ablation complications (the proportion of subjects in each treatment group with 1 or more of the following CSE components [PV stenosis, persistent PNP, AEF] will be compared between groups), (2) nonserious/serious CSEs, (3) postblanking cardioversions, (4) postblanking arrhythmia hospitalizations, (5) any related SAE, (6) any related stroke or TIA, (7) categorized PV dimensional changes, (8) control RFA/CBA safety assessment, and (9) learning curve safety assessment.
Additional effectiveness assessments comparing PFA and thermal subjects include (1) acute procedural success, (2) first-pass acute procedural success, (3) acute vein success, (4) chronic success, (5) chronic success allowing reablation, (6) chronic success allowing AADs, (7) treatment success allowing reablation, (8) treatment success allowing AADs, (9) treatment success with PVI/CTI only, (10) early recurrence of AF, (11) rate of reablation, (12) PVI durability at reablation, (13) CTI ablation failure, (14) control RFA/CBA effectiveness comparison, (15) AF symptom assessment, and (16) learning curve effectiveness assessment.
Other study outcome measures include relevant procedure durations, characterization of lesion sets, and 2 quality-of-life assessments—the EuroQol standardized questionnaire of health states (EQ-5D-3L) and the Atrial Fibrillation Effect on Quality of Life at baseline and 12 months.
Statistical analysis
Adaptive sample size determination
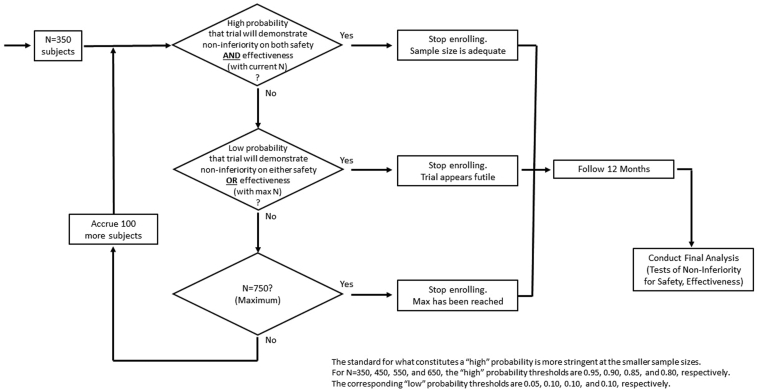
The study is designed using Bayesian statistical methods, with noninformative prior distributions. Sample size is determined adaptively via a Goldilocks14 design, with possible sizes of 350, 450, 550, 650, and 750 (Figure 3). At each enrollment milestone, the predictive probability that the trial will eventually demonstrate noninferiority for each primary endpoint is calculated; enrollment stops for predicted success if the probability of success is high for both primary endpoints when the current sample has been followed to 12 months; enrollment stops for futility if the probability of ultimate success is low for either primary endpoint, assuming that 750 are enrolled and followed to 12 months. If neither condition obtains, enrollment continues to the next specified size, subject to the maximum of 750, and the process repeats. A Data Safety Monitoring Board (DSMB) reviews each algorithmic recommendation and approves the implementation. Noninferiority for both endpoints is tested only when enrollment has stopped and all enrolled subjects have been followed to 12 months. In contrast to a traditional group sequential design, in which tests of the primary hypotheses occur at each interim analysis, this design requires full follow-up for all subjects before any noninferiority testing can occur, thereby giving even the last enrolled subject full weight in the study’s hypothesis tests.
Figure 3.
Adaptive sample size determination algorithm.
In computer simulation, this design achieves >95% power for the target scenario (8% primary safety event rate and 65% treatment success rate in each arm), while retaining >80% power for other plausible scenarios and allowing early enrollment stopping if the rates are very favorable or very unfavorable. Simulation also reveals that fast enrollment rates (>50/month) can be counterproductive, tending toward larger sample size without noticeably improving power or time to final analysis (see the Supplemental Data).
Primary statistical analyses
All primary and secondary endpoints will be analyzed using Bayesian methods with noninformative priors, though frequentist methods may be used for selected sensitivity analyses and a subset of the prespecified additional analyses. Both primary outcome assessments are noninferiority tests between the PFA and thermal groups.
The primary safety hypothesis is that the 12-month incidence of primary safety events for PFA is noninferior to thermal ablation, with an absolute margin of 8%. The primary effectiveness hypothesis is that the probability of treatment success at 12 months is noninferior to thermal ablation, with an absolute margin of 15%. Both are tested by assigning a noninformative Beta (0.5, 0.5) prior distribution to the rate and updating it based on observed 12-month binary outcomes. Subjects with censored follow-up are included via multiple imputation. The imputation model, as well as the predictive model used for sample size determination at the interim assessments, imputes unobserved final outcomes based on subject-specific censoring times and a Bayesian piecewise exponential survival model that is fitted to the currently available time-to-event data for each treatment arm. The standard of trial success is that posterior probabilities of noninferiority for safety and effectiveness exceed 0.966 and 0.956, respectively; these were demonstrated in simulation to control the trial’s false positive rate at the 0.05 level (see the Supplemental Data).
Secondary statistical analyses
Two formally tested secondary endpoints are included in the ADVENT trial, to be tested if and only if noninferiority is established for both safety and effectiveness. The secondary safety endpoint of aggregate PV cross-sectional area between baseline and 3 months will be compared between the PFA and thermal groups, testing whether the reduction in mean aggregate cross-sectional area is less in the PFA group. Within-subject changes will be compared via a Bayesian version of a t test, using noninformative priors for each group that are uniform on the scale of [μ, log(σ)]. The standard for success is a posterior probability exceeding 0.975. If the reduction in PV cross-sectional area is established to be lower in the PFA group, superiority of treatment success rates will be tested, using the same methods as the primary effectiveness analysis. The standard for success is a posterior probability of 0.977. The false positive rate for each secondary endpoint is controlled at the 0.025 level.
Additional analyses
Roll-in subjects will be summarized with descriptive statistics and will be analyzed separately from any populations composed of randomized subjects. Procedural characteristics and quality of life will be summarized with descriptive statistics and may be summarized with either Bayesian or frequentist inferential methods.
Sensitivity analysis
Planned sensitivity analyses include assessments of the impacts of missing data, imputation model, COVID-19, and subjects requiring ablation for accessory pathways, atrioventricular nodal re-entrant tachycardia, treatment-emergent left AFL, or incessant AT.
Assessment of blinding
At the time of index procedure discharge and at the 12-month follow-up visit, randomized subjects will be surveyed regarding their opinion as to which study group they were assigned. These self-assessments of treatment status will be evaluated according to the procedures described by Bang and colleagues15 and James and colleagues.16
Trial oversight
Key evaluators (Clinical Events Committee, Arrhythmia Core Laboratory, Cardiac Imaging Core Laboratory, and Brain Imaging Core Laboratory) for primary and secondary outcome assessments will be blinded. Follow-up Holter monitoring and event monitoring data, and all MRI/CT dimensions of PVs will be assessed objectively and without knowledge of subject treatment status by third parties according to standard protocols. Site monitoring, data management, and statistical analysis are performed by an independent contract research organization and sponsor personnel.
Clinical Events Committee
The Clinical Events Committee (CEC), an external group of 3 electrophysiologists, will convene regularly during the study to review, classify, and adjudicate adverse events reported during the study. All reported and potential SAEs as well as the occurrence of each primary effectiveness and safety endpoints will be reviewed. If unblinding should become necessary to complete adjudication, this will be documented for later review.
Data and Safety Monitoring Board
The DSMB will comprise of 3 independent physician experts and an independent statistician. The DSMB will convene regularly to review unblinded results to assess overall event rates, ensure the safety and welfare of the study subjects, and approve the adaptive design’s enrollment stopping recommendations.
Discussion
Significance of the trial
This multicenter, prospective, randomized controlled trial will evaluate a novel ablation modality, PFA, compared with conventional thermal ablation—RFA or CBA—for PVI ablation in patients with PAF. Since the introduction of PFA to European clinical practice, thousands of patients have been treated with the pentaspline PFA catheter, with numerous publications demonstrating positive safety and effectiveness outcomes.3,8, 10,17 In fact, early studies revealed acute procedural isolation in 100% using the PFA catheter alone, and initial 1-year freedom from atrial arrhythmia recurrence of 78.5 ± 3.8% and 84.5 ± 5.4% for the full cohort and subset using the optimized PF waveform, respectively,3 which is favorably aligned with outcomes previously reported using other ablation technologies.11,13,18,19 However, there are limited data in which PFA is prospectively compared with RFA or CBA, and to date, there have been no randomized clinical trials comparing the safety and efficacy of PFA against these well-established ablation modalities. While the Comparative Study of Two Ablation Procedures in Patients With Atrial Fibrillation (FIRE AND ICE) trial provided initial randomized clinical trial evidence of the effectiveness of CBA,13 the ADVENT trial is designed to provide a direct comparison of PFA with RFA and CBA.
While RFA and CBA are well established for treatment of PAF, even as first-line therapy,13,18, 19, 20, 21 there continues to be risk associated with these thermal ablation modalities. Nondiscriminant thermal ablation runs the risk of inadvertently impacting adjacent structures such as the esophagus and phrenic nerve. With the recent application of PFA, there is potential for these risks to be substantially reduced, if not eliminated, due to the selective nature of PFA. Notably, in the Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF) retrospective survey of more than 1,700 patients treated with PFA, there were no thermal-specific safety events such as esophageal injury, PV stenosis, or phrenic nerve paralysis.9 However, prospective, randomized data are lacking that allow this novel technology to be directly compared with the current standard of care.
This trial will also provide critical data regarding cerebral safety, comparing the incidence of SCEs and SCLs during PFA vs either RFA or CBA. With RF energy, tissue heating can result in char formation and subsequent embolization, with studies showing the prevalence of ischemic events ranging from 7% to 42%.22 Although there is a risk of air embolism during any catheter-based AF ablation procedure through catheter insertion and sheath flushing,23 there are limited data on the “embolic fingerprint” of PFA technologies.3,9,17 The neurological assessment subgroup in the ADVENT trial will provide valuable comparative data regarding the occurrence of cerebral events for all 3 ablative modalities used in this trial.
The direct comparison of changes in PV dimensions from baseline to day 90 should provide invaluable data regarding the potential for unfavorable PV remodeling and stenosis with PFA compared with thermal ablation. A preliminary nonrandomized comparison of PFA with RFA demonstrated that PV narrowing/stenosis was present in 0% and 0% vs 12.0% and 32.5% of PVs in patients who underwent PFA or RFA, respectively.6 However, the nonrandomized nature of this comparison raises the possibility of potential confounders; accordingly, the ADVENT trial is necessary to definitively conclude on this point.
Studies using PFA have demonstrated short learning curves and procedure times,9,10 which, for the right patients, may provide an optimal AF ablation approach. In clinical practice, procedural efficiency could also translate to the ability to treat more patients sooner after their initial AF diagnosis and ultimately earlier in the disease progression. Several recent studies have demonstrated the benefits of early rhythm control to intervene in the self-perpetuating effect of AF on atrial structural and electrical remodeling.18,19,24, 25, 26, 27
The ADVENT trial completed enrollment on June 3, 2022. Results are expected before the end of 2023. By providing a direct comparison between PFA and thermal ablation, the results of the ADVENT trial will fill a crucial gap in our understanding of this novel energy source and its potential impact on the safety and efficacy of catheter ablation for treating PAF.
Conclusion
The ADVENT trial is a multicenter, prospective, blinded, randomized controlled trial using a Bayesian adaptive design. By providing objective, comparative data, this study aims to assess with high scientific quality whether endocardial PFA using a pentaspline catheter is as safe and effective as conventional thermal ablation for performing PVI to treat drug-resistant PAF.
Acknowledgments
The authors acknowledge the Clinical Events Committee and Data Safety Monitoring Board members for their expertise and contributions to this study, and Elizabeth Albrecht, PhD (Boston Scientific Corporation, Scientific Communications), for assistance drafting, editing, and submitting the article.
Funding Sources
The ADVENT trial is sponsored and funded by FARAPULSE (now Boston Scientific).
Disclosures
Vivek Y. Reddy was a consultant to and had received equity from Farapulse Inc (now divested) and serves as a consultant to Boston Scientific Inc; he also has disclosures with companies unrelated to this article, which are listed in the Supplemental Data. John W. Lehmann was a consultant to and received equity from Farapulse Inc (now divested) and serves as a consultant to Boston Scientific Inc. Edward P. Gerstenfeld was a consultant to Farapulse Inc and serves as an unpaid consultant to Boston Scientific Inc. Andrew S. Mugglin was a consultant to Farapulse Inc and serves as a consultant to Boston Scientific Inc; unrelated to this manuscript, he has also provided statistical consulting and/or Data Safety Monitoring Board services for Atricure, Abbott, Biosense Webster, and Medtronic. Christopher W. Schneider is an employee of Boston Scientific Corporation. Anitha B. Achyutha is an employee of Boston Scientific Corporation. Moussa Mansour is a consultant for Boston Scientific, Biosense Webster, Abbott, Medtronic, Siemens, and Phillips; and has received equity from NewPace Ltd.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Informed written consent will be obtained from all trial participants before enrollment and randomization.
Ethics Statement
This study will be performed in accordance with the U.S. Code of Federal Regulations, Good Clinical Practice, and ethical principles consistent with the Declaration of Helsinki.
Appendix. Supplementary Data
References
- 1.Koruth J.S., Kuroki K., Iwasawa J., et al. Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation. Europace. 2020;22:434–439. doi: 10.1093/europace/euz341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koruth J.S., Kuroki K., Kawamura I., et al. Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy V.Y., Dukkipati S.R., Neuzil P., et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. J Am Coll Cardiol EP. 2021;7:614–627. doi: 10.1016/j.jacep.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Cochet H., Nakatani Y., Sridi-Cheniti S., et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace. 2021;23:1391–1399. doi: 10.1093/europace/euab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotnik T., Rems L., Tarek M., Miklavcic D. Membrane electroporation and electropermeabilization: mechanisms and models. Annu Rev Biophys. 2019;48:63–91. doi: 10.1146/annurev-biophys-052118-115451. [DOI] [PubMed] [Google Scholar]
- 6.Kuroki K., Whang W., Eggert C., et al. Ostial dimensional changes after pulmonary vein isolation: pulsed field ablation vs radiofrequency ablation. Heart Rhythm. 2020;17:1528–1535. doi: 10.1016/j.hrthm.2020.04.040. [DOI] [PubMed] [Google Scholar]
- 7.Nakatani Y., Sridi-Cheniti S., Cheniti G., et al. Pulsed field ablation prevents chronic atrial fibrotic changes and restrictive mechanics after catheter ablation for atrial fibrillation. Europace. 2021;23:1767–1776. doi: 10.1093/europace/euab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy V.Y., Anic A., Koruth J., et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol. 2020;76:1068–1080. doi: 10.1016/j.jacc.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Ekanem E., Reddy V.Y., Schmidt B., et al. Multi-national survey on the methods, efficacy, and safety on the postapproval clinical use of pulsed field ablation (MANIFEST-PF) Europace. 2022;24:1256–1266. doi: 10.1093/europace/euac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt B., Bordignon S., Tohoku S., et al. 5S study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol. 2022;15 doi: 10.1161/CIRCEP.121.010817. [DOI] [PubMed] [Google Scholar]
- 11.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Kuck K.H., Brugada J., Furnkranz A., et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 14.Broglio K.R., Connor J.T., Berry S.M. Not too big, not too small: a goldilocks approach to sample size selection. J Biopharm Stat. 2014;24:685–705. doi: 10.1080/10543406.2014.888569. [DOI] [PubMed] [Google Scholar]
- 15.Bang H., Ni L., Davis C.E. Assessment of blinding in clinical trials. Control Clin Trials. 2004;25:143–156. doi: 10.1016/j.cct.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 16.James K.E., Bloch D.A., Lee K.K., Kraemer H.C., Fuller R.K. An index for assessing blindness in a multi-centre clinical trial: disulfiram for alcohol cessation--a VA cooperative study. Stat Med. 1996;15:1421–1434. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1421::AID-SIM266>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 17.Reinsch N., Futing A., Howel D., Bell J., Lin Y., Neven K. Cerebral safety after pulsed field ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2022 doi: 10.1016/j.hrthm.2022.06.018. [E-pub ahead of print Jun 17] [DOI] [PubMed] [Google Scholar]
- 18.Wazni O.M., Dandamudi G., Sood N., et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384:316–324. doi: 10.1056/NEJMoa2029554. [DOI] [PubMed] [Google Scholar]
- 19.Andrade J.G., Wells G.A., Deyell M.W., et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. doi: 10.1056/NEJMoa2029980. [DOI] [PubMed] [Google Scholar]
- 20.Turagam M.K., Musikantow D., Whang W., et al. Assessment of catheter ablation or antiarrhythmic drugs for first-line therapy of atrial fibrillation: a meta-analysis of randomized clinical trials. JAMA Cardiol. 2021;6:697–705. doi: 10.1001/jamacardio.2021.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchhof P., Camm A.J., Goette A., et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 22.Deneke T., Jais P., Scaglione M., et al. Silent cerebral events/lesions related to atrial fibrillation ablation: a clinical review. J Cardiovasc Electrophysiol. 2015;26:455–463. doi: 10.1111/jce.12608. [DOI] [PubMed] [Google Scholar]
- 23.van Es R., Groen M.H.A., Stehouwer M., Doevendans P.A., Wittkampf F.H.M., Neven K. In vitro analysis of the origin and characteristics of gaseous microemboli during catheter electroporation ablation. J Cardiovasc Electrophysiol. 2019;30:2071–2079. doi: 10.1111/jce.14091. [DOI] [PubMed] [Google Scholar]
- 24.Packer D.L., Mark D.B., Robb R.A., et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade J.G., Deyell M.W., Macle L., et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023;388:105–116. doi: 10.1056/NEJMoa2212540. [DOI] [PubMed] [Google Scholar]
- 26.Kuck K.H., Lebedev D.S., Mikhaylov E.N., et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST) Europace. 2021;23:362–369. doi: 10.1093/europace/euaa298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camm A.J., Naccarelli G.V., Mittal S., et al. The increasing role of rhythm control in patients with atrial fibrillation: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79:1932–1948. doi: 10.1016/j.jacc.2022.03.337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.