Abstract
Anxiety disorders are currently a major psychiatric and social problem, the mechanisms of which have been only partially elucidated. The hippocampus serves as a major target of stress mediators and is closely related to anxiety modulation. Yet so far, its complex anatomy has been a challenge for research on the mechanisms of anxiety regulation. Recent advances in imaging, virus tracking, and optogenetics/chemogenetics have permitted elucidation of the activity, connectivity, and function of specific cell types within the hippocampus and its connected brain regions, providing mechanistic insights into the elaborate organization of the hippocampal circuitry underlying anxiety. Studies of hippocampal neurotransmitter systems, including glutamatergic, GABAergic, cholinergic, dopaminergic, and serotonergic systems, have contributed to the interpretation of the underlying neural mechanisms of anxiety. Neuropeptides and neuroinflammatory factors are also involved in anxiety modulation. This review comprehensively summarizes the hippocampal mechanisms associated with anxiety modulation, based on molecular, cellular, and circuit properties, to provide tailored targets for future anxiety treatment.
Keywords: Anxiety, Hippocampus, Excitatory neurons, Interneurons, Neural circuit
Introduction
The hippocampus is a major target of stress mediators which lead to multiple psychiatric disorders, especially anxiety disorders. Several studies have shown that the hippocampus is structurally and functionally differentiated between the dorsal and ventral portions [1–4]. The ventral hippocampus (vHPC) is closely related to emotion, and its injury particularly impacts anxiety-related functions, whereas the dorsal hippocampus (dHPC) plays a preferred role in learning and spatial memory [1, 5, 6]. Despite many animal and clinical studies suggesting that aberrant hippocampal activity is associated with anxiety disorders, the underlying mechanism is far from being conclusive. Intrahippocampal infusion studies have suggested that different neurotransmitter systems and receptor sub-types are responsible for anxiety or memory functions in the hippocampus [7]. In addition, hippocampal excitatory and inhibitory neurons have been described as targets of anxiety modulation. Many studies using optogenetics and chemogenetics have shown that the connection of neural circuits in the hippocampus and related brain regions is the physiological basis of the regulation of anxiety behavior. Therefore, revealing the molecular, cellular, and neural circuit underpinnings of anxiety would provide answers regarding the functions of the hippocampus in modulating anxiety.
In this review, we summarize the molecular features and cell types of the hippocampal sub-regions, as well as the intra-hippocampus network and connections made by the hippocampus and extra-hippocampus regions, and discuss the recent evidence on the molecular, cellular, and neural circuit mechanisms of anxiety.
Anxiety Disorders
Anxiety arises from a highly alert state in the unambiguous presence of immediate threats and consists of trait and state components [8–10]. Trait anxiety is an individual predisposition that is considered a stable long-term feature in daily life [11–13]. In comparison, state anxiety refers to an acute and transitory response to external stimuli [11, 13], and is usually a context-dependent behavior. State anxiety has evolved as an adaptive avoidance of potential dangers, while extensive and prolonged trait anxiety responses are considered to be pathological phenomena [11]. Anxiety disorders, including generalized anxiety disorder, social phobia, specific phobias, separation anxiety disorder, and panic disorder with or without agoraphobia, are among the most prevalent psychiatric disorders and social problems [14, 15]. The etiology of anxiety disorders is an interaction of genetic vulnerability and psychosocial stress factors, such as adversity in childhood and adolescence, or stressful life events [14–16]. At present, antidepressants are the primary therapeutics for most anxiety disorders, and among these drugs, selective serotonin re-uptake inhibitors and selective serotonin-norepinephrine re-uptake inhibitors are the first-line anxiolytic treatments [17]. However, these agents are not effective in relieving anxiety symptoms for all patients; the harmful side effects and delayed action onset also limit their use. A thorough understanding of the mechanisms underlying anxiety regulation, at the molecular, cellular, and neural circuit levels, will provide a conceptual framework for improving anxiety treatment strategies.
The classic idea that the hippocampus is associated with learning and memory began with the study of Henry Molaison who exhibited severe memory deficits after bilateral medial temporal lobectomy to alleviate drug-resistant seizures [18]. In recent years, the role of the hippocampus in emotion regulation has received more attention, and exciting progress has been made in understanding the mechanism of the hippocampus in anxiety.
Hippocampus
The hippocampus is a complex structure located in the medial temporal lobe, which is implicated in extensive cognitive functions, including declarative memory [19] and spatial navigation [20], as well as emotional responses, such as emotional memory and adaptation [21, 22], and is a model system for structure and function research in animals. Many studies on the anatomy and circuitry of the hippocampus have been done in rodents and primates. Current studies indicate that the extensive role of the hippocampus in cognition and emotion stems from its position in the center of the cerebral cortex and its special internal anatomical structure [23, 24].
Anatomical Features
Anatomically, the rodent hippocampal formation is mainly subdivided into three distinct divisions: the dentate gyrus (DG), the hippocampus (cornu ammonis, CA3, CA2, and CA1), and the subiculum (SUB) [25] (Fig. 1A), which correspond to human hippocampus DG, CA4, CA3, CA2, CA1, and SUB; the human CA4 subregion contains cells within the hilus of the DG [26, 27]. Neuronal somata, dendrites, and axons in all subfields are distributed into specific layers: the stratum pyramidale (SP), alveus, stratum moleculare (SM), stratum oriens (SO), stratum radiatum (SR), and stratum lacunosum (SL) [28].
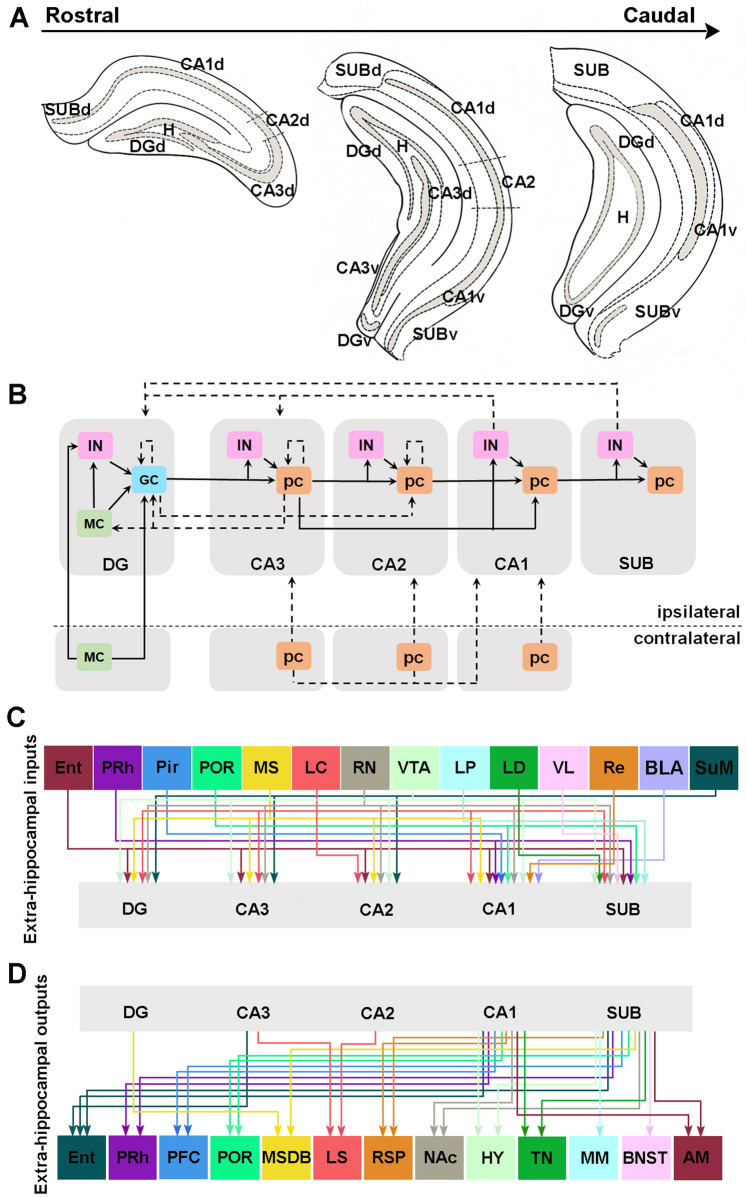
Fig. 1.
Anatomical features of the hippocampus. A Simplified diagram depicting the distribution of different subfields of the dorsal and ventral parts of the hippocampus from rostral to caudal. B The projected connections between different neuron types in subregions of the hippocampus, including ipsilateral and contralateral connections. Dashed arrows represent weak projections, solid arrows represent strong projections. C, D Hippocampal external input (C) and output (D) connectivity networks. DG, dentate gyrus; H, hilus; CA1-3, cornu ammonis 1-3; SUB, subiculum; d, dorsal; v, ventral; i, intermediate; GC, granule cell; MC, mossy cell; pc, pyramidal cell; IN, interneuron; EC, entorhinal cortex; PRh, perirhinal cortex; Pir, piriform cortex; POR, postrhinal cortex; MS, medial septum; LC, locus coeruleus; RN, raphe nuclei; VTA, ventral tegmental area; LP, lateral posterior thalamic nucleus; LD, laterodosal thalamic nucleus; VL, ventrolateral thalamic nucleus; Re, reuniens nucleus; SuM, supramammillary nucleus; BLA, basolateral amygdala; PFC, prefrontal cortex; DB, diagonal band; LS, lateral septum; RSP, retrosplenial cortex; NAc, nucleus accumbens; HY, hypothalamic nuclei; TN, thalamic nucleus; MM, mammillary bodies; BNST, bed nucleus of stria terminalis; AM, amygdala.
The DG subdivision is divided into five layers involving the outer SM (SMo), the middle SM (SMm), the inner SM (SMi), the stratum granulosum (SG), and the hilus. The principal glutamatergic neurons are granule cells (GCs) in the SG and mossy cells (MCs) in the hilus. The dendrites and axons of the GCs are located in the SO and hilus, respectively. The two types of glutamatergic neurons have distinct transcriptional characteristics. GCs have specific gene expression including Prox1, Stxbp6, Ctip2, Maml2, and Dock10 [29, 30], while MCs specifically express Calb2, Gria2/3, Cntn6, Drd2, Ociad2, and Nmb [31–33].
The hippocampus properly refers to three CA fields: CA3, CA2, and CA1. CA3 has a five-layered appearance which comprises the stratum lacunosum moleculare (SLM), SR, SL, SP, and SO. The laminar organization of CA1 and CA2 is similar to that of CA3 except for the absence of SL. The excitatory neurons within CA subdivisions are predominantly pyramidal neurons expressing the broad marker Sv2b [33–35]. The apical dendrites of pyramidal neurons are present in the SLM and SR, while their basal dendrites are ramified in the SO, and the axons enter the alveus.
In situ hybridization and next-generation sequencing have demonstrated that CA2 has unique molecular markers (Rgs14, Step, Pcp4, and Vcan) [33, 36–40], distinct from those of CA3 (Socs2, Cpne4, Tyro3, Lyd, and Coch) [33, 41] and those of CA1 (Satb2 and Wfs1) [33, 35].
The inside-to-outside anatomical structure of the SUB comprises three layers: the SM, SP, and polymorphic layer. The SP in the SUB is thicker than in CA3, CA2, and CA1; its principal cell type is pyramidal cells expressing the unique molecular marker Fn1 [29]. The SUB receives substantial projections from CA1 and other subfields of the hippocampus [42, 43], and outputs to multiple brain regions. Hence the SUB is the hub of information processing between the hippocampus and other brain regions through this different interconnectivity [44].
All hippocampal subregions have highly heterogeneous inhibitory interneurons, which can be categorized by molecular markers, electrophysiological properties, and morphological characteristics; they are further differentiated into at least eight defined GABAergic interneuron populations: positively stained for parvalbumin (PV+), neuropeptide Y (NPY+), somatostatin (SOM+), calbindin (CB+), calretinin (CR+), cholecystokinin (CCK+), vasoactive intestinal peptide (VIP+), and neuronal nitric oxide synthase (nNOS+) [28].
Hippocampal Intrinsic Circuitry
The anatomical connectivity of the hippocampus is described as a ‘tri-synaptic loop’. Neurons in the lateral and medial entorhinal cortex (LEC/MEC) provide primary cortical inputs to the dendrites of the GCs in the SMo and SMm of the DG via the perforant path, respectively [45]. GCs project to the SL apical dendrites of the CA3 pyramidal cells via the mossy fiber (MF) pathway [46]. CA3 pyramidal cells project to the proximal apical dendrites of the CA1 pyramidal cells in the SR via the Schaffer collateral (SC) pathway [47, 48]. Finally, CA1 projects to the SUB and back to the deep layers of the EC region, completing a processing loop. Neurons in the DG and CA3 also project to CA2 [49, 50]. In addition to the classical tri-synaptic circuit, the actual microcircuit connections within the hippocampus are quite complex (Fig. 1B).
Commissural systems connect ipsilateral and contralateral structures in the hippocampus. Contralaterally-projecting neurons mainly consist of glutamatergic cells in the dHPC; however, SOM+ and PV+ GABAergic interneurons also innervate the contralateral hippocampus, but with a lower density [51]. The hilar mossy cells project to the SMi of the contralateral DG [52, 53]. The CA3 pyramidal cells send commissural projections onto synapses in the SO of the contralateral CA3 [54]. CA1 pyramidal neurons receive contralateral CA3 or CA2 inputs in the SR, and contralateral CA1 inputs to the SO via commissural projections [40, 55].
In the ipsilateral hippocampus, the DG mainly receives excitatory inputs from MCs and CA3, and inhibitory inputs within the DG, plus a small number of long-distance inhibitory inputs from CA1 and the SUB [53]. In addition, GCs mainly interact indirectly with each other through interneurons or MCs, but there are still a few local direct projections between GCs [53]. Hilar MCs receive a large number of hippocampal inputs, mainly excitatory and inhibitory inputs from the DG and CA3 [56]. GC projections account for 57% of MC inputs. Excitatory inputs of pyramidal neurons from proximal CA3 (CA3c) and intermediate CA3 (CA3b) account for 4.9% and 1.5% of MC inputs [53]. Studies have shown that CA3c indirectly regulates DG GCs through their inputs to MCs [57]. Inhibitory inputs from the DG and CA3 non-pyramidal layers account for 3.7% and 2.5% of MCs presynaptic inputs, respectively [53]. CA3 pyramidal cells (CA3pcs) not only receive EC and DG excitatory inputs but also receives indirect inhibitory inputs from CA3 interneurons and extensive excitatory interconnection between CA3pcs [50, 58, 59]. Recently, retrovirus tracing has shown many direct projections of CA3 pyramidal neurons and inhibitory neurons in DG GCs, where they are distributed in CA3a, CA3b, and CA3c, the distal CA3a input being the strongest [53].
In CA2, the apical dendrites of pyramidal cells receive CA3 Schaffer collateral inputs in the SR [50], while the axons target the basal and apical dendrites of CA1pcs through SO and SR, respectively [60]. Studies have shown that both the basal and apical dendrites of CA2pcs receive projections from ipsilateral CA2 neurons [61] and that DG GCs send functional monosynaptic outputs to pyramidal cells of CA2 through longitudinal projections [37]. CA1 SO has a class of long-distance projection interneurons ("back projection cells"), whose axons project backward through the fissure to other hippocampal subfields, including CA3 and DG [62].
The Extra-hippocampal Connection Network
The hippocampal circuit is mainly modulated by extrinsic inputs, including various cortical areas, the medial septal region, the thalamus, the amygdaloid complex, the supramammillary nucleus, and monoaminergic brainstem nuclei (Fig. 1C).
The EC consists of two subregions with different cytoarchitectures and connectivity (LEC and MEC). The projection is strong to all subfields of the hippocampus, where the superficial cells (layer II) directly project to the DG and CA3, and the deeper cells (layer III) directly project to CA1 and the SUB [63]. There are differences in their direct projections to CA1, as the LEC projects to the region closer to the SUB, whereas the MEC innervates the region closer to CA3 [64]. In addition, hippocampal CA2 receives direct inputs from LEC and MEC [65, 66]. The perirhinal and postrhinal cortices project weakly and only to CA1 and the SUB. The perirhinal cortex preferentially projects to the ventral SUB, whereas the postrhinal cortex targets mainly the dorsal CA1 and SUB [67]. The piriform cortex also weakly contacts vCA1 [68].
The medial septal GABAergic neurons with low-rhythmic firing innervate the DG and CA3, and the connection between these neurons and CA1 regulates contextual memory retrieval [69, 70]. Meanwhile, the septal glutamatergic projections mainly contact interneurons in the alveus/oriens of CA1 [71]. The medial septal nucleus sends cholinergic outputs to CA2 and CA1 [72, 73]. Septo-hippocampal glutamatergic and GABAergic projections terminate predominantly on GABAergic neurons in the hippocampus [74, 75]. Conversely, the septal cholinergic projections primarily target the pyramidal neurons in the hippocampus [75].
The lateral posterior, laterodorsal, and ventrolateral thalamic nuclei send monosynaptic outputs to the SUB [76]. Moreover, the thalamic midline nucleus reuniens afferent inputs in the hippocampus modulate CA1 network activity via direct excitation and indirect inhibition [77]. Major inputs to the hippocampal formation also come from the amygdala. The basolateral amygdala (BLA) establishes monosynaptic and glutamatergic outputs to CA1 of the vHPC [78]. Under physiological conditions, the posterior BLA-vCA1 connection is more prominent than the anterior BLA-vCA1 connection [79].
The hippocampal formation also receives inputs from the hypothalamic supramammillary nucleus [80]. This supramammillary projection terminates in all hippocampal subfields via the fornix but is more prominent in the DG, CA2, and CA3. The hippocampus also receives projections from brainstem nuclei, including a major noradrenergic input from the locus coeruleus [81, 82], dopaminergic projections from the ventral tegmental area [83, 84], and serotonergic innervation from the raphe nuclei [85, 86] to all subfields of the hippocampus. A study suggests that the locus coeruleus also releases dopamine in the dorsal hippocampus [87].
In addition to the inputs from many regions, the hippocampus forms network connections through outputs to other regions outside the hippocampus, which affects overall brain network activity (Fig 1D). The DG, CA3, and CA2 subregions contribute relatively little to extra-hippocampal outputs; the DG provides GABAergic outputs to the medial septum [88], while CA3 and CA2 mainly project to the dorsolateral septum [89, 90]. Agster and colleagues first found a direct projection from ventral CA3 to the entorhinal cortex [67]. In contrast, the CA1 and SUB regional projections constitute the main network of extra-hippocampal connections. The SUB and CA1 project to the postrhinal, perirhinal, entorhinal, prefrontal, and retrosplenial cortices, as well as to the nucleus accumbens, amygdala, hypothalamic nuclei, and various midline thalamic nuclei [67, 91–95]. The SUB also projects to the septum and diagonal band [89], the bed nucleus of the stria terminalis [96], mammillary bodies, and anterior thalamic nuclei along with the postsubiculum [97].
The Hippocampus in the Pathophysiology of Anxiety
Studies have suggested that the hippocampus is closely involved in cognitive learning and the pathogenesis of mood and anxiety disorders, which may be mediated by the functional heterogeneity of its dorsoventral axis, with the dHPC mediating cognitive learning and the vHPC contributing to emotional regulation, especially in regulating anxiety [5, 98–102]. Moreover, some studies have also shown that dorsal hippocampal neurons are involved in anxiety regulation [103–105]. Here, we review the identified cell types, molecules, and projections in the hippocampus, especially the vHPC, that modulate anxiety-related behaviors.
Cellular Mechanisms of the Hippocampus During Anxiety
The hippocampus is extremely responsive to chronic stress and anxiety-inducing stimuli. In almost all hippocampal subregions, excitatory and inhibitory neurons provide a cellular substrate for anxiety modulation. Chronic stress causes changes in synaptic plasticity, including dendritic shortening and the debranching and spine loss of excitatory neurons, which further impacts excitability in the MF-CA3 and CA3-CA1 synapses. Chronic stress also causes a reduction in dendritic branches and the number of interneurons, thus affecting the excitation-inhibition balance in the hippocampus.
DG Granule Cells in Anxiety
In adult rodents and humans, the subgranular zone (SGZ) of the DG continuously generates new GCs [106–109]. Recently, several findings have demonstrated the important role of adult-born and mature GCs in influencing stress response and anxiety regulation. Increased neurogenesis in the DG reduces corticosteroid-induced anxiety-like behaviors [110], whereas inhibition of hippocampal neurogenesis increases anxiety-related behaviors [111]. Several important studies have proposed that adult-born GCs in the vDG, but not in the dDG, are key factors in mediating stress-induced anxiety-related behaviors. In mice, increasing neurogenesis in the vDG prevents the social defeat of stress-induced anxiety-like behaviors by inhibiting the activity of mature GCs [112]. Conversely, chemogenetic silencing of adult-born GCs induces an increased activity in mature GCs, leading to avoidance behavior in the social interaction test and a decrease in center exploration in the open field test [112]. However, another study from the same group found that optogenetic activation of mature vDG GCs contributes to the anxiolytic effects in mice [113]. In addition, chemogenetic activation of tactile experience-induced vDG GCs relieves anxiety [100]. Recently, with the application of single-cell sequencing and transcriptomics, heterogeneous cell populations with different molecular or functional characteristics have been found in different subregions of the hippocampus. Therefore, the above contradictory conclusions may be due to the different functions of GC subsets. In addition, a recent study indicates that activating osteocalcin-positive dDG GCs decreases anxiety-like behaviors by promoting adult DG neurogenesis [103].
GABAergic Interneurons in Anxiety
Besides the principal excitatory neurons, the hippocampus is also under the inhibitory control of diverse GABAergic interneurons, which are susceptible to chronic stress and play important roles in modulating anxiety-related behaviors. Disturbances in GABAergic neurotransmission are considered to be the pathological basis of anxiety disorders, as studies in patients and animal models suggest that an imbalance of excitatory and inhibitory neurotransmitters is the primary cause of anxiety disorders (Table 1).
Table 1.
Effects of chronic stress on the number and expression of marker protein in hippocampal interneurons.
| Chronic stress response | References | |
|---|---|---|
| PV-Parvalbumin | ||
| Reduced in vCA1 - Chronic mild stress | [115] | |
| Number | Loss of PV+ interneurons in vHPC results in | [121] |
| anxiety-like behavior in 5xFAD mice | ||
| Activity | Activation of PV+ interneurons in vDG produced | [120] |
| significant anxiolytic-like effects | ||
| Reduced intensity of PV in the vHPC - maternal | [123] | |
| separation with early weaning | ||
| Expression | Reduced in dHPC - Chronic mild stress | [119] |
| CR-Calretinin/CB-Calbindin | ||
| Number | Reduced in vCA1- Chronic mild stress | [115] |
| Increased in dCA1- Maternal separation | [125] | |
| Expression | Increased in CR and CB expression in vDG | [131] |
| - Chronic social defeat | ||
| NPY-Neuropeptide Y | ||
| Number | Reduced in vCA1-3 - Chronic mild stress | [115] |
| Expression | Reduced in dCA1, dCA3 - Chronic restrain stress | [128] |
| Reduced in dDG - Chronic mild stress | [127] | |
| SOM - Somatostatin | ||
| Number | Reduced in vDG and vCA1 - Chronic mild stress | [115] |
| Expression | Reduced in all vHPC - Chronic mild stress | [119] |
| CCK - Cholecystokinin | ||
| Number | No effects on vHPC - Chronic mild stress | [115] |
| No effects on vHPC - Chronic restrain stress | [122] | |
| Expression | Reduced in dCA1-3 - Chronic restrain stress | [133] |
dDG, dorsal dentate gyrus; dCA1-3, dorsal cornu ammonis 1-3; dHPC, dorsal hippocampus; vCA1, ventral CA1; vDG, ventral dentate gyrus; dCA3, dorsal CA3; vHPC, ventral hippocampus.
PV+ interneurons are particularly susceptible to chronic stress [114]. Studies indicate that exposure to multiple chronic stress significantly reduces the number of PV+ interneurons in vCA1 [115–118], and a decreased expression of PV has been reported in the dorsal hippocampus [119]. Moreover, activation of PV+ interneurons in the vDG produces significant anxiolytic-like effects [120], and loss of SST+ and PV+ interneurons in the vHPC leads to anxiety-like behavior in 5xFAD mice [121]. These changes may also relate to functional deficits like failure to generate the rhythmic spontaneous inhibitory postsynaptic currents recorded after chronic stress [122] and disruption of the balance between excitation and inhibition. Recently, a study has suggested that maternal separation with early weaning reduces the level of parvalbumin (PV) and increases the density of perineuronal nets around PV+ interneurons in the vHPC, as well as increasing theta power and enhancing theta-gamma coupling in the vHPC [123]. Contradictory results suggest that no changes in the number or expression of PV occur after chronic unpredictable stress or maternal separation [124, 125], and may be due to different types of stressors or stress times.
NPY+ interneurons, as endogenous ‘stress-resistant molecules’, have marked anxiolytic and anti-epilepsy effects [120, 126]. Evidence suggests that the number of NPY+ interneurons decreases in vCA1-3 of the hippocampus after chronic maternal stress (CMS) [115] and that CMS, as well as chronic restraint stress (CRS), reduce NPY expression in the dDG and dCA1-3 respectively [127, 128]. Early life stress events can lead to a reduction in the number of NPY+ interneurons in the hilus, which leads to overexcitation of the DG-CA3 circuit [129]. Anxiety-like behaviors induced by predator odor stress are due to reduced NPY release from CA1 NPY+ neurons [130]. The impairment of NPY release promotes glutamate release onto the CA1 pyramidal cells, thus increasing synaptic short-term facilitation [130].
CR+ interneurons are also reduced in vCA1 after CMS exposure [115], while chronic social defeat increases the CR and CB expression in the vDG [131]. CMS exposure significantly reduces the SOM+ interneurons in the vDG and vCA1 [115] and decreases the expression of SOM in the vHPC [119]. The hyperexcitability of SOM+ neurons leads to an enhancement of the inhibitory synaptic output onto the dendrites of CA1 pyramidal neurons and anxiolytic phenotypes [132]. In addition, although chronic stresses have no effect on the number of CCK+ interneurons [115, 122], CRS exposure reduces the CCK mRNA [133].
In addition, hippocampal vCA1 oriens-lacunosum moleculare (OLM) interneurons, which specifically express the nicotinic acetylcholine receptor α2 subunit, drive type 2 theta and are associated with increased risk-taking behavior in response to predator odor [98].
Chronic stress leads to changes in the number of inhibitory neurons or the expression of inhibitory neurotransmitters in the hippocampus, and affects the release of glutamate from excitatory neurons, supporting the role of excitation-inhibition imbalance in the pathology of anxiety disorders. Further research into the structural and functional connectivity between glutamatergic and GABAergic neurons in orchestrating hippocampal excitation-inhibition balance may provide a theoretical basis for anxiolytic therapy.
Stress-induced Hippocampal Plasticity in Anxiety
The precise pattern of dendrites is the major factor affecting the formation and function of neural circuits, and its morphology also affects the strength of synaptic connections [134]. Stress-induced changes in structural plasticity may lead to the expression of anxiety-related behaviors.
Many studies using magnetic resonance imaging have shown a lower hippocampal volume in patients suffering from anxiety disorders, and antidepressant treatments normalize it [135, 136]. Hippocampal volume loss has also been reported in individuals with adverse life events and chronic glucocorticoid treatment [137–139]. In rodent model studies, exposure to chronic stress and corticosterone also reduces hippocampal volume, which may be due to many cellular changes, including decreased glial number, inhibition of adult neurogenesis, and dendritic atrophy, particularly in the CA3 and CA1 pyramidal cells, as well as DG GCs [136]. Dendritic atrophy is considered to be a reversible loss of the total dendritic length, branching density of apical dendrites, and dendritic spines [140]. For example, it has been shown that CRS causes a significant reduction in branch points and length of the apical and basal dendrites in CA3 [141, 142]. Chronic immobilization stress (CIS) elicits an anxiety response accompanied by a pronounced shortening and debranching of the apical and basal dendrites of the CA3 pyramidal cells and the apical dendrites of CA1 pyramidal cells [143, 144].
Dendritic spines (small protrusions on dendrites) are crucial components in mediating the stress-induced structural plasticity of neurons. Both CRS and maternal separation (MS) stress significantly reduce the mature dendritic spine density of CA1 pyramidal cells [145, 146]. The neurotrophin brain-derived neurotrophic factor (BDNF) promotes the growth of dendrites and spines [147]. Both CIS and CRS decrease BDNF levels [147, 148], suggesting that BDNF is linked to the stress-induced alterations of dendrites and spines in the hippocampus [149].
Stress-induced abnormal long-term potentiation (LTP) in the MF-CA3 and SC-CA1 synapses is another cellular mechanism that may cause anxiety behaviors [150–152]. A study found that early deprivation induces anxiolytic behaviors by decreasing the threshold of LTP induction in the CA3-CA1 pathway [153]. In contrast, CRS increases anxiogenic behaviors due to the impairment of LTP in the SC-CA1 synapses [154]. These findings reveal that enhanced LTP in the SC synapses may be correlated with anxiolytic-like symptoms. Furthermore, another study showed that MS stress may induce anxiety-like behaviors through decreasing LTP and increasing paired-pulse facilitation ratios in the MF-CA3 pathway [152].
Hippocampus-related Molecular Mechanisms of Anxiety
Mounting evidence reveals that a diversity of molecules in the hippocampus are involved in anxiety modulation: channel proteins, receptors, neurotransmitters, neuropeptides, neuroinflammatory factors, and compounds associated with oxidative stress. These provide significant clues to hippocampus-related molecular mechanisms of anxiety (Table 2).
Table 2.
Summary of hippocampal molecules involved in anxiety modulation.
| Examples | Subfields | Anxiety-regulating functions | References |
|---|---|---|---|
| Channel protein | |||
| HCN1 | dCA1 | The anxiolytic effect of HCN1 knockdown. | [105] |
| HCN4 | dHPC | Anxiogenic effect of HCN4 knockdown. | [104] |
| Neurotransmitter systems | |||
| NMDAR | vHPC | NMDAR agonists increased anxiety. | [158] |
| NMDAR antagonists reduced anxiety. | |||
| GABAAR | vHPC | GABAAR agonists reduced anxiety. | [158] |
| GABAAR antagonists increased anxiety. | |||
| AChE | vHPC | AChE inhibitor alleviated anxiety. | [160] |
| HPC | knockdown of AChE increased anxiety | [161] | |
| D1/D2R | vHPC | The anxiolytic effect of D1/D2R antagonists. | [165] |
| vCA3 | Anxiogenic effect of D1/D2R antagonists. | [167] | |
| nNOS | HPC | Chronic stress increased the level and activity of hippocampal nNOS | [168] |
| Enhancement of nNOS-CAPON coupling is anxiogenic. | [168] | ||
| [169] | |||
| vHPC | Downregulating of nNOS is anxiolytic. | [171] | |
| AEA /CB1R | HPC | Enhancement of the ECBs signaling is anxiolytic. | [174] |
| [177] | |||
| vHPC | Reduced CB1R in GABAergic neurons and activated CB1R in glutamatergic neurons are anxiolytic | [178] | |
| Neuropeptide systems | |||
| CRH/CRHR1 | dHPC | Intervention with CRHR1 is anxiolytic. | [182] |
| vHPC | CRHR1 antagonist is anxiolytic. | [183] | |
| NPS | vHPC | The anxiolytic effect of NPS. | [184] |
| [185] | |||
| [186] | |||
| RXFP3 | vHPC | Anxiogenic effect of RXFP3 agonists. | [187] |
| Cytokine | |||
| IL-1β | DG | Increasing IL-1β is anxiogenic. | [189] |
| NLRP3 | vHPC | Deletion of NLRP3 is anxiogenic. | [192] [193] |
| vHPC | Activation of microglial NLRP3 in CMS. | [194] |
HCN, hyperpolarization-activated cyclic-nucleotide-gated channels; NMDAR, N-methyl-D-aspartate receptor; GABAAR, gamma amino butyric acid A receptor; AChE, acetylcholinesterase; D1/D2R, dopamine D1 and D2 receptor; 5-HT4R, 5-hydroxytryptamine 4 receptor; 5-HT1AR, 5-HT1A receptor; 5-HT2CR, 5-HT2C receptor; nNOS, neuronal nitric oxide synthase; CAPON, carboxy-terminal PDZ ligand; AEA, arachidonoyl ethanolamide or anandamide; CB1R, cannabinoid 1 receptor; ECB, endocannabinoid; CRH, corticotropin-releasing hormone; CRHR1, corticotropin-releasing hormone receptor 1; NPS, neuropeptide S; RXFP3, relaxin-family peptide 3 receptor; IL-1β, interleukin-1β; NLRP3, nucleotide binding and oligomerization domain-like receptor family pyrin domain-containing 3; HPC, hippocampus; dHPC, dorsal HPC; vHPC, ventral HPC; DG, dentate gyrus; dCA1, dorsal cornu ammonis 1; vCA3, ventral cornu ammonis 3.
The hyperpolarization-activated cyclic nucleotide-gated (HCN) channel family governs hippocampal neuronal excitability. Among these, HCN1 is the predominant isoform, while the HCN4 expression level is much lower in CA1 pyramidal neurons [104]. Although both isoforms have been shown to regulate hippocampus-related anxiety-like behaviors, the two proteins exert opposing functions. Knockdown of the HCN1 channel protein in the dCA1 enhances dCA1 activity, upregulates BDNF-mTOR signaling, and produces anxiolytic-like behaviors [105]. Conversely, the shRNA-mediated knockdown of HCN4 in the dHPC generates an anxiogenic effect [104]. A recent study found that HCN channel proteins participate in regulating synaptic transmission and glutamate release [155], suggesting that HCN deficiency might impair glutamatergic transmission.
The glutamatergic and GABAergic systems have crucial roles in the modulation of anxiety [156, 157]. Infusion of N-methyl-D-aspartate receptor (NMDAR) agonists and antagonists into the vHPC induces anxiogenic and anxiolytic responses, respectively [158]. In contrast, the direct injection of gamma amino butyric acid A receptor (GABAAR) agonists and antagonists into the vHPC have effects opposite to those of NMDAR agonists and antagonists [158]. Moreover, maintaining the excitation-inhibition balance might have a significant effect on regulating glutamate release from hippocampal pyramidal cells. The GABAAR antagonist blocks anxiolytic-like behaviors induced by the infusion of NMDAR antagonists into CA3 [159].
Hyperactivity of cholinergic systems is associated with the processes of hippocampus-modulated anxiety. One study showed that the administration of an acetylcholine esterase (AChE) inhibitor into the vHPC increases cholinergic activity and significantly alleviates anxiety responses [160]. In contrast, another study found that specific knockdown of AChE in the hippocampus promotes anxiety-like behaviors [161].
Dopamine (DA) is one of the neurotransmitters most active in anxiety responses, and its effects can be mediated by both D1 and D2 receptors expressed in the hippocampus [162, 163]. Several studies have revealed that the dopaminergic system modulates anxiety via its interaction with the glutamatergic or cholinergic system in the hippocampus. SCH23390, a DA D1 receptor antagonist, has synergistic anxiolytic effects with the NMDAR antagonist MK801 but is ineffective when injected into dCA1 alone [164]. However, the DA D2 receptor antagonist sulpiride suppresses the anxiolytic responses induced by MK801 in dCA1 [164]. Moreover, the D1 and D2 receptor antagonists alleviate the cholinergic anxiogenic effect of nicotine in the vHPC [165, 166]. Nicotine-induced DA release likely promotes anxiety responses, with DA antagonists reversing the anxiogenic effect by blocking D1 and D2 receptors. However, another study suggested that intra-hippocampal injection of SCH23390 or sulpiride into vCA3 blocks cholestasis-induced anxiolytic-like behaviors [167].
The neuronal messenger neuronal nitric oxide synthase (nNOS) is strongly expressed in the hippocampus and is involved in stress responses and anxiety-like behaviors. Chronic stress increases the expression and activity of nNOS in the hippocampus and contributes to stress-induced emotional behaviors [168]. Our previous studies have demonstrated that chronic stress enhances the coupling of nNOS with its carboxy-terminal PDZ ligand (CAPON) and induces anxious phenotypes [168, 169]. Blocking the nNOS-CAPON interaction ameliorates chronic stress-induced anxiety by promoting synaptogenesis [170]. Moreover, short-term running exercise exerts anxiolytic effects by increasing Nos1 DNA methylation and downregulating the expression of nNOS in the vHPC [171].
Endocannabinoids (ECBs), such as arachidonoyl ethanolamide (anandamide, AEA) and 2-arachidonoyl glycerol (2-AG), are relevant for anxiety regulation and stress responses. AEA and 2-AG levels in the dHPC are altered by diverse chronic stressors [172, 173]; elevation of AEA levels by inhibition of dHPC fatty-acid amide hydrolase and the ECB transporter attenuate anxiety responses [174]. Intra-vHPC injection of an AEA reuptake inhibitor has anxiogenic and anxiolytic effects in the elevated plus maze and the Vogel conflict tests, respectively; this discrepancy may be due to differences in the stress experienced by the subjects prior to the behavioral tests [175]. The hippocampus contains high levels of cannabinoid 1 (CB1) receptors, which are mainly expressed on the axon terminals of CCK+ interneurons, but at lower levels in glutamatergic, serotonergic, and cholinergic axon terminals. Evidence suggests that cannabinoid signaling in the hippocampus protects against stress-induced behavioral changes [176]. Injecting Δ9-tetrahydrocannabinol, a CB1 receptor agonist, into the vHPC increases the cAMP response element-binding protein (CREB) activation and attenuates anxiety behaviors [177]. Stimulation of the hippocampal CB1 receptors suppresses the release of neurotransmitters and increases BDNF expression [177]. A study has suggested that electroacupuncture exerts an anxiolytic effect via downregulating CB1Rs in GABAergic neurons and activating CB1Rs in glutamatergic neurons in the vHPC, thus reducing the release of glutamate and inhibiting the anxiety circuit related to the vHPC [178].
In addition to the diverse neurotransmitter systems, corticotropin-releasing hormone (CRH) acts as a neuropeptide that mediates anxiety and stress-related affective disorders in the hippocampus. Several studies have found that CRH exerts anxiety-regulating functions by affecting the expression or release of other neurotransmitters or neuropeptides. For instance, injecting CRH into the HPC suppresses the expression of Spexin, which plays an anxiolytic role [179]. CRH receptor type 1 (CRHR1), widely expressed in the hippocampus, has been demonstrated to be a potential drug target for anxiolytics in animals [180, 181]. Studies indicate that predator scent exposure stress increases the level of Crhr1 mRNA in the dorsal hippocampus, and inhibition of Crhr1 expression in the hippocampus significantly reverses the anxiety behaviors caused by chronic stress [182]. However, there is little research on whether CRHR1 in the vHPC is involved in the regulation of anxious behavior. Only one study has suggested that intra-vHPC injection of a CRHR1 antagonist increases the time spent in the open arms of an elevated plus maze test [183].
Neuropeptide S (NPS), acting via the NPS receptor (NPSR), has been shown to have anxiolytic effects. NPS significantly decreases anxiety-like behaviors when injected into the vHPC, possibly through its potential effects on short-term and long-term synaptic plasticity [184]. For instance, NPS reduces the magnitude of LTP and PPF at the vCA3-vCA1 synapses and induces anxiolytic response not only in normal mice but also in mice with high anxiety-related behavior [185, 186].
Activation of the relaxin family peptide 3 receptor (RXFP3) in the vHPC elicits anxiogenic phenotypes [187]. RXFP3 is mainly expressed in GABAergic SOM+ and PV+ neurons of all vHPC subregions, and its stimulation might inhibit interneuronal activity [188].
The hippocampus is vulnerable to neuroinflammation and oxidative stress, which are associated with anxiety disorders. Lipopolysaccharide (LPS) induces the downregulation of BDNF and the neuropeptide VGF, and of the neuroinflammatory and oxidative responses in the DG, which are attributable to the elevation of interleukin-1β (IL-1β) [189]. Inhibition of IL-1β activity ameliorates LPS-induced disorders in the DG and results in an anxiolytic effect. Recent studies indicate that IL-1β inhibits TrkB-mediated BDNF signaling and CREB, which regulate BDNF and VGF expression [190, 191]. Thus, BDNF and VGF downregulation appears to be the anxiogenic mechanisms of IL-1β. The NLRP3 (nucleotide binding and oligomerization domain-like receptor family pyrin domain-containing 3) inflammasome, is related to the onset and development of a variety of CNS diseases, including anxiety. Deletion of NLRP3 impairs synaptic transduction in vCA3-vCA1 and causes anxiety-like behaviors [192]. However, some studies have indicated that activation of NLRP3 inflammasome in the hippocampus induces anxiety-like behavior [193, 194].
Hippocampal Circuit Plasticity Underlies Anxiety
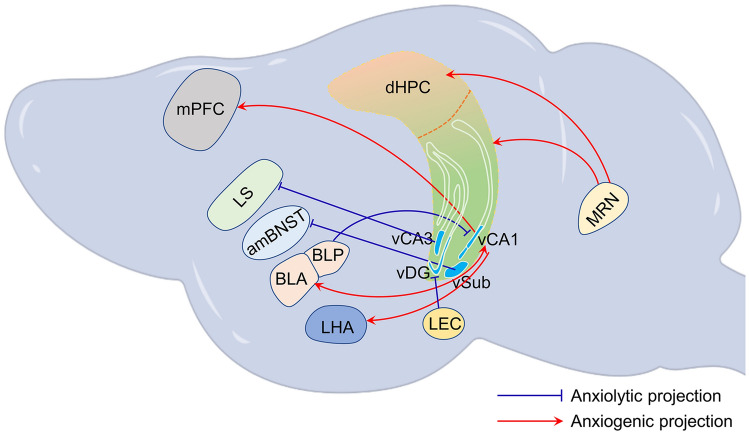
It is commonly recognized that the hippocampus and multiple brain regions coordinate to regulate anxiety. The vHPC is at the center of the complex neural circuits underlying anxiety regulation [99, 195, 196]. Recently, the application of optogenetics and chemogenetics have provided important insight into the anxiety circuits associated with the hippocampus. Hippocampal input and output nodes play anxiety-regulating roles together with connected upstream nuclei, including the EC, BLA, posterior basolateral amygdala (BLP), median raphe nucleus (MRN), and downstream nuclei, including the medial prefrontal cortex (mPFC), lateral septum (LS), anteromedial part of the bed nucleus of the stria terminalis (amBNST), lateral hypothalamic area (LHA), and amygdala (Fig. 2).
Fig. 2.
Upstream and downstream connection networks of the hippocampus that are implicated in anxiety-like behaviors. The sources of inputs into the hippocampus implicated in anxiety are the BLA, BLP, LEC, and MRN. The output regions of the hippocampus implicated in anxiety are the LHA, mPFC, BLA, amBNST, and LS. dHPC, dorsal hippocampus; vCA3, ventral CA3; vCA1, ventral CA1; vSub, ventral subiculum; amBNST, anteromedial part of the bed nucleus of the stria terminalis; BLA, basolateral amygdala; BLP, posterior basolateral amygdala; LHA, lateral hypothalamic area; LEC, lateral entorhinal cortex; LS, lateral septum; mPFC, medial prefrontal cortex; MRN, median raphe nucleus.
Hippocampal Afferent Circuits Underlie Anxiety
Entorhinal cortex-hippocampus circuits The EC (including the lateral and medial regions) is one of the major input sources to the hippocampus. However, there are relatively few reports on the relationship between EC and chronic stress or emotional disorders [100, 197, 198], especially anxiety disorders. One study suggested that tactile enrichment induces the activation of neurons in the vDG and increases the presynaptic input from the LEC. Chemogenetic activation of the projection reduces anxiety [100]. Therefore, its connection with the hippocampus in anxiety is not fully understood and needs further exploration.
Amygdala-hippocampus circuits The amygdala receives sensory stimuli associated with threats and plays a role in processing anxiety-associated events [199–201]. In addition, structural BLA and BLP connections to the vHPC have been confirmed [78, 79, 202, 203]. Robust connections and common functions between the amygdala and the vHPC facilitate the elucidation of the neural mechanism underlying anxiety at the circuit level. Felix-Ortiz et al. and Pi et al. showed that the BLA and BLP provide glutamatergic inputs to pyramidal cells in vCA1, revealing the structural and physiological heterogeneity between BLA-vCA1 and BLP-vCA1 inputs [78, 204].
CRS induces an increase in spine density and glutamatergic signaling in BLA-vHPC, leading to anxiety-like phenotypes in mice [205]. Transient optogenetic activation of the BLA terminals in vCA1 elicits significantly high anxiety behavior. In contrast, optogenetic inhibition of the BLA-vCA1 circuit attenuates the expression of anxiety-like behaviors [78].
By injecting anterograde monosynaptic viral tracers into BLP or BLA in a Calb1-IRES2-Cre-D: Ai9 transgenic mouse, Pi et al. found that the BLP and BLA innervate non-overlapping, lamina-specific vCA1 cell populations along the radial axis [204]. Specifically, BLP projects to calbindin1-positive vCA1 cells in the superficial pyramidal layer, and BLA projects to calbindin1-negative vCA1 cells in the deep pyramidal layer. Photoactivation of the BLP-vCA1 inputs elicits an anxiolytic effect, while photoinhibition drives an anxiogenic effect. By contrast, BLA-vCA1 inputs play an opposing role in modulating anxiety-like behaviors compared to the BLP-vCA1 circuit, which is consistent with the studies of Felix-Ortiz et al. [78, 204]. Calbindin1 expressed in superficial neurons may be a crucial molecule that contributes to the anxiolytic effect. To confirm the anxiolytic roles of calbindin1-positive vCA1 neurons in the BLP-vCA1 circuit, a Cre-responding ChR2 construct was expressed in the vCA1 of the Calb1-IRES2-Cre-D knock-in mouse and the photoactivation of vCA1Calb1+ somata induced an amelioration of anxiety [204].
Raphe nucleus - hippocampus circuits Serotonergic neurons originating in the raphe nuclei mainly project to the hippocampus, where almost all serotonin receptor subtypes are expressed [206, 207]. An in vivo microdialysis study found that anxiety-related aversive conditions increase extracellular serotonin within the vHPC [208]. Ohmura et al. found that blue light delivered to ChR2 variant-containing serotonergic terminals from the dorsal raphe nucleus and MRN in the vHPC induce serotonergic activation and generate anxiety-related behaviors [209]. Photostimulation of serotonin neurons in the MRN also induces anxiety-like behaviors [210]. However, serotonergic activation in the MRN is unable to induce anxiety-related behaviors in a 5-HT2C receptor knockout line. Thus, serotonergic MRN neurons play anxiogenic roles via the 5-HT2C receptors in the vHPC [209].
A recent finding showed that optogenetic manipulation of the serotonergic MRN neurons induces an anxiogenic-like effect in both male and female mice [210]. Another study suggested that the activation of serotonergic MRN neurons facilitates the anxiety response in female mice, partly through its input to the dHPC [211]. The serotonergic receptor underlying anxiety regulation in the dHPC might be the 5-HT1A receptor because the injection of its agonist into the dHPC drives the expression of anxiety behavior [212]. Therefore, the regulation of anxiety by serotonergic signaling in female and male mice may be mediated by different serotonergic receptor subtypes in the dHPC and vHPC.
Hippocampal Efferent Circuits Underlie Anxiety
Many studies have shown that glutamatergic projections from the vHPC to several downstream structures are involved in the regulation of anxiety.
vHPC-mPFC The direct single synaptic projection from vHPC to mPFC is a key component of anxiety-related circuits. Mounting evidence shows that the synchronization of the theta-frequency of the vHPC-mPFC circuit transmits anxiety-related information which is correlated with avoidance of the open arms in the elevated plus maze test [213–215]. vHPC-mPFC terminal inhibition using optogenetic techniques wipes out the theta-frequency synchronization, which is responsible for increased exploration in the open arms [216]. Inhibition of the theta-frequency communication of the vHPC-mPFC by abolishing the disinhibition functions of the mPFC results in open-arm exploration [217]. The anxiogenic role of the vHPC-mPFC circuit was also confirmed by the hM3Dq-mediated activation of the vCA1 neurons projecting to the mPFC, whereas hM4Di-mediated inactivation had the opposite effects [218].
vHPC-LS The glutamatergic vHPC fibers also largely innervate the LS, which is also implicated in anxiety [219, 220]. Disconnecting the ipsilateral input from the vHPC to the LS by an asymmetrical disconnection increases open-arm exploration by rats, suggesting that the vHPC and the LS synergistically regulate anxiety-like behaviors [221]. Moreover, the chemogenetic activation of projections from vHPC to LS suppresses the expression of anxiety-like behaviors, whereas their inhibition has opposite behavioral outcomes [218]. The LS receives dense glutamatergic fiber inputs from the vCA3 pyramidal layer, but few from the vCA1 pyramidal cells.
vHPC-amBNST The amBNST receives strong glutamatergic innervation from the ventral SUB/CA1. High-frequency vSUB/CA1 stimulation elicits NMDAR-mediated LTP in the amBNST, which promotes an anxiolytic effect [222]. Elaboration of the synaptic plasticity in the vSUB/CA1-amBNST circuit will advance our understanding of the role of the vHPC in anxiety regulation.
vHPC-LHA The LHA is implicated as an important brain region in the regulation of anxiety; it receives direct and exclusive outputs from pyramidal neurons in CA1 and the SUB of the vHPC. A recent study has found that a group of vCA1 pyramidal neurons send glutamatergic input into the LHA, and optogenetic activation of these neurons is anxiogenic [223].
vHPC-Amygdala vCA1 neurons make synaptic connections with the basal amygdala (BA) and lateral amygdala (LA) [224, 225]. Studies have shown that the theta frequency synchronization between vCA1 and LA is significantly enhanced in innate anxiety [224], while the synchronization between vCA1-BA and vCA1-mPFC conveys contextual information efficiently, contributing to contextual fear responses [225].
Conclusion
As a key region for information processing in the CNS, the hippocampus receives inputs from subcortical regions and sends outputs to a variety of downstream regions to mediate different behavioral and physiological functions, thus completing the adaptive regulation of the brain to the environment.
Anatomical and pharmacogenetic studies have shown extensive and close connections among the many subregions in the hippocampus and complex interconnections among its various neurons. These results suggest that the accurate regulation of microcircuitry in the hippocampus is an important basis for its physiological function. Most current studies on the hippocampus and anxiety regulation are limited to the connections between the subregions in the hippocampus and extra-hippocampal regions, as well as the regulation of the local inhibitory circuits in the subregions. However, the mechanism of neural networking between the subregions of the hippocampus in anxiety regulation remains unclear. The main challenges are as follows: (1) heterogeneity of the dorsal-ventral structure and gradient connections in the longitudinal axis; (2) complex anatomical connections between the ipsilateral and contralateral hippocampus; and (3) types and projection complexity of principal neurons and interneurons in the hippocampus. These problems make it difficult to dissect the neural networks in the hippocampus that regulate anxiety-related behavior. Recently, with the extensive application of single-cell transcriptomics and other new techniques, it has been shown that the hippocampus is a highly heterogeneous tissue with many cell subpopulations with different functional and connectivity characteristics. Therefore, future studies may focus on the synchronization of activities of hippocampal neural networks between different types of neurons in each subregion, especially the precise neural circuits mediated by different subsets of neurons in the vHPC, to understand the basis of neural circuits underlying different behaviors. This will also provide more accurate and effective targets for the development of new anxiolytic drugs.
In preclinical and clinical anxiety trials, the glutamatergic and GABAergic systems, the neuropeptide systems, and the endocannabinoid systems show positive prospects for future drug development. In the hippocampus of rodents, excitatory and inhibitory neurotransmitter systems, the cholinergic, dopaminergic, and serotonergic systems, have been established as critical components of anxiety-related behaviors. Therefore, these hippocampal molecules will also provide more reference points for the development of new and more effective therapeutic targets.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (31970951), the Six Talent Peaks Project of Jiangsu Province (YY-005), the Shanghai Rising-Star Program (21QA1407900), and “Zhong Ying Young Scholar” project of Cyrus Tang Foundation.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Hu-Jiang Shi and Shuang Wang contributed equally to this review.
References
- 1.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus—Memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- 4.Knierim JJ. The hippocampus. Curr Biol. 2015;25:R1116–R1121. doi: 10.1016/j.cub.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 5.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 6.Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engin E, Treit D. The role of hippocampus in anxiety: Intracerebral infusion studies. Behav Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- 8.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crocq MA. A history of anxiety: From Hippocrates to DSM. Dialogues Clin Neurosci. 2015;17:319–325. doi: 10.31887/DCNS.2015.17.3/macrocq. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endler NS, Kocovski NL. State and trait anxiety revisited. J Anxiety Disord. 2001;15:231–245. doi: 10.1016/S0887-6185(01)00060-3. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Ma C, Luo Y, Li J, Li Q, Liu Y, et al. Neural basis of uncertain cue processing in trait anxiety. Sci Rep. 2016;6:21298. doi: 10.1038/srep21298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goes TC, Almeida Souza TH, Marchioro M, Teixeira-Silva F. Excitotoxic lesion of the medial prefrontal cortex in Wistar rats: Effects on trait and state anxiety. Brain Res Bull. 2018;142:313–319. doi: 10.1016/j.brainresbull.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Thibaut F. Anxiety disorders: A review of current literature. Dialogues Clin Neurosci. 2017;19:87–88. doi: 10.31887/DCNS.2017.19.2/fthibaut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009;32:483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips AC, Carroll D, Der G. Negative life events and symptoms of depression and anxiety: Stress causation and/or stress generation. Anxiety Stress Coping. 2015;28:357–371. doi: 10.1080/10615806.2015.1005078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murrough JW, Yaqubi S, Sayed S, Charney DS. Emerging drugs for the treatment of anxiety. Expert Opin Emerg Drugs. 2015;20:393–406. doi: 10.1517/14728214.2015.1049996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. AMA Arch Neurol Psychiatry. 1958;79:475–497. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- 20.Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/S0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 21.Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Moscovitch M, Cabeza R, Winocur G, Nadel L. Episodic memory and beyond: The hippocampus and neocortex in transformation. Annu Rev Psychol. 2016;67:105–134. doi: 10.1146/annurev-psych-113011-143733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 25.Watson C, Paxinos G, Puelles L. The Mouse Nervous System. Amsterdam: Elsevier; 2012. pp. 783–795. [Google Scholar]
- 26.Schultz C, Engelhardt M. Anatomy of the hippocampal formation. Front Neurol Neurosci. 2014;34:6–17. doi: 10.1159/000360925. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Braak E, Yilmazer D, Bohl J. Functional anatomy of human hippocampal formation and related structures. J Child Neurol. 1996;11:265–275. doi: 10.1177/088307389601100402. [DOI] [PubMed] [Google Scholar]
- 28.Tribolet NAR, Crossman D. Neary, Neuroanatomy. An illustrated colour text. Acta Neurochir. 2009;2010:152. [Google Scholar]
- 29.Ayhan F, Kulkarni A, Berto S, Sivaprakasam K, Douglas C, Lega BC, et al. Resolving cellular and molecular diversity along the hippocampal anterior-to-posterior axis in humans. Neuron. 2021;109:2091–2105.e6. doi: 10.1016/j.neuron.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaeger BN, Linker SB, Parylak SL, Barron JJ, Gallina IS, Saavedra CD, et al. A novel environment-evoked transcriptional signature predicts reactivity in single dentate granule neurons. Nat Commun. 2018;9:3084. doi: 10.1038/s41467-018-05418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volz F, Bock HH, Gierthmuehlen M, Zentner J, Haas CA, Freiman TM. Stereologic estimation of hippocampal GluR2/3- and calretinin-immunoreactive hilar neurons (presumptive mossy cells) in two mouse models of temporal lobe epilepsy. Epilepsia. 2011;52:1579–1589. doi: 10.1111/j.1528-1167.2011.03086.x. [DOI] [PubMed] [Google Scholar]
- 32.Scharfman HE, Myers CE. Hilar mossy cells of the dentate gyrus: A historical perspective. Front Neural Circuits. 2013;6:106. doi: 10.3389/fncir.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cembrowski MS, Wang L, Sugino K, Shields BC, Spruston N. Hipposeq: A comprehensive RNA-seq database of gene expression in hippocampal principal neurons. eLife. 2016;5:e14997. doi: 10.7554/eLife.14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cembrowski MS, Wang L, Lemire AL, Copeland M, DiLisio SF, Clements J, et al. The subiculum is a patchwork of discrete subregions. eLife. 2018;7:e37701. doi: 10.7554/eLife.37701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lein ES, Zhao X, Gage FH. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J Neurosci. 2004;24:3879–3889. doi: 10.1523/JNEUROSCI.4710-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudek SM, Alexander GM, Farris S. Rediscovering area CA2: Unique properties and functions. Nat Rev Neurosci. 2016;17:89–102. doi: 10.1038/nrn.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, et al. Cell type–specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17:269–279. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SE, Simons SB, Heldt SA, Zhao M, Schroeder JP, Vellano CP, et al. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc Natl Acad Sci U S A. 2010;107:16994–16998. doi: 10.1073/pnas.1005362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lein ES, Callaway EM, Albright TD, Gage FH. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J Comp Neurol. 2005;485:1–10. doi: 10.1002/cne.20426. [DOI] [PubMed] [Google Scholar]
- 40.Shinohara Y, Hosoya A, Yahagi K, Ferecskó AS, Yaguchi K, Sík A, et al. Hippocampal CA3 and CA2 have distinct bilateral innervation patterns to CA1 in rodents. Eur J Neurosci. 2012;35:702–710. doi: 10.1111/j.1460-9568.2012.07993.x. [DOI] [PubMed] [Google Scholar]
- 41.Basrai HS, Turbic A, Christie KJ, Turnley AM. Suppressor of cytokine signalling 2 (SOCS2) regulates numbers of mature newborn adult hippocampal neurons and their dendritic spine maturation. Cell Mol Neurobiol. 2017;37:899–909. doi: 10.1007/s10571-016-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naber PA, LopesdaSilva FH, Witter MP. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- 43.Witter MP, Griffioen AW, Jorritsma-Byham B, Krijnen JL. Entorhinal projections to the hippocampal CA1 region in the rat: An underestimated pathway. Neurosci Lett. 1988;85:193–198. doi: 10.1016/0304-3940(88)90350-3. [DOI] [PubMed] [Google Scholar]
- 44.O'Mara SM, Commins S, Anderson M, Gigg J. The subiculum: A review of form, physiology and function. Prog Neurobiol. 2001;64:129–155. doi: 10.1016/S0301-0082(00)00054-X. [DOI] [PubMed] [Google Scholar]
- 45.van Groen T, Miettinen P, Kadish I. The entorhinal cortex of the mouse: Organization of the projection to the hippocampal formation. Hippocampus. 2003;13:133–149. doi: 10.1002/hipo.10037. [DOI] [PubMed] [Google Scholar]
- 46.Nakahara S, Matsumoto M, Ito H, Tajinda K. Ectopic mossy fiber pathfinding in the hippocampus caused the abnormal neuronal transmission in the mouse models of psychiatric disease. Biol Pharm Bull. 2018;41:138–141. doi: 10.1248/bpb.b17-00643. [DOI] [PubMed] [Google Scholar]
- 47.Speed HE, Dobrunz LE. Developmental changes in short-term facilitation are opposite at temporoammonic synapses compared to Schaffer collateral synapses onto CA1 pyramidal cells. Hippocampus. 2009;19:187–204. doi: 10.1002/hipo.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witter MP. Organization of the entorhinal-hippocampal system: A review of current anatomical data. Hippocampus 1993, 3 Spec No: 33–44. [PubMed]
- 49.Tóth K, Freund TF. Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: Their immunoreactivity for GABA and projection to the medial septum. Neuroscience. 1992;49:793–805. doi: 10.1016/0306-4522(92)90357-8. [DOI] [PubMed] [Google Scholar]
- 50.Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 51.Eyre MD, Bartos M. Somatostatin-expressing interneurons form axonal projections to the contralateral hippocampus. Front Neural Circuits. 2019;13:56. doi: 10.3389/fncir.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharfman HE. The enigmatic mossy cell of the dentate gyrus. Nat Rev Neurosci. 2016;17:562–575. doi: 10.1038/nrn.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Y, Grieco SF, Holmes TC, Xu X. Local and long-range circuit connections to hilar mossy cells in the dentate gyrus. eNeuro. 2017;4:ENEURO.0097–ENEURO.0017.2017. doi: 10.1523/ENEURO.0097-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Queiroz CMT, Mello LE. Synaptic plasticity of the CA3 commissural projection in epileptic rats: An in vivo electrophysiological study. Eur J Neurosci. 2007;25:3071–3079. doi: 10.1111/j.1460-9568.2007.05573.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhou H, Xiong GJ, Jing L, Song NN, Pu DL, Tang X, et al. The interhemispheric CA1 circuit governs rapid generalisation but not fear memory. Nat Commun. 2017;8:2190. doi: 10.1038/s41467-017-02315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson MB, Scharfman HE. Positive feedback from hilar mossy cells to granule cells in the dentate gyrus revealed by voltage-sensitive dye and microelectrode recording. J Neurophysiol. 1996;76:601–616. doi: 10.1152/jn.1996.76.1.601. [DOI] [PubMed] [Google Scholar]
- 57.Scharfman HE. The CA3 “backprojection” to the dentate gyrus. Prog Brain Res. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rolls ET. An attractor network in the hippocampus: Theory and neurophysiology. Learn Mem. 2007;14:714–731. doi: 10.1101/lm.631207. [DOI] [PubMed] [Google Scholar]
- 59.Lorente De Nó R. Studies on the structure of the cerebral cortex, II, Continuation of the study of the ammonic system. Journal für Psychologie und Neurologie. 1934;46:113–177. [Google Scholar]
- 60.Tamamaki N, Abe K, Nojyo Y. Three-dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique. Brain Res. 1988;452:255–272. doi: 10.1016/0006-8993(88)90030-3. [DOI] [PubMed] [Google Scholar]
- 61.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 62.Sik A, Ylinen A, Penttonen M, Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- 63.Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- 64.Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- 65.Dang R, Zhou Y, Zhang Y, Liu D, Wu M, Liu A, et al. Regulation of social memory by lateral entorhinal cortical projection to dorsal hippocampal CA2. Neurosci Bull. 2022;38:318–322. doi: 10.1007/s12264-021-00813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez-Rojas J, de Solis CA, Leroy F, Kandel ER, Siegelbaum SA. A direct lateral entorhinal cortex to hippocampal CA2 circuit conveys social information required for social memory. Neuron. 2022;110:1559–1572.e4. doi: 10.1016/j.neuron.2022.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agster KL, Burwell RD. Hippocampal and subicular efferents and afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Behav Brain Res. 2013;254:50–64. doi: 10.1016/j.bbr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tao S, Wang Y, Peng J, Zhao Y, He X, Yu X, et al. Whole-brain mapping the direct inputs of dorsal and ventral CA1 projection neurons. Front Neural Circuits. 2021;15:643230. doi: 10.3389/fncir.2021.643230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salib M, Joshi A, Katona L, Howarth M, Micklem BR, Somogyi P, et al. GABAergic medial septal neurons with low-rhythmic firing innervating the dentate gyrus and hippocampal area CA3. J Neurosci. 2019;39:4527–4549. doi: 10.1523/JNEUROSCI.3024-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sans-Dublanc A, Razzauti A, Desikan S, Pascual M, Monyer H, Sindreu C. Septal GABAergic inputs to CA1 govern contextual memory retrieval. Sci Adv. 2020;6:eaba5003. doi: 10.1126/sciadv.aba5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1998;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 72.Pimpinella D, Mastrorilli V, Giorgi C, Coemans S, Lecca S, Lalive AL, et al. Septal cholinergic input to CA2 hippocampal region controls social novelty discrimination via nicotinic receptor-mediated disinhibition. eLife. 2021;10:e65580. doi: 10.7554/eLife.65580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- 74.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 75.Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D, Choi J, et al. Cell-type-specific circuit connectivity of hippocampal CA1 revealed through cre-dependent rabies tracing. Cell Rep. 2014;7:269–280. doi: 10.1016/j.celrep.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bohne P, Schwarz MK, Herlitze S, Mark MD. A new projection from the deep cerebellar nuclei to the hippocampus via the ventrolateral and laterodorsal thalamus in mice. Front Neural Circuits. 2019;13:51. doi: 10.3389/fncir.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goswamee P, Leggett E, McQuiston AR. Nucleus reuniens afferents in hippocampus modulate CA1 network function via monosynaptic excitation and polysynaptic inhibition. Front Cell Neurosci. 2021;15:660897. doi: 10.3389/fncel.2021.660897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y, Wang ZH, Jin S, Gao D, Liu N, Chen SP, et al. Opposite monosynaptic scaling of BLP–vCA1 inputs governs hopefulness- and helplessness-modulated spatial learning and memory. Nat Commun. 2016;7:11935. doi: 10.1038/ncomms11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. J Comp Neurol. 1980;189:573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- 81.Oleskevich S, Descarries L, Lacaille JC. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci. 1989;9:3803–3815. doi: 10.1523/JNEUROSCI.09-11-03803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 1980;189:699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- 83.Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuro Psychopharmacol Biol Psychiatry. 1997;21:1–22. doi: 10.1016/S0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- 84.Swanson LW, Sawchenko PE, Cowan WM. Evidence that the commissural, associational and septal projections of the regio inferior of the hippocampus arise from the same neurons. Brain Res. 1980;197:207–212. doi: 10.1016/0006-8993(80)90446-1. [DOI] [PubMed] [Google Scholar]
- 85.Luchetti A, Bota A, Weitemier A, Mizuta K, Sato M, Islam T, et al. Two functionally distinct serotonergic projections into hippocampus. J Neurosci. 2020;40:4936–4944. doi: 10.1523/JNEUROSCI.2724-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aznar S, Qian ZX, Knudsen GM. Non-serotonergic dorsal and Median raphe projection onto parvalbumin- and calbindin-containing neurons in hippocampus and septum. Neuroscience. 2004;124:573–581. doi: 10.1016/j.neuroscience.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 87.Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci USA. 2016;113:14835–14840. doi: 10.1073/pnas.1616515114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan M, Meyer T, Benkowitz C, Savanthrapadian S, Ansel-Bollepalli L, Foggetti A, et al. Somatostatin-positive interneurons in the dentate gyrus of mice provide local- and long-range septal synaptic inhibition. eLife. 2017;6:e21105. doi: 10.7554/eLife.21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Besnard A, Gao Y, Kim MT, Twarkowski H, Reed AK, Langberg T, et al. Dorsolateral septum somatostatin interneurons gate mobility to calibrate context-specific behavioral fear responses. Nat Neurosci. 2019;22:436–446. doi: 10.1038/s41593-018-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leroy F, Park J, Asok A, Brann DH, Meira T, Boyle LM, et al. A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature. 2018;564:213–218. doi: 10.1038/s41586-018-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kloosterman F, Witter MP, Van Haeften T. Topographical and laminar organization of subicular projections to the parahippocampal region of the rat. J Comp Neurol. 2003;455:156–171. doi: 10.1002/cne.10472. [DOI] [PubMed] [Google Scholar]
- 93.Witter MP. Connections of the subiculum of the rat: Topography in relation to columnar and laminar organization. Behav Brain Res. 2006;174:251–264. doi: 10.1016/j.bbr.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 94.Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, et al. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 95.Fu JY, Yu XD, Zhu Y, Xie SZ, Tang MY, Yu B, et al. Whole-brain map of long-range monosynaptic inputs to different cell types in the amygdala of the mouse. Neurosci Bull. 2020;36:1381–1394. doi: 10.1007/s12264-020-00545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cole AB, Montgomery K, Bale TL, Thompson SM. What the hippocampus tells the HPA axis: Hippocampal output attenuates acute stress responses via disynaptic inhibition of CRF+ PVN neurons. Neurobiol Stress. 2022;20:100473. doi: 10.1016/j.ynstr.2022.100473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christiansen K, Dillingham CM, Wright NF, Saunders RC, Vann SD, Aggleton JP. Complementary subicular pathways to the anterior thalamic nuclei and mammillary bodies in the rat and macaque monkey brain. Eur J Neurosci. 2016;43:1044–1061. doi: 10.1111/ejn.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mikulovic S, Restrepo CE, Siwani S, Bauer P, Pupe S, Tort ABL, et al. Ventral hippocampal OLM cells control type 2 theta oscillations and response to predator odor. Nat Commun. 2018;9:3638. doi: 10.1038/s41467-018-05907-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xia F, Kheirbek MA. Circuit-based biomarkers for mood and anxiety disorders. Trends Neurosci. 2020;43:902–915. doi: 10.1016/j.tins.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang C, Liu H, Li K, Wu ZZ, Wu C, Yu JY, et al. Tactile modulation of memory and anxiety requires dentate granule cells along the dorsoventral axis. Nat Commun. 2020;11:6045. doi: 10.1038/s41467-020-19874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 102.Kemp GM, Altimimi HF, Nho Y, Heir R, Klyczek A, Stellwagen D. Sustained TNF signaling is required for the synaptic and anxiety-like behavioral response to acute stress. Mol Psychiatry. 2022;27:4474–4484. doi: 10.1038/s41380-022-01737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun D, Milibari L, Pan JX, Ren X, Yao LL, Zhao Y, et al. Critical roles of embryonic born dorsal dentate granule neurons for activity-dependent increases in BDNF, adult hippocampal neurogenesis, and antianxiety-like behaviors. Biol Psychiatry. 2021;89:600–614. doi: 10.1016/j.biopsych.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Günther A, Luczak V, Gruteser N, Abel T, Baumann A. HCN4 knockdown in dorsal hippocampus promotes anxiety-like behavior in mice. Genes Brain Behav. 2019;18:e12550. doi: 10.1111/gbb.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim CS, Chang PY, Johnston D. Enhancement of dorsal hippocampal activity by knockdown of HCN1 channels leads to anxiolytic- and antidepressant-like behaviors. Neuron. 2012;75:503–516. doi: 10.1016/j.neuron.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 107.Song J, Olsen RHJ, Sun J, Ming GL, Song H. Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harb Perspect Biol. 2016;8:a018937. doi: 10.1101/cshperspect.a018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 110.Hill AS, Sahay A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40:2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 112.Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, et al. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559:98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77:955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zaletel I, Filipović D, Puškaš N. Chronic stress, hippocampus and parvalbumin-positive interneurons: What do we know so far? Rev Neurosci. 2016;27:397–409. doi: 10.1515/revneuro-2015-0042. [DOI] [PubMed] [Google Scholar]
- 115.Czéh B, Varga ZK, Henningsen K, Kovács GL, Miseta A, Wiborg O. Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus. 2015;25:393–405. doi: 10.1002/hipo.22382. [DOI] [PubMed] [Google Scholar]
- 116.Filipović D, Zlatković J, Gass P, Inta D. The differential effects of acute vs. chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience. 2013;236:47–54. doi: 10.1016/j.neuroscience.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 117.Filipović D, Stanisavljević A, Jasnić N, Bernardi RE, Inta D, Perić I, et al. Chronic treatment with fluoxetine or clozapine of socially isolated rats prevents subsector-specific reduction of parvalbumin immunoreactive cells in the hippocampus. Neuroscience. 2018;371:384–394. doi: 10.1016/j.neuroscience.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 118.Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: Prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology. 2005;30:67–79. doi: 10.1038/sj.npp.1300581. [DOI] [PubMed] [Google Scholar]
- 119.Rossetti AC, Paladini MS, Colombo M, Gruca P, Lason-Tyburkiewicz M, Tota-Glowczyk K, et al. Chronic stress exposure reduces parvalbumin expression in the rat hippocampus through an imbalance of redox mechanisms: Restorative effect of the antipsychotic lurasidone. Int J Neuropsychopharmacol. 2018;21:883–893. doi: 10.1093/ijnp/pyy046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zou D, Chen L, Deng D, Jiang D, Dong F, McSweeney C, et al. DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr Mol Med. 2016;16:91–102. doi: 10.2174/1566524016666151222150024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li H, Zhao J, Lai L, Xia Y, Wan C, Wei S, et al. Loss of SST and PV positive interneurons in the ventral hippocampus results in anxiety-like behavior in 5xFAD mice. Neurobiol Aging. 2022;117:165–178. doi: 10.1016/j.neurobiolaging.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 122.Hu W, Zhang M, Czéh B, Flügge G, Zhang W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology. 2010;35:1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Murthy S, Kane GA, Katchur NJ, Lara Mejia PS, Obiofuma G, Buschman TJ, et al. Perineuronal nets, inhibitory interneurons, and anxiety-related ventral hippocampal neuronal oscillations are altered by early life adversity. Biol Psychiatry. 2019;85:1011–1020. doi: 10.1016/j.biopsych.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Banasr M, Lepack A, Fee C, Duric V, Maldonado-Aviles J, DiLeone R, et al. Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress (Thousand Oaks) 2017;1:2470547017720459. doi: 10.1177/2470547017720459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Giachino C, Canalia N, Capone F, Fasolo A, Alleva E, Riva MA, et al. Maternal deprivation and early handling affect density of calcium binding protein-containing neurons in selected brain regions and emotional behavior in periadolescent rats. Neuroscience. 2007;145:568–578. doi: 10.1016/j.neuroscience.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 126.Heilig M, Zachrisson O, Thorsell A, Ehnvall A, Mottagui-Tabar S, Sjögren M, et al. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: Preliminary evidence for association with preproNPY gene polymorphism. J Psychiatr Res. 2004;38:113–121. doi: 10.1016/S0022-3956(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 127.Sergeyev V, Fetissov S, Mathé AA, Jimenez PA, Bartfai T, Mortas P, et al. Neuropeptide expression in rats exposed to chronic mild stresses. Psychopharmacology. 2005;178:115–124. doi: 10.1007/s00213-004-2015-3. [DOI] [PubMed] [Google Scholar]
- 128.Sweerts BW, Jarrott B, Lawrence AJ. The effect of acute and chronic restraint on the central expression of prepro-neuropeptide Y mRNA in normotensive and hypertensive rats. J Neuroendocrinol. 2001;13:608–617. doi: 10.1046/j.1365-2826.2001.00674.x. [DOI] [PubMed] [Google Scholar]
- 129.Alviña K, Farshbaf MJ, Mondal AK. Long term effects of stress on hippocampal function: Emphasis on early life stress paradigms and potential involvement of neuropeptide Y. J Neurosci Res. 2021;99:57–66. doi: 10.1002/jnr.24614. [DOI] [PubMed] [Google Scholar]
- 130.Li Q, Bartley AF, Dobrunz LE. Endogenously released neuropeptide Y suppresses hippocampal short-term facilitation and is impaired by stress-induced anxiety. J Neurosci. 2017;37:23–37. doi: 10.1523/JNEUROSCI.2599-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.LaGamma CT, Tang WW, Morgan AA, McGowan JC, Brachman RA, Denny CA. Antidepressant but not prophylactic ketamine administration alters calretinin and calbindin expression in the ventral hippocampus. Front Mol Neurosci. 2018;11:404. doi: 10.3389/fnmol.2018.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry. 2017;22:920–930. doi: 10.1038/mp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giardino L, Bettelli C, Pozza M, Calzà L. Regulation of CCK mRNA expression in the rat brain by stress and treatment with sertraline, a selective serotonin re-uptake inhibitor. Brain Res. 1999;824:304–307. doi: 10.1016/S0006-8993(99)01242-1. [DOI] [PubMed] [Google Scholar]
- 134.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 135.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 136.Schoenfeld TJ, McCausland HC, Morris HD, Padmanaban V, Cameron HA. Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol Psychiatry. 2017;82:914–923. doi: 10.1016/j.biopsych.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van der Flier WM, Scheltens P. Hippocampal volume loss and Alzheimer disease progression. Nat Rev Neurol. 2009;5:361–362. doi: 10.1038/nrneurol.2009.94. [DOI] [PubMed] [Google Scholar]
- 139.Noorlander CW, Tijsseling D, Hessel EVS, de Vries WB, Derks JB, Visser GHA, et al. Antenatal glucocorticoid treatment affects hippocampal development in mice. PLoS One. 2014;9:e85671. doi: 10.1371/journal.pone.0085671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/S0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 141.McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Orlowski D, Elfving B, Müller HK, Wegener G, Bjarkam CR. Wistar rats subjected to chronic restraint stress display increased hippocampal spine density paralleled by increased expression levels of synaptic scaffolding proteins. Stress. 2012;15:514–523. doi: 10.3109/10253890.2011.643516. [DOI] [PubMed] [Google Scholar]
- 143.Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Christian KM, Miracle AD, Wellman CL, Nakazawa K. Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience. 2011;174:26–36. doi: 10.1016/j.neuroscience.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Papadakakis A, Sidiropoulou K, Panagis G. Music exposure attenuates anxiety- and depression-like behaviors and increases hippocampal spine density in male rats. Behav Brain Res. 2019;372:112023. doi: 10.1016/j.bbr.2019.112023. [DOI] [PubMed] [Google Scholar]
- 146.Magariños AM, Li CJ, Gal Toth J, Bath KG, Jing D, Lee FS, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 148.Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012;7:e30481. doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.von Bohlen und Halbach O, von Bohlen und Halbach V. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res. 2018;373:729–741. doi: 10.1007/s00441-017-2782-x. [DOI] [PubMed] [Google Scholar]
- 150.Kumar A. Long-term potentiation at CA3-CA1 hippocampal synapses with special emphasis on aging, disease, and stress. Front Aging Neurosci. 2011;3:7. doi: 10.3389/fnagi.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pan E, Puranam RS, McNamara JO. Long-term potentiation of mossy fiber feedforward inhibition of CA3 pyramidal cells maintains E/I balance in epilepsy model. Neuro. 2022;9:ENEURO.0375–ENEURO.0321.2021. doi: 10.1523/ENEURO.0375-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shin SY, Han SH, Woo RS, Jang SH, Min SS. Adolescent mice show anxiety- and aggressive-like behavior and the reduction of long-term potentiation in mossy fiber-CA3 synapses after neonatal maternal separation. Neuroscience. 2016;316:221–231. doi: 10.1016/j.neuroscience.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 153.Zhang X, Wang B, Jin J, An S, Zeng Q, Duan Y, et al. Early deprivation reduced anxiety and enhanced memory in adult male rats. Brain Res Bull. 2014;108:44–50. doi: 10.1016/j.brainresbull.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 154.Bhagya VR, Srikumar BN, Veena J, Shankaranarayana Rao BS. Short-term exposure to enriched environment rescues chronic stress-induced impaired hippocampal synaptic plasticity, anxiety, and memory deficits. J Neurosci Res. 2017;95:1602–1610. doi: 10.1002/jnr.23992. [DOI] [PubMed] [Google Scholar]
- 155.Neitz A, Mergia E, Imbrosci B, Petrasch-Parwez E, Eysel UT, Koesling D, et al. Postsynaptic NO/cGMP increases NMDA receptor currents via hyperpolarization-activated cyclic nucleotide-gated channels in the hippocampus. Cereb Cortex. 2014;24:1923–1936. doi: 10.1093/cercor/bht048. [DOI] [PubMed] [Google Scholar]
- 156.Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS Spectr. 2005;10:820–830. doi: 10.1017/S1092852900010427. [DOI] [PubMed] [Google Scholar]
- 157.Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiatry. 2003;64:21–27. [PubMed] [Google Scholar]
- 158.Rezvanfard M, Zarrindast MR, Bina P. Role of ventral hippocampal GABA(A) and NMDA receptors in the anxiolytic effect of carbamazepine in rats using the elevated plus maze test. Pharmacology. 2009;84:356–366. doi: 10.1159/000256666. [DOI] [PubMed] [Google Scholar]
- 159.Zarrabian S, Farahizadeh M, Nasehi M, Zarrindast MR. The role of CA3 GABAA receptors on anxiolytic-like behaviors and avoidance memory deficit induced by NMDA receptor antagonists. J Psychopharmacol. 2016;30:215–223. doi: 10.1177/0269881115622239. [DOI] [PubMed] [Google Scholar]
- 160.Degroot A, Treit D. Dorsal and ventral hippocampal cholinergic systems modulate anxiety in the plus-maze and shock-probe tests. Brain Res. 2002;949:60–70. doi: 10.1016/S0006-8993(02)02965-7. [DOI] [PubMed] [Google Scholar]
- 161.Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci USA. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Köhler C, Ericson H, Radesäter AC. Different laminar distributions of dopamine D1 and D2 receptors in the rat hippocampal region. Neurosci Lett. 1991;126:107–109. doi: 10.1016/0304-3940(91)90530-7. [DOI] [PubMed] [Google Scholar]
- 163.Hasbi A, Nguyen T, Rahal H, Manduca JD, Miksys S, Tyndale RF, et al. Sex difference in dopamine D1–D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol Sex Differ. 2020;11:8. doi: 10.1186/s13293-020-00285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zarrindast MR, Nasehi M, Pournaghshband M, Yekta BG. Dopaminergic system in CA1 modulates MK-801 induced anxiolytic-like responses. Pharmacol Biochem Behav. 2012;103:102–110. doi: 10.1016/j.pbb.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 165.Zarrindast MR, Naghdi-Sedeh N, Nasehi M, Sahraei H, Bahrami F, Asadi F. The effects of dopaminergic drugs in the ventral hippocampus of rats in the nicotine-induced anxiogenic-like response. Neurosci Lett. 2010;475:156–160. doi: 10.1016/j.neulet.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 166.Nasehi M, Mafi F, Oryan S, Nasri S, Zarrindast MR. The effects of dopaminergic drugs in the dorsal hippocampus of mice in the nicotine-induced anxiogenic-like response. Pharmacol Biochem Behav. 2011;98:468–473. doi: 10.1016/j.pbb.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 167.Nasehi M, Kafi F, Zarrindast MR. Differential mechanisms of opioidergic and dopaminergic systems of the ventral hippocampus (CA3) in anxiolytic-like behaviors induced by cholestasis in mice. Eur J Pharmacol. 2013;714:352–358. doi: 10.1016/j.ejphar.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 168.Zhou QG, Zhu LJ, Chen C, Wu HY, Luo CX, Chang L, et al. Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J Neurosci. 2011;31:7579–7590. doi: 10.1523/JNEUROSCI.0004-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu LJ, Li TY, Luo CX, Jiang N, Chang L, Lin YH, et al. CAPON-nNOS coupling can serve as a target for developing new anxiolytics. Nat Med. 2014;20:1050–1054. doi: 10.1038/nm.3644. [DOI] [PubMed] [Google Scholar]
- 170.Zhu LJ, Shi HJ, Chang L, Zhang CC, Si M, Li N, et al. nNOS-CAPON blockers produce anxiolytic effects by promoting synaptogenesis in chronic stress-induced animal models of anxiety. Br J Pharmacol. 2020;177:3674–3690. doi: 10.1111/bph.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tomiga Y, Sakai K, Ra SG, Kusano M, Ito A, Uehara Y, et al. Short-term running exercise alters DNA methylation patterns in neuronal nitric oxide synthase and brain-derived neurotrophic factor genes in the mouse hippocampus and reduces anxiety-like behaviors. FASEB J. 2021;35:e21767. doi: 10.1096/fj.202100630R. [DOI] [PubMed] [Google Scholar]
- 172.Dubreucq S, Matias I, Cardinal P, Häring M, Lutz B, Marsicano G, et al. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology. 2012;37:1885–1900. doi: 10.1038/npp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ, et al. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology. 2014;39:1763–1776. doi: 10.1038/npp.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lisboa SF, Borges AA, Nejo P, Fassini A, Guimarães FS, Resstel LB. Cannabinoid CB1 receptors in the dorsal hippocampus and prelimbic medial prefrontal cortex modulate anxiety-like behavior in rats: Additional evidence. Prog Neuro Psychopharmacol Biol Psychiatry. 2015;59:76–83. doi: 10.1016/j.pnpbp.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 175.Campos AC, Ferreira FR, Guimarães FS, Lemos JI. Facilitation of endocannabinoid effects in the ventral hippocampus modulates anxiety-like behaviors depending on previous stress experience. Neuroscience. 2010;167:238–246. doi: 10.1016/j.neuroscience.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 176.Scarante FF, Vila-Verde C, Detoni VL, Ferreira-Junior NC, Guimarães FS, Campos AC. Cannabinoid modulation of the stressed hippocampus. Front Mol Neurosci. 2017;10:411. doi: 10.3389/fnmol.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, et al. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology. 2008;54:151–160. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 178.Hu XF, Zhang H, Yu LL, Ge WQ, Zhan-Mu OY, Li YZ, et al. Electroacupuncture reduces anxiety associated with inflammatory bowel disease by acting on cannabinoid CB1 receptors in the ventral hippocampus in mice. Front Pharmacol. 2022;13:919553. doi: 10.3389/fphar.2022.919553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhuang M, Lai Q, Yang C, Ma Y, Fan B, Bian Z, et al. Spexin as an anxiety regulator in mouse hippocampus: Mechanisms for transcriptional regulation of spexin gene expression by corticotropin releasing factor. Biochem Biophys Res Commun. 2020;525:326–333. doi: 10.1016/j.bbrc.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 180.Philbert J, Pichat P, Palme R, Belzung C, Griebel G. The CRF1 receptor antagonist SSR125543 attenuates long-term cognitive deficit induced by acute inescapable stress in mice, independently from the hypothalamic pituitary adrenal axis. Pharmacol Biochem Behav. 2012;102:415–422. doi: 10.1016/j.pbb.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 181.Chen Y, Brunson KL, Müller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: Light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(SICI)1096-9861(20000508)420:3<305::AID-CNE3>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kozlovsky N, Zohar J, Kaplan Z, Cohen H. Microinfusion of a corticotrophin-releasing hormone receptor 1 antisense oligodeoxynucleotide into the dorsal hippocampus attenuates stress responses at specific times after stress exposure. J Neuroendocrinol. 2012;24:489–503. doi: 10.1111/j.1365-2826.2011.02267.x. [DOI] [PubMed] [Google Scholar]
- 183.Bertagna NB, dos Santos PGC, Queiroz RM, Fernandes GJD, Cruz FC, Miguel TT. Involvement of the ventral, but not dorsal, hippocampus in anxiety-like behaviors in mice exposed to the elevated plus maze: Participation of CRF1 receptor and PKA pathway. Pharmacol Rep. 2021;73:57–72. doi: 10.1007/s43440-020-00182-3. [DOI] [PubMed] [Google Scholar]
- 184.Dine J, Ionescu IA, Stepan J, Yen YC, Holsboer F, Landgraf R, et al. Identification of a role for the ventral hippocampus in neuropeptide S-elicited anxiolysis. PLoS One. 2013;8:e60219. doi: 10.1371/journal.pone.0060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ionescu IA, Dine J, Yen YC, Buell DR, Herrmann L, Holsboer F, et al. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology. 2012;37:1323–1337. doi: 10.1038/npp.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dine J, Ionescu IA, Avrabos C, Yen YC, Holsboer F, Landgraf R, et al. Intranasally applied neuropeptide S shifts a high-anxiety electrophysiological endophenotype in the ventral hippocampus towards a normal-anxiety one. PLoS One. 2015;10:e0120272. doi: 10.1371/journal.pone.0120272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rytova V, Ganella DE, Hawkes D, Bathgate RAD, Ma S, Gundlach AL. Chronic activation of the relaxin-3 receptor on GABA neurons in rat ventral hippocampus promotes anxiety and social avoidance. Hippocampus. 2019;29:905–920. doi: 10.1002/hipo.23089. [DOI] [PubMed] [Google Scholar]
- 188.Engin E, Stellbrink J, Treit D, Dickson CT. Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: Behavioral and neurophysiological evidence. Neuroscience. 2008;157:666–676. doi: 10.1016/j.neuroscience.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 189.Li M, Li C, Yu H, Cai X, Shen X, Sun X, et al. Lentivirus-mediated interleukin-1β (IL-1β) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J Neuroinflammation. 2017;14:190. doi: 10.1186/s12974-017-0964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rodríguez-Seoane C, Ramos A, Korth C, Requena JR. DISC1 regulates expression of the neurotrophin VGF through the PI3K/AKT/CREB pathway. J Neurochem. 2015;135:598–605. doi: 10.1111/jnc.13258. [DOI] [PubMed] [Google Scholar]
- 191.Guo J, Lin P, Zhao X, Zhang J, Wei X, Wang Q, et al. Etazolate abrogates the lipopolysaccharide (LPS)-induced downregulation of the cAMP/pCREB/BDNF signaling, neuroinflammatory response and depressive-like behavior in mice. Neuroscience. 2014;263:1–14. doi: 10.1016/j.neuroscience.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 192.Komleva YK, Lopatina OL, Gorina IV, Shuvaev AN, Chernykh A, Potapenko IV, et al. NLRP3 deficiency-induced hippocampal dysfunction and anxiety-like behavior in mice. Brain Res. 2021;1752:147220. doi: 10.1016/j.brainres.2020.147220. [DOI] [PubMed] [Google Scholar]
- 193.Xu Y, Sheng H, Bao Q, Wang Y, Lu J, Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun. 2016;56:175–186. doi: 10.1016/j.bbi.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 194.Wang YL, Han QQ, Gong WQ, Pan DH, Wang LZ, Hu W, et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation. 2018;15:21. doi: 10.1186/s12974-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wheeler DW, White CM, Rees CL, Komendantov AO, Hamilton DJ, Ascoli GA. Hippocampome.org: A knowledge base of neuron types in the rodent hippocampus. Life. 2015;4:e09960. doi: 10.7554/eLife.09960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Avital A, Ram E, Maayan R, Weizman A, Richter-Levin G. Effects of early-life stress on behavior and neurosteroid levels in the rat hypothalamus and entorhinal cortex. Brain Res Bull. 2006;68:419–424. doi: 10.1016/j.brainresbull.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 198.Lu J, Zhang Z, Yin X, Tang Y, Ji R, Chen H, et al. An entorhinal-visual cortical circuit regulates depression-like behaviors. Mol Psychiatry. 2022;27:3807–3820. doi: 10.1038/s41380-022-01540-8. [DOI] [PubMed] [Google Scholar]
- 199.Davidson RJ. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/S0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 200.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Novaes LS, Dos Santos NB, Batalhote RFP, Malta MB, Camarini R, Scavone C, et al. Environmental enrichment protects against stress-induced anxiety: Role of glucocorticoid receptor, ERK, and CREB signaling in the basolateral amygdala. Neuropharmacology. 2017;113:457–466. doi: 10.1016/j.neuropharm.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 202.Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. doi: 10.1002/(SICI)1096-9861(19990111)403:2<229::AID-CNE7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 203.Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 204.Pi G, Gao D, Wu D, Wang Y, Lei H, Zeng W, et al. Posterior basolateral amygdala to ventral hippocampal CA1 drives approach behaviour to exert an anxiolytic effect. Nat Commun. 2020;11:183. doi: 10.1038/s41467-019-13919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang JY, Liu TH, He Y, Pan HQ, Zhang WH, Yin XP, et al. Chronic stress remodels synapses in an amygdala circuit-specific manner. Biol Psychiatry. 2019;85:189–201. doi: 10.1016/j.biopsych.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 207.Berumen LC, Rodríguez A, Miledi R, García-Alcocer G. Serotonin receptors in hippocampus. Sci World J. 2012;2012:823493. doi: 10.1100/2012/823493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rex A, Voigt JP, Fink H. Anxiety but not arousal increases 5-hydroxytryptamine release in the rat ventral hippocampus in vivo. Eur J Neurosci. 2005;22:1185–1189. doi: 10.1111/j.1460-9568.2005.04251.x. [DOI] [PubMed] [Google Scholar]
- 209.Ohmura Y, Tsutsui-Kimura I, Sasamori H, Nebuka M, Nishitani N, Tanaka KF, et al. Different roles of distinct serotonergic pathways in anxiety-like behavior, antidepressant-like, and anti-impulsive effects. Neuropharmacology. 2020;167:107703. doi: 10.1016/j.neuropharm.2019.107703. [DOI] [PubMed] [Google Scholar]
- 210.Ohmura Y, Tanaka KF, Tsunematsu T, Yamanaka A, Yoshioka M. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int J Neuropsychopharmacol. 2014;17:1777–1783. doi: 10.1017/S1461145714000637. [DOI] [PubMed] [Google Scholar]
- 211.Abela AR, Browne CJ, Sargin D, Prevot TD, Ji XD, Li Z, et al. Median raphe serotonin neurons promote anxiety-like behavior via inputs to the dorsal hippocampus. Neuropharmacology. 2020;168:107985. doi: 10.1016/j.neuropharm.2020.107985. [DOI] [PubMed] [Google Scholar]
- 212.dos Santos L, de Andrade TG, Zangrossi Junior H. 5-HT1A receptors in the dorsal hippocampus mediate the anxiogenic effect induced by the stimulation of 5-HT neurons in the Median raphe nucleus. Eur Neuropsychopharmacol. 2008;18:286–294. doi: 10.1016/j.euroneuro.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 213.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Padilla-Coreano N, Canetta S, Mikofsky RM, Alway E, Passecker J, Myroshnychenko MV, et al. Hippocampal-prefrontal Theta transmission regulates avoidance behavior. Neuron. 2019;104:601–610.e4. doi: 10.1016/j.neuron.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Adhikari A, Topiwala MA, Gordon JA. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron. 2011;71:898–910. doi: 10.1016/j.neuron.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 2016;89:857–866. doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lee AT, Cunniff MM, See JZ, Wilke SA, Luongo FJ, Ellwood IT, et al. VIP interneurons contribute to avoidance behavior by regulating information flow across hippocampal-prefrontal networks. Neuron. 2019;102:1223–1234.e4. doi: 10.1016/j.neuron.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Parfitt GM, Nguyen R, Bang JY, Aqrabawi AJ, Tran MM, Seo DK, et al. Bidirectional control of anxiety-related behaviors in mice: Role of inputs arising from the ventral hippocampus to the lateral septum and medial prefrontal cortex. Neuropsychopharmacology. 2017;42:1715–1728. doi: 10.1038/npp.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zarrindast MR, Valizadegan F, Rostami P, Rezayof A. Histaminergic system of the lateral septum in the modulation of anxiety-like behaviour in rats. Eur J Pharmacol. 2008;583:108–114. doi: 10.1016/j.ejphar.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 220.Troyano-Rodriguez E, Wirsig-Wiechmann CR, Ahmad M. Neuroligin-2 determines inhibitory synaptic transmission in the lateral septum to optimize stress-induced neuronal activation and avoidance behavior. Biol Psychiatry. 2019;85:1046–1055. doi: 10.1016/j.biopsych.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Trent NL, Menard JL. The ventral hippocampus and the lateral septum work in tandem to regulate rats' open-arm exploration in the elevated plus-maze. Physiol Behav. 2010;101:141–152. doi: 10.1016/j.physbeh.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 222.Glangetas C, Massi L, Fois GR, Jalabert M, Girard D, Diana M, et al. NMDA-receptor-dependent plasticity in the bed nucleus of the stria terminalis triggers long-term anxiolysis. Nat Commun. 2017;8:14456. doi: 10.1038/ncomms14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron. 2018;97:670–683.e6. doi: 10.1016/j.neuron.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kim WB, Cho JH. Synaptic targeting of double-projecting ventral CA1 hippocampal neurons to the medial prefrontal cortex and basal amygdala. J Neurosci. 2017;37:4868–4882. doi: 10.1523/JNEUROSCI.3579-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]