Abstract
Introduction
This study aimed to retrospectively examine the drug survival of tumor necrosis factor (TNF)-alpha inhibitors and switched subsequent biologic agents after discontinuation of TNF inhibitors.
Methods
This real-world setting study was conducted at a single academic center. We included patients who were treated with adalimumab (n = 111), certolizumab pegol (n = 12), and infliximab (n = 74) at Jichi Medical University Hospital from 1 January 2010 to 31 July 2021.
Results
No significant differences were noted in drug survival between the three TNF inhibitors. The 10-year drug survival rate for adalimumab and infliximab was 14% and 18%, respectively. Of the patients who discontinued TNF inhibitors for any reason (n = 137), 105 chose biologics as their subsequent treatment. The subsequent biologics included 31 cases of TNF inhibitors (adalimumab in 20, certolizumab pegol in 1, and infliximab in 10), 19 of interleukin-12/23 inhibitor (ustekinumab), 42 of interleukin-17 inhibitors (secukinumab in 19, brodalumab in 9, and ixekizumab in 14) and 13 of interleukin-23 inhibitors (guselkumab in 11, risankizumab in 1, and tildrakizumab in 1). Cox proportional hazards analysis for the subsequent drugs in cases of discontinuation due to inadequate efficacy revealed that female sex was a predictor of drug discontinuation (hazard ratio 2.58, 95% confidence interval 1.17–5.70) and that taking interleukin-17 inhibitors rather than TNF inhibitors was a predictor of drug persistence (hazard ratio 0.37, 95% confidence interval 0.15–0.93).
Conclusions
Interleukin-17 inhibitors may be a favorable option for patients who need to switch from TNF inhibitors due to inadequate efficacy. However, this study is limited by the small number of cases and its retrospective design.
Keywords: Adalimumab, Biologics, Certolizumab pegol, Drug switching, Infliximab, Kaplan–Meier survival curves, Psoriasis, TNF inhibitors
Plain Language Summary
With many biologic options available for the treatment of psoriasis, choosing the optimal drug can be challenging, especially when switching drugs. Tumor necrosis factor (TNF) inhibitors are the oldest category of biologics used for psoriasis, with adalimumab and infliximab being available since 2010 and certolizumab pegol since 2019 in Japan. In this study, we examined the drug survival of TNF inhibitors in patients treated with adalimumab (n = 111), certolizumab pegol (n = 12), and infliximab (n = 74) at Jichi Medical University Hospital from 1 January 2010 to 31 July 2021. No significant differences were noted in drug survival between the three TNF inhibitors, and the 10-year drug survival rate for adalimumab and infliximab was 14% and 18%, respectively. We examined the drug survival of subsequent biologics used by patients who discontinued TNF inhibitors for any reason (n = 137) and found that among patients who discontinued TNF inhibitors due to inadequate efficacy, female sex was a predictor of drug discontinuation and that taking interleukin-17 inhibitors rather than TNF inhibitors was a predictor of drug continuation. The study results suggest that interleukin-17 inhibitors is a favorable option for patients who discontinue TNF inhibitors due to inadequate efficacy and need to switch to other agents. However, this study has limitations, including the small number of cases and the single-center and retrospective study design.
Key Summary Points
| Why carry out this study? |
| There is no clear consensus on the order of preference for biologics in psoriasis. |
| Appropriate selection of the next therapeutic agent following discontinuation of a drug due to ineffectiveness or adverse events is particularly challenging. |
| We examined drug survival of the subsequent biologics selected for patients with psoriasis who had discontinued TNF inhibitors to determine which drugs were more likely to be continued. |
| What was learned from the study? |
| Among patients who discontinued TNF inhibitors due to inadequate efficacy, female sex was a predictor of drug discontinuation and taking interleukin-17 inhibitors rather than TNF inhibitors was a predictor of drug continuation. |
| Interleukin-17 inhibitors may be a favorable option for patients who discontinue TNF inhibitors due to inadequate efficacy and need to switch to other agents. |
Introduction
With many biologics now available for the treatment of moderate-to-severe psoriasis, clinicians are challenged to select the optimal treatment to improve the patient’s quality of life. This is because while first-line drug selection is important, it is also necessary to have a strategy for switching drugs, as there are many cases of drug discontinuation [1, 2]. In Japan, tumor necrosis factor (TNF)-alpha inhibitors have been used for more than a decade for the treatment of psoriasis since adalimumab and infliximab became available in 2010. Moreover, with the approval of certolizumab pegol in December 2019, three TNF inhibitors are now available for the treatment of psoriasis and psoriatic arthritis. TNF inhibitors are more effective than conventional medications, and they substantially improve the symptoms of psoriasis; however, a significant number of patients treated with TNF inhibitors experience primary/secondary failure or adverse events [3]. Patients who experience therapeutic failure or who are intolerant of a certain biologic agent often make a joint decision with their physician to switch to a subsequent biologic alternative; however, which therapy is optimal after failure or intolerance of the preceding biologic agent has not been established [2]. In addition, when switching biologics, there is no consensus as to which category of inhibitors to select from for the subsequent drug. This study examined how patients with long psoriasis continued on TNF inhibitors and the drug survival of their subsequent biologic therapy if they discontinued TNF inhibitors in a real-world setting by means of a retrospective single center study, from 1 January 2010, to 31 July 2021.
Methods
Patients and Treatment
We extracted retrospective information from the medical records of patients with psoriasis treated with adalimumab, certolizumab pegol, and infliximab at the Department of Dermatology, Jichi Medical University Hospital, from 1 January 2010, to 31 July 2021. All patients were Japanese and had a moderate-to-severe psoriasis diagnosis based on clinical findings with/without histological examination by experienced dermatologists. The study included all patients who received at least one dose of TNF inhibitors and had at least one follow-up visit during the study period. Treatment discontinuation was defined as drug withdrawal, switching to a different drug, or having a treatment gap of more than 6 months. When a patient discontinued a TNF inhibitor and then started any biologic agent within the study period, that agent was defined as the “subsequent biologic agent.” If a patient switched to more than one biologic agent during the study period, only the first drug switched from a TNF inhibitor was examined.
Statistical Analyses
The procedure for calculating drug survival is identical to that of our previous paper [4]. Comparisons of categorical variables among the groups were examined using the chi-squared test, and comparisons of continuous variables were examined using one-way analysis of variance. Drug survival rates for each biologic agent were examined using the Kaplan–Meier method, and log-rank tests were used for univariate analysis. The Bonferroni method was used for multiple-testing corrections of the log-rank test. The multivariable Cox proportional hazard model (forced entry method) was used to identify factors associated with discontinuation of the switched subsequent biologic agents. On the basis of previous studies, we chose the following factors as covariates: sex, body mass index (BMI), psoriasis subtypes, and biologic drug classes. TNF inhibitors were used as a reference treatment class for comparison with other treatment classes. On the basis of BMI, patients were categorized into two groups: BMI ≥ 25 kg/m2 and BMI < 25 kg/m2 according to the Japanese standards for obesity. The proportional hazard assumption was evaluated by visual inspection of the log-minus-log plot and found to be fulfilled. Statistical significance was set at p < 0.05. Statistical analysis was performed using IBM SPSS version 26.0 software.
Ethical Approval
Our study was performed in accordance with the Declaration of Helsinki of 1964 and its later amendments. All patients provided informed consent to participate in the study. The study was approved on 26 November 2021 by the Ethical Committee of Jichi Medical University (approval no. 21-050).
Results
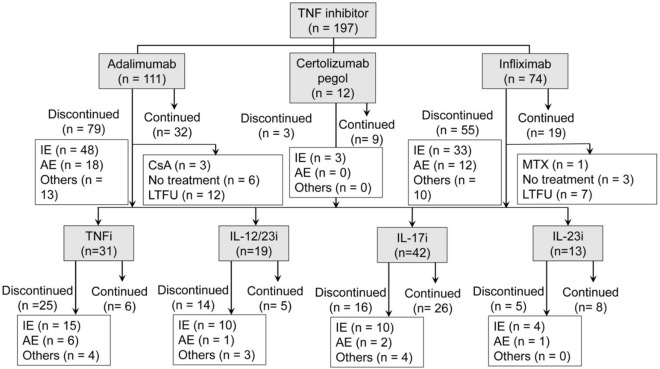
In a total of 197 treatment case series, 111, 12, and 74 patients were treated with adalimumab, certolizumab pegol, and infliximab, respectively (Fig. 1). Table 1 presents patient characteristics of patients treated with TNF inhibitors. The mean age of patients was significantly higher for certolizumab pegol than for infliximab. The proportion of bio-naïve patients was lower for certolizumab pegol than for adalimumab and infliximab and higher for infliximab than for adalimumab, with statistical significance. No significant differences were noted between the three drugs in terms of the proportion of male patients, mean BMI, and proportion of patients with psoriatic arthritis and generalized pustular psoriasis. TNF inhibitors were discontinued during the study period in 137 patients (70%); among these patients, the inhibitors were discontinued due to inadequate efficacy in 84 (61%), adverse events in 30 (22%), and other reasons in 23 (18%) patients. Other reasons included hospital change (n = 7), loss to follow-up (n = 4), financial issues (n = 4), patient request (n = 3), inconvenience (n = 3), remission (n = 1), and pregnancy (n = 1).
Fig. 1.
Patient flow chart. IE inadequate efficacy, AE adverse event, CsA cyclosporine A, LTFU loss to follow-up, MTX methotrexate, TNF tumor necrosis factor, TNFi TNF inhibitor, IL-12/23i IL-12/23 inhibitor, IL-17i IL-17 inhibitor, IL-23i IL-23 inhibitor
Table 1.
Demographic data for patients treated with TNF inhibitors
| All patients n = 197 |
Adalimumab n = 111 |
Certolizumab pegol n = 12 |
Infliximab n = 74 |
|
|---|---|---|---|---|
| Age, mean ± SD, years | 49.2 ± 14.3 | 49.1 ± 15.3 | 57.5 ± 12.0 | 48.1 ± 12.6 |
| Sex (male), n (%) | 120 (61) | 63 (57) | 7 (58) | 54 (73) |
| Median treatment duration, days (interquartile range, days) | 644 (180–1977) | 1053 (232–2162.5) | 131 (81.75–187) | 499 (150.75–1589.25) |
| BMI, mean ± SD | 25.2 ± 4.2 (n = 177) | 25.1 ± 4.3 (n = 97) | 25.1 ± 2.9 (n = 10) | 25.5 ± 4.3 (n = 70) |
| Bio-naïve patients, n (%) | 137 (70) | 73 (67) | 3 (25) | 61 (84) |
| PsA, n (%) | 120 (61) | 66 (59) | 11 (92) | 43 (58) |
| GPP, n (%) | 16 (8) | 6 (5) | 0 | 10 (14) |
| Concomitant use of MTX, n (%) | 13 (7) | 8 (7) | 1 (8) | 4 (5) |
| Drug discontinuation, n (%) | 137 (70) | 79 (71) | 3 (25) | 55 (74) |
| Reasons for drug discontinuation | ||||
| Inadequate efficacy, n (%) | 84 (43) | 48 (43) | 3 (25) | 33 (44) |
| Adverse events, n (%) | 30 (15) | 18 (16) | 0 (0) | 12 (16) |
| Others, n (%) | 23 (12) | 13 (12) | 0 (0) | 10 (14) |
| Subsequent biologic treatment | ||||
| Adalimumab | 20 | 1 | 1 | 18 |
| Certolizumab pegol | 1 | 1 | 0 | 0 |
| Infliximab | 10 | 9 | 0 | 1 |
| Ustekinumab | 19 | 10 | 0 | 9 |
| Secukinumab | 19 | 9 | 1 | 9 |
| Brodalumab | 9 | 8 | 0 | 1 |
| Ixekizumab | 14 | 10 | 0 | 4 |
| Guselkumab | 11 | 9 | 0 | 2 |
| Risankizumab | 1 | 0 | 1 | 0 |
| Tildrakizumab | 1 | 1 | 0 | 0 |
BMI body mass index, PsA psoriatic arthritis, GPP generalized pustular psoriasis, MTX methotrexate, TNF tumor necrosis factor
Table 2 lists the adverse events that were the reasons for discontinuation. There was no trend in the types of adverse events and no significant difference between adalimumab and infliximab in the incidence of comorbid malignancies.
Table 2.
Adverse events leading to discontinuation of the drug
| Adalimumab (n = 17) | Infliximab (n = 13) | |
|---|---|---|
| Malignant tumor, n | 7 (4 lung cancer, 1 colon cancer, 1 prostate cancer, 1 tongue cancer) | 1 (1 gastric cancer) |
| Drug eruption (including suspected cases), n | 2 | 1 |
| Infusion reaction, n | 1 | 2 |
| Interstitial pneumonia, n | 2 | 0 |
| Cerebral infarction, n | 1 | 1 |
| Hypertension, n | 0 | 2 |
| Malaise, n | 2 | 0 |
| Tuberculous lymphadenitis, n | 0 | 1 |
| Acute hepatitis B infection, n | 0 | 1 |
| IgA vasculitis, n | 1 | 0 |
| Thrombocytopenia, n | 1 | 0 |
| Pneumocystis pneumonia, n | 0 | 1 |
| Bacterial pneumonia, n | 0 | 1 |
| Panic attack, n | 0 | 1 |
| Proteinuria, n | 0 | 1 |
| Severe headache, n | 0 | 1 |
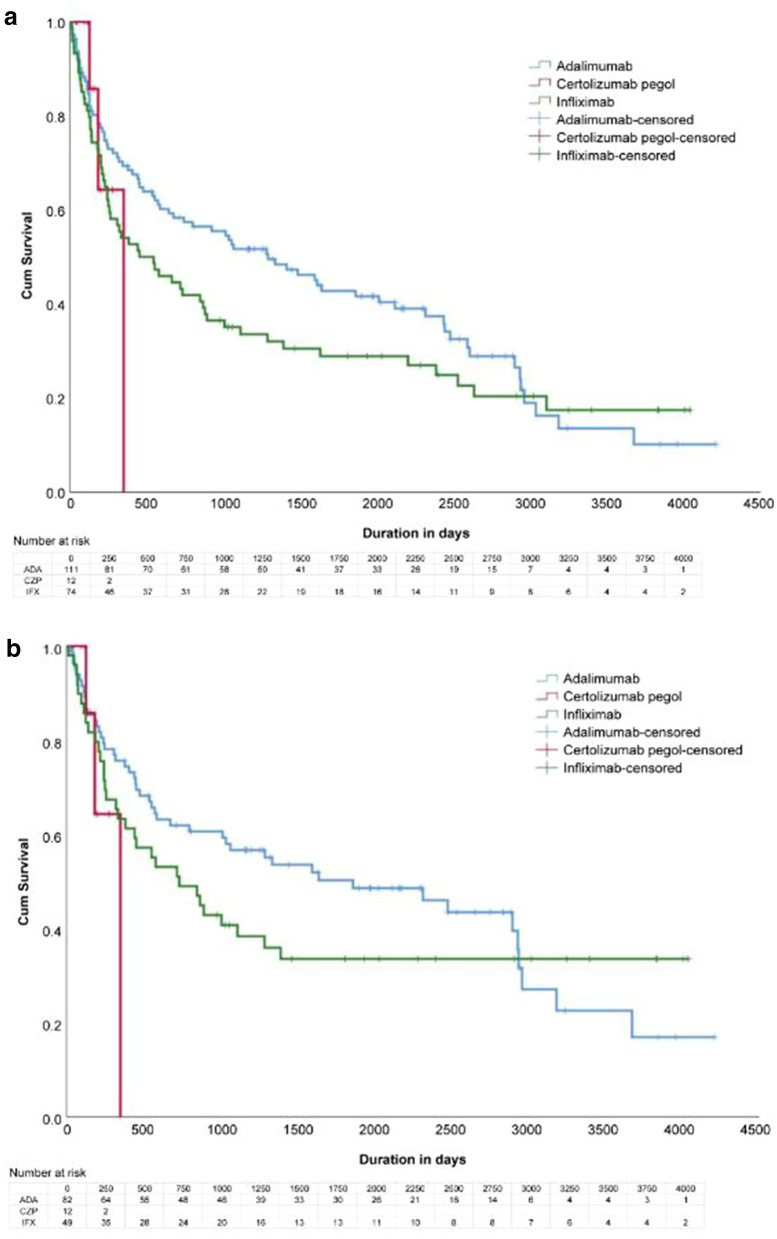
The drug survival curve of adalimumab, certolizumab pegol, and infliximab is shown in Fig. 2a (all cases) and Fig. 2b (only cases in which the drugs were discontinued due to inadequate efficacy). No significant differences were noted in drug survival between the three TNF inhibitors or between psoriasis subtypes by log-rank test. This was shown in all cases and in cases of inadequate efficacy. The drug survival was significantly lower in female individuals than in male individuals in all cases, although no significant difference was noted between sexes in cases of inadequate efficacy.
Fig. 2.
a Drug survival rate for three TNF inhibitors (all cases). b Drug survival rate for three TNF inhibitors (cases of discontinuation due to inadequate efficacy). TNF tumor necrosis factor
When comparing drug survival rates between bio-naïve patients (n = 137, 70%) and non-naïve patients (n = 60, 30%) for all patients treated with the three TNF inhibitors, drug survival was significantly superior in bio-naïve patients (p = 0.003). For each drug, no significant difference was noted in drug survival between bio-naïve and non-naïve patients for infliximab and certolizumab pegol; however, the drug survival rate was significantly higher in bio-naïve patients than in non-naïve patients for adalimumab (p = 0.006).
The 3-year, 5-year, and 10-year survival rates of adalimumab and infliximab are presented in Table 3. Of the 84 patients who discontinued TNF inhibitors due to inadequate efficacy, 81 chose biologics as their following therapy, two chose cyclosporine, and one chose methotrexate. Of the 30 patients who discontinued TNF inhibitors due to adverse events, 20 chose biologics as their following therapy, two chose apremilast, two chose etretinate, one chose cyclosporine, one chose topical therapy, and the remainder stopped treatment for psoriasis.
Table 3.
The drug survival rate at 3, 5, and 10 years of adalimumab and infliximab use
| Reason for discontinuation | All reasons | Inadequate efficacy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adalimumab (n = 111) |
Infliximab (n = 57) |
Adalimumab (n = 82) |
Infliximab (n = 49) |
|||||||||
| Estimate | SE | n | Estimate | SE | n | Estimate | SE | n | Estimate | SE | N | |
| 3-year | 0.52 | 0.48 | 54 | 0.35 | 0.56 | 25 | 0.57 | 0.55 | 43 | 0.40 | 0.70 | 17 |
| 5-year | 0.43 | 0.49 | 37 | 0.30 | 0.54 | 18 | 0.50 | 0.58 | 30 | 0.34 | 0.69 | 12 |
| 10-year | 0.14 | 0.50 | 5 | 0.18 | 0.52 | 6 | 0.22 | 0.75 | 4 | 0.34 | 0.69 | 4 |
SE standard error
Table 4 presents the characteristics of patients who switched to the subsequent biologic agents after discontinuation of TNF inhibitors for any reason (n = 105). The subsequent biologics included 31 cases of TNF inhibitors (adalimumab in 20, certolizumab pegol in 1, and infliximab in 10), 19 of IL-12/23 inhibitor (ustekinumab), 42 of IL-17 inhibitors (secukinumab in 19, brodalumab in 9, and ixekizumab in 14), and 13 of IL-23 inhibitors (guselkumab in 11, risankizumab in 1, and tildrakizumab in 1). There were no significant differences in terms of the mean age, proportion of male patients, mean BMI, or proportion of patients with generalized pustular psoriasis, although the proportion of those with psoriatic arthritis was significantly higher in patients treated with TNF inhibitors than in those treated with IL-23 inhibitors.
Table 4.
Demographic data for patients who switched biologic agents from TNF inhibitors to subsequent agents
| All patients n = 105 |
TNF inhibitors n = 31 |
IL-12/23 inhibitor n = 19 |
IL-17 inhibitors n = 42 |
IL-23 inhibitors n = 13 |
|
|---|---|---|---|---|---|
| Drug, n |
Adalimumab, 20 Certolizumab pegol, 1 Infliximab, 10 |
Ustekinumab, 19 |
Secukinumab, 19 Brodalumab, 9 Ixekizumab, 14 |
Guselkumab, 11 Risankizumab, 1 Tildrakizumab, 1 |
|
| Age, mean ± SD, years | 51.7 ± 14.2 | 50.0 ± 12.7 | 49.5 ± 16.1 | 53.1 ± 13.5 | 54.2 ± 15.9 |
| Sex (male), n (%) | 68 (65) | 22 (70) | 12 (63) | 27 (64) | 7 (54) |
| Median treatment duration, days (interquartile range, days) | 766 (273–1561) | 607 (168.5–1758.5) | 1916 (1372–2569.5) | 677 (272.5–979) | 759 (122–1067) |
| BMI, mean ± SD | 25.8 ± 4.4 (n = 97) | 26.8 ± 3.8 (n = 31) | 25.4 ± 4.4 (n = 18) | 25.4 ± 4.4 (n = 37) | 26.6 ± 5.6 (n = 11) |
| Previous drug, n |
Adalimumab, 58 Certolizumab pegol, 3 Infliximab, 44 |
Adalimumab, 11 Certolizumab pegol, 1 Infliximab, 19 |
Adalimumab, 10 Certolizumab pegol, 0 Infliximab, 9 |
Adalimumab, 27 Certolizumab pegol, 1 Infliximab, 14 |
Adalimumab, 10 Certolizumab pegol, 1 Infliximab, 2 |
| PsA, n (%) | 58 (55) | 23 (74) | 8 (42) | 23 (55) | 4 (31) |
| GPP, n (%) | 9 (9) | 3 (10) | 2 (11) | 3 (7) | 1 (2) |
| Drug discontinuation, n (%) | 60 (57) | 25 (80) | 14 (73) | 16 (38) | 5 (38) |
| Reasons for drug discontinuation | |||||
| Ineffectiveness, n | n = 39 | n = 15 | n = 10 | n = 10 | n = 4 |
| Adverse events, n | n = 10 | n = 6 | n = 1 | n = 2 | n = 1 |
| Other, n | n = 11 | n = 4 | n = 3 | n = 4 | n = 0 |
BMI body mass index, PsA psoriatic arthritis, GPP generalized pustular psoriasis, TNF tumor necrosis factor
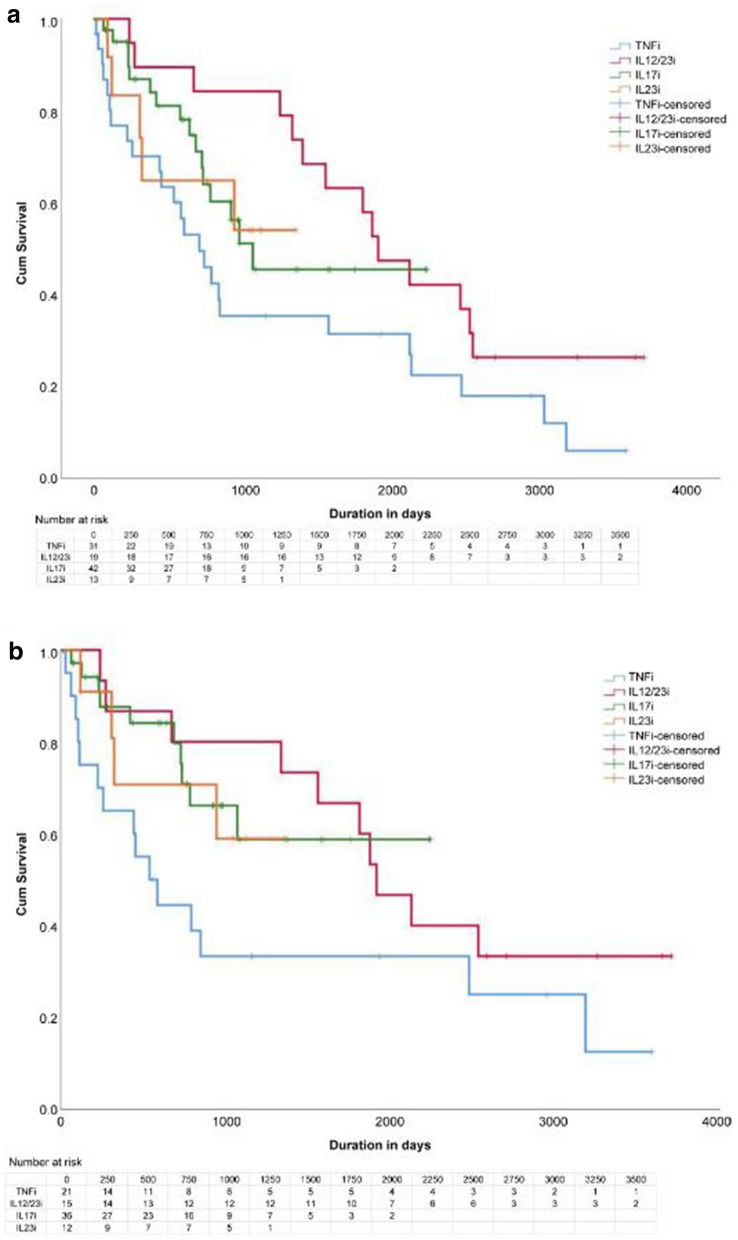
Figures 3a and b show the drug survival for each class of the subsequent biologic agents. No significant differences were noted in drug survival compared by sex, psoriasis subtypes, or drug classes by log-rank test, either for all patients or for cases of inadequate efficacy.
Fig. 3.
a Drug survival rate for subsequent drugs (all cases). b Drug survival rate for subsequent drugs (cases of discontinuation due to inadequate efficacy). TNF tumor necrosis factor, TNFi TNF inhibitor, IL12/23i IL-12/23 inhibitor, IL17i IL-17 inhibitor, IL23i IL-23 inhibitor
Table 5 presents the results of multivariate Cox proportional hazards analysis for the switched subsequent biologics. For overall discontinuation of the subsequent biologic therapy, female sex was a predictor of discontinuation [hazard ratio (HR) 1.91, 95% confidence interval (CI) 1.01–3.62, p = 0.046]. For discontinuation due to inadequate efficacy, female sex was a predictor of discontinuation (HR 2.58, CI 1.17–5.70, p = 0.019) and taking IL-17 inhibitors rather than TNF inhibitors was a predictor of drug persistence (HR 0.37, CI 0.15–0.93, p = 0.034).
Table 5.
Multivariate Cox proportional hazard analyses for switched subsequent drug discontinuation
| Overall discontinuation | Discontinuation due to inadequate efficacy | |||
|---|---|---|---|---|
| Covariates | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Female | 1.94 (1.01–3.62) | 0.046 | 2.58 (1.17–5.70) | 0.019 |
| Obese (BMI ≥ 25 kg/m2) | 1.35 (0.74–2.46) | 0.32 | 1.66 (0.76–3.52) | 0.21 |
| PsA versus PsO | 1.26 (0.68–2.33) | 0.47 | 1.11 (0.52–2.36) | 0.78 |
| GPP versus PsO | 0.79 (0.26–2.40) | 0.69 | 0.24 (0.04–1.35) | 0.11 |
| IL-12/23i versus TNFi | 0.56 (0.27–1.18) | 0.56 | 0.56 (0.23–1.40) | 0.22 |
| IL-17i versus TNFi | 0.59 (0.29–1.20) | 0.15 | 0.37 (0.15–0.93) | 0.034 |
| IL-23i versus TNFi | 0.93 (0.33–2.59) | 0.88 | 0.67 (0.21–2.20) | 0.51 |
Bold letters indicate a p-value of less than 0.05
HR hazard ratio, CI confidence interval, BMI body mass index, PsA psoriatic arthritis, PsO plaque psoriasis, GPP generalized pustular psoriasis, IL-12/23i IL-12/23 inhibitor, TNFi TNF inhibitor, IL-17i IL-17 inhibitor, IL-23i IL-23 inhibitor
Discussion
In 2019, we reported on drug survival of biologics for psoriasis [4]. Herein, we examined the drug survival of TNF inhibitors with an extended follow-up period and that of subsequent biologics for patients who had discontinued TNF inhibitors.
The log-rank test showed no significant difference in drug survival between the three TNF inhibitors; however, there is a limitation in that certolizumab pegol has only been available for a short period of time, and the sample size is small (n = 12). As presented in Table 3, the 10-year drug survival rate for adalimumab and infliximab in this study was 14% and 18% (all patients) and 22% and 34% (patients who discontinued due to inadequate efficacy), respectively. Previous studies [1, 5–11] have reported 10-year drug survival rates for biologics used to treat psoriasis, wherein the drug survival rates tended to decrease over time, with the 10-year drug survival rates for TNF inhibitors ranging from < 5% to 50%. Some cohort studies of psoriatic arthritis have reported high survival rates for TNF inhibitors of approximately 40–50% [9, 10], which is higher than that reported in our study (14% and 18% for adalimumab and infliximab, respectively), although our data showed no significant difference in drug survival between plaque psoriasis and psoriatic arthritis (data not shown). The lower drug survival rate for TNF inhibitors in this study may be partially due to the lower rate of concomitant use of methotrexate in our department (7%) than in rheumatology departments reported in previous studies (approximately 35–65%) [9, 10].
In this study, the proportion of patients with concomitant psoriatic arthritis was very high at 61%. This is presumably due to the fact that our department receives many patients with severe psoriatic arthritis from the community and that TNF inhibitors were the first-line drug of choice until IL-17 inhibitors became available. Furthermore, for patients with severe psoriasis, infliximab, which is administered intravenously and is fast-acting, had been the first-line TNF inhibitor, especially in the early 2010s. This may also be the reason for the higher proportion of bio-naïve patients for infliximab than for adalimumab.
Interestingly, the shapes of the drug survival curves of adalimumab and infliximab in patients who discontinued drugs due to inadequate efficacy in our study (Fig. 2b) were similar to those reported by Egeberg et al. [1]. The drug survival rate of adalimumab was higher than that of infliximab from induction to treatment days 2500–3000; however, after that point, the drug survival rate of infliximab tended to remain stable, resulting in a crossing of the two drug survival curves. It is unclear why the drug survival rates of infliximab become constant with fewer secondary failures after a certain point. The proportion of bio-naïve patients differed between the three TNF inhibitors, and overall, drug survival was superior in bio-naïve patients, which is consistent with the findings of previous reports [1, 6, 8, 11]. The lower proportion of bio-naïve patients for adalimumab than for infliximab and the significantly lower drug survival rate in non-naïve patients for adalimumab than for infliximab may explain the lower drug survival for adalimumab than for infliximab in this study.
In our study, 4 (4/111, 3.6%) and 7 (7/74, 9.5%) long-term responders continued adalimumab and infliximab for more than 3000 days, respectively. The percentage of discontinuations due to inadequate efficacy or adverse events in this study was roughly similar to that reported in previous studies with similar study durations [6, 12–14]. Three of the patients who discontinued TNF inhibitors due to inadequate efficacy did not choose biologics as their subsequent therapy. This is because cyclosporine, which was used for treatment before biologics, was more effective for the two patients who chose cyclosporin. In one case, methotrexate was chosen because the patient preferred oral medication.
In this study, the switched subsequent biologic agents were generally selected in a physician–patient discussion based on each patient’s symptoms, comorbidities, and preferences. However, when an adverse event appeared specifically related to TNF inhibitors, the subsequent drug was selected from other classes of biologics in most cases. For example, adverse events with onset or exacerbation related to TNF inhibitors [15–18] included three cases of infusion reaction, one case of tuberculous lymphadenitis, one case of immunoglobulin A (IgA) vasculitis, and one case of thrombocytopenia. In addition to those cases, in one case with acute hepatitis B [19], a non-TNF inhibitor was selected as the subsequent drug to reduce the risk of reactivation of the hepatitis B virus [20].
According to reports in the literature, when patients need to change TNF inhibitors, switching to another TNF inhibitor or switching to inhibitors of different classes can be both safe and effective options in cases of insufficient response or intolerance [21, 22], especially when the first TNF inhibitor did not show primary failure [23]. Moreover, there are many reports of successful intra-class switches among TNF inhibitors and among IL-17 inhibitors [21, 22, 24–26]. In contrast, in a study of psoriatic arthritis, the response rate was significantly lower for the second switcher than for the first switcher when the patient switched among TNF inhibitors more than once [27]. In that report, the authors recommended that patients who do not respond adequately to two TNF inhibitors should choose a third drug from another class of biologics.
With regard to drug survival of subsequent agents following TNF inhibitor discontinuation in this study, female sex was a predictor of drug discontinuation in all cases and in cases of discontinuation due to inadequate efficacy. In previous reports on drug survival of biologics for psoriasis, several studies reported that female sex was a predictor of drug discontinuation [2, 11, 13, 28–34]. In addition, in this study, we observed that choosing an IL-17 inhibitor after discontinuation of a TNF inhibitor due to inadequate efficacy was a predictor of drug persistence, indicating that IL-17 inhibitors may be the preferred subsequent drug option when TNF inhibitors are discontinued due to inadequate efficacy. Nevertheless, one study reported a different result from ours in that drug survival of second-line biologics were not significantly different between intra-class and inter-class switching [13]. Future studies need to establish a strategy for drug switching.
This study has some limitations. First, the study design was retrospective, and the study was conducted at a single center with a limited number of cases. Second, the choice of biologics was influenced by the timing of introduction and physician/patient preference. Third, as the number of patients who switched to IL-23 inhibitors was very small, the efficacy of the subsequent IL-23 inhibitors not adequately evaluated. Finally, as the study included real-world data, the psoriasis area and severity index (PASI) scores were unavailable for all cases, making it impossible to include an accurate discussion of disease severity or treatment efficacy.
Conclusions
Our study demonstrated the 10-year drug survival of TNF inhibitors for psoriasis and evaluated the predictors of drug discontinuation after switching from a TNF inhibitor. These results should provide useful information for drug selection when switching biologic agents in the treatment of psoriasis.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Megumi Kishimoto and Makiko Mieno. The first draft of the manuscript was written by Megumi Kishimoto, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Prior Presentation
The manuscript contains content presented at the 36th Annual Meeting of the Japanese Society for Psoriasis Research held in Chiba, Japan on September 4, 2021.
Disclosures
Mamitaro Ohtsuki has received honoraria or fees for serving on advisory boards or speakers’ bureaus, fees for consulting, and grants for investigator activities from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Janssen Pharmaceutical, Kyowa Kirin, LEO Pharma, Eli Lilly, Maruho, Mitsubishi Tanabe Pharma, Novartis, Pfizer, Sun Pharma, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB. Mayumi Komine received a research grant from Boehringer Ingelheim. Megumi Kishimoto, Koji Kamiya, Junichi Sugai, Aya Kuwahara, and Makiko Mieno have no conflict of interest to declare.
Compliance with Ethics Guidelines
Our study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All patients provided informed consent to participate in the study. The study was approved on November 26, 2021, by the Ethical Committee of Jichi Medical University (approval number: 21-050).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178:509–519. doi: 10.1111/bjd.16102. [DOI] [PubMed] [Google Scholar]
- 2.Iskandar IYK, Warren RB, Lunt M, et al. Differential drug survival of second-line biologic therapies in patients with psoriasis: observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Invest Dermatol. 2018;138:775–784. doi: 10.1016/j.jid.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atiqi S, Hooijberg F, Loeff FC, Rispens T, Wolbink GJ. Immunogenicity of TNF-inhibitors. Front Immunol. 2020;11:312. doi: 10.3389/fimmu.2020.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishimoto M, Komine M, Kamiya K, Sugai J, Mieno M, Ohtsuki M. Drug survival of biologic agents for psoriatic patients in a real-world setting in Japan. J Dermatol. 2020;47:33–40. doi: 10.1111/1346-8138.15146. [DOI] [PubMed] [Google Scholar]
- 5.Geale K, Lindberg I, Paulsson EC, et al. Persistence of biologic treatments in psoriatic arthritis: a population-based study in Sweden. Rheumatol Adv Pract. 2020;4:rkaa070. doi: 10.1093/rap/rkaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooderham MJ, Lynde C, Turchin I, Avadisian M, Labelle M, Papp KA. Real-world, long-term treatment patterns of commonly used biologics in Canadian patients with moderate-to-severe chronic plaque psoriasis. J Dermatol. 2022;49:95–105. doi: 10.1111/1346-8138.16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glintborg B, Østergaard M, Dreyer L, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum. 2011;63:382–390. doi: 10.1002/art.30117. [DOI] [PubMed] [Google Scholar]
- 8.Vieira-Sousa E, Eusébio M, Ávila-Ribeiro P, et al. Real-world longterm effectiveness of tumor necrosis factor inhibitors in psoriatic arthritis patients from the Rheumatic Diseases Portuguese Register. J Rheumatol. 2020;47:690–700. doi: 10.3899/jrheum.181272. [DOI] [PubMed] [Google Scholar]
- 9.Thomas ML, Shaddick G, Charlton R, et al. Tumor necrosis factor inhibitor monotherapy versus combination therapy for the treatment of psoriatic arthritis: combined analysis of European biologics databases. J Rheumatol. 2021;48:48–57. doi: 10.3899/jrheum.190815. [DOI] [PubMed] [Google Scholar]
- 10.Flouri ID, Markatseli TE, Boki KA, et al. Comparative analysis and predictors of 10-year tumor necrosis factor inhibitors drug survival in patients with spondyloarthritis: first-year response predicts longterm drug persistence. J Rheumatol. 2018;45:785–794. doi: 10.3899/jrheum.170477. [DOI] [PubMed] [Google Scholar]
- 11.Kojanova M, Cetkovska P, Strosova D, et al. Real-world evidence from more than 1000 patients treated with adalimumab for moderate-to-severe psoriasis in the Czech Republic. Dermatol Ther (Heidelb) 2021;11:543–553. doi: 10.1007/s13555-021-00499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayaraa B, Imafuku S. Sustainability and switching of biologics for psoriasis and psoriatic arthritis at Fukuoka University Psoriasis Registry. J Dermatol. 2019;46:389–398. doi: 10.1111/1346-8138.14834. [DOI] [PubMed] [Google Scholar]
- 13.Lorenzin M, Ortolan A, Cozzi G, et al. Predictive factors for switching in patients with psoriatic arthritis undergoing Anti-TNFα, anti-IL12/23, or anti-IL17 drugs: a 15-year monocentric real-life study. Clin Rheumatol. 2021;40:4569–4580. doi: 10.1007/s10067-021-05799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Özkur E, Kıvanç Altunay İ, Oğuz Topal İ, et al. Switching biologics in the treatment of psoriasis: a multicenter experience. Dermatology. 2021;237:22–30. doi: 10.1159/000504839. [DOI] [PubMed] [Google Scholar]
- 15.Shivaji UN, Sharratt CL, Thomas T, et al. Review article: managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:664–680. doi: 10.1111/apt.15097. [DOI] [PubMed] [Google Scholar]
- 16.Murdaca G, Negrini S, Pellecchio M, et al. Update upon the infection risk in patients receiving TNF alpha inhibitors. Expert Opin Drug Saf. 2019;18:219–229. doi: 10.1080/14740338.2019.1577817. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen C, Abitbol V, El Karoui K, et al. IgA vasculitis in patients with inflammatory bowel disease: new insights into the role of TNF-α blockers. Rheumatol (Oxf, Engl) 2022;61:1957–1965. doi: 10.1093/rheumatology/keab662. [DOI] [PubMed] [Google Scholar]
- 18.Bessissow T, Renard M, Hoffman I, Vermeire S, Rutgeerts P, Van Assche G. Review article: non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment Pharmacol Ther. 2012;36:312–323. doi: 10.1111/j.1365-2036.2012.05189.x. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto M, Komine M, Kamiya K, et al. Case of psoriasis with hepatitis B virus infection during tumor necrosis factor inhibitor treatment successfully treated with ixekizumab and tenofovir alafenamide fumarate. J Dermatol. 2022;49:e193–e194. doi: 10.1111/1346-8138.16320. [DOI] [PubMed] [Google Scholar]
- 20.Piaserico S, Messina F, Russo FP. Managing psoriasis in patients with HBV or HCV infection: practical considerations. Am J Clin Dermatol. 2019;20:829–845. doi: 10.1007/s40257-019-00457-3. [DOI] [PubMed] [Google Scholar]
- 21.Tsai YC, Tsai TF. Switching biologics in psoriasis - practical guidance and evidence to support. Expert Rev Clin Pharmacol. 2020;13:493–503. doi: 10.1080/17512433.2020.1767590. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Chen Z, Gong Y, Shi Y. A review of switching biologic agents in the treatment of moderate-to-severe plaque psoriasis. Clin Drug Investig. 2018;38:191–199. doi: 10.1007/s40261-017-0603-3. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi PS, Bissonnette R, Teixeira HD, Valdecantos WC. Systematic review of efficacy of anti-tumor necrosis factor (TNF) therapy in patients with psoriasis previously treated with a different anti-TNF agent. J Am Acad Dermatol. 2016;75:612–8.e6. doi: 10.1016/j.jaad.2016.02.1221. [DOI] [PubMed] [Google Scholar]
- 24.Kromer C, Wilsmann-Theis D, Gerdes S, et al. Changing within the same class: efficacy of brodalumab in plaque psoriasis after treatment with an IL-17A blocker - a retrospective multicenter study. J Dermatolog Treat. 2021;32:878–882. doi: 10.1080/09546634.2020.1716932. [DOI] [PubMed] [Google Scholar]
- 25.Bokor-Billmann T, Schäkel K. No need to change the drug class: ixekizumab- following secukinumab-therapy in psoriasis. J Dermatolog Treat. 2019;30:216–220. doi: 10.1080/09546634.2018.1506081. [DOI] [PubMed] [Google Scholar]
- 26.Tichy M, Kojanova M, Velackova B, et al. Efficacy of switches within the class of IL-17 inhibitors: an analysis of data from the Czech nationwide registry of psoriatic patients receiving biological/targeted therapy (BIOREP) Dermatol Ther. 2022;35:e15772. doi: 10.1111/dth.15772. [DOI] [PubMed] [Google Scholar]
- 27.Kristensen LE, Lie E, Jacobsson LT, et al. Effectiveness and feasibility associated with switching to a second or third TNF inhibitor in patients with psoriatic arthritis: a cohort study from southern Sweden. J Rheumatol. 2016;43:81–87. doi: 10.3899/jrheum.150744. [DOI] [PubMed] [Google Scholar]
- 28.Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Invest Dermatol. 2015;135:2632–2640. doi: 10.1038/jid.2015.208. [DOI] [PubMed] [Google Scholar]
- 29.Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172:244–252. doi: 10.1111/bjd.13343. [DOI] [PubMed] [Google Scholar]
- 30.Roche H, Bouiller K, Puzenat E, et al. Efficacy and survival of biologic agents in psoriasis: a practical real-life 12-year experience in a French dermatology department. J Dermatolog Treat. 2019;30:540–544. doi: 10.1080/09546634.2018.1480746. [DOI] [PubMed] [Google Scholar]
- 31.Zweegers J, van den Reek JM, van de Kerkhof PC, et al. Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side-effects in patients with psoriasis treated with adalimumab, etanercept or ustekinumab in daily practice: a prospective, comparative, long-term drug-survival study from the BioCAPTURE registry. Br J Dermatol. 2016;175:340–347. doi: 10.1111/bjd.14552. [DOI] [PubMed] [Google Scholar]
- 32.Shalom G, Cohen AD, Ziv M, et al. Biologic drug survival in Israeli psoriasis patients. J Am Acad Dermatol. 2017;76:662–9.e1. doi: 10.1016/j.jaad.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 33.Esposito M, Gisondi P, Cassano N, et al. Survival rate of antitumour necrosis factor-α treatments for psoriasis in routine dermatological practice: a multicentre observational study. Br J Dermatol. 2013;169:666–672. doi: 10.1111/bjd.12422. [DOI] [PubMed] [Google Scholar]
- 34.Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Eur Acad Dermatol Venereol. 2016;30:1148–1158. doi: 10.1111/jdv.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.