Temporal lobe epilepsy (TLE), one of the most common drug-resistant epilepsies, is characterized by abnormal recurrent excitatory activity originating from the temporal lobe. In the dentate gyrus (DG) of the hippocampus, a common seizure focus of TLE, the glutamatergic mossy cells (MCs) make synaptic connections with the granule cells (GCs) and form recurrent MC-GC-MC excitatory circuits. Cross-layer projections of MCs are able to modulate the excitability of the DG and even the whole hippocampus, and they may be closely involved in TLE. Injury and loss of MCs during chronic seizures have been widely reported in both human patients and animal models of TLE, and the surviving MCs have been disclosed to play an anti-epileptic role [1]. However, the functions and the underlying mechanisms of MCs in the early stage of epileptogenesis remain unclear.
Recently, Nasrallah et al. have focused on the connection of the MC-GC excitatory circuit with epilepsy, and revealed that seizure-induced strengthening of the MC-GC circuit plays a detrimental role during the early stage of epileptogenesis [2]. First, by the use of a Drd2-cre mouse line combined with a Cre-recombinase–dependent virus under the CaMKII promoter, they selectively studied the role of MCs in the DG. They verified that chemogenetic silencing of MCs in the DG significantly reduced the amplitude of evoked MC-GC excitatory postsynaptic currents in acute slices from healthy mice, and reduced the susceptibility to seizure stage 3 in mice induced by acute intraperitoneal injection of kainic acid (KA). This is consistent with the results in another acute pilocarpine mouse model reported by Botterill et al, in which inhibiting MCs during epileptogenesis not only reduced the severity of early seizures but also ameliorated the following chronic seizures [3]. Meanwhile, neither of them specifically investigated whether there was any functional difference between the MCs in the dorsal DG and those in the ventral DG during epileptogenesis, given that their axon distributions are heterogeneous and their roles in chronic seizures are distinct [1, 4, 5].
Then Nasrallah et al. used two-photon imaging to monitor the activity of the MC-GC circuit in vivo and found that both the MCs and GCs are activated after the initial convulsive seizures. More importantly, the enhanced activity of MCs precedes that of GCs, indicating the potential of MCs for driving the activation of GCs. Since the repetitive activity of MCs induced long-term potentiation (LTP) in MC-GC circuits of healthy mice, they further verified that LTP in MC-GC circuits was induced in epileptic mice after an initial stage 3 seizure, the mechanism being enhanced presynaptic MC-GC transmission. In addition, they also found that KA-induced seizures induced LTP in the medial perforant path (MPP)-GC circuit, but mainly through postsynaptic mechanisms. Furthermore, they applied repeated optogenetic activation of MCs to induce LTP in MC-GC circuits in advance and found that this further increased the seizure susceptibility of healthy mice induced by a lower dose of KA (20 mg/kg). Understandably, a lower dose of KA may lower the bar for the MC-GC LTP induction to show an effect. However, whether optogenetically-induced LTP in advance would further aggravate seizure severity induced by a normal dose of KA (30 mg/kg) was not discussed in this article. Above all, these results indicate that enhanced MC-GC synaptic transmission in the early seizure stage promotes epileptogenesis. Further studies could be applied to verify the role of LTP in MPP-GC circuits in seizures and to compare its importance with that of the MC-GC circuits.
It is known that brain-derived neurotrophic factor (BDNF)/tropomyosin receptor kinase B signaling is vital for the formation of LTP. Next, Nasrallah et al. further tested in mice in which BDNF was conditional knocked out from GCs, and found that seizure-induced MC-GC and MPP-GC synaptic strengthening required postsynaptic BDNF. Therefore, they further applied genetic removal of BDNF from hippocampal excitatory neurons and found that this reduced the susceptibility to acute seizures. However, considering the inhibitory role in MPP-GC synaptic strengthening by knocking out BDNF from GCs, selectively knocking out BDNF from presynaptic MCs could provide more direct evidence for the role of the MC-GC circuit in early epileptogenesis. It is notable that, just like the paradoxical function of MCs in different periods of seizures, BDNF may play opposite roles in acute and chronic epilepsy [6], since BDNF in the epileptic hippocampus reduces the frequency of spontaneous generalized seizures and improves cognitive performance [7]. Above all, Nasrallah and colleagues found that in the acute stage but not the chronic stage of seizures, the BDNF-dependent LTP in the MC-GC circuit promotes epileptogenesis, and reducing BDNF in the hippocampus would be an effective therapeutic strategy for early epileptogenesis and a potential molecular target for pharmacological treatment of TLE. As for the long way for genetic modulation in clinical therapy, drugs targeting BDNF signals may be further tested for potential translational medicine.
In general, by using chemogenetics, in vivo Ca2+ imaging, electrophysiology, and a gene-knockout strategy, Nasrallah et al disclosed a detrimental role of MCs in the DG of the hippocampus during early epileptogenesis, with a potential mechanism involving BDNF-dependent synaptic enhancement of MC-GC circuits. Notwithstanding, several further issues remain to be resolved (Fig. 1): (1) MCs in the dorsal and ventral DG have been found to be functionally heterogeneous not only in memory and emotion but also in chronic epileptic seizures [1, 8]. Are the roles and the underlying downstream circuit foundation of the MCs in the dorsal DG consistent with those in the ventral part in acute seizures? This could be further examined by selectively injecting a virus into a particular subregion of the DG or directly expressing the virus under the calretinin promoter, a special marker of MCs in the ventral DG but not the dorsal DG of mice. (2) Apart from the direct input from MCs to GCs, MCs also provide synaptic connections to the basket cells in the hippocampus, thereby forming both a directly excitatory circuit and an indirectly inhibitory microcircuit to modify the balance of the hippocampus [9]. It has been reported that chemogenetic inhibition but not activation of MCs induces an increase in c-fos expression in GC layers under normal physiological conditions [10]. So, what happened to this indirect inhibitory microcircuit after the initial seizure is an important question that deserves further investigation. (3) The MCs play an important role in connecting the bilateral hippocampus in the two cerebral hemispheres by sending both ipsilateral and contralateral projections, and the function of the local connection may be different from that of the remote connection. It is hard for Nasrallah et al. and Botterill et al. to study the functional difference between the MCs ipsilateral and contralateral to the seizure focus, as they induced seizures by intraperitoneal injection of epileptic drugs. To reveal the role of MCs in the propagation from focal to generalized seizures, further studies could be applied in epileptic models induced unilaterally. (4) Most interestingly and importantly, how did the MCs change from a detrimental pro-epileptic role during the early stage to a beneficial anti-epileptic role during chronic seizures? Are there any characteristic changes or circuit reorganizations that happen to these surviving MCs? These questions deserve further research.
Fig. 1.
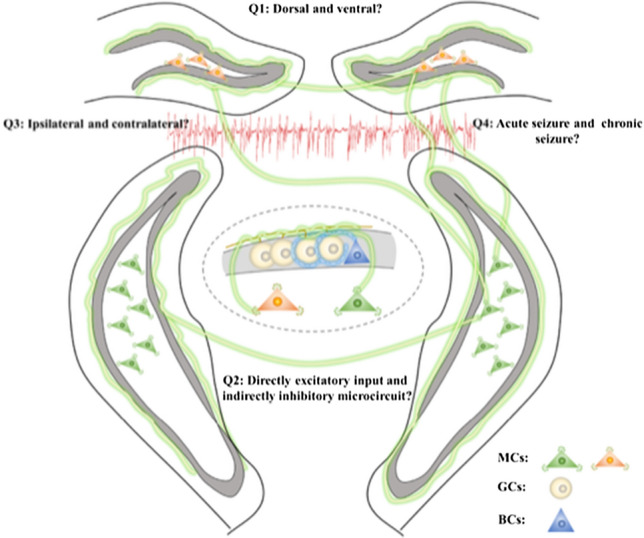
Further directions to reveal the precise roles of mossy cells in epilepsy. Question 1: Are the roles and the underlying downstream circuit foundation of the MCs in the dorsal DG consistent with those in the ventral part in acute seizures? Question 2: What happens to the MC-BC-GC microcircuits after the initial seizure? Question 3: Is there any functional difference between the MCs ipsilateral and contralateral to the seizure focus? Question 4: How do the MCs change from a detrimental pro-epileptic role during the early stage to a beneficial anti-epileptic role during chronic seizures? Abbreviations: MCs, mossy cells; GCs, granule cells; BCs, basket cells.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82204353) and the Research Project of Zhejiang Chinese Medical University (2022JKZKTS07).
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Heming Cheng and Qiuwen Lou have contributed equally to this article.
References
- 1.Bui AD, Nguyen TM, Limouse C, Kim HK, Szabo GG, Felong S, et al. Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science. 2018;359:787–790. doi: 10.1126/science.aan4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasrallah K, Frechou MA, Yoon YJ, Persaud S, Gonçalves JT, Castillo PE. Seizure-induced strengthening of a recurrent excitatory circuit in the dentate gyrus is proconvulsant. Proc Natl Acad Sci U S A. 2022;119:e2201151119. doi: 10.1073/pnas.2201151119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botterill JJ, Lu YL, LaFrancois JJ, Bernstein HL, Alcantara-Gonzalez D, Jain S, et al. An excitatory and epileptogenic effect of dentate gyrus mossy cells in a mouse model of epilepsy. Cell Rep. 2019;29:2875–2889.e6. doi: 10.1016/j.celrep.2019.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houser CR, Peng Z, Wei X, Huang CS, Mody I. Mossy cells in the dorsal and ventral dentate gyrus differ in their patterns of axonal projections. J Neurosci. 2021;41:991–1004. doi: 10.1523/JNEUROSCI.2455-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botterill JJ, Gerencer KJ, Vinod KY, Alcantara-Gonzalez D, Scharfman HE. Dorsal and ventral mossy cells differ in their axonal projections throughout the dentate gyrus of the mouse hippocampus. Hippocampus. 2021;31:522–539. doi: 10.1002/hipo.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Liu J, Liu J, Liu XL, Jin LY, Fan W, et al. BDNF-TrkB signaling pathway is involved in pentylenetetrazole-evoked progression of epileptiform activity in hippocampal neurons in anesthetized rats. Neurosci Bull. 2013;29:565–575. doi: 10.1007/s12264-013-1326-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin TW, Harward SC, Huang YZ, McNamara JO. Targeting BDNF/TrkB pathways for preventing or suppressing epilepsy. Neuropharmacology. 2020;167:107734. doi: 10.1016/j.neuropharm.2019.107734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang KY, Wu JW, Cheng JK, Chen CC, Wong WY, Averkin RG, et al. Elevation of hilar mossy cell activity suppresses hippocampal excitability and avoidance behavior. Cell Rep. 2021;36:109702. doi: 10.1016/j.celrep.2021.109702. [DOI] [PubMed] [Google Scholar]
- 9.Scharfman HE. The enigmatic mossy cell of the dentate gyrus. Nat Rev Neurosci. 2016;17:562–575. doi: 10.1038/nrn.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botterill JJ, Vinod KY, Gerencer KJ, Teixeira CM, LaFrancois JJ, Scharfman HE. Bidirectional regulation of cognitive and anxiety-like behaviors by dentate gyrus mossy cells in male and female mice. J Neurosci. 2021;41:2475–2495. doi: 10.1523/JNEUROSCI.1724-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


