Abstract
Objectives
Underlying immunodeficiency has been associated with worse clinical presentation and increased mortality in patients with COVID-19. We evaluated the mortality of solid organ transplant (SOT) recipients (SOTR) hospitalized in Spain due to COVID-19.
Methods
Nationwide, retrospective, observational analysis of all adults hospitalized because of COVID-19 in Spain during 2020. Stratification was made according to SOT status. The National Registry of Hospital Discharges was used, using the International Classification of Diseases, 10th revision coding list.
Results
Of the 117,694 adults hospitalized during this period, 491 were SOTR: kidney 390 (79.4%), liver 59 (12%), lung 27 (5.5%), and heart 19 (3.9%). Overall, the mortality of SOTR was 13.8%. After adjustment for baseline characteristics, SOTR was not associated with higher mortality risk (odds ratio [OR] = 0.79, 95% confidence interval [CI] 0.60-1.03). However, lung transplantation was an independent factor related to mortality (OR = 3.26, 95% CI 1.33-7.43), while kidney, liver, and heart transplantation were not. Being a lung transplant recipient was the strongest prognostic factor in SOT patients (OR = 5.12, 95% CI 1.88-13.98).
Conclusion
This nationwide study supports that the COVID-19 mortality rate in SOTR in Spain during 2020 did not differ from the general population, except for lung transplant recipients, who presented worse outcomes. Efforts should be focused on the optimal management of lung transplant recipients with COVID-19.
Keywords: COVID-19, SOT, Lung transplantation, Lymphoma, Mortality, Hospitalization
Introduction
The surge of SARS-CoV-2 infection at the end of 2019 in China and its rapid spread worldwide is an unprecedented medical phenomenon. By the end of 2021, estimates for excess mortality due to COVID-19 were over 18 million people globally [1]. During the first wave after the pandemic onset, predictors of severe disease were soon unveiled and included older age, male gender, and certain comorbidities including hypertension, obesity, diabetes, heart failure, and chronic kidney disease [2]. Moreover, special attention was paid to people with immune deficiencies or prolonged immunosuppressive therapy. Accordingly, the exacerbated inflammatory response after SARS-CoV-2 infection led to fears of a worse prognosis in people with cancer, autoimmune diseases, or solid organ transplant (SOT) recipients (SOTR), among others [3], [4], [5], [6], [7], [8].
Spain is a world leader in SOT. In the last 10 years, more than 45,000 transplants have been made, significantly improving the survival rate in a group of young patients with otherwise limited life expectancy (www.ont.es). Nevertheless, SOTRs are at an increased risk of some infections due to immunosuppressive therapy [9]. Furthermore, in addition to baseline clinical conditions in this population, long-term exposure to immunosuppressive therapy leads to increased comorbidities such as chronic kidney disease, hypertension, and diabetes, among others [9], [10], [11]. In this context, SARS-CoV-2 infection could lead to severe COVID-19 in a higher proportion of patients, justifying the excess mortality identified in this population during the initial stages of the pandemic [7,8].
Accordingly, the aim of this study was to evaluate the impact of SOT on COVID-19 mortality. Hence, we evaluated the mortality of SOT patients hospitalized in Spain due to COVID-19 before the introduction of the SARS-CoV-2 vaccine.
Methods
A retrospective study with data from population-based hospital discharge diagnoses at the Minimum Basic Data Set (MBDS) of the Spanish National Registry of Hospital Discharges (SNRHD) was performed. This national public registry belongs to the Spanish Ministry of Health and records information from all patients discharged from hospitals/clinics across the country since the 1990s. Previous studies have been performed on this registry for other illnesses, including infectious diseases, and have recognized its high value for producing estimates of current burden and time trends for different clinical conditions at the national level [4,5].
Study population
Our study was performed using all the data from January 01 to December 31, 2020, included in the SNRHD. During this period, the main circulating variants in our setting were the original Wuhan, Epsilon, and Alfa. The criteria for diseases and procedures were defined according to the International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM). We selected all hospital admissions assigned with the code U07.1 (COVID-19) as the main diagnosis.
Data regarding demographics and outcomes, including age, gender, ethnicity, length of admission, and intensive care unit admission or in-hospital mortality were retrieved from the database. Baseline conditions, as well as the presence of interstitial pneumonia and acute respiratory insufficiency, were recorded from other ICD-10 codes in the dataset, regardless of position, for each episode of hospital admission. In addition, the age-adjusted Charlson Comorbidity Index (CCI), which is a well-validated composite that predicts clinical outcomes in multiple illnesses, was calculated from the previous data [12]. Among other medical conditions, it included diabetes, heart failure, dementia, chronic kidney disease, liver disease, and cancer, most of which have been associated with severe COVID-19 [2,3]. Finally, patients were tagged as SOTR according to the Z94 code, which also identifies the type of organ transplanted.
Statistical analysis
Quantitative variables are described as mean with SD or as median with interquartile ranges (IQR). Qualitative variables were summarized as counts and percentages. Bivariate comparisons of quantitative and qualitative variables were performed using the Kruskal-Wallis test, Mann-Whitney U test, and the chi-square test. Factors related to in-hospital mortality, both in the overall population and SOTR, were determined by binary logistic regression analyses. In addition, the 95% confidence interval (CI) was provided. All statistical analyses were performed using the IBM SPSS package for Windows v25.0 (IBM Corp, Armonk, New York). All tests were 2-tailed and only P-values <0.05 were considered as significant.
Ethical aspects
The Spanish Ministry of Health provided the database after removal of all potential patient identifiers. In accordance with Spanish legislation, the patient's informed consent was not needed for this analysis. The study protocol was approved by the Clinical Research Ethics Committee of Puerta de Hierro University Hospital (Madrid, Spain) (ref. PI_134-20). The procedures described here were carried out in accordance with the ethical standards described in the 2013 Revised Declaration of Helsinki.
Results
Population characteristics
A total of 117,694 adults were hospitalized due to COVID-19 in Spain during 2020 (Table 1 ). Overall, 56.7% were male and 73% Caucasian, with a mean age at admission of 66.5 years. Only 491 of these patients (0.4%) were SOTRs (391 kidney, 59 liver, 27 lung, and 19 heart). When compared, SOTRs were younger (mean age 56.8 vs 66.6 years old, P <0.001), more frequently male (65% vs 56.6%, P <0.001), and presented more baseline hypertension (67% vs 48.1%, P <0.001), diabetes (33% vs 23.8%, P <0.001), and chronic kidney disease (39.1% vs 11.1%, P <0.001) than non-SOTR patients. In contrast, the rate of obesity (5.9% vs 11.9%; P <0.001), ischemic heart disease (4.1% vs 6.7%, P = 0.013), cerebrovascular disease (2.4% vs 6.2%; P <0.001), dementia (0.8% vs 8.7%, P <0.001), and chronic lung disease (8.1% vs 14.3%; P <0.001) were lower among SOTRs. In addition, CCI was significantly lower in SOTR compared with the rest (median three points [IQR 2-5] vs 3 [IQR 1-5], P = 0.004).
Table 1.
Distribution of baseline characteristics and major comorbidities in the study population.
| Total (N, %) | SOT (n, %) | Non-SOT (n, %) | P-value | |
|---|---|---|---|---|
| COVID-19 hospitalized patients | 117,694 | 491 (0.4) | 117,201(99.6) | - |
| Mean age (SD) | 66.5 (18) | 56.8 (15.4) | 66.6 (18) | <0.001 |
| Male gender | 66,685 (56.7) | 319 (65) | 66,366 (56.6) | <0.001 |
| Hypertension | 56,701 (48.2) | 329 (67) | 56,372 (48.1) | <0.001 |
| Diabetes mellitus | 28,094 (23.9) | 162 (33) | 27,932 (23.8) | <0.001 |
| Obesity | 13,966 (11.90) | 29 (5.9) | 13,937 (11.9) | <0.001 |
| Ischemic heart disease | 7,858 (6.7) | 20 (4.1) | 7,838 (6.7) | 0.013 |
| Heart failure | 14,199 (12.1) | 59 (12) | 14,140 (12.1) | 0.515 |
| Peripheral vascular disease | 5,059 (4.3) | 23 (4.7) | 5,036 (4.3) | 0.378 |
| Cerebrovascular disease (cerebrovascular accident or transient ischemic attack) | 7,308 (6.2) | 12 (2.4) | 7,296 (6.2) | <0.001 |
| Hemiplegia | 1,717 (1.5) | 3 (0.6) | 1,714 (1.5) | 0.131 |
| Dementia | 10,146 (8.6) | 4 (0.8) | 10,142 (8.7) | <0.001 |
| Chronic lung disease | 16,814 (14.3) | 40 (8.1) | 16,774 (14.3) | <0.001 |
| Liver disease | 6,001 (5.1) | 24 (4.9) | 5,161 (4.4) | 0.340 |
| Chronic kidney disease | 13,232 (11.2) | 192 (39.1) | 13,040 (11.1) | <0.001 |
| Connective tissue disease | 2,019 (1.7) | 5 (1) | 2014 (1.7) | 0.295 |
| Tumor | ||||
| Localized | 346 (0.3) | 1 (0.2) | 345 (0.3) | 0.492 |
| Metastatic | 701 (0.6) | 1 (0.2) | 700 (0.6) | 0.258 |
| Leukemia | 697 (0.6) | 1 (0.2) | 696 (0.6) | 0.380 |
| Lymphoma | 610 (0.5) | 5 (1) | 605 (0.5) | 0.114 |
| HIV | 234 (0.2) | 0 | 234 (0.2) | 0.376 |
| Charlson comorbidity index (median, interquartile range) | 3 (1-5) | 3 (2-5) | 3 (1-5) | 0.004 |
SOT, solid organ transplantation.
Outcomes and mortality risk
The clinical outcomes of patients hospitalized with COVID-19 in Spain are shown in Table 2 . SOTR presented a higher rate of interstitial pneumonia (73.1% vs 64.3%, P <0.001) but a lower rate of respiratory insufficiency (20.6% vs 40.5%, P <0.001). Interestingly, no differences regarding intensive care unit admission (9.6% vs 9.7%) or in-hospital mortality (13.8% vs 16%) were found.
Table 2.
Clinical outcomes in patients hospitalized with COVID-19 according to SOT status.
| Total (N, %) |
SOT (N, %) |
Non-SOT (N, %) |
P-value | |
|---|---|---|---|---|
| Interstitial pneumonia | 75,739 (64.4) | 359 (73.1) | 75,380 (64.3) | <0.001 |
| Respiratory insufficiency | 47,529 (40.4) | 101 (20.6) | 46,818 (39.9) | <0.001 |
| ICU admission | 11,449 (9.7) | 47 (9.6) | 11,400 (9.7) | 0.484 |
| In-hospital mortality | 18,858 (16) | 68 (13.8) | 18,790 (16) | 0.105 |
| Admission length (days) (Mean, SD) |
10.6 (11.7) | 9.9 (7.5) | 10.6 (11.8) | 0.210 |
| ICU admission length (days) (Mean, SD) |
15.6 (17.6) | 9.5 (6.7) | 15.6 (17.6) | <0.001 |
ICU, intensive care unit; SOT, solid organ transplantation.
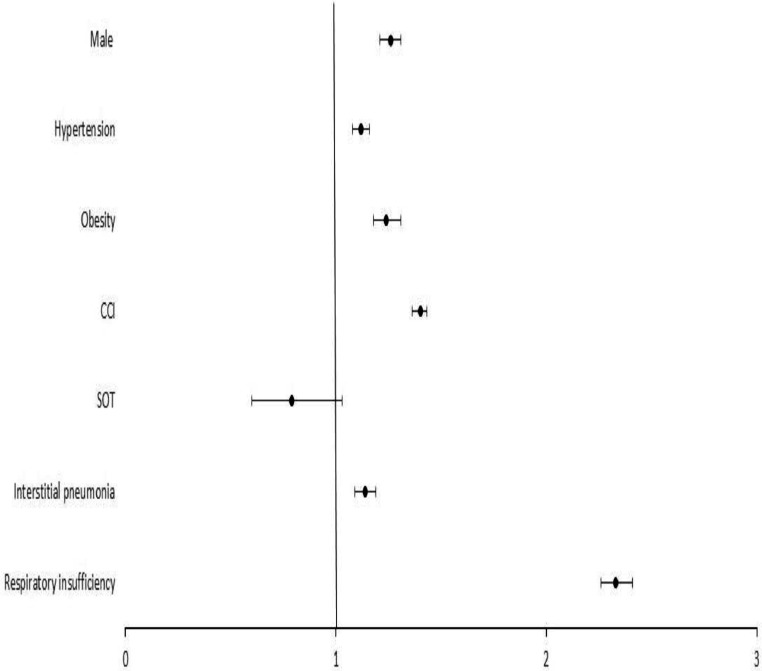
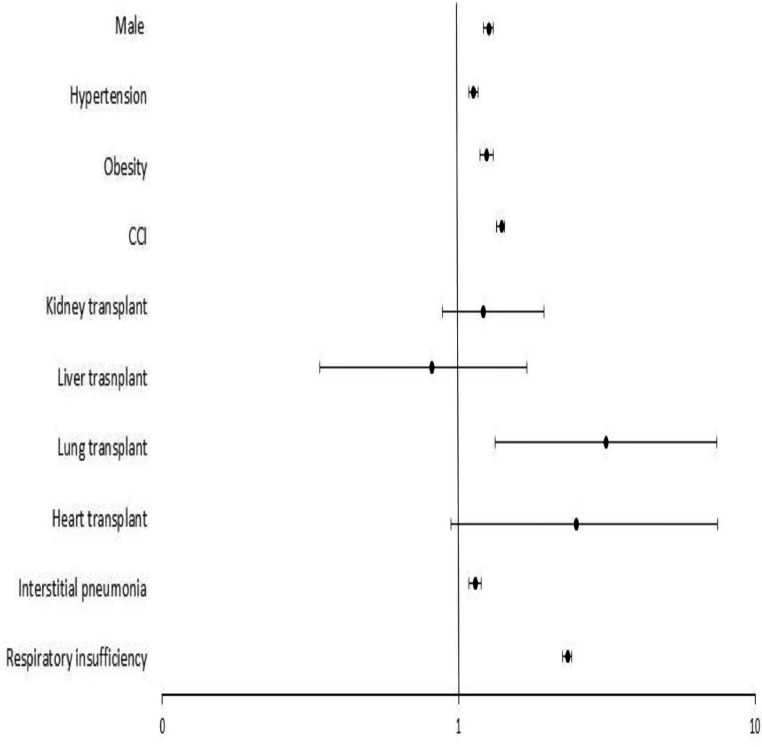
In order to determine the impact of SOTR status on COVID-19 in-hospital mortality, a multivariate analysis was performed (Figure 1 ). Male gender (odds ratio [OR] = 1.26, 95% CI 1.22-1.31), hypertension (OR = 1.12, 95% CI 1.08-1.16), obesity (OR = 1.24, 95% CI 1.18-1.31), CCI (OR = 1.40, 95% CI 1.39-1.42), interstitial pneumonia (OR = 1.14, 95% CI 1.10-1.19) and respiratory insufficiency (OR = 2.33, 95% CI 2.26-2.41) were predictors of COVID-19 mortality, while SOT did not (OR = 0.79, 95% CI 0.60-1.03). However, when each type of organ transplanted was considered individually, lung transplantation did indeed entail a higher mortality risk during COVID-19 admission (OR = 3.14, 95% CI 1.33-7.43), (Figure 2 ). Therefore, kidney (OR = 1.21, 95% CI 0.88-1.65), liver (OR = 0.81, 95% CI 0.34-1.94), or heart transplantation (OR = 2.50, 95% CI 0.94-7.48) were not associated with death after adjustment.
Figure 1.
Predictors of COVID-19 in-hospitality mortality for the Spanish population in 2020. Forrest-plot of multivariable logistic regression of factors associated with in-hospital mortality for the Spanish population in 2020. The odds ratio, 95% confidence interval, and P-value for being SOT recipient were 0.79 (0.60-1.03, P = 0.083). The rest of covariables were: male 1.26 (1.22-1.31, P <0.001); hypertension 1.12 (1.08-1.16, P <0.001); obesity 1.24 (1.18-1.31, P <0.001), CCI (per point) 1.40 (1.39-1.42, P <0.001); interstitial pneumonia 1.14 (1.10-1.19, P <0.001); and respiratory insufficiency 2.33 (2.26-2.41, P <0.001). CCI, Charlson Comorbidity Index; SOT, solid organ transplantation.
Figure 2.
Predictors of COVID-19 in-hospitality mortality for the Spanish population in 2020, considering the type of organ transplanted. Forrest-plot of multivariable logistic regression of factors associated with in-hospital mortality for the Spanish population in 2020, considering the type of organ transplanted. The odds ratio, 95% confidence interval, and P-value for each solid organ were: lung 3.14 (1.33-7.43, P <0.001); heart 2.50 (0.94-7.48, P = 0.100); kidney 1.21 (0.88-1.65, P = 0.237); and liver 0.81 (0.34-1.94, P = 0.221). The rest of covariables were: male 1.26 (1.22-1.31, P <0.001); hypertension 1.12 (1.08-1.16, P <0.001); obesity 1.24 (1.18-1.31, P <0.001), CCI (per point) 1.40 (1.39-1.42, P <0.001); interstitial pneumonia 1.14 (1.10-1.19, P <0.001); and respiratory insufficiency 2.33 (2.26-2.41, P <0.001). CCI, Charlson Comorbidity Index; SOT, solid organ transplantation.
Finally, we performed a logistic binary regression to determine the factors related to in-hospital mortality in SOT patients, confirming that age (OR = 1.09, 95% CI 1.06-1.12), male gender (OR = 2.20, 95% CI 1.21-4.01), heart failure (OR = 2.34, 95% CI 1.06-5.13), lymphoma (OR = 15.7, 95% CI 1.20-206. 57), interstitial pneumonia (OR = 2.74, 95% CI 1.19-6.34), respiratory insufficiency (OR = 2.21, 95% CI 1.18-4.15) and lung transplant (OR = 5.12, 95% CI 1.88-13.98) determined a higher mortality risk in this population (Supplemental Table S1).
Differences according to the transplanted organ
Because COVID-19 prognosis varied depending on the transplanted organ, we compared baseline conditions, clinical characteristics, and outcomes between kidney, liver, lung, and heart transplant recipients (Supplemental Table S2). It is noteworthy that lung transplant recipients were less hypertensive (22.2% vs 69.6%, P <0.001) but presented more baseline cerebrovascular disease (14.8% vs 1.7%, P = 0.003), hemiplegia (11% vs 0%, P <0.001), and chronic lung disease (18.5% vs 7.5%, P = 0.05) than other SOTR. In addition, these groups of patients developed more respiratory insufficiency (48.1% vs 19%, P = 0.001) and presented a higher mortality rate (33.3% vs 12.7%, P = 0.007) than kidney, liver, and heart transplant patients, as previously confirmed in the multivariate analysis. No differences between the groups were found regarding CCI score or the prevalence of lymphoma.
Discussion
Our results confirmed that lung transplants entailed a higher mortality risk than the general population, while kidney, liver, and heart transplants did not. The variation in the mortality rates identified for each organ transplanted could be related to the differences in the clinical phenotypes and comorbidities for each population, at the same time probably conditioned by the transplantation inclusion or exclusion criteria as well as baseline drug toxicity.
In the 117,694 patients hospitalized due to COVID-19, in-hospital mortality was 16%, similar numbers to those published by us and other Spanish researchers during the first year of the pandemic [13,14]. Furthermore, the mortality rate in SOT patients was 13.8%. Our findings are also concordant with the previous studies [6,[15], [16], [17], [18]. Even so, and regardless of the strength and size of some of these reports, there is still conflicting evidence regarding COVID-19 prognosis in SOTR. Some studies have shown that SOT is related to higher COVID-19 severity and mortality, suggesting that this population might be indeed at higher risk [17,19]. However, the majority have shown otherwise, emphasizing that mortality in this population is related to age and comorbidities, and not to SOT status [6,15,[20], [21], [22], [23], [24]. Our results confirm the latest, and, in our opinion, might help to justify the aforementioned discrepancies. First, significant differences regarding comorbidities were found when the two groups were compared, including conditions that are related to calcineurin inhibitors, steroids, and other immunosuppressant drugs used to prevent graft rejection [9], [10], [11]. Second, it is remarkable that SOTR developed less frequently acute respiratory insufficiency, despite presenting a higher interstitial pneumonia rate than non-SOTR. Respiratory insufficiency was the strongest related factor to mortality in the overall population. Accordingly, it must be considered that, because SOTR presented less respiratory insufficiency than not-SOT, other factors might justify the equal mortality seen in the two groups, such as the higher risk of acute kidney injury, bacterial and fungal superinfection, and mechanical-ventilation complications in SOTR than in the general population [17,20,21,23,25]. In addition, and probably related to the previous, COVID-19 management and treatment (antibiotics, steroids, remdesivir, or tocilizumab) during 2020 has been different in SOTR than in non-SOTR [6,17,26]. Altogether, we believe that all these factors might explain the similar mortality rate identified in hospitalized patients with COVID-19 in both groups, despite the differences in baseline comorbidities and severity.
One of the main findings of our study refers to the worse prognosis of lung transplanted recipients. Other researchers have tried to identify different outcomes according to the type of transplant [8,15,18,24]. In fact, Schaenman et al. pointed out that non-kidney transplanted patients were most likely to die because of COVID-19 [19]. In parallel, due to their lower prevalence, lung and heart transplants have been worse characterized than liver and kidney transplants [6,7,[20], [21], [22], [23], [24]. To our knowledge, only Heldman et al. have been able to find a higher COVID-19-related mortality risk in lung transplant recipients [16]. In this interesting work, chronic lung allograft dysfunction (CLAD), but not age or comorbidities, was the only independent risk factor for mortality in these patients. Our large population-based study allowed us to confirm that lung transplant recipients indeed presented a higher mortality risk after adjustment by comorbidities, while others SOTR did not. However, it is noteworthy that cardiac transplant recipients also presented a higher mortality trend when compared to kidney and liver transplant recipients, although this difference did not reach statistical significance probably due to the small population size.
The comparison between different SOTRs revealed distinct clinical profiles that might justify their different outcomes. Accordingly, lung transplant recipients presented a higher rate of chronic lung disease, including probably a high CLAD rate, which could explain the higher presence of respiratory insufficiency and the subsequent worse COVID-19 mortality identified in these patients. Furthermore, the worse outcomes seen in lung transplant recipients might be also conditioned by the intrinsic impaired pulmonary lymph clearance, the alveolocapillary membrane disruption, and the worse immunity response to microorganisms or injuries that lung grafts characteristically present [16,27].
Another factor that can influence the worse prognosis of lung transplant recipients is the higher baseline immunosuppression generally used in this organ. Unfortunately, we were not able to analyze immunosuppressant drugs that patients were receiving before admission, because no information regarding treatments could be retrieved from the database. Although there is no strong evidence supporting that other immunosuppressants than steroids, such as calcineurin inhibitors, mycophenolate, or mammalian target of rapamycin (mTOR) inhibitors, increase SARS-CoV-2 severity and mortality [6,19,23,24,26,28], we believe that the higher doses of steroids frequently used in lung transplant when compared to kidney or liver transplant might also justify the worse outcomes described, as others have previously inferred [16,17]. It should be highlighted that the presence of lymphoma, a surrogate marker of long-term immunosuppression after transplantation, was the strongest factor related to mortality in SOT patients, which supports the theory that higher baseline immunosuppression confers higher mortality risk, as described by other authors [29].
In summary, our nationwide multicentric study highlights that COVID-19 prognosis in SOTR is mainly related to baseline organ damage, either due to the different conditions that led or were related to transplant, as well as pharmacologic toxicity from drugs to prevent rejection.
Altogether, our findings reinforce the importance of preventive measures in order to attenuate certain comorbidities in SOTR and minimize immunosuppression or drug toxicity. Moreover, this study highlights lung transplant recipients, as well as those who develop post-transplant lymphoproliferative disorders, as a population particularly at risk because of all the aforementioned, where not only these measures might be intensified but also clinical outcomes, treatments, and vaccine efficacy might be specifically analyzed in further studies.
Our study has several limitations. While the SNRHD records hospital discharge diagnoses along with demographic data, this database lacks information related to certain previous clinical conditions, as well as treatment or time from transplantation. Therefore, we have not been able to determine the impact of short-term vs long-term transplantation and the severity of baseline conditions (baseline glomerular filtration rate, MELD or CHILD scores, ventricular ejection fraction, or lung functional tests, among others). Similarly, we could not elucidate if respiratory chronic disease in lung transplant recipients referred to the baseline condition that led to transplantation or if it indeed assessed CLAD, which has proven to be a major prognostic factor in this setting [16,27]. In parallel, we were not able to precisely define COVID-19 severity considering clinical and laboratory parameters, as well as the presence of superinfection, thrombo-embolic disease, and acute kidney injury. However, we believe that the lack of these data is compensated by the size of the study population, the nationwide spectrum of the study population, and the statistical power of the analysis. Furthermore, certain inclusion or selection bias might occur because we only analyzed admitted patients. During 2020, hospital admission criteria differed significantly among SOTR and the rest of the population, as might be reflected in the different proportions of interstitial pneumonia and respiratory insufficiency between the two groups. However, we performed a multivariate analysis including both variables in order to attenuate these biases. Furthermore, it should be highlighted that we only analyzed patients whose admission was attributable to COVID-19, and not asymptomatic carriers or those patients who presented pauci-symptomatic SARS-CoV-2 infection during admission or outpatient surveillance. Secondly, we only analyzed the year 2020 to avoid selection or information bias regarding vaccination in this population. However, nowadays, the majority of the population in Spain has received the complete vaccine cycle and new variants have been discovered [30]. During our study period, the main circulating variants were Wuhan and Alpha. Accordingly, our study does not cover the impact of new variants, such as Omicron, on the prognosis of SARS-CoV2 infection in SOTR. Therefore, further studies are needed to evaluate the impact of the vaccine and new variants in this population, as well as COVID-19 outcomes in SOTR after vaccination.
In conclusion, this nationwide study supports that the COVID-19 mortality rate in SOTR in Spain during 2020 did not differ from the general population except for lung transplant recipients, who presented more respiratory comorbidity leading to a higher respiratory insufficiency rate and death. Therefore, efforts should be focused on the optimal management of lung transplantation, minimizing the impact of organ damage, immunosuppression, and drug toxicity that has been shown to determine COVID-19 prognosis in these patients.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The study protocol was approved by the Clinical Research Ethics Committee of Puerta de Hierro University Hospital (Madrid, Spain) (ref. PI_134-20)
Author contributions
Study design: VMT, CM. Data Collection: VMT. Data analysis: VMT. Writing (original draft): VMT. Writing (revision and manuscript preparation): VMT, MMU, JCP, PM, AMS, AAM, LB, MAP, EMR, ARM, AFC, VCM, CM.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.06.007.
Appendix. Supplementary materials
References
- 1.COVID-19 Excess Mortality Collaborators Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno-Torres V, de la Fuente S, Mills P, Muñoz A, Muñez E, Ramos A, et al. Major determinants of death in patients hospitalized with COVID-19 during the first epidemic wave in Madrid, Spain. Medicine. 2021;100:e25634. doi: 10.1097/MD.0000000000025634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez-Urbistondo M, Gutiérrez-Rojas Á, Andrés A, Gutiérrez I, Escudero G, García S, et al. Severe lymphopenia as a predictor of COVID-19 mortality in immunosuppressed patients. J Clin Med. 2021;10:3595. doi: 10.3390/jcm10163595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno-Torres V, de Mendoza C, Mellor-Pita S, Martínez-Urbistondo M, Durán-del Campo P, Tutor-Ureta P, et al. Systemic autoimmune diseases in patients hospitalized with COVID-19 in Spain: a nation-wide registry study. Viruses. 2022;14:1631. doi: 10.3390/v14081631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno-Torres V, de Mendoza C, Martínez-Urbistondo M, Mills P, Treviño A, de la Fuente S, et al. Predictors of in-hospital mortality in HIV-infected patients with COVID-19. QJM An Int J Med. 2023;116:57–62. doi: 10.1093/qjmed/hcac215. [DOI] [PubMed] [Google Scholar]
- 6.Belsky JA, Tullius BP, Lamb MG, Sayegh R, Stanek JR, Auletta JJ. COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. 2021;82:329–338. doi: 10.1016/j.jinf.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jering KS, McGrath MM, Mc Causland FR, Claggett B, Cunningham JW, Solomon SD. Excess mortality in solid organ transplant recipients hospitalized with COVID-19: a large-scale comparison of SOT recipients hospitalized with or without COVID-19. Clin Transplant. 2022;36:e14492. doi: 10.1111/ctr.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke JA, Wiemken TL, Korenblat KM. Excess mortality among solid organ transplant recipients in the United States during the COVID-19 pandemic. Transplantation. 2022;106:2399–2407. doi: 10.1097/TP.0000000000004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalisvaart M, Schlegel A, Trivedi PJ, Roberts K, Mirza DF, Perera T, et al. Chronic kidney disease after liver transplantation: impact of extended criteria grafts. Liver Transpl. 2019;25:922–933. doi: 10.1002/lt.25468. [DOI] [PubMed] [Google Scholar]
- 10.Nassar M, Nso N, Lakhdar S, Kondaveeti R, Buttar C, Bhangoo H, et al. New onset hypertension after transplantation. World J Transplant. 2022;12:42–54. doi: 10.5500/wjt.v12.i3.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.dos Santos Q, Hornum M, Terrones-Campos C, Crone CG, Wareham NE, Soeborg A, et al. Posttransplantation diabetes mellitus among solid organ recipients in a Danish cohort. Transpl Int. 2022;35:10352. doi: 10.3389/ti.2022.10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Moreno-Torres V, Muñoz-Serrano A, Calderón-Parra J, Mills-Sánchez P, Pintos-Pascual I, Rodríguez-Olleros C, et al. Mortality by COVID-19 before vaccination - one year experience of hospitalized patients in Madrid. Int J Infect Dis. 2022;116:339–343. doi: 10.1016/j.ijid.2022.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano V, de Mendoza C, Gómez-Gallego F, Corral O, Barreiro P. Third wave of COVID-19 in Madrid, Spain. Int J Infect Dis. 2021;107:212–214. doi: 10.1016/j.ijid.2021.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An W, Wang Q, Kim TE, Kang JS. Clinical characteristics and outcome of coronavirus disease 2019 infection in patients with solid organ transplants: a systematic review and meta-analysis. J Infect Public Health. 2022;15:365–372. doi: 10.1016/j.jiph.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldman MR, Kates OS, Safa K, Kotton CN, Georgia SJ, Steinbrink JM, et al. COVID-19 in hospitalized lung and non-lung solid organ transplant recipients: a comparative analysis from a multicenter study. Am J Transplant. 2021;21:2774–2784. doi: 10.1111/ajt.16692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher AM, Schlauch D, Mulloy M, Dao A, Reyad AI, Correll M, et al. Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States. Clin Transplant. 2021;35:e14216. doi: 10.1111/ctr.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, Martínez-Fernández JR, Crespo M, Gayoso J, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–1837. doi: 10.1111/ajt.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaenman J, Byford H, Grogan T, Motwani Y, Beaird OE, Kamath M, et al. Impact of solid organ transplant status on outcomes of hospitalized patients with COVID-19 infection. Transpl Infect Dis. 2022;24:e13853. doi: 10.1111/tid.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatti M, Rinaldi M, Bussini L, Bonazzetti C, Pascale R, Pasquini Z, et al. Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:1057–1065. doi: 10.1016/j.cmi.2022.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinaldi M, Bartoletti M, Bussini L, Pancaldi L, Pascale R, Comai G, et al. COVID-19 in solid organ transplant recipients: no difference in survival compared to general population. Transpl Infect Dis. 2021;23:e13421. doi: 10.1111/tid.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linares L, Cofan F, Diekmann F, Herrera S, Marcos MA, Castel MA, et al. A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105:1365–1371. doi: 10.1097/TP.0000000000003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahota A, Tien A, Yao J, Dong E, Herald J, Javaherifar S, et al. Incidence, risk factors, and outcomes of COVID-19 infection in a large cohort of solid organ transplant recipients. Transplantation. 2022;106:2426–2434. doi: 10.1097/TP.0000000000004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno-Torres V, de Mendoza C, de la Fuente S, Sánchez E, Martínez-Urbistondo M, Herráiz J, et al. Bacterial infections in patients hospitalized with COVID-19. Intern Emerg Med. 2022;17:431–438. doi: 10.1007/s11739-021-02824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartelt L, van Duin D. An overview of COVID-19 in solid organ transplantation. Clin Microbiol Infect. 2022;28:779–784. doi: 10.1016/j.cmi.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saez-Giménez B, Berastegui C, Barrecheguren M, Revilla-López E, Los Arcos I, Alonso R, et al. COVID-19 in lung transplant recipients: a multicenter study. Am J Transplant. 2021;21:1816–1824. doi: 10.1111/ajt.16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calderón-Parra J, Cuervas-Mons V, Moreno-Torres V, Rubio-Rivas M, de Blas PA, Pinilla-Llorente B, et al. Influence of chronic use of corticosteroids and calcineurin inhibitors on COVID-19 clinical outcomes: analysis of a nationwide registry. Int J Infect Dis. 2022;116:51–58. doi: 10.1016/j.ijid.2021.12.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeWolf S, Laracy JC, Perales MA, Kamboj M, van den Brink MRM, Vardhana S. SARS-CoV-2 in immunocompromised individuals. Immunity. 2022;55:1779–1798. doi: 10.1016/j.immuni.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barandalla I, Alvarez C, Barreiro P, de Mendoza C, González-Crespo R, Soriano V. Impact of scaling up SARS-CoV-2 vaccination on COVID-19 hospitalizations in Spain. Int J Infect Dis. 2021;112:81–88. doi: 10.1016/j.ijid.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.