Abstract
Cell-penetrating peptides (CPPs) are small amino acid sequences with the potential to enter cell membranes. Along with nucleic acids, large proteins, and other chemical compounds, they can deliver several bioactive cargos inside cells. Numerous CPPs have been extracted from natural or synthetic materials since the discovery of the first CPP. In the past few decades, a significant variety of studies have shown the potential of CPPs to cure different diseases. The low toxicity in peptide compared to other drug delivery carriers is a significant benefit of CPP-based therapy, in addition to the high efficacy brought about by swift and effective delivery. A significant tendency for intracellular DNA delivery may also be observed when nanoparticles and the cell penetration peptide are combined. CPPs are frequently used to increase intracellular absorption of nucleic acid, and other therapeutic agents inside the cell. Due to long-term side effects and possible toxicity, its implementation is restricted. The use of cell-permeating peptides is a commonly used technique to increase their intracellular absorption. Additionally, CPPs have lately been sought for application in vivo, following their success in cellular studies. This review will go through the numerous CPPs, the chemical modifications that improve their cellular uptake, the various means for getting them across cell membranes, and the biological activity they acquire after being conjugate with specific chemicals.
Keywords: Cellular uptake, Cell membrane, Conjugated, CPP, Nanoparticles
Introduction
Around 7 million deaths worldwide each year from cancer-related causes and greater than 16 million additional cases of cancer are expected to occur per year worldwide (Jemal et al. 2011). Cancer is distinguished by uncontrolled cell division as well as the tendency of these cells to enter other tissues, which contributes to the growth of cancerous tumour clusters, vascularization, and metastases (Some areas of the body are infected with cancer) (Vogelstein and Kinzler 2004). Although angiogenesis (fresh blood vessel formation from previously formed vessels) is a crucial and natural process of advancement, it has been a crucial phase in the progression from quiescence to malignant tumours (Pérez-Herrero and Fernández-Medarde 2015). Important chemotherapeutic challenges include low drug penetration into cancerous tissues, the prevalence of strong doses or lengthy therapy-resistant tumours, and dose-dependent side effects. High blood pressure, which blocks molecules from entering tumour tissue (such as in pancreatic cancer), and the involvement of lymph arteries in cancer contribute to the poor surgical implant of cancer treatment in tissues. By promoting extravasation and penetration of cancer cells, CPPs may enhance the delivery of drugs to tumour cells, although other tissues remain untouched by the treatment. Therapeutic techniques using antibodies or peptides that recognize tumour cell-specific target molecules cause several medications capable of inhibiting cancer growth to be concentrated in tumour tissue. As a consequence, where the drug is placed and optimally obtained at the tumour site, increased activity and decreased toxicity to normal tissues are expected (Ruoslahti 2017). The cell specificities are boosted by endogenous stimuli such as specific enzyme activation and pH values that characterize different cancer cells. External stimuli, such as moderate light, have also seen promising effects on improving the delivery of cargo and increasing the retention of drugs supplied by CPP. In different biological applications, an important technique for the intracellular delivery of bioactive materials is provided by cell-penetrating peptides (CPPs). Latest innovations in the usage of CPPs to provide cancerous cells with anticancer therapy and imaging reagents, together with CPP contribute to innovative tumour-targeting strategies. Despite the absence of cell specificity and limited time of action, CPPs are already commonly used to provide several therapeutics. Resolving these limitations to allow improved specificity of cancer cells and /or tumours may enhance CPP-based drug delivery techniques, extend the possibility of integrated drug delivery, and improve possible therapeutic applications of these peptides (Raucher and Ryu 2015). Effective cell penetration is necessary to pass the plasma membrane, minimize, side effects and deliver the dosage needed for operation. The need for peptides in drug delivery as cargo carriers is motivated by the peptide’s potential to improve drug absorption and thereby offer a significant therapeutic benefit. Most peptides known as possible vectors of cargo come under the group of Cell-penetrating peptides (CPPs) (Vivès et al. 2008). We concentrate on recent developments in CPPs and nanoparticles based on the anticancer drug delivery system in this review.
Cell-penetrating peptide
CPPs strengthen the incorporation of cells. The protein transduction domains are often known as CPPs. Protein transduction domains (PTDs) are referred to as small peptides (just around 30 residues), mostly with the capacity to enter the cellular membranes in a vitality-dependent or individualistic manner (Swain et al. 2016; Moreno et al. 2021; Ye et al. 2016). The CPP is a small amino acid sequence characterized by the capacity to cross the membrane. It carries many bioactive loads, as well as nucleic acids, big proteins, and other chemicals, inside of cells (Habault and Poyet 2019). These hurdles, which led to the introduction of new tumour-specific molecular therapies, have been resolved with the recent development of CPPs. Also called PTDs, they are small amino acid sequences that translocate a conjugated cargo across the cell membrane. Once inside the cell, CPP-related cargo may associate and obstruct their tumours with their intracellular targets. In particular, the nontoxic cell penetration mechanism enables the secure and efficient systemic delivery of anticancer therapy (Bitler and Schroeder 2010). some examples of the Cell-penetrating peptide are shown in Table.1.
Table 1.
Cell-penetrating peptides with their sequence based on amino acids
| Name | Sequence | References |
|---|---|---|
| Oligoarginine | R8, R9, R10, R12 | Hu et al. (2012) |
| NLS | CGGGPKKKRKVGG CGGFSTSLRARKA CKKKKKKSEDEYPYVPN | Delaroche et al. (2007) |
| Antennapedia Penetrating (43–58) | RQIKIWFQNRRMKWKK | Su et al. (2020) |
| Azurin-p28 | LSTAADMQGVVTDGMASGLDKD | Johansson et al. (2011) |
| HIV-1 TAT protein (48–60) | GRKKRRQRRRPPQ | Mai et al. (2002) |
| ARF (1–22) | MVRRFLVTLRIRRACGPPRVRV | Mai et al. (2002) |
| pVEC Cadherin (615–632) | LLIILRRRIRKQAHAHSK | Tat Protein from Human Immunodeficiency Virus Forms a Metal-Linked Dimer on JSTOR 2021) |
| 8-lysines | KKKKKKKK | Lindgren et al. (2004) |
| Transportan Galanine/Mastoparan | GWTLNSAGYLLGKINLKAL AALAKKIL | Ragin et al. (2002) |
| Oligoarginine (e.g., R8) RRRRRRRR | RRRRRRRR | Futaki et al. (2001a) |
| Tat (48–56) GRKKRRQRR | GRKKRRQRR | Vivès et al. (1997) |
| Penetrating | RQIKIWFQNRRMKWKK | Derossi et al. (1994) |
| CyLoP-1 | CRWRWKCCKK | El-Sayed et al. (2009; Regberg et al. (2012) |
| sC18 | GLRKRLRKFRNKIKEK | Neundorf et al. (2009) |
| VP22 | RPRAPARSASRPRRPVE | Elliott and O’Hare (1997) |
| MAP | KLALKLALKALKAALKLA | Squires et al. (2013) |
| Pept1 | PLILLRLLRGQF | Macchi et al. 2015) |
| PF14 | AGYLLGKLLOOLAAAALOOLL | (PDF) Peptide Nanoparticle Delivery of Charge-Neutral Splice-Switching Morpholino Oligonucleotides | Suzan Hammond and Graham McClorey - Academia.edu (2021) |
Classification of cell-penetrating peptides
CPP may have a variety of sources, ranging from peptide collections and random aa or peptide-based variants with unique architecture from known proteins/peptides (de Figueiredo et al. 2014). It is based on peptide variants shown in Fig.1.
Fig. 1.
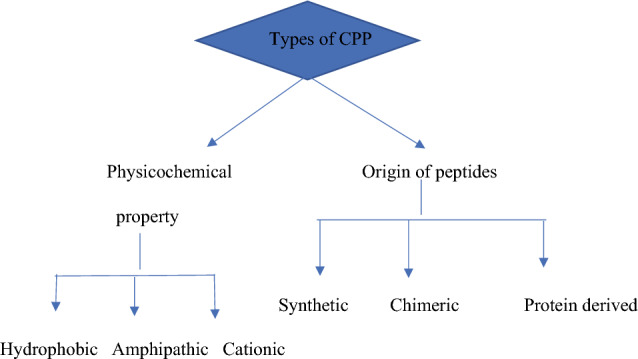
Classifications of CPPs based on peptide variants
Types of cell-penetrating peptides (CPP)
Cationic: CPP is a common peptide that generates substantially positive net charges from simple short arginine and lysine strains, both of which are important in influencing the idealisation of various therapeutic loads. The two most commonly used cationic CPPs are Tat-derived peptides, homeodomain-derived peptides (Antp), and non-arginine peptides. These have nine amino acids, including Arginine (Koren and Torchilin 2012). Peptides of this type also comprise short sequences of amino acids rich in arginine, lysine, or histidine, and they electrostatically connect on the plasma membrane with the net negative charge of phosphates and sulphates, contributing to independent receptor internalisation (Futaki et al. 2001b). Because histidine and lysine have only been partially protonated, CPPs with an Olga arginine sequence are the most successful in this type of carrier-cell contact. Furthermore, cationic CPPs require at least eight positive cellular uptake charges (Dokka et al. 1997).
Hydrophobic peptides: These are differentiated by their low loading and high hydrophobicity. The hydrophobic peptides, which are primarily composed of the second type of CPPs. These CPPs have hydrophobic amino acid groups, which, along with a low netload, are critical for cellular absorption. The Kaposi fibroblast growth factor (K-FGF) 43 signal series and the Fibroblast growth factor 12 (FGF-12) Hydrophobic CPPs are examples of these (Nakayama et al. 2011; Deshayes et al. 2008). It has been demonstrated that combining lipophilic amino acids with short cationic peptides improves cellular absorption; non-polar amino acids are also found in amphipathic CPPs (Milletti 2012).
Amphipathic: CPPs have a succession of lipophilic and hydrophilic segments and are further split into primary amphipathic CPPs. (Pep-1) CPPs, dual amphipathic alpha-helical CPPs (hCT18-32), -sheet amphipathic CPPs (VT5), and cysteine amphipathic CPPs (Bac7) (Marty et al. 2004). The search for protein transcriptional mechanisms in natural proteins continued (Morris et al. 2008). As a result, the development of monoclonal proteins comprising domains isolated from other proteins is an example of the fast-expanding search for alternative, highly competitive CPPs.
Approaches to drug targeting
Peptides have been utilised as drugs to treat a wide range of medical conditions. Peptides have increasingly been utilised in drug delivery to route drug molecules to particular cell groups (e.g., immune cells, cancer cells) due to their capacity to target specific receptors and reduce drug side effects. Peptides are also being synthesised to assist medications in crossing the intestinal mucosa barrier (IMB) and the blood–brain barrier (BBB). Drug distribution over the IMB and BBB via paracellular pathways is improved for all of these peptides by occluding, claudins, and cadherins, which are generated from intercellular junction proteins such as occluding, claudins, and cadherins. Cancer cells can be killed using two techniques: active and passive targeting (Ulapane et al. 2017), as shown in Fig. 2.
Fig. 2.
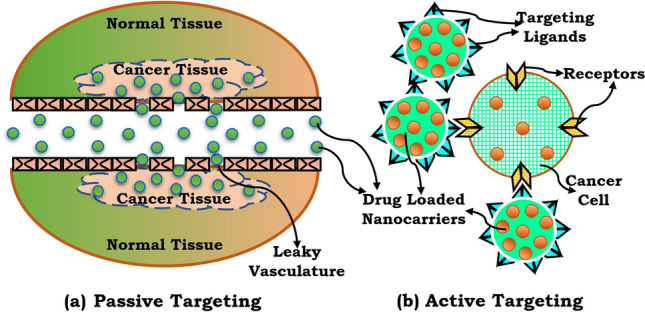
a Active and passive targeting methods. Passive targeting is accomplished using the enhanced permeation and retention (EPR) effect b active targeting is achieved through ligand receptor-mediated interaction
Passive targeting
Targeted cancer treatment is considered an irreplaceable part of the new production of anticancer drugs (Ghaz-Jahanian et al. 2015; Prabhakar et al. 2013). The best method for increasing the potency and reducing the adverse effects of an anticancer medication is to achieve a particular objective and sustain adequate doses for a sufficient period, allowing the drug to deliver the intended therapeutic result. Passive targeting refers to the extravasation of dosage formed by leaky vasculature/tumour capillaries as a result of pathological angiogenesis at tumour sites, leading to accumulation and persistence. This mechanism is called the improved influence of permeability and retention (Kharkar et al. 2020; Ulbrich et al. 2016). Furthermore, the drug is released from pH-sensitive formulations into the acidic cellular environment within the cancer cells (Thanki et al. 2015). Passive targeting by targeting mononuclear phagocytosis, systems are also called phagocytosis and the privileged role of reticuloendothelial system organ particulate carriers (Adams et al. 2001; Gosk et al. 2008).
Active targeting
The receptor at the target site is bound to the surface of the nanocarriers, which contain the inactive ligand. Ligands are chosen to bind to a tumour cell or tumour vasculature super-expressed receptor that is absent in healthy cells. Additionally, specific receptors must be uniformly expressed on all targeted cells. Antibodies that are examples of targeted ligands (peptides or not) include monoclonal antibodies (mAbs) and antibody clusters, also known as nanoantibody ligands. Because of their binding affinity, the tumour affects the ligands. “Due to the dynamic flow state of the bloodstream, high-affinity binding remains ideal for targets where cells are widely accessible, typically the vasculature of tumours.” (Allen 2002). Various anticancer therapies, grouped under the term “ligand-targeted therapies,” are classified into distinct categories based on the drug distribution strategy (Kirpotin et al. 2006). The delivery of medications to cancerous cells is the fundamental principle underlying both of these therapies. Targeting cancer cells with both ligands and medications. The active molecule targets both ligands and medications. Antibodies (or fragments) can take on the role of the attacking ligand when combined with the therapeutic particle. Rapid focusing on internalised, overexpressed cell-surface receptors is intended to improve cellular (Bitler and Schroeder 2010).
Protein constructs in CPP
CPP networks for the supply of peptides and proteins for oncology antiproliferation programs have been actively developed for many years now. These are commonly chimerical conjugates with CPP sequencing and a therapy-effective peptide or protein strand (Zhang et al. 2018). These structures are very commonly used to reach protein–protein (PPI) connections, which play a vital part in cancer growth. One of the most recent examples was a leviathan in which the newly developed Mut3DPT serine/threonine phosphatase PP2A was used in combination with CPP, which can trigger apoptosis by disrupting SET oncoprotein associations and thereby activate apoptosis (Kuhlmann et al. 2017). Sirt2 is a sirtuin deacetylase that deacetylates RhoGDIa and was one other protein–protein interaction that has been effectively resolved by a CPP chimera. According to this article, therapy of cervical cancer cells with K52-trifluoroacetate, a substrate peptide of the sirtuin receptor that is derived from RhoGDIa, significantly reduced the cells’ (Kurrikoff et al. 2019).
Peptide therapies
A host of diseases have been treated with peptide-based medicine. Animal models in vitro and in vivo, peptide-based therapies were studied, with promising results in some cases. Peptide-based cancer treatments, such as peptide vaccines, have increasingly gained prominence(Saif et al. 2014). Sipuleucel-T was approved by the US Food and Drug Administration (FDA) as the first standard peptide vaccine for prostate tumours, there have been increased numbers of clinical trials for certain different forms of cancer, such as melanoma, glioblastoma, breast cancer, and gastric cancer(Cheever and Higano 2011).
Clinical use
A variety of peptides are currently being used as cancer treatments or screening tools. Examples are: The first alternative drugs for the prevention of mature prostate cancer are gonadotropin-releasing hormone (GnRH) agonists for androgen reduction (androgen depletion therapy) and the diagnosis of breast cancer(Singh et al. 2015).
Anticancer peptide
ACPs are active and harmful to cancer cells as short peptides including amino acid sequences (Chiangjong et al. 2020; Otvos 2008; Sw et al. 2016; Huang et al. 2015). The high degree of selectivity, high penetration, and simple modification of anticancer peptides are the better options compared to antibodies and tiny molecules(Peyressatre et al. 2015). Cancer peptides act as molecular peptides, capable of entering the cell or chromatin membrane and attaching them directly to it or binding peptides that are linked to the drugs against cancer (Chu et al. 2015; Huang et al. 2017; Casazza and Fairchild 1996; Fuertes et al. 2012). In cancerous cells, anticancer peptides penetrate the cellular membrane, the nuclear membrane, as molecularly targeted peptides, particularly in the alpha-helical process and/or mitochondrial membrane performing pharmacological activity through various methods (such as inactivation of DNA production or cell division) to promote cancer cell proliferation (Casazza and Fairchild 1996). Nevertheless, bonded peptides that do not possess anti-cancer properties can also detect and infiltrate the cancerous cell membrane, called Peptides that target tumors or penetrate cells (Huang et al. 2017). There are more than 100 distinct CPPs, with their series differing enormously. There are also many different methods, as well as for origin, pattern, function, therapeutic, and absorption processes, to distinguish these peptides. the use, however, hydrophobic and amphipathic, cationic, CPPs are quickly divided into three groups based on their physiological chemical properties (Ramsey and Flynn 2015).
Mechanism of cellular uptake of CPP
Mechanism of cellular uptake of CPP is divided into two parts: Endocytosis, Direct penetration are shown in Fig. 3.
Fig. 3.
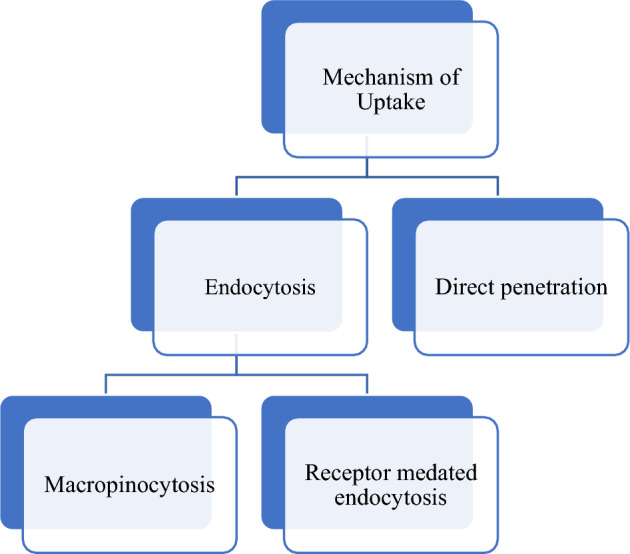
Mechanism of uptake
Cellular membranes are the structure that already separates living cells from the atmosphere and usually only allow small molecular molecules to pass into the cell through the hurdle. Any medications are massive lipophilic molecules that have substantial cell membrane penetrating limits. A community of small peptides that act as transport vectors for vast molecules has been invented. They may have been named by various names like membrane translocating sequence, Trojan peptide, A protein translocation domain, or more colloquially, a CPP (Kapoor et al. 2012). CPPs are generally categorized as low water-soluble, slightly lipophilic, and/or polybasic peptides (with a maximum of 30–35 amino acid particulates with a net gain physiological pH load (Gräslund et al. 2011; Letoha et al. 2010). Cellular uptake of CPPs and their conjugates are shown in Fig. 4. The main benefit of CPPs is they can be capable of reaching the membrane of the cell at small micromolar concentration amounts in vivo and in vitro without the use of any chiral receptors, while also inducing severe cell damage. Besides, these peptides can internalize naturally occurring cargoes, including high and poor toxic medications, electrostatically or covalently (Cell-Penetrating Peptides: Methods and Protocols | Ülo Langel | download 2021).
Fig. 4.
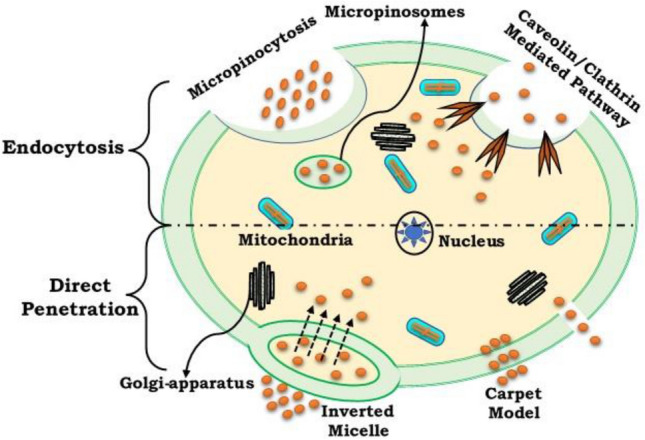
Cellular uptake of CPPs and their conjugates
Direct penetration (energy-independent pathway)
There are different processes involved, for example, the creation of pores, the method carpet-like, and the model membrane dilution (Vijakumaran et al. 2020; Xie et al. 2020) Energy-independent direct penetration is accomplished at minimum temperatures requiring many input routes initially dependent on cell institutionalization (Derossi et al. 1996). The use of endocytose CPP-peptide complexity is an energy-dependent endocytose and micropinocytosis operation (Matsuzaki et al. 1996). Different processes defined as micelle forming inverted can involve direct penetration through individual energy pathways (Lee et al. 2005), pore formation (Elson-Schwab et al. 2007), Models like the tapestry, and the diluting membrane (Stalmans et al. 2015). The interaction of positively charged CPP with negatively charged membrane molecules such as heparan sulphate (HS) and the phospholipid bilayer is the first step in both of these processes (Stalmans et al. 2015). Conceptualization is strongly dependent on the concentration of peptide, peptide series, and lipid composition of every membrane model sample. Direct permeation is more common at high CPP concentrations and for primary amphipathic CPPs such as transportation analogs and MPG. The “inverted micelle” is a method for direct penetration still proposed at the initial level. This process is also included in the relationship of positively loaded CPP and negatively loaded lipid membrane elements, such as tryptophan, between hydrophobic remains and the hydrophobia portion of the membrane. As a consequence, for highly cationic CPPs like TAT, this mechanism is not feasible. The barrel stave model and the tribal model are used to prepare the pores. Helical CPPs form a vessel through which lipid chains are nearly lipophilic residues and central pores are formed by water-soluble residues inside the barrel stave configuration. In the model of toroid lipids, they can curve to such an extent that the CPP is near the functional group always. Pores happen in both mechanisms if the concentration of the peptide is greater than a specific amount, various and of different peptides. The interactions between the phospholipid of the negative load and the cationic CPPs within the taper process model and membrane thickening approach result in the membrane thickening and dilution accordingly (Jones 2007; Mayor and Pagano 2007). CPP transport is obtained afterward when CPP concentration is greater than a threshold (Jones 2007; Mayor and Pagano 2007).
Endocytosis pathway (energy-dependent pathway)
Cells collect molecules from outside of the membrane during the endocytosis process and consume them. There are several endocytosis pathways, phagocytosis is used for massive particle uptake, and pinocytosis for solvent uptake. Pinocytosis is also called micropinocytosis, endocytosis which is based on Clathrin or caveolin endocytosis (Richard et al. 2003). Micropinocytosis is synonymous with the internal pliage of the plasma membrane surface, causing macropinosomes, vesicles to grow. The membrane is close to the cell membrane surrounding macropinosomes, for membrane invagination, dynamite protein is essential. Clathrin or caveolin pits are included in the absorption pathway in receptor-mediated endocytosis. The intracellular portion of the membrane is covered by both clathrin and caveolin proteins. They are needed for membrane invagination and the formation of vesicles following extracellular molecules binding to the membrane receptor. The vesicles are covered in Clathrin with a diameter of about a few hundred nanometres, while the diameter of the caveolin is about 50–80 nm (Ozcelikkale et al. 2017; Chen et al. 2016). Previous research indicated the absorption process of most CPPs was direct penetration. This hypothesis was focused on the discovery by the energy-independent pathway that peptides reach the cell also at 4 °C. After experiments have shown that this inference is caused by experimental artifacts. Methanol or formaldehyde can lead to experimental objects using the assessed cells for confocal microscopy, nowadays when using trypsin to derive externally linked peptides and live-cell confocal microscopy this issue is usually avoided (Tang et al. 2015). For most CPPs, endocytosis is now usually assumed to be implicated in the process of translocation. It is most possible, although, that different pathways function in all CPPs under different circumstances.
Methods for researching the molecular mechanisms of uptake
Cell culture-based biological approaches
To measurably examine the cellular absorption process, there are already several other research approaches. In general, the mechanism responsible for the beginning can be identified by blocking one or even more pathways. One typical method of assessing endocytic involvement is to treat 4 °C peptide cells that hinder all energy-dependent pathways (Kapoor et al. 2012). To establish the mechanism of absorption of CPPs, particular endocytosis blockers are commonly used, and using these inhibitors, it has been shown that TAT absorption (Allen 2002; Kirpotin et al. 2006; Zhang et al. 2018; Kuhlmann et al. 2017; Kurrikoff et al. 2019; Saif et al. 2014; Cheever and Higano 2011; Singh et al. 2015; Chiangjong et al. 2020; Otvos 2008; Sw et al. 2016; Huang et al. 2015; Peyressatre et al. 2015) has been hindered by cytochalasin D, a micropinocytosis inhibitor (Wadia et al. 2004). These findings were corroborated by another analysis, in which amiloride, another micropinocytosis promoter, inhibited TAT fusion protein uptake(Vercauteren et al. 2010). The use of inhibitors in establishing the process of absorption is complicated since the inhibitor is not entirely precise (Sieczkarski and Whittaker 2002; Sandgren et al. 2002). Closing down one uptake path may also lead to an ineffective uptake of another process. Colocation with endocytose markers has also been investigated for the investigative process of absorption processes. The intracellular destiny of CPPs can also be calculated using this process. To decide if CPPs collocate with lysosomes, lysotracker red, a material that transmits light in alkaline conditions, can be used (Canton and Grinstein 2015; Ord et al. 1983). The impact of chloroquine (CQ) has now been used in many trials as an agent of endosomal eutrophication and to facilitate the efficacy of CPP. CQ is also used for the proof of an endocytosis pathway. A moderately small hydrophobic core with two simple categories is the lysosomotropic agent CQ. CQ acts to prohibit endosome/lysosome merger by resisting the pH decrease within the endosome. The macromolecule then persists in the endosome for a longer time (Magzoub and Gräslund 2004). If CQ is impacted by the results of a practical experiment, it can be inferred that endocytosis is at least part of the process of the CPP.
Biophysical methods
Analysis of the relationship between cell-penetrating peptide CPPs and model membranes or lipid bilayers is useful in recognizing the CPP transcriptional mechanisms (Kapoor et al. 2012). In lipid-peptide interaction studies, membrane models more commonly used are, in particular, large uniflagellar phospholipid vesicles (LUV’s). In physiological studies of the uptake process, experimental parameter factors such as the membrane structure of peptide strength and lipid load are essential. Physiological LUV leakage experiments are measures of the level of membrane disruption induced by various CPPs. The results are often linked to CPPs' direct penetration or exhaust. Spherical dichroism, fluorescence, and nuclear magnetic resonance are some of the methods used, which offer more precise details on the secondary layer, the membrane, and 3D structures, respectively. Another approach used to predict CPP’s relationship with the membranes is molecular modelling (Derakhshankhah and Jafari 2018).
Limitations of CPPs
Despite the numerous benefits of CPPs, it should be remembered that they have multiple disadvantages due to their chemical and mechanical properties. The poor cell, tissue, and organ specificity of a significant number of first-generational CPPs. As a result, for medicinal purposes, Those CPPs should be added to the target tissue directly. Cell, organ, or Disease-specific peptide carriers have only been in progress for a few years and will take time to prove themselves in clinical trials. Since this differs from one CPP to the next, from freight to cargo, and from cell type to cell type, each CPP freight complex needs to be measured for its transportability, cytotoxicity, and necessary aids for each purpose. As a consequence, CPPs do not represent the Philosopher's Stone. Each implementation must be meticulously optimized in terms of internalization efficiency and cytotoxicity. One of the most serious disadvantages arises from the intracellular absorption of endosomes. Auxiliary compounds release or loaded polymers, like(polyetherimide) PEIs, of all these cell organelles, requiring their destabilization. Many of these auxiliary compounds can be cytotoxic. New CPPs should be formed with non-endosomal pathways to avoid endosomal absorption (Reissmann 2014).
Nanoparticles
The ability to continuously upgrade nanoscale manufacturing materials with tunable physicochemical properties has culminated in the unavoidable application of NPs that are used in a variety of facets of our daily lives, including medicine NPs have not only been engineered to allow rapid identification of faulty cellular pathways for biosensors and bioimaging but also therapeutic purposes, with the ability to revolutionize our ongoing medication and visualization of numerous diseases, including cancers (Chu et al. 2015). The supervised killing of a cancer cell is usually accomplished using heat (photothermal therapy, hyperthermia), ultrasound, radiation, reactive oxygen species (photodynamic therapy), gene and immune modulation, transmission, or combinations of high drug carriers (Stephens and Allan 2003), by its highly efficient surface relative to their limited length, NPs are not only effective vectors for therapeutic (bio)molecules but also have inherent properties which permit intracellular or therapeutic activity dependent on structure. nanoparticles filed patents on cancer are shown in Table.2.
Table 2.
List of patents
| Work done | Patent no. | References |
|---|---|---|
| 1. The procedure consists of applying many nanoparticles to a patient to locate a tumour, nanoparticles are covered with antitumor antibodies and cell-penetrating peptides (CPPs), and a polymer, as well as nanoparticles incorporating medication and/or a gene, as well as a dye or indicator in the polymer coating, at least some of the nanoparticles attaching to surface antigens of tumour cells to form a tumour cell/nanoparticle complex | US20190091350A1 | US20190091350A1 (2021) |
| 2. A peptide POD is equipped with the capacity to penetrate and supply fluorophores, siRNA, DNA, and quantum dots to cultured cells as well as in vivo retinal and eyepiece tissues. Adenovirus-decorated POD couples enhance the tropism of some cells and can provide a safer and more effective route for molecular delivery in vivo to the eye and other tissues. POD constructs are a restorative means of treatment of cells and tissues, including all ocular and retinal deterioration tissue | US8778886B2 | Methods of making and using a cell penetrating peptide for enhanced delivery of nucleic acids, proteins, drugs, and adenovirus to tissues and cells, and compositions and kits (2010) |
| 3. The invention relates to a preparation method of a difunctional nanoparticle preparation entrapping vincristine sulphate, which belongs to the technical field of medicine. The difunctional nanoparticle preparation is prepared by entrapping the vincristine sulphate in a PLGA-PEG polymer carrier modified by folic acid/cell-penetrating peptide through a multiple emulsion method. The difunctional nanoparticle preparation shows favourable pharmacokinetic behaviour in vitro and vivo. The diameter of the prepared PLGA-PEG difunctional nanoparticles modified by folic acid/cell-penetrating peptide is 287.2 ± 0.8 nm, and the difunctional nanoparticle preparation has a high drug loading rate, high entrapment rate, and good stability | CN101926775B | CN101926775B (2021) |
| 4. The invention relates to the modification of a cell-penetrating peptide for realizing a low-toxicity administration system with a positive targeting selecting function. The invention aims to actively convey an antitumor medicament to tumour tissues in a targeted way and make the antitumor medicament enter tumours cells to a larger extent using the administration system which can be used for activating a cell-penetrating function so that the toxicity at a nontumor position is lowered while the antitumor effect of the medicament is enhanced | CN102552929A | CN102552929A (2021) |
CPPs and nanoparticles formulations
The non-covalent interaction of cargo and CPP has acquired traction with the increase in nano preparations in cancer science (Tabujew et al. 2015). Non-covalent cargo fixtures have the simplicity of scale and extensibility, but of course, any nanoformulation has an advantage: physical characteristics and chemical structure that have not been well established. Vaseem Shaikh Muhammad et al. The formulation and optimization of polymer-loaded doxorubicin nanomaterials were studied was conducted based on the configuration of Box-Behnken and important process parameters were chosen. The Box-Behnken architecture (BBD) was used to assess the effect of the chosen CPP on essential quality attributes (CQA) and to build a design space. Lyophilization strengthened the improved formulation which was used on the A549 cancer cell for in vitro release of drugs and in vitro action. Besides, the colloidal stabilization of NPs in the biological setting was tested. According to the researcher, BBD optimization of DOX-PLGA-NPs resulted in a successful doxorubicin drug carrier that could provide a novel cancer treatment choice.
Delivery of nanoparticles using CPP
Nanomaterials are chemical substances or particle-size materials in at least one dimension ranging from 1 to 100 nm. Due to the increased volume of the specific surface area, nanomaterials can differ from identical materials without nanoscale properties. The nanomaterial used in CPP is shown in Fig. 5.
Fig. 5.
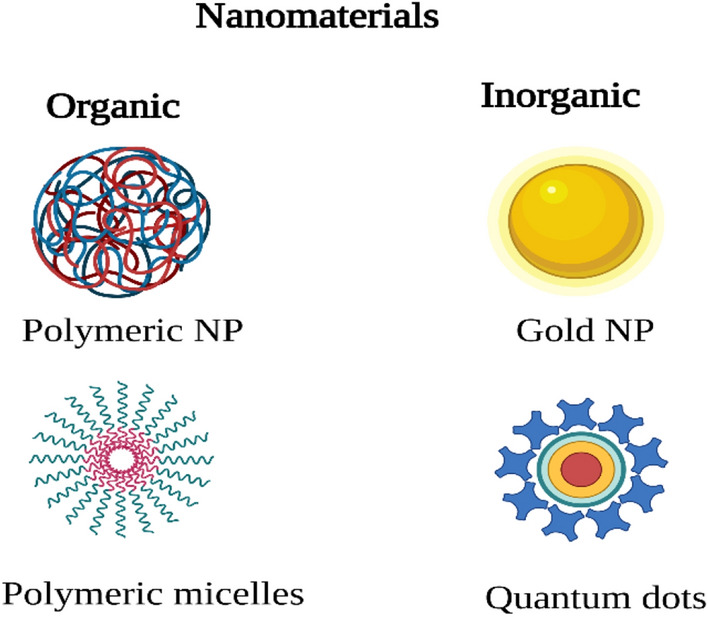
Nanomaterials used in CPPs
Quantum dots
Since their intracellular or exogenous molecules may illuminate, fluorescent markers are a useful method for studying cells in living conditions and are often used to detect and analyse multiple complex cellular mechanisms (Tsoi et al. 2013) Nanocrystals with a centre diameter of 1–6 nm are quantum dots (QDs). They can consist of different components (groups II to IV or III to V), but the most widely found in life science are CdSe and CdTe (Delehanty et al. 2009). However, its use of non-biodegradable high metals for medicinal purposes demands that their biodistribution and long-term toxic effects be carefully considered (Xue et al. 2007). Thus, by using a little finished hydrodynamic radius (< 5.5 nm) and composition with fully nontoxic and even degreasing coating materials it is necessary to ensure quick kidney clearance of QDs. These composites are typically also applied in the case of aqueous incentives to improve their solubility and equipping quantum points with functional groups, which are already in turn required to conjugate the biological compounds. Ds are also represented as an excellent fluorescent labelling candidate particularly for extended observations because they not only have photobleaching resistance, high quantum yields, and can be tuned for photo excitement (Lei et al. 2008), but still clearly defined intensity peaks. Xue et al. discussed for e.g., Tat peptide was conjugated with thiol-capped CdTe QDs (2–4 nm in diameter) in human and human breast cancer (MCF7) cells and attributed their intracellular efficacy with unmodified CdTe QDs by the confocal microscopy laser scanner. The writers were able to show that intracellular transport in both cell lines improved with Tat conjugation (Liu et al. 2013). The same goes for CdSe-ZnS QDs encompassed with PEG and were covalently attached to Tat to be used in mesenchymal stem cells effectively (Kumari et al. 2010) Liu et, al. have shown their ability to the competitive position of the complex inside cells as the result of the association of QDs with chimerical IR9 CPP (IR9: mixed INF7 peptide and none-arginine, R9) and stable IR9 + QD complexes. Owing to the cationic nature of IR9, the plasma membrane is of considerable significance for cell internalization because of the electrostatic attraction of IR9/ cargo configuration. In their low concentration in cells, IR9 and IR9 cargo compounds are not cytotoxic; thus, a good tool for biological processes, including gene expression, can be the latest chimerical CPP (Hans and Lowman 2002).
Polymeric nanoparticles
Structures with a diameter of < 1 micron and made of natural or synthetic polymers can be classified as nanoparticles in the polymer. Because of their low toxicity and bioavailability, Polysaccharides, for example, are natural polymers that are well suited for drug delivery (Feiner-Gracia et al. 2018). However, batch-to-batch variations are different and vary in purity, rendering synthetic polymers, such as poly (lactic acid) (PLA) and poly (glycolic acid) are biocompatible and biodegradable (PGA), or their copolymers the favoured alternative [poly (lactide Co-glycolide) (PLGA)] (Layek and Singh 2013). Their use and study, particularly in biomedicine, have increased exponentially since several generations earlier. Polymeric nanoparticles have been founded (Li et al. 2010; Banks 2008). N. Fine-Gracia et al. for the formulation of Dell penetrator peptide (CPP) process—practical PLGA nanoparticles capable of effectively crossing the plasma membrane and releasing their loads in the cell where even the most involved ingredients have to be therapeutic. Penetration pAntp peptide is a peptide series originating from the Drosophila antennapedia homeodomain, with 43.75% essential amino acids. Linoleic acid and dual functionalized chitosan (CS-Lin-Pen) penetrated the skin, according to the findings, a modified CS was successfully used for plasmid DNA transfection (pDNA) (Demeule et al. 2014; Jafari et al. 2015). In brain drug distribution, the blood–brain barrier (BBB) is a big hurdle. The Blood–Brain Barrier (BBB) is made up of near endothelial junctions that essentially block therapeutic molecules from passing into the central nervous system (Silva et al. 2019). CPPs combined with nanoparticles have been added as an appealing vehicle for maximizing brain-targeted transport; however, due to their positive charge, the brain delivery efficacy of these vehicles may be canceled out by their gradual systematic clearance (Park et al. 2013).
Gold nanoparticles
Gold nanoparticles (GNPs) are a type of metal nanoparticle that is commonly used in drug delivery systems. Many medical researchers have been inspired by their unusual properties including mediated minimal toxicity, good solubility, fast synthesis, bioconjugation, good absorption, effective bloodstream clearance, and scattering (Qin et al. 2021). GNPs have a favourable effect, on the other hand; they can also be followed by an energy-independent approach to translocate cationic CPPs into the cells. The anticancer drug doxorubicin (DOX) is transported using -helix peptide 17-amino acids combined with gold nanoparticles as vectors; this approach has been used to deliver more efficiently than free DOX for cellulite-selective internalization activities of the chosen peptides. It is also shown to be more effective than free DOX (Gessner and Neundorf 2020).
CPP-NP conjugates for cancer treatment
Most chemotherapeutic medications have low pharmacokinetics and should be used to maintain a high degree of clearing and minimal retention at the goal location. Especially due to their small scale, some NPs can also bypass the barrier of the blood–brain, thus providing diagnosis and treatment of difficult targets, including brain tumours (Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties 2021). Multimodal structures can also be built as a network within one NP, for example, it may include medicinal objects and decorate the surface in several functions, including housing equipment, CPPs, and PEG chains. We have given some examples of years of multifunctionality in various fields of cancer research in the following article (Perillo et al. 2017). The application of the cell-penetrating peptide is shown in Fig. 6.
Fig. 6.
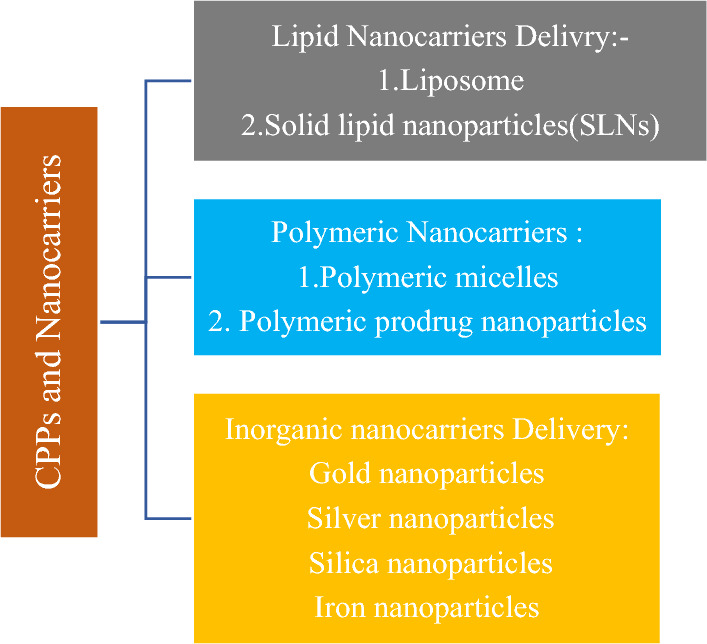
Application of the cell-penetrating peptide as nanocarriers delivery vehicles
Cancer targeting
CPPs are typically nonselective and are primarily capable of joining all types of cells, CPP-NP cancer diagnostic or treatment mechanisms are usually paired with specific groups, Biomolecules that recognize cancerous cells' surface or membrane receptors are most commonly used. Besides, the addition of specific affinity labels can allow crossing the blood–brain barrier (BBB), as seen currently by dos Santos, Rodrigues, etc., who created lipid nanoparticles and used CPPs to design them and an overexpressed TF pattern for transfer receptors on BBB. The addition of selective affinity labels is also possible. The findings showed that Tf and Tat-modified liposomes are more capable of transmitting the boundary layer in an in vivo configuration and of transferring neural cells to sustain the high importance of the current brain-oriented gene deliverable (Dowaidar et al. 2018).
Cancer imaging
NPs can be adapted for high clinical instruments and pose impressive opportunities for tumour diagnostics due to their varying physicochemical structure and properties, Furthermore, based on their features and ease of adjustment for usable classes, NPs have the potential to be used as multipurpose imaging platforms, including optical imaging, radionuclide imaging, magnetic resonance imaging or ultrasound imaging. As newly started by Perillo et al., these properties are also combined to design multifunctional NP structures consisting of fluorescent teeth that have been added to superparamagnetic iron oxide nanoparticles (Bolhassani 2011).
Cancer therapy
The utilization of CPP-adjusted NP drug transporters draws extraordinary interest in creating novel promising malignant growth medicines. Surprisingly, the disadvantages of CPPs and inorganic particles can be mitigated by mixing multiple composites. For example, CPPs, as other peptide treatments, are proteolytically unstable, which hinders, for example, oral administering and affects ultimate bioavailability. Previously, one way to boost CPP’s productivity was demonstrated when iron oxide NPs with CPP cross-linked chitosan were not covalently customized. The drug mounting capability's efficiency, in this situation SiRNA, was significantly improved by chitosan reconfiguration, along with the overall biocompatibility and bioavailability of the system (Regberg et al. 2012).
Cell-targeting peptides
Cell-targeting peptides (CTPs) with the ability to recognize cancer cells are particularly appealing for cancer care (Kersemans and Cornelissen 2010; Veloria et al. 2018). The need for these peptides, while minimizing adverse effects in a model system, has improved the versatility and effectiveness of drug delivery (Arrouss et al. 2013). Such a tracer peptide was the cyclic peptide PEGA, already shown to persist in mouse tissue of the breast tumour. In vitro, numerous breast cancer cells were picked up by PEGA peptide covalently attached to the cell-penetrating peptide pVEC. In the case of the conjugate in vivo, which accumulates primarily in the brain tubers and the breast cancer vascular system, tumour cells are consumed. The PEGA-pVEC tracer capability has also been preserved. Moreover, the combination of the anticancer medication chlorambucil with pVEC-PEGA has been shown to improve drug effectiveness more than four times, thus decreasing MCF-7 cell clonogenic sustainability (Veloria et al. 2018). The peptide has recently been introduced by Rebello and her group to imitate an important connection between caspase 9 and serine/threonine phosphatase PP2A in cancer development. The combination of CPP and interfering peptides resulted in a therapeutic peptide building capable of entering cells, inhibiting PP2A's connection with caspase-9, and inducing cultured cells apoptosis(Zhang et al. 2012).
Activatable CPPs
One approach to the issue of CPPs is to activate CPPs (ACPPs). The most recent polycationic CPP in vivo monitoring agents were addressed by Emilia S Olson et.al, which was linked through a severable connection to a safely neutralized polyanion. Absorber and cell absorption are blocked before the connector has been proteolyzed. The first one to be shown to function in an in vivo tumour prototype was a CPP, a metalloproteinase-2 (MMP-2) matrix cleavable, but only HT-1080 xenografts and human squamate cell carcinomas were tested. Further characterization is necessary for specific other types of cancer, in vivo MMP specificity, and spatial resolution of CPPs. In addition to a well-studied transgenic method of spontaneously breast cancer, we now demonstrate that ACPPs can treat multiple xenograft tumour models from various cancer sites (Promotor of mouse mammary tumour virus driving middle T antigen polyoma, MMTV-PyMT) (Snyder and Dowdy 2004). In a variety of cancers, which include breast (MMP-11), colon (MMP-1), stomach (MMP-2 and MMP-9), non-small cell lung cancer (MMP-13), oesophageal lung cancer, many reports have shown a link between elevated MMP interpretation and poor treatment outcomes (MMP-7), And lung cancer of small cells (MMP-3, MMP-11, and MMP-14). Furthermore, the expression of specific MMPs has been used as a prognostic therapeutic impact factor as well as a tumour growth marker in several tumour types (Höckel and Vaupel 2001).
CPPs transfusible agents
While a subset of fatal tumours is regarded with local chemotherapy, several tumors are spread across the body, requiring systemic distribution of anticancer agents. Recent studies in vivo have shown that after intraperitoneal (IP) injection, TAT proteins are distributed to a wide number of tissues, indicating that a regular transmission to single or numerous metastases with transfusible agents could be probable (Höckel and Vaupel 2001; Harada et al. 2006). A fused protein was specifically optimized in hypoxemic tumour cells to build a possible therapeutic protein drug, particularly unique to solid tumors (Harada et al. 2006). Strong tumors in organisms produce a slightly lower oxygen pressure in hyperglycaemic regions than in ordinary tissues. This includes resilience to radiation and cancer therapy to avoid a rise in tumour metastases. These may also prevent an increased risk of cancer (Harada et al. 2006). Hence, tumour hypoxemia as a particular neovascular has been identified (Shi et al. 2012). A Hypoxia-Inducible-1 a transcript factor (HIF-1), the hypoxic reaction master regulator, induces different genes linked to angiogenesis and gluconeogenesis and contributes to the intrusive and metastatic characteristics of tumour (Shi et al. 2012). TAT-ODD-β-Gal protein was seen mostly in the hypoxic areas of the tumors. TAT-β-galactosidase protein may, by comparison, be found in all tumors after administering IP (US20190091350A1 2021).
Conclusion
In this review, we stressed the promise for the diagnosis of cancer with therapeutic peptides. Targeting drugs is important in the treatment of cancer when a drug is selectively and quantitatively accumulated in the targeted organ or tissue. There are passive and active targeting mechanisms available, but NP-based targeting is critical. NPs had immense benefits in decreasing specific cell absorption and adverse effects, stretching circulation, and allowing for the safe release and encapsulation of several mixed-care medications. Several methods are used, such as using CPP to efficiently distribute anticancer peptides to their tumour cell targets, for resolving peptide restrictions. A new and innovative strategy for cancer treatment is therapeutic peptides. Some examples of formulation of nanoparticles using cell-penetrating peptide are shown in Table. 2.
Author contributions
SY and PS are the principal investigators of the study. SY and PS prepared the concept and design. Both authors participated in preparing the final draft of the manuscript, revised the manuscript, and critically evaluated the academic contents.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Data availability
Data is available on request.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
We affirm that all the authors have seen, prepared, and agreed to the submission of the paper and their inclusion of name(s) as co-author(s). We also declare that there are no conflicts of interest for the same.
References
- Adams GP et al (2001) High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules 1. Accessed 21 Jan 2021 (Online) [PubMed]
- Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2(10):750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- Arrouss I, et al. Specific targeting of caspase-9/PP2A interaction as potential new anti-cancer therapy. PLoS ONE. 2013;8(4):e60816. doi: 10.1371/journal.pone.0060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA. Delivery of peptides to the brain: emphasis on therapeutic development. Biopolymers. 2008;90(5):589–594. doi: 10.1002/bip.20980. [DOI] [PubMed] [Google Scholar]
- Bitler B, Schroeder J. Anti-cancer therapies that utilize cell penetrating peptides. Recent Pat Anti-Cancer Drug Discovery. 2010;5(2):99–108. doi: 10.2174/157489210790936252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhassani A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim Biophys Acta Rev Cancer. 2011;1816(2):232–246. doi: 10.1016/j.bbcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Canton J, Grinstein S. Measuring lysosomal pH by fluorescence microscopy. Methods Cell Biol. 2015;126:85–99. doi: 10.1016/bs.mcb.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Casazza AM, Fairchild CR. Paclitaxel (Taxol): mechanisms of resistance. Cancer Treat Res. 1996;87:149–171. doi: 10.1007/978-1-4613-1267-3_6. [DOI] [PubMed] [Google Scholar]
- Cell-Penetrating Peptides: Methods and Protocols | Ülo Langel | download (2021) https://b-ok.cc/book/2575108/841916. Accessed 27 Jan 2021
- Cheever MA, Higano CS. PROVENGE (sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116(5):2826–2885. doi: 10.1021/acs.chemrev.5b00148. [DOI] [PubMed] [Google Scholar]
- Chiangjong W, Chutipongtanate S, Hongeng S. Anticancer peptide: physicochemical property, functional aspect and trend in clinical application (Review) Int J Oncol. 2020;57(3):678. doi: 10.3892/IJO.2020.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H-L, Yip B-S, Chen K-H, Yu H-Y, Chih Y-H, Cheng H-T, et al. Novel antimicrobial peptides with high anticancer activity and selectivity. PLoS ONE. 2015;10(5):e0126390. doi: 10.1371/journal.pone.0126390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CN101926775B (2021) Preparation and application methods of difunctional naonparticle preparation entrapping vincristine sulphate - Google Patents. https://patents.google.com/patent/CN101926775B/en?q=Preparation+and+application+methods+of+difunctional+naonparticle+preparation+entrapping+vincristine+sulphate&oq=Preparation+and+application+methods+of+difunctional+naonparticle+preparation+entrapping+vincristine+sulphate. Accessed 02 Apr 2021
- CN102552929A (2021) Method for enhancing targeting selectivity of administration system by modifying cell penetrating peptide - Google Patents.” https://patents.google.com/patent/CN102552929A/en?q=cell+penetrating+peptide+anticancer+nanoparticles&oq=cell+penetrating+peptide+anticancer+nanoparticles&page=11. Accessed 25 Mar 2021
- de Figueiredo IR, Freire JM, Flores L, Veiga AS, Castanho MARB. Cell-penetrating peptides: a tool for effective delivery in gene-targeted therapies. IUBMB Life. 2014;66(3):182–194. doi: 10.1002/iub.1257. [DOI] [PubMed] [Google Scholar]
- Delaroche D, et al. Tracking a new cell-penetrating (W/R) nonapeptide, through an enzyme-stable mass spectrometry reporter tag. Anal Chem. 2007;79(5):1932–1938. doi: 10.1021/ac061108l. [DOI] [PubMed] [Google Scholar]
- Delehanty JB, Mattoussi H, Medintz IL. Delivering quantum dots into cells: strategies, progress and remaining issues. Anal Bioanal Chem. 2009;393(4):1091–1105. doi: 10.1007/s00216-008-2410-4. [DOI] [PubMed] [Google Scholar]
- Demeule M, et al. Conjugation of a brain-penetrant peptide with neurotensin provides antinociceptive properties. J Clin Investig. 2014;124(3):1199–1213. doi: 10.1172/JCI70647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshankhah H, Jafari S. Cell penetrating peptides: a concise review with emphasis on biomedical applications. Biomed Pharmacother. 2018;108:1090–1096. doi: 10.1016/j.biopha.2018.09.097. [DOI] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269(14):10444–10450. doi: 10.1016/s0021-9258(17)34080-2. [DOI] [PubMed] [Google Scholar]
- Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271(30):18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- Deshayes S, Morris M, Heitz F, Divita G. Delivery of proteins and nucleic acids using a non-covalent peptide-based strategy. Adv Drug Deliv Rev. 2008;60(4–5):537–547. doi: 10.1016/j.addr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties. - Abstract - Europe PMC (2021) https://europepmc.org/article/MED/31616141. Accessed 28 Jan 2021 [DOI] [PMC free article] [PubMed]
- Dokka S, Toledo-Velasquez D, Shi X, Wang L, Rojanasakul Y. Cellular delivery of oligonucleotides by synthetic import peptide carrier. Pharm Res. 1997;14(12):1759–1764. doi: 10.1023/A:1012188014919. [DOI] [PubMed] [Google Scholar]
- Dowaidar M, Nasser Abdelhamid H, Hällbrink M, Langel Ü, Zou X. Chitosan enhances gene delivery of oligonucleotide complexes with magnetic nanoparticles–cell-penetrating peptide. J Biomater Appl. 2018;33(3):392–401. doi: 10.1177/0885328218796623. [DOI] [PubMed] [Google Scholar]
- Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88(2):223–233. doi: 10.1016/S0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- El-Sayed A, Futaki S, Harashima H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: ways to overcome endosomal entrapment. AAPS J. 2009;11(1):13–22. doi: 10.1208/S12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson-Schwab L, Garner OB, Schuksz M, Crawford BE, Esko JD, Tor Y. Guanidinylated neomycin delivers large, bioactive cargo into cells through a heparan sulfate-dependent pathway. J Biol Chem. 2007;282(18):13585–13591. doi: 10.1074/jbc.M700463200. [DOI] [PubMed] [Google Scholar]
- Feiner-Gracia N, Dols-Perez A, Royo M, Solans C, Garcia-Celma MJ, Fornaguera C. Cell penetrating peptide grafting of PLGA nanoparticles to enhance cell uptake. Eur Polymer J. 2018;108:429–438. doi: 10.1016/j.eurpolymj.2018.09.026. [DOI] [Google Scholar]
- Fuertes M, Castilla J, Alonso C, Pérez J. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 2012;10(3):257–266. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- Futaki S, et al. Arginine-rich peptides. J Biol Chem. 2001;276(8):5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, Sugiura Y. Arginine-rich peptides: an abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276(8):5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Gessner I, Neundorf I. Nanoparticles modified with cell-penetrating peptides: conjugation mechanisms, physicochemical properties, and application in cancer diagnosis and therapy. Int J Mol Sci. 2020;21(7):2536. doi: 10.3390/ijms21072536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaz-Jahanian MA, Abbaspour-Aghdam F, Anarjan N, Berenjian A, Jafarizadeh-Malmiri H. Application of chitosan-based nanocarriers in tumor-targeted drug delivery. Mol Biotechnol. 2015;57(3):201–218. doi: 10.1007/s12033-014-9816-3. [DOI] [PubMed] [Google Scholar]
- Gosk S, Moos T, Gottstein C, Bendas G. VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochimica Et Biophysica Acta - Biomembranes. 2008;1778(4):854–863. doi: 10.1016/j.bbamem.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Gräslund A, Madani F, Lindberg S, Langel Ü, Futaki S. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys. 2011 doi: 10.1155/2011/414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habault J, Poyet JL. Recent advances in cell penetrating peptide-based anticancer therapies. Molecules. 2019;24(5):927. doi: 10.3390/molecules24050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6(4):319–327. doi: 10.1016/S1359-0286(02)00117-1. [DOI] [Google Scholar]
- Harada H, Kizaka-Kondoh S, Hiraoka M. Antitumor protein therapy; application of the protein transduction domain to the development of a protein drug for cancer treatment. Breast Cancer. 2006;13(1):16–26. doi: 10.2325/jbcs.13.16. [DOI] [PubMed] [Google Scholar]
- Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- Hu Q, et al. Intracellular pathways and nuclear localization signal peptide-mediated gene transfection by cationic polymeric nanovectors. Biomaterials. 2012;33(4):1135–1145. doi: 10.1016/j.biomaterials.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Huang Y-W, Lee H-J, Tolliver LM, Aronstam RS. Delivery of nucleic acids and nanomaterials by cell-penetrating peptides: opportunities and challenges. Biomed Res Int. 2015;2015:1–16. doi: 10.1155/2015/834079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, et al. Tumor-penetrating peptide fused to a pro-Apoptotic peptide facilitates effective gastric cancer therapy. Oncol Rep. 2017;37(4):2063–2070. doi: 10.3892/or.2017.5440. [DOI] [PubMed] [Google Scholar]
- Jafari S, Dizaj SM, Adibkia K. Cell-penetrating peptides and their analogues as novel nanocarriers for drug delivery. BioImpacts. 2015;5(2):103–111. doi: 10.15171/bi.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Johansson HJ, Andaloussi SEL, Langel U (2011) Mimicry of protein function with cell-penetrating peptides. In: Methods in molecular biology (Clifton, N.J.), vol 683. Humana Press, pp 233–247. 10.1007/978-1-60761-919-2_17 [DOI] [PubMed]
- Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med. 2007;11(4):670–684. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor P, Singh H, Gautam A, Chaudhary K, Kumar R, Raghava GPS. TumorHoPe: a database of tumor homing peptides. PLoS ONE. 2012;7(4):e35187. doi: 10.1371/JOURNAL.PONE.0035187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersemans V, Cornelissen B. Targeting the tumour: cell penetrating peptides for molecular imaging and radiotherapy. Pharmaceuticals. 2010;3(3):600–620. doi: 10.3390/ph3030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkar PS, Soni G, Rathod V, Shetty S, Gupta MK, Yadav KS. An outlook on procedures of conjugating folate to (co)polymers and drugs for effective cancer targeting. Drug Dev Res. 2020;81(7):823–836. doi: 10.1002/ddr.21698. [DOI] [PubMed] [Google Scholar]
- Kirpotin DB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Can Res. 2006;66(13):6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18(7):385–393. doi: 10.1016/J.MOLMED.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Kuhlmann N, Chollet C, Baldus L, Neundorf I, Lammers M. Development of substrate-derived sirtuin inhibitors with potential anticancer activity. ChemMedChem. 2017;12(20):1703–1714. doi: 10.1002/cmdc.201700414. [DOI] [PubMed] [Google Scholar]
- Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Kurrikoff K, Aphkhazava D, Langel Ü. The future of peptides in cancer treatment. Curr Opin Pharmacol. 2019;47:27–32. doi: 10.1016/j.coph.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Layek B, Singh J. Cell penetrating peptide conjugated polymeric micelles as a high performance versatile nonviral gene carrier. Biomacromol. 2013;14(11):4071–4081. doi: 10.1021/bm401204n. [DOI] [PubMed] [Google Scholar]
- Lee MT, Hung WC, Chen FY, Huang HW. Many-body effect of antimicrobial peptides: on the correlation between lipid’s spontaneous curvature and pore formation. Biophys J. 2005;89(6):4006–4016. doi: 10.1529/biophysj.105.068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Tang H, Yao L, Yu R, Feng M, Zou B. Applications of mesenchymal stem cells labeled with tat peptide conjugated quantum dots to cell tracking in mouse body. Bioconjug Chem. 2008;19(2):421–427. doi: 10.1021/bc0700685. [DOI] [PubMed] [Google Scholar]
- Letoha T, et al. Cell-penetrating peptide exploited syndecans. Biochimica Et Biophysica Acta - Biomembranes. 2010;1798(12):2258–2265. doi: 10.1016/j.bbamem.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Li G, et al. Permeability of endothelial and astrocyte cocultures: In vitro Blood-brain barrier models for drug delivery studies. Ann Biomed Eng. 2010;38(8):2499–2511. doi: 10.1007/s10439-010-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren ME, Hällbrink MM, Elmquist AM, Langel Ü. Passage of cell-penetrating peptides across a human epithelial cell layer in vitro. Biochem J. 2004;377(1):69–76. doi: 10.1042/BJ20030760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BR, Liou J-S, Huang Y-W, Aronstam RS, Lee H-J. Intracellular delivery of nanoparticles and DNAs by IR9 cell-penetrating peptides. PLoS ONE. 2013;8(5):64205. doi: 10.1371/journal.pone.0064205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi S, Signore G, Boccardi C, di Rienzo C, Beltram F, Cardarelli F. Spontaneous membrane-translocating peptides: influence of peptide self-aggregation and cargo polarity. Sci Rep. 2015;5(1):16914. doi: 10.1038/srep16914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magzoub M, Gräslund A. Cell-penetrating peptides: small from inception to application. Q Rev Biophys. 2004;37(2):147–195. doi: 10.1017/S0033583505004014. [DOI] [PubMed] [Google Scholar]
- Mai JC, Shen H, Watkins SC, Cheng T, Robbins PD. Efficiency of protein transduction is cell type-dependent and is enhanced by dextran sulfate. J Biol Chem. 2002;277(33):30208–30218. doi: 10.1074/jbc.M204202200. [DOI] [PubMed] [Google Scholar]
- Marty C, Meylan C, Schott H, Ballmer-Hofer K, Schwendener RA. Enhanced heparan sulfate proteoglycan-mediated uptake of cell-penetrating peptide-modified liposomes. Cell Mol Life Sci. 2004;61(14):1785–1794. doi: 10.1007/s00018-004-4166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K, Yoneyama S, Murase O, Miyajima K. Transbilayer transport of ions and lipids coupled with mastoparan X translocation. Biochemistry. 1996;35(25):8450–8456. doi: 10.1021/bi960342a. [DOI] [PubMed] [Google Scholar]
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methods of making and using a cell penetrating peptide for enhanced delivery of nucleic acids, proteins, drugs, and adenovirus to tissues and cells, and compositions and kits (2010) Accessed 15 Mar 2021 (Online)
- Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discovery Today. 2012;17(15–16):850–860. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Moreno VM, Baeza A, Vallet-Regí M. Evaluation of the penetration process of fluorescent collagenase nanocapsules in a 3D collagen gel. Acta Biomater. 2021;121:263–274. doi: 10.1016/J.ACTBIO.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100(4):201–217. doi: 10.1042/bc20070116. [DOI] [PubMed] [Google Scholar]
- Nakayama F, et al. Fibroblast growth factor-12 (FGF12) translocation into intestinal epithelial cells is dependent on a novel cell-penetrating peptide domain: Involvement of internalization in the in vivo role of exogenous FGF12. J Biol Chem. 2011;286(29):25823–25834. doi: 10.1074/jbc.M110.198267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neundorf I, et al. Fusion of a short HA2-derived peptide sequence to cell-penetrating peptides improves cytosolic uptake, but enhances cytotoxic activity. Pharmaceuticals. 2009;2(2):49–65. doi: 10.3390/ph2020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord JM, Wakeland JR, Crie JS, Wildenthal K. Mechanisms of degradation of myofibrillar and nonmyofibrillar protein in heart. Adv Myocardiol. 1983;4:195–199. doi: 10.1007/978-1-4757-4441-5_17. [DOI] [PubMed] [Google Scholar]
- Otvos L. Peptide-based drug design: here and now. Methods Mol Biol. 2008;494:1–8. doi: 10.1007/978-1-59745-419-3_1. [DOI] [PubMed] [Google Scholar]
- Ozcelikkale A, Moon H, Linnes M, Han B. In vitro microfluidic models of tumor microenvironment to screen transport of drugs and nanoparticles. Wiley Interdisciplinary Rev Nanomed Nanobiotechnol. 2017;9(5):e1460. doi: 10.1002/wnan.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Tsutsumi H, Mihara H. Cell penetration and cell-selective drug delivery using α-helix peptides conjugated with gold nanoparticles. Biomaterials. 2013;34(20):4872–4879. doi: 10.1016/j.biomaterials.2013.03.049. [DOI] [PubMed] [Google Scholar]
- (PDF) Peptide Nanoparticle Delivery of Charge-Neutral Splice-Switching Morpholino Oligonucleotides | Suzan Hammond and Graham McClorey - Academia.edu (2021) https://www.academia.edu/14399089/Peptide_Nanoparticle_Delivery_of_Charge_Neutral_Splice_Switching_Morpholino_Oligonucleotides. Accessed 24 Mar 2021 [DOI] [PMC free article] [PubMed]
- Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Perillo E, Hervé-Aubert K, Allard-Vannier E, Falanga A, Galdiero S, Chourpa I. Synthesis and in vitro evaluation of fluorescent and magnetic nanoparticles functionalized with a cell penetrating peptide for cancer theranosis. J Colloid Interface Sci. 2017;499:209–217. doi: 10.1016/j.jcis.2017.03.106. [DOI] [PubMed] [Google Scholar]
- Peyressatre M, Prével C, Pellerano M, Morris MC. Targeting cyclin-dependent kinases in human cancers: from small molecules to peptide inhibitors. Cancers. 2015;7(1):179–237. doi: 10.3390/CANCERS7010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar U, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Can Res. 2013;73(8):2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Zhu Y, Zheng D, Zhao Q. pH-sensitive polymeric nanocarriers for antitumor biotherapeutic molecules targeting delivery. Bio-Design Manuf. 2021 doi: 10.1007/s42242-020-00105-4. [DOI] [Google Scholar]
- Ragin AD, Morgan RA, Chmielewski J. Cellular import mediated by nuclear localization signal peptide sequences. Chem Biol. 2002;9(8):943–948. doi: 10.1016/S1074-5521(02)00189-8. [DOI] [PubMed] [Google Scholar]
- Ramsey JD, Flynn NH. Cell-penetrating peptides transport therapeutics into cells. Pharmacol Ther. 2015;154:78–86. doi: 10.1016/j.pharmthera.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Raucher D, Ryu JS. Cell-penetrating peptides: strategies for anticancer treatment. Trends Mol Med. 2015;21(9):560–570. doi: 10.1016/j.molmed.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Regberg J, Srimanee A, Langel Ü. Applications of cell-penetrating peptides for tumor targeting and future cancer therapies. Pharmaceuticals. 2012;5(9):991–1007. doi: 10.3390/PH5090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissmann S. Cell penetration: scope and limitations by the application of cell-penetrating peptides. J Pept Sci. 2014;20(10):760–784. doi: 10.1002/psc.2672. [DOI] [PubMed] [Google Scholar]
- Richard JP, et al. Cell-penetrating peptides. J Biol Chem. 2003;278(1):585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev. 2017;110–111:3–12. doi: 10.1016/j.addr.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif JMS, et al. Novel prostate acid phosphatase-based peptide vaccination strategy induces antigen-specific T-cell responses and limits tumour growth in mice. Eur J Immunol. 2014;44(4):994–1004. doi: 10.1002/EJI.201343863. [DOI] [PubMed] [Google Scholar]
- Sandgren S, Cheng F, Belting M. Nuclear targeting of macromolecular polyanions by an HIV-Tat derived peptide: role for cell-surface proteoglycans. J Biol Chem. 2002;277(41):38877–38883. doi: 10.1074/jbc.M205395200. [DOI] [PubMed] [Google Scholar]
- Shi NQ, Gao W, Xiang B, Qi XR. Enhancing cellular uptake of activable cell-penetrating peptide–doxorubicin conjugate by enzymatic cleavage. Int J Nanomed. 2012;7:1613. doi: 10.2147/IJN.S30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83(7):1535–1545. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- Silva S, Almeida AJ, Vale N. Combination of cell-penetrating peptides with nanoparticles for therapeutic application: a review. Biomolecules. 2019;9(1):22. doi: 10.3390/biom9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, et al. SATPdb: a database of structurally annotated therapeutic peptides. Nucleic Acids Res. 2015;44(D1):D1119–D1126. doi: 10.1093/nar/gkv1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharm Res. 2004;21(3):389–393. doi: 10.1023/B:PHAM.0000019289.61978.f5. [DOI] [PubMed] [Google Scholar]
- Squires S, et al. Effects of redox state on the efficient uptake of cell permeable peptide in mammalian cells. Open Biochem J. 2013;7:54–65. doi: 10.2174/1874091x20130531001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmans S, Bracke N, Wynendaele E, Gevaert B, Peremans K, Burvenich C, et al. Cell-penetrating peptides selectively cross the blood-brain barrier in vivo. PLoS ONE. 2015;10(10):e0139652. doi: 10.1371/journal.pone.0139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DJ, Allan VJ. Light microscopy techniques for live cell imaging. Science. 2003;300(5616):82–86. doi: 10.1126/science.1082160. [DOI] [PubMed] [Google Scholar]
- Su R, Hu J, Zou Q, Manavalan B, Wei L. Empirical comparison and analysis of web-based cell-penetrating peptide prediction tools. Brief Bioinform. 2020;21(2):408–420. doi: 10.1093/bib/bby124. [DOI] [PubMed] [Google Scholar]
- Sw B, et al. Large gliadin peptides detected in the pancreas of NOD and healthy mice following oral administration. J Diabetes Res. 2016;2016:2424306–2424306. doi: 10.1155/2016/2424306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S, Sahu P, Beg S, Babu S. Nanoparticles for cancer targeting: current and future directions. Curr Drug Deliv. 2016;13(8):1290–1302. doi: 10.2174/1567201813666160713121122. [DOI] [PubMed] [Google Scholar]
- Tabujew I, Lelle M, Peneva K. Cell-penetrating peptides for nanomedicine-how to choose the right peptide. BioNanoMaterials. 2015;16(1):59–72. doi: 10.1515/bnm-2015-0001. [DOI] [Google Scholar]
- Tang Y, Hu J, Elmenoufy AH, Yang X. Highly efficient FRET system capable of deep photodynamic therapy established on X-ray excited mesoporous LaF3: Tb scintillating nanoparticles. ACS Appl Mater Interfaces. 2015;7(22):12261–12269. doi: 10.1021/ACSAMI.5B03067. [DOI] [PubMed] [Google Scholar]
- Tat Protein from Human Immunodeficiency Virus Forms a Metal-Linked Dimer on JSTOR (2021) https://www.jstor.org/stable/1701694?seq=1. Accessed 24 Mar 2021 [DOI] [PubMed]
- Thanki K, Kushwah V, Jain S. Recent advances in tumor targeting approaches. Cham: Springer; 2015. pp. 41–112. [Google Scholar]
- Tsoi KM, Dai Q, Alman BA, Chan WCW. Are quantum dots toxic? Exploring the discrepancy between cell culture and animal studies. Acc Chem Res. 2013;46(3):662–671. doi: 10.1021/ar300040z. [DOI] [PubMed] [Google Scholar]
- Ulapane KR, Kopec BM, Moral MEG, Siahaan TJ. Peptides and drug delivery. Adv Exp Med Biol. 2017;1030:167–184. doi: 10.1007/978-3-319-66095-0_8. [DOI] [PubMed] [Google Scholar]
- Ulbrich K, Holá K, Šubr V, Bakandritsos A, Tuček J, Zbořil R. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem Rev. 2016;116(9):5338–5431. doi: 10.1021/acs.chemrev.5b00589. [DOI] [PubMed] [Google Scholar]
- US20190091350A1 (2021) Cancer Treatment Methods Using Thermotherapy And/Or Enhanced Immunotherapy - Google Patents. https://patents.google.com/patent/US20190091350A1/en?oq=US20190091350A1. Accessed 13 Mar 2021
- Veloria JR, Chen L, Li L, Breen GAM, Lee J, Goux WJ. Novel cell-penetrating-amyloid peptide conjugates preferentially kill cancer cells. MedChemComm. 2018;9(1):121–130. doi: 10.1039/c7md00321h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren D, et al. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol Ther. 2010;18(3):561–569. doi: 10.1038/mt.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijakumaran U, Nordin F, Hamid ZA, Abdullah M, Jun TG. Development of cell penetrating peptides for effective delivery of recombinant factors into target cells. Protein Pept Lett. 2020;27(11):1092–1101. doi: 10.2174/0929866527666200525164135. [DOI] [PubMed] [Google Scholar]
- Vivès E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272(25):16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Vivès E, Schmidt J, Pèlegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta Rev Cancer. 2008;1786(2):126–138. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10(3):310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Xie J, et al. Cell-penetrating peptides in diagnosis and treatment of human diseases: from preclinical research to clinical application. Front Pharmacol. 2020;11:697. doi: 10.3389/fphar.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue FL, et al. Enhancement of intracellular delivery of CdTe quantum dots (QDs) to living cells by tat conjugation. J Fluoresc. 2007;17(2):149–154. doi: 10.1007/s10895-006-0152-2. [DOI] [PubMed] [Google Scholar]
- Ye J, et al. CPP-assisted intracellular drug delivery, what is next? Int J Mol Sci. 2016;17(11):1892. doi: 10.3390/IJMS17111892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XX, Eden HS, Chen X. Peptides in cancer nanomedicine: drug carriers, targeting ligands and protease substrates. J Control Release. 2012;159(1):2–13. doi: 10.1016/j.jconrel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Brossas JY, Parizot C, Zini JM, Rebollo A. Identification and characterization of novel enhanced cell penetrating peptides for anti-cancer cargo delivery. Oncotarget. 2018;9(5):5944–5957. doi: 10.18632/oncotarget.23179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available on request.


