Abstract
Evading or escaping from predators is one of the most crucial issues for survival across the animal kingdom. The timely detection of predators and the initiation of appropriate fight-or-flight responses are innate capabilities of the nervous system. Here we review recent progress in our understanding of innate visually-triggered defensive behaviors and the underlying neural circuit mechanisms, and a comparison among vinegar flies, zebrafish, and mice is included. This overview covers the anatomical and functional aspects of the neural circuits involved in this process, including visual threat processing and identification, the selection of appropriate behavioral responses, and the initiation of these innate defensive behaviors. The emphasis of this review is on the early stages of this pathway, namely, threat identification from complex visual inputs and how behavioral choices are influenced by differences in visual threats. We also briefly cover how the innate defensive response is processed centrally. Based on these summaries, we discuss coding strategies for visual threats and propose a common prototypical pathway for rapid innate defensive responses.
Keywords: Looming, Innate defensive behavior, Escape, Freezing, Circuit mechanism, Encoding
Introduction
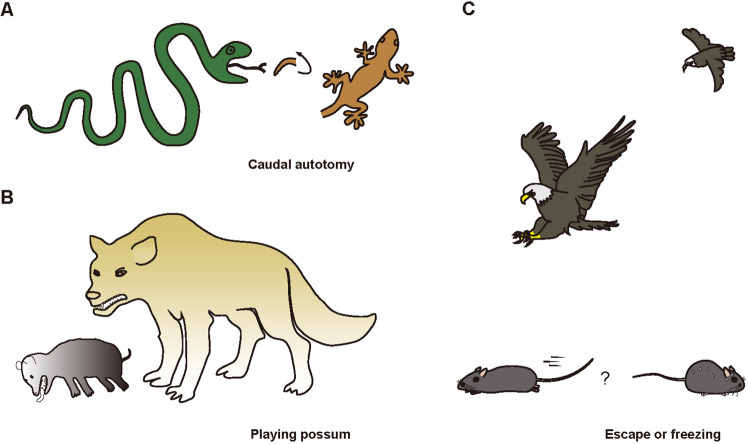
The most important thing for any nervous system is to respond appropriately to external changes. Avoidance of danger is essential for survival and future reproduction. Animals have evolved surprising skills to evade predators. For example, geckos voluntarily sacrifice their tails to distract predators and buy themselves time to escape (Fig. 1A) [1, 2]; possums mislead predators by feigning death from poisoning hence making them unsuitable as food (Fig. 1B) [3]. For most animals, including mice [4, 5], escape or freezing responses are evoked depending on the imminence of the threat (Fig. 1C) [6, 7]. The neural processing of these innate responses to threats forms a closed loop that comprises threat detection, action initiation, action execution, and post-action resetting (preparing for the next threat) [8]. Recent studies have made significant progress toward understanding the neural mechanisms of animals’ responses to visual threats. In this review, we summarize the current understanding of the innate defensive responses in insects, zebrafish, and mice; primarily on the neural circuits for the processing and identification of visual threats. After cross-species comparisons, we conclude our overview by proposing a conserved core pathway for the innate response to visual threats.
Fig. 1.
Repertoire of predator avoidance behaviors. A A gecko escapes from a snake by forcefully contracting the tail muscle to break its caudal vertebra then shed the still-wriggling tail to distract the snake. B A Virginia possum, when confronted by a wolf, pretends to be dead from poisoning, with its tongue sticking out, baring its teeth, its mouth foaming, and a foul smell released from its anal glands. C A mouse escapes rapidly to a refuge upon seeing a fast-approaching eagle or freezes to avoid detection if the eagle is just sweeping by.
Visual Threat Processing in Insects
Detection of Looming Objects by the Vinegar Fly
The neural mechanisms of visual threat detection and processing are well-studied in insects [9–13]. The vinegar fly (Drosophila melanogaster) has tractable neural circuits that are easier to manipulate compared to vertebrates, thus much of the recent progress has been made in these flies [13, 14]. A dark looming disk is typically used as the visual stimulus to mimic an incoming threat [11, 12]. Sophisticated motor programs for flight take-off can be elicited in flies presented with such a looming stimulus [15, 16]. One neural pathway mediating looming-induced escape behaviors has been mapped for the most part (Fig. 2A). In this pathway, T4/T5 neurons projecting to the lobule plate respond to this looming stimulus in a directionally-selective (DS) manner [17]. Silencing T4/T5 neurons abolishes looming-elicited landing and avoidance behavior, thus they are essential for the detection of looming stimuli [18]. The DS outputs from T4/T5 neurons are then integrated by the lobula plate/lobula columnar type II (LPLC2) visual projection neurons. The primary dendrite of each LPLC2 neuron ramifies in one of four layers innervated by T4/T5 neurons and extends along that layer’s preferred motion direction to align with the excitatory DS inputs from T4/T5. Each dendritic branch of the LPLC2 neuron also receives local inhibitory DS inputs for inward motion (i.e., towards the center of the neuron’s receptive field). Together, the selectivity of the excitatory and inhibitory inputs form radial motion opponency so that the LPLC2 neuron responds to expanding motion but not to receding motion [19]. Another type of visual projection neuron, lobula columnar subtype 4 (LC4) is also implicated in the detection of a looming stimulus, but the mechanisms underlying its response are not yet understood. The angular velocity of the looming disk is encoded by the LC4 neurons [20], and the size of the looming disk is encoded by the LPLC2 neurons [21]. Combined, information carried by these two types of neurons fully describes the looming disk stimulus at any given moment.
Fig. 2.
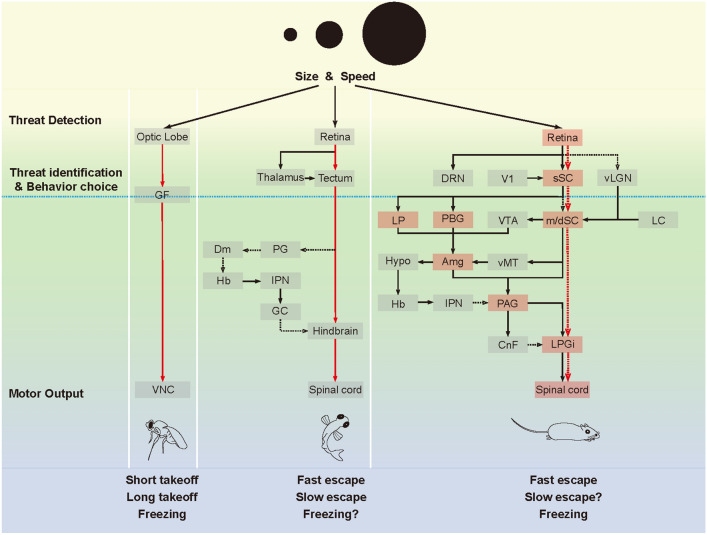
Neural circuits for visual threat processing and response across species, in Drosophila (A), zebrafish (B), and mouse C). A Core pathways for escape (left) and freezing (right) in response to visual threats in Drosophila. Lo, lobula; Lp, lobula plate; Me, medulla. Dashed line for DNp09: possible connection. The dashed line on the left indicates a non-GF pathway for long take-off. Black arrows in the Lp: the preferred motion directions of T5 neurons. B Core escape circuit in zebrafish. Light brown shaded region: thalamus. SINs, superficial inhibitory interneurons; PVNs, periventricular neurons; M-cell, hindbrain Mauthner cell. The dashed line indicates that non-M cells participate in slow escape. Other colored lines indicate modulation of the tectum and M cells. C Core pathways for escape (red) and freezing (blue) in response to visual threats in the mouse. The four modulatory systems are involved in visual threat-evoked innate defensive behaviors, but the scope of their effects and their target brain regions are not fully understood yet. Red/blue: circuit components of the escape/freezing pathway, respectively; purple: participants in both pathways; other colors: modulation of the SC; dotted lines: other sensory inputs. sSC superficial superior colliculus, m/dSC intermediate/deep superior colliculus, LP lateral posterior nucleus, PBG parabigeminal nucleus, Amg amygdala, Hypo hypothalamus, PAG periaqueductal grey, LPGi lateral paragigantocellular nucleus.
A Common Strategy for Looming Detection in Insects
Other insects seem to share a similar circuit composition for visual threat detection [9, 10]. That is, visual projection neurons convey information on different parameters of the incoming object to downstream neurons. Then the downstream neurons combine this information to decide whether the incoming object is a threat, and direct motor neurons or related interneurons to elicit defensive behaviors if necessary. The lobula giant movement detector (LGMD) neuron and the downstream descending contralateral movement detector (DCMD) neuron found in the locust, for example, are well known for such a composition. Splitting looming information into pathways for size and speed was first described in the context of the LGMD [11]. The LGMD neuron receives inputs from visual neurons conveying information about looming angular speed at its dendritic subfield A and information about looming size at dendritic subfields B and C [11, 22–24]. It combines these inputs nonlinearly to detect visual threats [25, 26], Then, the DCMD neuron receives this threat information and directs the jumping and flight steering motion in the locust to escape the incoming object [11]. Aside from size and speed, encoding of looming information in the LGMD neuron is also assisted by presynaptic mechanisms that preferentially boost the response to the coherent motion, thereby helping to segregate the looming object from the background [27, 28]. It would be interesting to investigate if similar mechanisms exist in other systems.
Diverse Response Mechanisms to Visual Threats
The outputs from LC4 and LPLC2 neurons are summed nonlinearly in the downstream giant fiber (GF) neuron, a wide-axon descending neuron [13], and the spike timing of the GF determines a fast mode of escape [29]. Direct activation of the GF induces take-off that vinegar flies execute under threat [29–31], and inactivation of the GF abolishes this type of emergency take-off but leaves normal flight take-off unaffected [16, 29, 32]. So, the GF is considered to be an essential part of the neural pathway for threat response in vinegar flies [9, 10, 13].
In addition to the escape pathway via GF-mediated fast take-off, there is also a non-GF take-off pathway in the vinegar fly [29, 32, 33]. The Drosophila non-GF take-off allows for more controlled motor planning, the same as what occurs in locust escape jumps [15, 16, 29]. The LGMD-mediated escape response in locusts requires > 100 ms of preparation time [11], thus it may resemble more the non-GF response than the GF-mediated response in Drosophila. The vinegar flies tend to select the GF pathway when the danger is more imminent, and the GF pathway can even override the non-GF pathway sometimes [29]. But the mechanisms underlying the choice between the two pathways are not yet understood. The detection of other types of visual threats may involve different visual neurons, with the less urgent threat going through the non-GF pathway or a combination of both pathways [34]. A recent study systematically screened many visual projection neurons and revealed that optogenetic activation of lobula columnar subtype 6 (LC6) and lobula plate/lobula columnar, type I (LPLC1) neurons in the lobula elicit avoidance behaviors [35]. Whether these neurons are important for detecting different types of visual threats remains to be seen.
Freezing is also a behavioral response to visual threats in flies, although the circuit mechanisms are less well studied. Escape can be triggered by looming objects but not by small moving objects, while freezing can be triggered by both [36, 37]. The lobula columnar subtype 11 (LC11) neuron, which receives input from T2/T3 neurons sensitive to small objects, is essential for brief freezing behavior in vinegar flies [36, 38], but not for escape [21]. In addition, silencing T4/T5 neurons, which do not provide inputs to LC11 but are important for the detection of a looming stimulus [18, 19, 38], also significantly weakens freezing behavior [36]. Thus, pathways for processing looming objects and for processing small objects are both implicated in freezing behavior. One may wonder whether the freezing response after seeing small objects is identical to that after seeing a looming stimulus. The former is active freezing to avoid being detected by potential distant predators and the latter is passive freezing to inescapable, imminent danger. How much the neural circuits for freezing under different stimulus conditions differ is a question that needs answering in the future.
Briefly, visual threat detection and response circuits in insects are complex, and much is still unknown. But the pathways detecting imminent danger, simulated by looming disk stimuli, seem to share some common components among different insect species: the encoding of looming size and looming velocity by different visual neurons to represent the visual threat; the nonlinear summation of these two inputs in one neuron to detect the threat; and a direct projection to motor execution neurons for escape responses.
Visual Threat Processing in Zebrafish
Detection of Visual Threats
A dark looming disk elicits a defensive response in almost all the vertebrate species studied, such as fish, amphibians, reptiles, birds, and mammals (including humans) [39–45]. Larval zebrafish have received increasing attention as a useful model in which to study the neural mechanisms underlying behaviors. With the development of better in vivo whole-brain functional imaging techniques [46, 47] and single-cell tracing techniques [48, 49], a comprehensive understanding of single neuron responses to circuit-wide functions, even to whole brain activity during a specific behavior is now feasible in zebrafish [50, 51]. Hence, much progress has been made in larval zebrafish on the mechanisms of visual threat processing and its neural modulation. After retinal projections to the brain stabilize [52], larval zebrafish readily show escape responses to simple looming stimuli [53, 54], animated images [55], robotic predators [56], and live predators [57, 58]. Testing variants of looming stimuli on zebrafish has shown that the expanding motion is necessary to drive the escape behavior, while the luminance change (aka dimming) increases the escape probability [54, 59, 60]. Thus, similar to insects, the encoding and processing of looming stimuli in zebrafish can also be separated into two branches: the expanding/looming motion, which is determined by looming velocity; and the luminance change, which is a function of the size of the looming stimulus.
Although there are light-sensitive neurons in the skin and brain of the zebrafish [61, 62], the detection of visual threats by the retinal ganglion cells (RGCs) is necessary for the innate defensive responses to looming stimuli [54, 63]. There are more than 30 distinct types of RGC in the zebrafish retina [63]. Some RGC types are sensitive to looming motion [54]. Some exhibit sensitivity to stimulus size or changes in luminance [54, 64–66]. Due to the limitation of the Ca2+ imaging method used in the functional studies, whether any of these RGCs show encoding capabilities similar to the size-encoding or velocity-encoding neurons in insects remains unknown. Furthermore, a causal connection between any specific RGC type and the visually-triggered defensive responses has not been established yet. Thus, there is still much to be resolved on how visual threat is processed in the zebrafish retina and brain.
RGCs project ten distinct arborization fields in the fish brain, including the optic tectum [52, 67]. Selective ablation of RGC axon bundles in the tectum completely abolishes escape behaviors but does not affect optomotor responses [54]. Further, optogenetic activation of tectal neurons triggers escape behaviors [68, 69]. Thus, the retinotectal connection is critical in visual threat processing. Axons of RGC subtypes terminate in specific layers of the tectum, delivering different aspects of visual information [64, 70–74]. Based on the responses of axon terminals of the RGCs to different variants of the looming stimuli, looming and dimming information are delivered to different parts of the tectum and thus likely processed by different tectal neurons [52, 54, 60, 66, 70, 75]. But whether and how these two channels of information on the same looming stimulus combine in the tectum neurons is still unknown.
A looming (expanding) object on the retina may be either a predator or a potential prey. Zebrafish discriminate between the two mainly based on size information [64]. Looming objects need to surpass a size threshold to elicit escape behaviors [54, 59, 76, 77]. A similar computation also occurs in the optic tectum: tectal neurons in different layers receive different size-selective RGC inputs, information on smaller objects is sent to superficial layers [78], and information on larger objects is sent to deeper layers [64]. The response of the size-selective tectal neurons is gated by superficial inhibitory interneurons (SINs) [64, 74], which serve as computational modules to separate large predators from small prey [59]. A group of tectal neurons in the innermost layer of the optic tectum, the periventricular neurons, receive inputs from SINs and encode the threshold angular size associated with escape latency [59, 74].
Response Mechanisms to Visual Threats
After the processing of visual threat information in the retina and the tectum, the decision to escape is conveyed by the tectal output neurons to the well-known Mauthner cell (MC) and other reticulospinal neurons in the premotor areas (Fig. 2B) [53, 68, 79]. The MC is a critical element in threat-relevant escape behaviors [42, 53, 73, 76, 80, 81]. Activation of a single MC spike elicits a fast escape response [80–82]; ablation of the MC soma results in a significantly longer escape latency [53, 76, 80, 81, 83–85], while ablation of the MC and its axon abolishes the fast escape behavior [86].
The visually triggered looming pathway can be modulated by many factors. Luminance change information from the thalamus to the tectum helps to direct escape behaviors and likely increases the response probability [60]. Hunger, through serotonergic modulation, alters the neural representation of prey-like stimuli in the tectum, and the behavioral response accordingly [87]. Dopaminergic neurons in the hypothalamus modulate and gate the MC response through hindbrain glycinergic interneurons [53]. Both serotonergic and dopaminergic neurons can be modulated by the habenula [88, 89], and the habenula can also modulate defensive behaviors through the habenula—interpeduncular nucleus—griseum centrale pathway [90–92]. Many other neuromodulatory neurons also respond to a looming stimulus and are thus implicated in the process, such as somatostatin neurons, neuropeptide Y neurons, cocaine- and amphetamine-regulated transcript-positive neurons in the hypothalamus, noradrenergic neurons in the locus coeruleus (LC), and cholinergic neurons in the tegmentum [93–96]. But how these neurons modulate the looming pathway remains largely unknown.
In summary, the detection of looming threats in zebrafish shares some similarities with that in insects, the most significant being the separate processing of looming size and looming motion by different visual neurons. Also worth noting is the shortcut circuit via the MC, directly connecting visual detection results to motor outputs [97, 98]. Zebrafish also freeze occasionally under threat [55, 57]. Regrettably, little is known about the mechanisms underlying this behavior, thus preventing comparison to the insects.
Neural Mechanisms for Visually-Evoked Defensive Behavior in Mice
Mice have evolved two types of innate defensive behavior to evade predators [5]. When the threats are remote, such as an eagle sweeping high above in the sky, mice stop all movements (freeze) and wait for the predator to leave. Freezing helps the mouse avoid being identified as prey at a distance, and is also more energy-efficient than escape. But if the threat is imminent, such as in the case of a fast-approaching predator, escape to a nearby refuge is more likely to ensure survival. In this section, we summarize the current understanding of the neural circuits involved in the generation of visually-triggered freezing and escape behaviors. The contents cover stimulus detection and threat identification in the retina and the superior colliculus (SC), then the circuits in various subcortical regions involved in the decision between freezing and escape responses, and finally the generation of the behaviors in the brainstem.
Visual Threat Detection in the Retina
As the first stage of vision, the retina determines what the brain sees of the outside world, and the detection of visual danger signals starts in the retina. The output neurons of the retina, the RGCs, are responsible for transmitting danger signals to the brain rapidly and correctly as soon as the danger emerges in the visual field. Related to research performed in insects and fish, repeated presentation of a dark expanding disk on an overhead screen is efficient in eliciting a robust defensive response in mice [4], and is often used as a visual threat stimulus, although it is different from the looming stimulus commonly used in insects and fish [11, 12]. An RGC subtype that selectively responds to this dark expanding stimulus was first reported in 2009 [99]. It is a subset of the RGCs labeled by the parvalbumin-Cre transgene (PV-5 RGCs). The morphology and function of the PV-5 RGCs indicate that they are a subtype best known as OFF-transient alpha RGCs (OFFt αRGCs). Alpha RGCs are conserved across mammalian species [100]. They have the largest receptive fields and fastest axonal conductance among all RGC subtypes [101–104]. This makes them ideal for transmitting visual threat signals to the brain. Recently, more evidence emerged that placed the OFFt αRGCs firmly in the center of looming detection in the retina: ablation of either OFFt αRGCs themselves or their input interneurons, vGlut3+ amacrine cells, severely diminishes looming-evoked defensive responses [105, 106]; and optogenetic activation of these RGCs in the absence of any visual inputs is sufficient to induce defensive responses [106]. These results are consistent with OFFt αRGC activation being a necessary and sufficient retinal component of the neural pathway for looming evoked innate defensive responses. It is worth noting that a specific molecular marker, Kcnip2, has been identified in the OFFt αRGCs, and a CreER knock-in mouse strain has been created [106]. This will greatly facilitate future targeted functional studies on these RGCs.
In addition to the OFFt αRGCs, other RGC subtypes also play important roles in processing looming stimuli. In one report, the dorsal raphe nucleus (DRN) was shown to modulate looming-evoked defensive responses, and RGCs that project to the DRN are necessary for these responses [107]. In a more recent report, it was found that some RGCs are GABAergic, and ablation of the GABAergic RGCs projecting to the SC diminished looming-induced defensive responses [108]. The subtype identities of these two groups of RGCs, and how they participate in the processing of looming information remain to be explored. Neither OFFt αRGCs nor GABAergic RGCs project to the DRN [106, 108], thus they do not overlap with the DRN-projecting RGCs. But whether a significant proportion of OFFt αRGCs are also GABAergic is unknown, and needs to be clarified in the future.
Interestingly, OFFt αRGCs encode the size of a looming object [106], which is reminiscent of the size coding neuron LPLC2 in vinegar flies [21]. LC4 neurons in the vinegar fly encode looming speed [20]. Together, the activity of LPLC2 and LC neurons fully describes the looming stimulus at any given time. Whether there are RGCs in the mouse retina that encode other parameters of the looming stimulus, such as velocity, would be an intriguing question to investigate.
We have summarized so far how RGCs participate in the detection of looming stimuli. The looming stimuli used in these experiments were dark disks quickly expanding to occupy a large visual angle (typically expanding to 30°–40° in a fraction of a second), thus they are likely perceived as an imminent danger by the animals. Mice respond to imminent danger and remote threats in different ways: escape from imminent danger, but freeze and observe if the threat is from a distance [5, 109]. It is reasonable to infer that there could be another neural pathway to detect and respond to remote dangers, such as a cruising eagle in the sky. This pathway may involve RGCs other than the OFFt αRGCs, as the visual stimulus is different. So far, no clear picture has emerged of how remote danger is detected and processed in the retina. A small and slow-moving disk overhead can be used to mimic a remote threat, and mice freeze when presented with this stimulus [5]. This is also an ideal stimulus for W3-RGCs, an RGC subtype that is selectively activated by slow-moving small objects on a clean background like the sky [110]. Yet no connection between W3-RGCs and freezing behavior has been discovered, due to a lack of molecular tools with which to study W3-RGCs. More molecular tools to manipulate different RGC subtypes will help to answer whether W3-RGCs are indeed involved in this behavior, or if other RGCs with a similar response profile are responsible.
Recently, RGC inputs to SC neurons that project to either the lateral posterior nucleus (LP) or the parabigeminal nucleus (PBG) have been identified [111]. The LP and PBG are involved in different aspects of innate defensive responses, as we further discuss in the next part. Thus this result provides a more comprehensive picture of RGC subtypes closely connected to visual threat detection. Among these RGC subtypes are the ON-OFF direction-selective RGCs (ooDSGCs) and the OFF-sustained RGCs. The functional similarity (direction selectivity) between ooDSGCs and the T4/T5 neurons in vinegar flies may imply that they play a similar role in looming detection [19]. Meanwhile, OFF sustained αRGCs, being fast-conducting αRGCs that respond to a large moving object, may work synergistically with OFFt αRGCs to signal imminent looming danger. Further examination is needed to fully describe the roles these 14 subtypes of RGC play in visual threat detection and processing.
RGCs may also influence the choice of innate defensive behaviors. The behavioral response of mice to looming stimuli is predominantly escaped, with a small fraction of freezing. One interesting study demonstrated that the loss of Brn3b in the retina significantly reduces the escape probability, while keeping freezing intact in response to a looming stimulus, indicating that there are already distinctions between flight and freezing from the retina [112]. Further investigations of which RGC subtypes play roles in it, and how the loss of Brn3b impacts their roles, are needed to fully understand this phenotype. In zebrafish, dimming information sent to the tectum through the retino-thalamic pathway helps to determine the direction of escape and increases the response probability [60]. Similarly, some mouse RGCs that encode luminance information indirectly excite SC neurons through neurons in the ventral lateral geniculate nucleus (vLGN) that project to the SC [113, 114]. This retino-thalamo-colliculus pathway may modulate escape behaviors in mice just like in zebrafish.
Threat Identification and Response Choice in the Superior Colliculus
The SC is believed to be critical for the innate defensive response to visual threats [115–118]. It is a laminated midbrain structure implicated in diverse physiological functions [117, 119–123]. The lamination in the SC is conserved across mammalian species, although the relative thickness of the layers varies [121]. The layers can be roughly divided into two major parts, the superficial SC (sSC) and the intermediate/deep SC (m/dSC), receiving visual and multisensory inputs, respectively. The anatomical structure [121], functional connectivity [123, 124], and behavioral outputs [115, 117, 119, 120, 122, 125, 126] are different between the sSC and the m/dSC. In this section, we discuss recent findings on neural circuits for the innate defensive behaviors in the sSC and m/dSC separately.
The Superficial SC
The sSC is immediately downstream of the retina and is considered to be a place mostly for visual information processing. Almost all of the RGCs project to the sSC whereas only 20–50% of the RGCs project to the dorsal LGN (dLGN) in rodents [127, 128], thus visual processing in sSC is a critical component of vision-related functions. The processed visual information is then used by the sSC to direct a multitude of innate behaviors.
Neurons in the sSC can be classified into four major types: wide-field (WF) cells, narrow-field (NF) cells, horizontal cells, and stellate cells, each with distinct morphological and electrophysiological properties [129]. Freezing and escape behaviors can be elicited rapidly after optogenetic activation of neurons in the sSC [123, 130–133]. The molecular label for the escape-related neurons is parvalbumin (PV), and they act via their projections to the PBG: activation of the PV+ neurons projecting to the PBG triggers immediate escape behavior, and the activity of these neurons may be correlated with the time-to-collision with the approaching object [131]. One interesting fact about these neurons is that they are excitatory and not inhibitory, unlike most PV+ neurons [134]. PV+ neurons in the sSC are heterogeneous based on their morphology, projections, electrophysiological properties, and connections to relevant behaviors [130, 131, 135], but those that project to the PBG may belong to a single type, NF neurons [130, 131]. Although their activation readily triggers escape behavior, it has not been tested whether these PV+ neurons that project to the PBG are required for the behavior, thus the participation of other SC neuronal types is still possible. It was first reported that the SC neurons projecting to the LP drive freezing behavior upon optogenetic activation [136], although the report indicated that the neurons were in the m/dSC. Later, neurotensin receptor 1 (Ntsr1) and cerebellin 2 precursors (Cbln2) were found to be potential markers for the sSC neurons that drive freezing behavior [132, 133]. Ntsr1+ neurons are WF neurons with large receptive fields [129, 137]. Interestingly, these neurons best respond to small slow-moving objects [129, 137]. This makes Ntsr1+ neurons good candidates for alarm neurons that can warn animals of potential predators at a distance. Ntsr1+ neurons project exclusively to the LP. Cbln2 expression probably partially overlaps with Ntsr1 expression, and Cbln2+ neurons include mostly LP-projecting neurons and a small portion of non-LP-projecting neurons [133]. The exact relationship between the two markers is not yet clear, but the same subset of freezing-driving neurons is likely labeled by both markers. Ablation or activation experiments with more specificity will help to clarify this.
Thus, two different populations of neurons in the sSC drive the escape and freezing behaviors separately: WF neurons that project to the LP for the freezing behavior and PV+ neurons that project to the PBG for the escape behavior. So far, there has been no report of interactions between the two pathways in the sSC; they seem to operate relatively independently (see [130]). Also of note is that the peak response time of WF neurons is correlated with the time-to-collision during a looming stimulus [137], thus they may also contribute to the processing of the looming stimulus, and so interact with the escape pathway. The sSC and the primary visual cortex (V1) have robust information exchange and modulate each other’s visual functions. SC neurons in the freezing pathway receive V1 inputs [138], and V1 modulates the magnitude of looming-evoked responses in the SC [139]. Meanwhile, the SC influences the tuning of V1 via the tectogeniculate pathway [140]. The tectogeniculate pathway also participates in visual threat processing. Some GABAergic neurons in the sSC project to the dLGN and modulate the choice between freezing and escape through this tectogeniculate pathway [141]. Hence, three out of four outputs of the sSC are connected to visually-evoked defensive behaviors: the sSC-LP pathway for freezing, the sSC-PBG pathway for escape, and the sSC-LGN pathway for modulating the probability of freezing. Whether the sSC-mSC pathway also participates in visually-triggered defensive behaviors remains an interesting question.
Decoding visual information correctly is important for the SC to make appropriate behavioral choices. Visual threat information delivered by the RGCs is not specific enough to use directly as go signals for the behavioral responses. For example, OFFt αRGCs not only respond to dark looming stimuli, but also respond robustly to dark objects appearing or bright objects disappearing inside their receptive fields, yet the latter types of stimuli do not induce defensive behaviors in mice. Therefore, SC circuits downstream of OFFt αRGCs must distinguish the two and only respond when looming stimuli are presented. To understand how sSC neurons further extract and identify visual threat information from the RGC inputs, one needs to first understand how RGCs and sSC neurons are connected. Although efforts have been made to map the connections between the retina and the SC [111], the input RGCs for the Ntsr1+ neurons and the PV+ neurons, and the circuit mechanisms underlying the processing of RGC inputs by these SC neurons are not yet clear (but see the most recent report [142] while this review was being edited). Nevertheless, we know enough now to form a few hypotheses. For example, activity manipulations of the OFFt αRGCs impact both the escape and the freezing behaviors [106], thus these RGCs likely make connections with both the Ntsr1+ neurons and the PV+ neurons. Further, inputs from some other RGCs are likely needed to help these SC neurons to distinguish different stimuli and only respond to those considered potential threats. Similarly, if W3-RGCs are indeed connected to the detection of remote danger, and thus the freezing response, then they should preferentially connect with Ntsr1+ neurons. Investigations into these predictions will help to connect the innate defensive-related pathways between the retina and the SC.
Another aspect of visual processing also needs to be investigated to fully understand the role of the SC in visually-triggered innate defensive behaviors: the context-dependent interpretation of the visual information. For example, the same small and slow-moving objects on the retina may signify either a large predator at a distance or a small potential prey close by. How does the SC distinguish the two? Interestingly, it has been demonstrated that WF neurons (aka Ntsr1+ neurons), in addition to mediating freezing behavior, are also necessary for prey detection in mice [143]. Incidentally, they also respond robustly to small slow-moving objects [137]. Thus, these neurons receive information from the retina about small and slow-moving objects, identify if it represents a predator or prey, and then trigger completely different behavior outputs based on that identification. One likely method to distinguish predator from prey is to use the location of the object: the prey is present almost exclusively in the lower half of the visual field for the mouse, whereas the predators are mainly located in the upper visual field. Retinotopically, lower and upper visual fields correspond to lateral and medial parts of the SC, respectively. It would be illuminating to test whether activation of Ntsr1+ cells at medial and lateral SC locations leads to different behavioral outputs.
The Intermediate and Deep SC (m/dSC)
The m/dSC is one of the most well-studied regions in the brain. It is considered a place for sensory integration and the initiation of motor outputs. It receives inputs from visual, auditory, and somatosensory modules and performs sensory-motor transformation [122, 144, 145]. Here, we mainly focus on the motor output aspect of its functions, more specifically, the circuits involved in the generation of innate defensive behaviors. Unlike the superficial layer, which has limited input and output regions, m/dSC neurons receive inputs from almost the entire brain and part of the spinal cord, and project to multiple thalamic nuclei and the spinal cord [125, 146]. Direct activation of the m/dSC neurons or their axon terminals elicits freezing, escape, orienting, or preying behaviors [116, 133, 136, 147–152]. It is worth noting that activating neurons that project to the ipsilateral brainstem reticular formation (uncrossed pathway) mainly elicit defensive behaviors while activating neurons that descend to the contralateral brainstem reticular formation (crossed pathway) only induce orienting behaviors [116, 121, 149]. Many studies indicate that neurons in the medial part of the m/dSC are important in defensive behaviors and those in the lateral part are more involved in prey capture [148, 153]. But little is known about the mechanism of divergence between the medial and the lateral parts of the m/dSC. In addition to visual threats, auditory crescendo inputs also evoke defensive behaviors via an m/dSC pathway [154]. To generate fast responses to threats, one would expect a direct pathway from the looming-responsive neurons in the sSC to the defensive behavior-related neurons in the m/dSC. Although this has not been tested, with the discovery of more cell-type markers in the SC, we hope this important question will be addressed soon.
Behavior Modulation and Execution: Beyond the SC
Besides the m/dSC, the sSC also projects to the LGN through stellate and horizontal neurons, to the LP through WF neurons, and to the PBG through non-WF neurons [129]. Except for the vLGN, all other targets contain few inhibitory neurons in mice. The vLGN provides important information about changes in luminance to the m/dSC and enhances defensive behaviors [113, 114]. The LP is the lateral posterior part of the thalamus and a homologue of the pulvinar in the primate brain. The LP mediates visually-evoked freezing behavior through projections to the lateral amygdala [130, 136]. The PBG receives inputs from both the sSC and the m/dSC, and forms reciprocal connections with the sSC [155]. The PBG mediates looming-evoked escape behavior [130, 131]. Interestingly, the PBG also projects to the central part of the amygdala [131]. Projections from the m/dSC through the ventral tegmental area (VTA) or ventral midline thalamus (vMT) to the amygdala are also reported to modulate looming-evoked innate defensive responses [147, 156]. Whether connections from the LP, PBG, vMT, and VTA converge or interact in the amygdala remains largely unknown [157–160].
From the amygdala, the pathway likely becomes common for innate defensive responses to threats from all sensory modalities [158, 159, 161–165]. As an aversive stimulus, looming also activates the hypothalamus [166–169], all the way to the periaqueductal grey (PAG) [158, 170–175]. Signals from the m/dSC and the amygdala converge on the dorsal and ventrolateral PAG, respectively [150]. Further downstream, the lateral paragigantocellular nucleus (LPGi) is the major output nucleus to initiates defensive behaviors. Both the deep SC and the PAG have connections to motor-related midbrain areas such as the cuneiform nucleus and medullary regions such as the LPGi [146, 170, 176, 177]. The LPGi then sends commands to motor neurons in the spinal cord to generate movements [178, 179]. Freezing and flight responses are mediated by different subsets of neurons in this pathway, but there is also evidence of interactions between the two behavioral options (Fig. 2C) [157, 170].
The entire innate defensive pathway is heavily modulated. The serotonergic DRN, noradrenergic LC, dopaminergic A13, cholinergic PBG, and pedunculopontine tegmental nucleus all provide inputs to the SC [146, 180–182], influence the responses of SC neurons [182, 183], and regulate visually-evoked defensive behaviors [107, 147, 184]. Anatomical data also show that the m/dSC sends projections to dopaminergic systems in the substantia nigra pars compacta and the VTA, the cholinergic system in the pedunculopontine nucleus, and the lateral dorsal tegmental area [146]. Furthermore, the habenula receives visual threat inputs from the hypothalamus and targets almost all midbrain neuromodulatory systems to modulate escape and freezing behaviors [168, 185, 186]. The mechanisms by which these modulation systems are involved in visually-evoked defensive behaviors and how they interact require further study.
An Evolutionarily Conserved Pathway Across Species
To conclude, we summarize the neural mechanisms involved in visual threat responses, focusing on common features among species. First, there appears to be a common scheme for the identification of looming objects that signal danger. Animals need to identify visual threats quickly and correctly to evade danger in time and at the same time not waste energy on non-threatening objects in the visual field. In insects and fish, perhaps also in mice, the same two parameters are used to identify looming objects: object size, and looming speed. The two parameters are encoded in parallel by different sets of visual sensory neurons [21, 25, 106, 112]. In insects, the neurons downstream then combine the size and speed information to correctly identify a looming object, then initiate appropriate behavioral responses [11, 20, 21, 25, 26, 77]. How these two types of information converge in fish and mice is less clear, although the convergence likely also occurs in neurons directly downstream of the size- and speed-encoding RGCs, in the optic tectum (fish) or sSC (mice).
Second, the mechanisms underlying behavior choice show significant similarities. Freezing and escape are two consistent behavioral responses to visual threats across species [187]. Freezing behavior in zebrafish is much less studied, so we can only compare results in insects and mice. In both insects and mice, looming objects induce either escape or freezing [4, 29, 37], while small moving objects induce freezing [5, 36]. Also common to insects and mice, freezing and fleeing are mediated by separate neural circuits, so many manipulations affect only one behavior and not the other [21, 112, 130]. Different visual threats activate the two pathways differently: looming activates both pathways [19, 36, 130, 137]. Small moving objects likely only activate the freezing pathway, although this has not been rigorously tested yet (see [20]). When both pathways are activated, internal states and environmental conditions help bias the behavioral choice toward one or the other [37, 95, 166, 184].
Third, the underlying circuits share a similar overall structure that favors reaction speed. Incoming threats are mainly identified by their size and looming velocity, without consideration of details such as texture or shape. The encoding of size and velocity does not require complex computations; thus, the central process of visual threat identification occurs in two abstracted layers of visual processing (Fig. 3, threat detection layer, threat identification, and behavior choice layer): The first layer encodes looming velocity and object size, and the second layer combines the information and identifies the threat. In vinegar flies and zebrafish, this second layer, GF in vinegar flies and optic tectum neurons in fish, then connects directly to the motor output neurons for behavior execution (Fig. 3). This direct connection ensures a fast response as soon as a threat is identified. In mice, however, such a direct pathway has not been described. This pathway, should it exist, likely connects the visual SC directly to the motor output part of the SC, then to the LPGi in the medulla (Fig. 3, red dashed lines in mouse). Projections and connections between the sSC and the m/dSC are plentiful [129, 146, 188]. In addition, optogenetic activation of Ntsr1+ and PV+ neurons in the sSC induces responses in m/dSC neurons, indicating functional connections [132]. Further downstream, the m/dSC has projections to the medulla [146, 189, 190], and activation of m/dSC neurons projecting to the medulla induces defensive behaviors [149], suggesting a direct connection between the m/dSC and the defensive behavior-related medullary structure LPGi. Thus, this sSC-m/dSC-LPGi pathway is anatomically plausible. Based on these, we propose that a retina-to-medulla shortcut pathway exists in mice for visually triggered innate defensive responses (Fig. 3).
Fig. 3.
Schematic overview and cross-species comparison of the neural pathways involved in visual threat processing. Black arrows: known descending connections. Black dashed arrows: suspected descending connections. Red arrows: direct descending pathways in Drosophila and zebrafish. Red dashed arrows: hypothesized short-cut pathway in the mouse. Blue dashed line: completion of visual threat processing. Orange areas: brain regions that are indispensable to visual threat responses. GF giant fiber, VNC ventral nerve cord, PG preglomerular nucleus, Dm dorsal pallium, Hb habenula, IPN interpeduncular nucleus, GC griseum centrale, V1 primary visual cortex, vLGN ventral lateral geniculate nucleus, sSC superficial superior colliculus, DRN dorsal raphe nucleus, LP lateral posterior nucleus, PBG parabigeminal nucleus, VTA ventral tegmental area, m/dSC intermediate/deep superior colliculus, LC locus coeruleus, Hypo hypothalamus, Amg amygdala, vMT ventral midline thalamus, PAG periaqueductal grey, CnF cuneiform nucleus, LPGi lateral paragigantocellular nucleus.
Thus, for visually triggered innate defensive responses in different species, although their neural circuits differ drastically in complexity and details, the overall structure and underlying principles appear to be conserved in evolution. Further comparative studies across species may help to better understand the circuit mechanisms underlying visual threat processing and innate defensive responses.
Acknowledgements
We thank members of Dr. Yifeng Zhang’s and Dr. Jun Yan’s lab for their help and support. This review was supported by a general project of the National Natural Science Foundation of China (31871052).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Clause AR, Capaldi EA. Caudal autotomy and regeneration in lizards. J Exp Zool A Comp Exp Biol. 2006;305:965–973. doi: 10.1002/jez.a.346. [DOI] [PubMed] [Google Scholar]
- 2.Higham TE, Russell AP. Flip, flop and fly: Modulated motor control and highly variable movement patterns of autotomized gecko tails. Biol Lett. 2010;6:70–73. doi: 10.1098/rsbl.2009.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabrielsen GW, Smith EN. Physiological responses associated with feigned death in the American opossum. Acta Physiol Scand. 1985;123:393–398. doi: 10.1111/j.1748-1716.1985.tb07605.x. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz M, Meister M. Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol. 2013;23:2011–2015. doi: 10.1016/j.cub.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Franceschi G, Vivattanasarn T, Saleem AB, Solomon SG. Vision guides selection of freeze or flight defense strategies in mice. Curr Biol. 2016;26:2150–2154. doi: 10.1016/j.cub.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 7.Ydenberg RC, Dill LM. The economics of fleeing from predators. Adv Study Behav. 1986;16:229–249. doi: 10.1016/S0065-3454(08)60192-8. [DOI] [Google Scholar]
- 8.Evans DA, Stempel AV, Vale R, Branco T. Cognitive control of escape behaviour. Trends Cogn Sci. 2019;23:334–348. doi: 10.1016/j.tics.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card GM. Escape behaviors in insects. Curr Opin Neurobiol. 2012;22:180–186. doi: 10.1016/j.conb.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Herberholz J, Marquart GD. Decision making and behavioral choice during predator avoidance. Front Neurosci. 2012;6:125. doi: 10.3389/fnins.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fotowat H, Gabbiani F. Collision detection as a model for sensory-motor integration. Annu Rev Neurosci. 2011;34:1–19. doi: 10.1146/annurev-neuro-061010-113632. [DOI] [PubMed] [Google Scholar]
- 12.Peek MY, Card GM. Comparative approaches to escape. Curr Opin Neurobiol. 2016;41:167–173. doi: 10.1016/j.conb.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Allen MJ, Godenschwege TA, Tanouye MA, Phelan P. Making an escape: Development and function of the Drosophila giant fibre system. Semin Cell Dev Biol. 2006;17:31–41. doi: 10.1016/j.semcdb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Modi MN, Shuai Y, Turner GC. The Drosophila mushroom body: From architecture to algorithm in a learning circuit. Annu Rev Neurosci. 2020;43:465–484. doi: 10.1146/annurev-neuro-080317-0621333. [DOI] [PubMed] [Google Scholar]
- 15.Card G, Dickinson MH. Visually mediated motor planning in the escape response of Drosophila. Curr Biol. 2008;18:1300–1307. doi: 10.1016/j.cub.2008.07.094. [DOI] [PubMed] [Google Scholar]
- 16.Card G, Dickinson M. Performance trade-offs in the flight initiation of Drosophila. J Exp Biol. 2008;211:341–353. doi: 10.1242/jeb.012682. [DOI] [PubMed] [Google Scholar]
- 17.Maisak MS, Haag J, Ammer G, Serbe E, Meier M, Leonhardt A, et al. A directional tuning map of Drosophila elementary motion detectors. Nature. 2013;500:212–216. doi: 10.1038/nature12320. [DOI] [PubMed] [Google Scholar]
- 18.Schilling T, Borst A. Local motion detectors are required for the computation of expansion flow-fields. Biol Open. 2015;4:1105–1108. doi: 10.1242/bio.012690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klapoetke NC, Nern A, Peek MY, Rogers EM, Breads P, Rubin GM, et al. Ultra-selective looming detection from radial motion opponency. Nature. 2017;551:237–241. doi: 10.1038/nature24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Reyn CR, Nern A, Williamson WR, Breads P, Wu M, Namiki S, et al. Feature integration drives probabilistic behavior in the Drosophila escape response. Neuron. 2017;94:1190–1204.e6. doi: 10.1016/j.neuron.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Ache JM, Polsky J, Alghailani S, Parekh R, Breads P, Peek MY, et al. Neural basis for looming size and velocity encoding in the Drosophila giant fiber escape pathway. Curr Biol. 2019;29:1073–1081.e4. doi: 10.1016/j.cub.2019.01.079. [DOI] [PubMed] [Google Scholar]
- 22.Krapp HG, Gabbiani F. Spatial distribution of inputs and local receptive field properties of a wide-field, looming sensitive neuron. J Neurophysiol. 2005;93:2240–2253. doi: 10.1152/jn.00965.2004. [DOI] [PubMed] [Google Scholar]
- 23.Gabbiani F, Cohen I, Laurent G. Time-dependent activation of feed-forward inhibition in a looming-sensitive neuron. J Neurophysiol. 2005;94:2150–2161. doi: 10.1152/jn.00411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Dewell RB, Zhu Y, Gabbiani F. Feedforward inhibition conveys time-varying stimulus information in a collision detection circuit. Curr Biol. 2018;28:1509–1521.e3. doi: 10.1016/j.cub.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabbiani F, Krapp HG, Koch C, Laurent G. Multiplicative computation in a visual neuron sensitive to looming. Nature. 2002;420:320–324. doi: 10.1038/nature01190. [DOI] [PubMed] [Google Scholar]
- 26.Hatsopoulos N, Gabbiani F, Laurent G. Elementary computation of object approach by wide-field visual neuron. Science. 1995;270:1000–1003. doi: 10.1126/science.270.5238.1000. [DOI] [PubMed] [Google Scholar]
- 27.Dewell RB, Gabbiani F. Biophysics of object segmentation in a collision-detecting neuron. eLife. 2018;7:e34238. doi: 10.7554/eLife.34238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y, Dewell RB, Wang H, Gabbiani F. Pre-synaptic muscarinic excitation enhances the discrimination of looming stimuli in a collision-detection neuron. Cell Rep. 2018;23:2365–2378. doi: 10.1016/j.celrep.2018.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Reyn CR, Breads P, Peek MY, Zheng GZ, Williamson WR, Yee AL, et al. A spike-timing mechanism for action selection. Nat Neurosci. 2014;17:962–970. doi: 10.1038/nn.3741. [DOI] [PubMed] [Google Scholar]
- 30.Tanouye MA, Wyman RJ. Motor outputs of giant nerve fiber in Drosophila. J Neurophysiol. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- 31.Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Fotowat H, Fayyazuddin A, Bellen HJ, Gabbiani F. A novel neuronal pathway for visually guided escape in Drosophila melanogaster. J Neurophysiol. 2009;102:875–885. doi: 10.1152/jn.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheong HS, Siwanowicz I, Card GM. Multi-regional circuits underlying visually guided decision-making in Drosophila. Curr Opin Neurobiol. 2020;65:77–87. doi: 10.1016/j.conb.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 34.de Vries SEJ, Clandinin TR. Loom-sensitive neurons link computation to action in the Drosophila visual system. Curr Biol. 2012;22:353–362. doi: 10.1016/j.cub.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu M, Nern A, Wu M, Morimoto MM, Reiser MB, Card GM, et al. Visual projection neurons in the Drosophila lobula link feature detection to distinct behavioral programs. eLife. 2016;5:e21022. doi: 10.7554/eLife.21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka R, Clark DA. Object-displacement-sensitive visual neurons drive freezing in Drosophila. Curr Biol. 2020;30:2532–2550.e8. doi: 10.1016/j.cub.2020.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zacarias R, Namiki S, Card GM, Vasconcelos ML, Moita MA. Speed dependent descending control of freezing behavior in Drosophila melanogaster. Nat Commun. 2018;9:3697. doi: 10.1038/s41467-018-05875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keleş MF, Hardcastle BJ, Städele C, Xiao Q, Frye MA. Inhibitory interactions and columnar inputs to an object motion detector in Drosophila. Cell Rep. 2020;30:2115–2124.e5. doi: 10.1016/j.celrep.2020.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ball W, Tronick E. Infant responses to impending collision: Optical and real. Science. 1971;171:818–820. doi: 10.1126/science.171.3973.818. [DOI] [PubMed] [Google Scholar]
- 40.Schiff W, Caviness JA, Gibson JJ. Persistent fear responses in rhesus monkeys to the optical stimulus of looming. Science. 1962;136:982–983. doi: 10.1126/science.136.3520.982. [DOI] [PubMed] [Google Scholar]
- 41.Sun H, Frost BJ. Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat Neurosci. 1998;1:296–303. doi: 10.1038/1110. [DOI] [PubMed] [Google Scholar]
- 42.Preuss T, Osei-Bonsu PE, Weiss SA, Wang C, Faber DS. Neural representation of object approach in a decision-making motor circuit. J Neurosci. 2006;26:3454–3464. doi: 10.1523/JNEUROSCI.5259-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes WN, Saiff EI. Visual alarm reactions in turtles. Animal Behav. 1967;15:102–106. doi: 10.1016/S0003-3472(67)80018-6. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto K, Nakata M, Nakagawa H. Input and output characteristics of collision avoidance behavior in the frog Rana catesbeiana. Brain Behav Evol. 2003;62:201–211. doi: 10.1159/000073272. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Frost BJ. Time to collision is signalled by neurons in the nucleus rotundus of pigeons. Nature. 1992;356:236–238. doi: 10.1038/356236a0. [DOI] [PubMed] [Google Scholar]
- 46.Cong L, Wang Z, Chai Y, Hang W, Shang C, Yang W, et al. Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio) eLife. 2017;6:e28158. doi: 10.7554/eLife.28158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim DH, Kim J, Marques JC, Grama A, Hildebrand DGC, Gu W, et al. Pan-neuronal calcium imaging with cellular resolution in freely swimming zebrafish. Nat Methods. 2017;14:1107–1114. doi: 10.1038/nmeth.4429. [DOI] [PubMed] [Google Scholar]
- 48.Fosque BF, Sun Y, Dana H, Yang CT, Ohyama T, Tadross MR, et al. Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science. 2015;347:755–760. doi: 10.1126/science.1260922. [DOI] [PubMed] [Google Scholar]
- 49.Förster D, Kramer A, Baier H, Kubo F. Optogenetic precision toolkit to reveal form, function and connectivity of single neurons. Methods. 2018;150:42–48. doi: 10.1016/j.ymeth.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Orger MB. The cellular organization of zebrafish visuomotor circuits. Curr Biol. 2016;26:R377–R385. doi: 10.1016/j.cub.2016.03.054. [DOI] [PubMed] [Google Scholar]
- 51.Orger MB, de Polavieja GG. Zebrafish behavior: Opportunities and challenges. Annu Rev Neurosci. 2017;40:125–147. doi: 10.1146/annurev-neuro-071714-033857. [DOI] [PubMed] [Google Scholar]
- 52.Baier H, Wullimann MF. Anatomy and function of retinorecipient arborization fields in zebrafish. J Comp Neurol. 2021;529:3454–3476. doi: 10.1002/cne.25204. [DOI] [PubMed] [Google Scholar]
- 53.Yao Y, Li X, Zhang B, Yin C, Liu Y, Chen W, et al. Visual cue-discriminative dopaminergic control of visuomotor transformation and behavior selection. Neuron. 2016;89:598–612. doi: 10.1016/j.neuron.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 54.Temizer I, Donovan JC, Baier H, Semmelhack JL. A visual pathway for looming-evoked escape in larval zebrafish. Curr Biol. 2015;25:1823–1834. doi: 10.1016/j.cub.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Luca RM, Gerlai R. In search of optimal fear inducing stimuli: Differential behavioral responses to computer animated images in zebrafish. Behav Brain Res. 2012;226:66–76. doi: 10.1016/j.bbr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spinello C, Yang Y, Macrì S, Porfiri M. Zebrafish adjust their behavior in response to an interactive robotic predator. Front Robot AI. 2019;6:38. doi: 10.3389/frobt.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bass SLS, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 58.Ladu F, Bartolini T, Panitz SG, Chiarotti F, Butail S, Macrì S, et al. Live predators, robots, and computer-animated images elicit differential avoidance responses in zebrafish. Zebrafish. 2015;12:205–214. doi: 10.1089/zeb.2014.1041. [DOI] [PubMed] [Google Scholar]
- 59.Dunn TW, Gebhardt C, Naumann EA, Riegler C, Ahrens MB, Engert F, et al. Neural circuits underlying visually evoked escapes in larval zebrafish. Neuron. 2016;89:613–628. doi: 10.1016/j.neuron.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heap LAL, Vanwalleghem G, Thompson AW, Favre-Bulle IA, Scott EK. Luminance changes drive directional startle through a thalamic pathway. Neuron. 2018;99:293–301.e4. doi: 10.1016/j.neuron.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Bellingham J, Whitmore D, Philp AR, Wells DJ, Foster RG. Zebrafish melanopsin: Isolation, tissue localisation and phylogenetic position. Mol Brain Res. 2002;107:128–136. doi: 10.1016/S0169-328X(02)00454-0. [DOI] [PubMed] [Google Scholar]
- 62.Fernandes AM, Fero K, Arrenberg AB, Bergeron SA, Driever W, Burgess HA. Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr Biol. 2012;22:2042–2047. doi: 10.1016/j.cub.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kölsch Y, Hahn J, Sappington A, Stemmer M, Fernandes AM, Helmbrecht TO, et al. Molecular classification of zebrafish retinal ganglion cells links genes to cell types to behavior. Neuron. 2021;109:645–662.e9. doi: 10.1016/j.neuron.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Preuss SJ, Trivedi CA, vom Berg-Maurer CM, Ryu S, Bollmann JH. Classification of object size in retinotectal microcircuits. Curr Biol. 2014;24:2376–2385. doi: 10.1016/j.cub.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 65.Robles E, Fields NP, Baier H. The zebrafish visual system transmits dimming information via multiple segregated pathways. J Comp Neurol. 2021;529:539–552. doi: 10.1002/cne.24964. [DOI] [PubMed] [Google Scholar]
- 66.Förster D, Helmbrecht TO, Mearns DS, Jordan L, Mokayes N, Baier H. Retinotectal circuitry of larval zebrafish is adapted to detection and pursuit of prey. eLife. 2020;9:e58596. doi: 10.7554/eLife.58596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burrill JD, Easter SS., Jr Development of the retinofugal projections in the embryonic and larval zebrafish (Brachydanio rerio) J Comp Neurol. 1994;346:583–600. doi: 10.1002/cne.903460410. [DOI] [PubMed] [Google Scholar]
- 68.Helmbrecht TO, dal Maschio M, Donovan JC, Koutsouli S, Baier H. Topography of a visuomotor transformation. Neuron. 2018;100:1429–1445.e4. doi: 10.1016/j.neuron.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 69.Fajardo O, Zhu P, Friedrich RW. Control of a specific motor program by a small brain area in zebrafish. Front Neural Circuits. 2013;7:67. doi: 10.3389/fncir.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robles E, Laurell E, Baier H. The retinal projectome reveals brain-area-specific visual representations generated by ganglion cell diversity. Curr Biol. 2014;24:2085–2096. doi: 10.1016/j.cub.2014.07.080. [DOI] [PubMed] [Google Scholar]
- 71.Lowe AS, Nikolaou N, Hunter PR, Thompson ID, Meyer MP. A systems-based dissection of retinal inputs to the zebrafish tectum reveals different rules for different functional classes during development. J Neurosci. 2013;33:13946–13956. doi: 10.1523/JNEUROSCI.1866-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nikolaou N, Lowe AS, Walker AS, Abbas F, Hunter PR, Thompson ID, et al. Parametric functional maps of visual inputs to the tectum. Neuron. 2012;76:317–324. doi: 10.1016/j.neuron.2012.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marquez-Legorreta E, Piber M, Scott EK. Visual escape in larval zebrafish: Stimuli, circuits, and behavior. In: Gerlai RT, editor. Behavioral and Neural Genetics of Zebrafish. Amsterdam: Elsevier; 2020. pp. 49–71. [Google Scholar]
- 74.Del Bene F, Wyart C, Robles E, Tran A, Looger L, Scott EK, et al. Filtering of visual information in the tectum by an identified neural circuit. Science. 2010;330:669–673. doi: 10.1126/science.1192949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bollmann JH. The zebrafish visual system: From circuits to behavior. Annu Rev Vis Sci. 2019;5:269–293. doi: 10.1146/annurev-vision-091718-014723. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharyya K, McLean DL, MacIver MA. Visual threat assessment and reticulospinal encoding of calibrated responses in larval zebrafish. Curr Biol. 2017;27:2751–2762.e6. doi: 10.1016/j.cub.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fotowat H, Harrison RR, Gabbiani F. Multiplexing of motor information in the discharge of a collision detecting neuron during escape behaviors. Neuron. 2011;69:147–158. doi: 10.1016/j.neuron.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Semmelhack JL, Donovan JC, Thiele TR, Kuehn E, Laurell E, Baier H. A dedicated visual pathway for prey detection in larval zebrafish. eLife. 2014;3:e04878. doi: 10.7554/eLife.04878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato T, Hamaoka T, Aizawa H, Hosoya T, Okamoto H. Genetic single-cell mosaic analysis implicates ephrinB2 reverse signaling in projections from the posterior tectum to the hindbrain in zebrafish. J Neurosci. 2007;27:5271–5279. doi: 10.1523/JNEUROSCI.0883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korn H, Faber DS. The mauthner cell half a century later: A neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 81.Eaton RC, Lee RKK, Foreman MB. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol. 2001;63:467–485. doi: 10.1016/S0301-0082(00)00047-2. [DOI] [PubMed] [Google Scholar]
- 82.Tabor KM, Bergeron SA, Horstick EJ, Jordan DC, Aho V, Porkka-Heiskanen T, et al. Direct activation of the Mauthner cell by electric field pulses drives ultrarapid escape responses. J Neurophysiol. 2014;112:834–844. doi: 10.1152/jn.00228.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kohashi T, Oda Y. Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J Neurosci. 2008;28:10641–10653. doi: 10.1523/JNEUROSCI.1435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/S0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 85.Lacoste AMB, Schoppik D, Robson DN, Haesemeyer M, Portugues R, Li JM, et al. A convergent and essential interneuron pathway for mauthner-cell-mediated escapes. Curr Biol. 2015;25:1526–1534. doi: 10.1016/j.cub.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hecker A, Schulze W, Oster J, Richter DO, Schuster S. Removing a single neuron in a vertebrate brain forever abolishes an essential behavior. Proc Natl Acad Sci U S A. 2020;117:3254–3260. doi: 10.1073/pnas.1918578117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Filosa A, Barker AJ, Dal Maschio M, Baier H. Feeding state modulates behavioral choice and processing of prey stimuli in the zebrafish tectum. Neuron. 2016;90:596–608. doi: 10.1016/j.neuron.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 88.Hikosaka O. The habenula: From stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suryanarayana SM, Pérez-Fernández J, Robertson B, Grillner S. The lamprey forebrain—Evolutionary implications. Brain Behav Evol. 2022;96:318–333. doi: 10.1159/000517492. [DOI] [PubMed] [Google Scholar]
- 90.Agetsuma M, Aizawa H, Aoki T, Nakayama R, Takahoko M, Goto M, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- 91.Duboué ER, Hong E, Eldred KC, Halpern ME. Left habenular activity attenuates fear responses in larval zebrafish. Curr Biol. 2017;27:2154–2162.e3. doi: 10.1016/j.cub.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okamoto H, Agetsuma M, Aizawa H. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev Neurobiol. 2012;72:386–394. doi: 10.1002/dneu.20913. [DOI] [PubMed] [Google Scholar]
- 93.Lovett-Barron M, Andalman AS, Allen WE, Vesuna S, Kauvar I, Burns VM, et al. Ancestral circuits for the coordinated modulation of brain state. Cell. 2017;171:1411–1423.e17. doi: 10.1016/j.cell.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feng J, Zhang C, Lischinsky JE, Jing M, Zhou J, Wang H, et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron. 2019;102:745–761.e8. doi: 10.1016/j.neuron.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corradi L, Filosa A. Neuromodulation and behavioral flexibility in larval zebrafish: From neurotransmitters to circuits. Front Mol Neurosci. 2021;14:718951. doi: 10.3389/fnmol.2021.718951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lovett-Barron M, Chen R, Bradbury S, Andalman AS, Wagle M, Guo S, et al. Multiple convergent hypothalamus–brainstem circuits drive defensive behavior. Nat Neurosci. 2020;23:959–967. doi: 10.1038/s41593-020-0655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.do Carmo Silva RX, Lima-Maximino MG, Maximino C. The aversive brain system of teleosts: Implications for neuroscience and biological psychiatry. Neurosci Biobehav Rev. 2018;95:123–135. doi: 10.1016/j.neubiorev.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Carr JA. I'll take the low road: The evolutionary underpinnings of visually triggered fear. Front Neurosci. 2015;9:414. doi: 10.3389/fnins.2015.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Münch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci. 2009;12:1308–1316. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- 100.Peichl L. Alpha ganglion cells in mammalian retinae: Common properties, species differences, and some comments on other ganglion cells. Vis Neurosci. 1991;7:155–169. doi: 10.1017/S0952523800011020. [DOI] [PubMed] [Google Scholar]
- 101.Cleland BG, Levick WR, Wässle H. Physiological identification of a morphological class of cat retinal ganglion cells. J Physiol. 1975;248:151–171. doi: 10.1113/jphysiol.1975.sp010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cleland BG, Levick WR. Brisk and sluggish concentrically organized ganglion cells in the cat’s retina. J Physiol. 1974;240:421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krieger B, Qiao M, Rousso DL, Sanes JR, Meister M. Four alpha ganglion cell types in mouse retina: Function, structure, and molecular signatures. PLoS ONE. 2017;12:e0180091. doi: 10.1371/journal.pone.0180091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boycott BB, Wässle H. The morphological types of ganglion cells of the domestic cat’s retina. J Physiol. 1974;240:397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim T, Shen N, Hsiang JC, Johnson KP, Kerschensteiner D. Dendritic and parallel processing of visual threats in the retina control defensive responses. Sci Adv. 2020;6:eabc9920. doi: 10.1126/sciadv.abc9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang F, Li E, De L, Wu Q, Zhang Y. OFF-transient alpha RGCs mediate looming triggered innate defensive response. Curr Biol. 2021;31:2263–2273.e3. doi: 10.1016/j.cub.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 107.Huang L, Yuan T, Tan M, Xi Y, Hu Y, Tao Q, et al. A retinoraphe projection regulates serotonergic activity and looming-evoked defensive behaviour. Nat Commun. 2017;8:14908. doi: 10.1038/ncomms14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cai D, Luo X, Shen K, Shen Y. GABAergic retinal ganglion cells regulate innate defensive responses. Neuroreport. 2021;32:643–649. doi: 10.1097/WNR.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 109.Yang X, Liu Q, Zhong J, Song R, Zhang L, Wang L. A simple threat-detection strategy in mice. BMC Biol. 2020;18:93. doi: 10.1186/s12915-020-00825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y, Kim IJ, Sanes JR, Meister M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc Natl Acad Sci U S A. 2012;109:E2391–E2398. doi: 10.1073/pnas.1211547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reinhard K, Li C, Do Q, Burke EG, Heynderickx S, Farrow K. A projection specific logic to sampling visual inputs in mouse superior colliculus. eLife. 2019;8:e50697. doi: 10.7554/eLife.50697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lees RN, Akbar AF, Badea TC. Retinal ganglion cell defects cause decision shifts in visually evoked defense responses. J Neurophysiol. 2020;124:1530–1549. doi: 10.1152/jn.00474.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salay LD, Huberman AD. Divergent outputs of the ventral lateral geniculate nucleus mediate visually evoked defensive behaviors. Cell Rep. 2021;37:109792. doi: 10.1016/j.celrep.2021.109792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fratzl A, Koltchev AM, Vissers N, Tan YL, Marques-Smith A, Vanessa Stempel A, et al. Flexible inhibitory control of visually evoked defensive behavior by the ventral lateral geniculate nucleus. Neuron. 2021;109:3810–3822.e9. doi: 10.1016/j.neuron.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Isa T, Marquez-Legorreta E, Grillner S, Scott EK. The tectum/superior colliculus as the vertebrate solution for spatial sensory integration and action. Curr Biol. 2021;31:R741–R762. doi: 10.1016/j.cub.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dean P, Redgrave P, Westby GWM. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–147. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- 117.Oliveira AF, Yonehara K. The mouse superior Colliculus as a model system for investigating cell type-based mechanisms of visual motor transformation. Front Neural Circuits. 2018;12:59. doi: 10.3389/fncir.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu X, Huang H, Snutch TP, Cao P, Wang L, Wang F. The superior Colliculus: Cell types, connectivity, and behavior. Neurosci Bull. 2022;38:1519–1540. doi: 10.1007/s12264-022-00858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krauzlis RJ, Lovejoy LP, Zénon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165–182. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci. 2011;34:205–231. doi: 10.1146/annurev-neuro-061010-113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.May PJ. The mammalian superior colliculus: Laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- 122.Ito S, Feldheim DA. The mouse superior Colliculus: An emerging model for studying circuit formation and function. Front Neural Circuits. 2018;12:10. doi: 10.3389/fncir.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wheatcroft T, Saleem AB, Solomon SG. Functional organisation of the mouse superior Colliculus. Front Neural Circuits. 2022;16:792959. doi: 10.3389/fncir.2022.792959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee KH, Tran A, Turan Z, Meister M. The sifting of visual information in the superior colliculus. eLife. 2020;9:e50678. doi: 10.7554/eLife.50678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Basso MA, May PJ. Circuits for action and cognition: A view from the superior Colliculus. Annu Rev Vis Sci. 2017;3:197–226. doi: 10.1146/annurev-vision-102016-061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cang J, Savier E, Barchini J, Liu X. Visual function, organization, and development of the mouse superior Colliculus. Annu Rev Vis Sci. 2018;4:239–262. doi: 10.1146/annurev-vision-091517-034142. [DOI] [PubMed] [Google Scholar]
- 127.Ellis EM, Gauvain G, Sivyer B, Murphy GJ. Shared and distinct retinal input to the mouse superior colliculus and dorsal lateral geniculate nucleus. J Neurophysiol. 2016;116:602–610. doi: 10.1152/jn.00227.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seabrook TA, Burbridge TJ, Crair MC, Huberman AD. Architecture, function, and assembly of the mouse visual system. Annu Rev Neurosci. 2017;40:499–538. doi: 10.1146/annurev-neuro-071714-033842. [DOI] [PubMed] [Google Scholar]
- 129.Gale SD, Murphy GJ. Distinct representation and distribution of visual information by specific cell types in mouse superficial superior colliculus. J Neurosci. 2014;34:13458–13471. doi: 10.1523/JNEUROSCI.2768-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shang C, Chen Z, Liu A, Li Y, Zhang J, Qu B, et al. Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nat Commun. 2018;9:1232. doi: 10.1038/s41467-018-03580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shang C, Liu Z, Chen Z, Shi Y, Wang Q, Liu S, et al. BRAIN CIRCUITS. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science. 2015;348:1472–1477. doi: 10.1126/science.aaa8694. [DOI] [PubMed] [Google Scholar]
- 132.Sans-Dublanc A, Chrzanowska A, Reinhard K, Lemmon D, Nuttin B, Lambert T, et al. Optogenetic fUSI for brain-wide mapping of neural activity mediating collicular-dependent behaviors. Neuron. 2021;109:1888–1905.e10. doi: 10.1016/j.neuron.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 133.Xie Z, Wang M, Liu Z, Shang C, Zhang C, Sun L, et al. Transcriptomic encoding of sensorimotor transformation in the midbrain. eLife. 2021;10:e69825. doi: 10.7554/eLife.69825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 135.Villalobos CA, Wu Q, Lee PH, May PJ, Basso MA. Parvalbumin and GABA microcircuits in the mouse superior Colliculus. Front Neural Circuits. 2018;12:35. doi: 10.3389/fncir.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wei P, Liu N, Zhang Z, Liu X, Tang Y, He X, et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun. 2015;6:6756. doi: 10.1038/ncomms7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gale SD, Murphy GJ. Active dendritic properties and local inhibitory input enable selectivity for object motion in mouse superior Colliculus neurons. J Neurosci. 2016;36:9111–9123. doi: 10.1523/JNEUROSCI.0645-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, et al. AAV-mediated anterograde transsynaptic tagging: Mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron. 2017;93:33–47. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao X, Liu M, Cang J. Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior Colliculus of awake mice. Neuron. 2014;84:202–213. doi: 10.1016/j.neuron.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ahmadlou M, Zweifel LS, Heimel JA. Functional modulation of primary visual cortex by the superior colliculus in the mouse. Nat Commun. 2018;9:3895. doi: 10.1038/s41467-018-06389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li C, Kühn NK, Alkislar I, Dublanc AS, Zemmouri F, Paesmans S, et al. Pathway-specific inputs to the superior colliculus support flexible triggering of innate behaviors. bioRxiv. 2022 doi: 10.1101/2022.07.08.499294. [DOI] [Google Scholar]
- 142.Wu Q, Li E, Zhang Y. A synaptic filtering mechanism in visual threat identification in mouse. Proc Natl Acad Sci U S A. 2023;120:e2212786120. doi: 10.1073/pnas.2212786120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hoy JL, Bishop HI, Niell CM. Defined cell types in superior Colliculus make distinct contributions to prey capture behavior in the mouse. Curr Biol. 2019;29:4130–4138.e5. doi: 10.1016/j.cub.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dräger UC, Hubel DH. Topography of visual and somatosensory projections to mouse superior colliculus. J Neurophysiol. 1976;39:91–101. doi: 10.1152/jn.1976.39.1.91. [DOI] [PubMed] [Google Scholar]
- 145.Holmes NP, Spence C. Multisensory integration: Space, time and superadditivity. Curr Biol. 2005;15:R762–R764. doi: 10.1016/j.cub.2005.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Benavidez NL, Bienkowski MS, Zhu M, Garcia LH, Fayzullina M, Gao L, et al. Organization of the inputs and outputs of the mouse superior colliculus. Nat Commun. 2021;12:4004. doi: 10.1038/s41467-021-24241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou Z, Liu X, Chen S, Zhang Z, Liu Y, Montardy Q, et al. A VTA GABAergic neural circuit mediates visually evoked innate defensive responses. Neuron. 2019;103:473–488.e6. doi: 10.1016/j.neuron.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 148.Shang C, Liu A, Li D, Xie Z, Chen Z, Huang M, et al. A subcortical excitatory circuit for sensory-triggered predatory hunting in mice. Nat Neurosci. 2019;22:909–920. doi: 10.1038/s41593-019-0405-4. [DOI] [PubMed] [Google Scholar]
- 149.Isa K, Sooksawate T, Kobayashi K, Kobayashi K, Redgrave P, Isa T. Dissecting the tectal output channels for orienting and defense responses. eNeuro. 2020 doi: 10.1523/ENEURO.0271-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Evans DA, Stempel AV, Vale R, Ruehle S, Lefler Y, Branco T. A synaptic threshold mechanism for computing escape decisions. Nature. 2018;558:590–594. doi: 10.1038/s41586-018-0244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stubblefield EA, Costabile JD, Felsen G. Optogenetic investigation of the role of the superior colliculus in orienting movements. Behav Brain Res. 2013;255:55–63. doi: 10.1016/j.bbr.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Masullo L, Mariotti L, Alexandre N, Freire-Pritchett P, Boulanger J, Tripodi M. Genetically defined functional modules for spatial orienting in the mouse superior Colliculus. Curr Biol. 2019;29:2892–2904.e8. doi: 10.1016/j.cub.2019.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Comoli E, Das Neves Favaro P, Vautrelle N, Leriche M, Overton PG, Redgrave P. Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense. Front Neuroanat. 2012;6:9. doi: 10.3389/fnana.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li Z, Wei JX, Zhang GW, Huang JJ, Zingg B, Wang X, et al. Corticostriatal control of defense behavior in mice induced by auditory looming cues. Nat Commun. 2021;12:1040. doi: 10.1038/s41467-021-21248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tokuoka K, Kasai M, Kobayashi K, Isa T. Anatomical and electrophysiological analysis of cholinergic inputs from the parabigeminal nucleus to the superficial superior colliculus. J Neurophysiol. 2020;124:1968–1985. doi: 10.1152/jn.00148.2020. [DOI] [PubMed] [Google Scholar]
- 156.Salay LD, Ishiko N, Huberman AD. A midline thalamic circuit determines reactions to visual threat. Nature. 2018;557:183–189. doi: 10.1038/s41586-018-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–100. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- 158.Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- 159.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 160.Liu J, Lin L, Wang DV. Representation of fear of heights by basolateral amygdala neurons. J Neurosci. 2021;41:1080–1091. doi: 10.1523/JNEUROSCI.0483-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Silva BA, Gross CT, Gräff J. The neural circuits of innate fear: Detection, integration, action, and memorization. Learn Mem. 2016;23:544–555. doi: 10.1101/lm.042812.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 164.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 165.Gothard KM. Multidimensional processing in the amygdala. Nat Rev Neurosci. 2020;21:565–575. doi: 10.1038/s41583-020-0350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Daviu N, Füzesi T, Rosenegger DG, Rasiah NP, Sterley TL, Peringod G, et al. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat Neurosci. 2020;23:398–410. doi: 10.1038/s41593-020-0591-0. [DOI] [PubMed] [Google Scholar]
- 167.Kim J, Lee S, Fang YY, Shin A, Park S, Hashikawa K, et al. Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat Neurosci. 2019;22:576–585. doi: 10.1038/s41593-019-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lecca S, Meye FJ, Trusel M, Tchenio A, Harris J, Schwarz MK, et al. Aversive stimuli drive hypothalamus-to-habenula excitation to promote escape behavior. eLife. 2017;6:e30697. doi: 10.7554/eLife.30697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barbano MF, Wang HL, Zhang S, Miranda-Barrientos J, Estrin DJ, Figueroa-González A, et al. VTA glutamatergic neurons mediate innate defensive behaviors. Neuron. 2020;107:368–382.e8. doi: 10.1016/j.neuron.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, et al. Midbrain circuits for defensive behaviour. Nature. 2016;534:206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- 171.Silva C, McNaughton N. Are periaqueductal gray and dorsal raphe the foundation of appetitive and aversive control? A comprehensive review. Prog Neurobiol. 2019;177:33–72. doi: 10.1016/j.pneurobio.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 172.Brandão ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC, Landeira-Fernandez J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: Association with different types of anxiety. Behav Brain Res. 2008;188:1–13. doi: 10.1016/j.bbr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 173.Misslin R. The defense system of fear: Behavior and neurocircuitry. Neurophysiol Clin/Clin Neurophysiol. 2003;33:55–66. doi: 10.1016/S0987-7053(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 174.Franklin TB. Recent advancements surrounding the role of the periaqueductal gray in predators and prey. Front Behav Neurosci. 2019;13:60. doi: 10.3389/fnbeh.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lefler Y, Campagner D, Branco T. The role of the periaqueductal gray in escape behavior. Curr Opin Neurobiol. 2020;60:115–121. doi: 10.1016/j.conb.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 176.Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature. 2014;508:351–356. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- 177.Capelli P, Pivetta C, Soledad Esposito M, Arber S. Locomotor speed control circuits in the caudal brainstem. Nature. 2017;551:373–377. doi: 10.1038/nature24064. [DOI] [PubMed] [Google Scholar]
- 178.Ferreira-Pinto MJ, Ruder L, Capelli P, Arber S. Connecting circuits for supraspinal control of locomotion. Neuron. 2018;100:361–374. doi: 10.1016/j.neuron.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 179.Ruder L, Arber S. Brainstem circuits controlling action diversification. Annu Rev Neurosci. 2019;42:485–504. doi: 10.1146/annurev-neuro-070918-050201. [DOI] [PubMed] [Google Scholar]
- 180.Bolton AD, Murata Y, Kirchner R, Kim SY, Young A, Dang T, et al. A diencephalic dopamine source provides input to the superior Colliculus, where D1 and D2 receptors segregate to distinct functional zones. Cell Rep. 2015;13:1003–1015. doi: 10.1016/j.celrep.2015.09.046. [DOI] [PubMed] [Google Scholar]
- 181.Deichler A, Carrasco D, Lopez-Jury L, Vega-Zuniga T, Márquez N, Mpodozis J, et al. A specialized reciprocal connectivity suggests a link between the mechanisms by which the superior colliculus and parabigeminal nucleus produce defensive behaviors in rodents. Sci Rep. 2020;10:16220. doi: 10.1038/s41598-020-72848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stubblefield EA, Thompson JA, Felsen G. Optogenetic cholinergic modulation of the mouse superior colliculus in vivo. J Neurophysiol. 2015;114:978–988. doi: 10.1152/jn.00917.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Endo T, Yanagawa Y, Obata K, Isa T. Nicotinic acetylcholine receptor subtypes involved in facilitation of GABAergic inhibition in mouse superficial superior colliculus. J Neurophysiol. 2005;94:3893–3902. doi: 10.1152/jn.00211.2005. [DOI] [PubMed] [Google Scholar]
- 184.Li L, Feng X, Zhou Z, Zhang H, Shi Q, Lei Z, et al. Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior Colliculus projection. Curr Biol. 2018;28:859–871.e5. doi: 10.1016/j.cub.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 185.Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci. 2020;21:277–295. doi: 10.1038/s41583-020-0292-4. [DOI] [PubMed] [Google Scholar]
- 186.Lecca S, Namboodiri VMK, Restivo L, Gervasi N, Pillolla G, Stuber GD, et al. Heterogeneous habenular neuronal ensembles during selection of defensive behaviors. Cell Rep. 2020;31:107752. doi: 10.1016/j.celrep.2020.107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Eilam D. Die hard: A blend of freezing and fleeing as a dynamic defense—Implications for the control of defensive behavior. Neurosci Biobehav Rev. 2005;29:1181–1191. doi: 10.1016/j.neubiorev.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 188.Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol. 2009;102:2581–2593. doi: 10.1152/jn.00498.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Redgrave P, Mitchell IJ, Dean P. Further evidence for segregated output channels from superior colliculus in rat: Ipsilateral tecto-pontine and tecto-cuneiform projections have different cells of origin. Brain Res. 1987;413:170–174. doi: 10.1016/0006-8993(87)90165-X. [DOI] [PubMed] [Google Scholar]
- 190.Andrezik JA, Chan-Palay V, Palay SL. The nucleus paragigantocellularis lateralis in the rat. Demonstration of afferents by the retrograde transport of horseradish peroxidase. Anat Embryol (Berl) 1981;161:373–390. doi: 10.1007/BF00316049. [DOI] [PubMed] [Google Scholar]