Abstract
Digital Breast Tomosynthesis (DBT) is a cutting-edge technology introduced in recent years as an in-depth analysis of breast cancer diagnostics. Compared with 2D Full-Field Digital Mammography, DBT has demonstrated greater sensitivity and specificity in detecting breast tumors. This work aims to quantitatively evaluate the impact of the systematic introduction of DBT in terms of Biopsy Rate and Positive Predictive Values for the number of biopsies performed (PPV-3). For this purpose, we collected 69,384 mammograms and 7894 biopsies, of which 6484 were Core Biopsies and 1410 were stereotactic Vacuum-assisted Breast Biopsies (VABBs), performed on female patients afferent to the Breast Unit of the Istituto Tumori “Giovanni Paolo II” of Bari from 2012 to 2021, thus, in the period before, during and after the systematic introduction of DBT. Linear regression analysis was then implemented to investigate how the Biopsy Rate had changed over the 10 year screening. The next step was to focus on VABBs, which were generally performed during in-depth examinations of mammogram detected lesions. Finally, three radiologists from the institute’s Breast Unit underwent a comparative study to ascertain their performances in terms of breast cancer detection rates before and after the introduction of DBT. As a result, it was demonstrated that both the overall Biopsy Rate and the VABBs Biopsy Rate significantly decreased following the introduction of DBT, with the diagnosis of an equal number of tumors. Besides, no statistically significant differences were observed among the three operators evaluated. In conclusion, this work highlights how the systematic introduction of DBT has significantly impacted the breast cancer diagnostic procedure, by improving the diagnostic quality and thereby reducing needless biopsies, resulting in a consequent reduction in costs.
Keywords: Breast neoplasm, Full-field digital mammography, Digital breast tomosynthesis, Breast core biopsy, VABB
1. Introduction
The American Cancer Society (ACS) [40] estimates that cancer represents the second major cause of death in the USA and a primary health concern worldwide. Findings published by the ACS in 2022 show that breast cancer incidence in the USA has risen, that is to say, there was a 0.5% annual increase between 2010 and 2019, whereas mortality rates have declined [17]. Although mammography screening programs have contributed to reducing breast cancer mortality by up to 30%, by encouraging early diagnosis [42], the mammogram’s sensitivity is significantly reduced in the case of dense breasts, which are breasts characterized by a higher component of fibro-glandular matter which require closer follow-up and the employment of further methods, such as manual or automated ultrasound [14, 19, 30]. Currently, the presence of dense tissue is the main reason for failed early diagnosis in mammography screening programs, increasing the likelihood of a diagnosis of advanced breast cancer [8]. Over the years, the issue of dense breasts has gained more importance in the scientific community since legislation was introduced in the USA to assess both the density levels and the risks associated with reduced mammographic sensitivity [25, 27]: Differently from dense breasts, the non-dense ones have recently been associated with increased cardiovascular risk [37].
Moreover, in recent years, several cutting-edge techniques have been developed to overcome problems associated with the sensitivity of 2D FFDM (Full-Field Digital Mammography). An example is DBT (Digital Breast Tomosynthesis), which generates three-dimensional breast images through a series of acquisitions at different angles, which partly solves the masking effect linked to the tissue density and increases both the sensitivity and specificity of the investigation [16, 22, 33]. Specifically, DBT can highlight small masses and distortions better than 2D FFDM [44], though there seems to be no significant improvement in detecting microcalcifications [26].
The US Food and Drug Administration originally approved the use of DBT equipment for screening, but only in combination with 2D mammography (FFDM or synthesized) [12, 29]. Nonetheless, the combined use of DBT and 2D FFDM would significantly increase the amount of radiation exposure per examination and the execution time compared with the implementation of the 2D FFDM screening alone. Furthermore, current DBT protocols involve radiation doses comparable to those used in 2D FFDM, depending on the equipment used, the imaging protocol, and the breast thickness [13, 15, 26, 31, 32]. Therefore, to improve the diagnostic accuracy without significantly increasing the patients’ radiation exposure, several centers, including the Breast Unit of the Istituto Tumori “Giovanni Paolo II” of Bari, decided on the exclusive and systematic use of the DBT.
Thus far, several state-of-the-art retrospective studies have focused on comparing FFDM and DBT performances in terms of either sensitivity or specificity or both [18]. Furthermore, in many studies, the performances of radiologists in breast cancer screening were compared both before and after the systematic introduction of DBT in terms of recall rate and cancer detection rate [41]. Finally, Sharma et al. in their work [39] investigated variations in the benign biopsy rate (the number of benign tumors diagnosed out of the total number of biopsies performed).
Nonetheless, to the best of our knowledge, the B5 Biopsy Rate, that is, the number of malignant tumors diagnosed out of the total number of biopsies performed, along with Positive Predictive Values for biopsies performed (PPV-3), that is the ratio between the detected cancers and the number of biopsies performed [22], has yet to be investigated in order to compare the performances of FFDM and DBT.
The aim of this work is, therefore, to evaluate the variations of both the Biopsy Rate and the PPV-3 over a 10 year period of diagnostic activity at a Breast Unit of an Italian Oncological Institute regarding the exclusive use of FFDM and the exclusive use of DBT to verify if the systematic introduction of DBT produced any improvement in terms of better diagnostic accuracy and reduction in needless biopsy samples.
Materials and methods
Experimental data
For this retrospective study (approved by the Scientific Board of the Istituto Tumori "Giovanni Paolo II” of Bari, Italy), 69,384 consecutive mammograms of women participants were collected from January 1, 2012, to December 31, 2021, and clinical diagnostic mastology checkups were carried out from Monday to Friday, by 2 teams, each consisting of 1 radiologist, 1 nurse and 1 radiology technician dedicated to first-level diagnostics on a total of 40 patients (20 per team) divided as follows: first access (n = 6), patients with oncological family history (n = 6), controls (n = 5), oncological patients in follow-up (n = 17), and emergencies (n = 6). It follows that 6/40 were symptomatic patients, while 34/40 were asymptomatic (on the day): Among the latter, there were 17 cancer patients who had already undergone surgery in follow-up, 6 patients with oncological family history linked to breast cancer and 11 with no particular risks. All mammograms were acquired and analyzed by radiologists with an experience of over 15 years and afferent to the Breast Unit of our institute. More specifically, FFDMs were acquired from 2012 to 2015, whereas DBTs were acquired from 2017 to 2021. Meanwhile, a combined acquirement of FFDMs and DBT was performed in 2016, before the exclusive and systematic introduction of the DBT. In addition, we collected data on the number of mammograms performed, the total number of biopsies required, the estimated BIRADS, the average patient’s age, and the bioptic histological class, the results of which were classified from B1 to B5, according to European Guidelines and supplements [7, 34, 35]: B1, normal tissue; B2, benign abnormalities; B3, a heterogeneous group of lesions of unknown biologic potential; B4, suspicious findings but insufficient for a definite diagnosis of malignancy; and B5, unequivocal malignancy.
Characteristics of radiological techniques
DBT involves multiple projections acquired across an arc which are reconstructed into a series of stacked images [5]. Depending on the manufacturer, differently from FFDM, during DBT image acquisition, the x-ray tube pivots in an arc that varies between 15° (narrow range) and 60° (wide range) in a plane aligned with the chest wall. In general, the wider angular range of X-ray tube motion produces more tomographic information and enables better section separation or better vertical resolution (z-axis). Increasing the angular range for the tube movement requires more projections for sufficient sampling [5].
Mammography investigations were conducted on the same device throughout the observation period (GE Healthcare Senographe Essential™) before and after the introduction of the tomosynthesis module. During the period of the introduction of tomosynthesis (2016–2021), investigations were performed by the same dedicated radiologists.
On average, the radiation dose was about 30% higher in the DBT plus SM (Synthesized Mammography) protocol compared to the FFDM data, which was however, considered acceptable and in line with the European guidelines for quality assurance in mammography screening [1, 3, 24, 28, 34].
Statistical analysis
For each year from 2012 to 2021, we evaluated both the Biopsy Rate and PPV-3. Specifically, we focused on B5 tumors. Therefore, to evaluate how the Biopsy Rate changed over the 10 year period, we performed a linear regression analysis, considering the estimated linear association as statistically significant when the p-value resulted as less than 0.05. Furthermore, we investigated whether or not there was any significant interobserver variability in detecting neoplasms, by comparing the performances of three different operators by means of the Wilcoxon statistical test. All analyses were performed using RStudio 2022.02.3 statistical software.
Results
According to the data reported in the Materials and Methods section, the examined population consisted of 85% asymptomatic patients and 15% symptomatic patients. More specifically, 42.5% of the patients were on oncological follow-up, 15% of the patients presented with familiar risk and 27.5% of the patients with no significant risk at all.
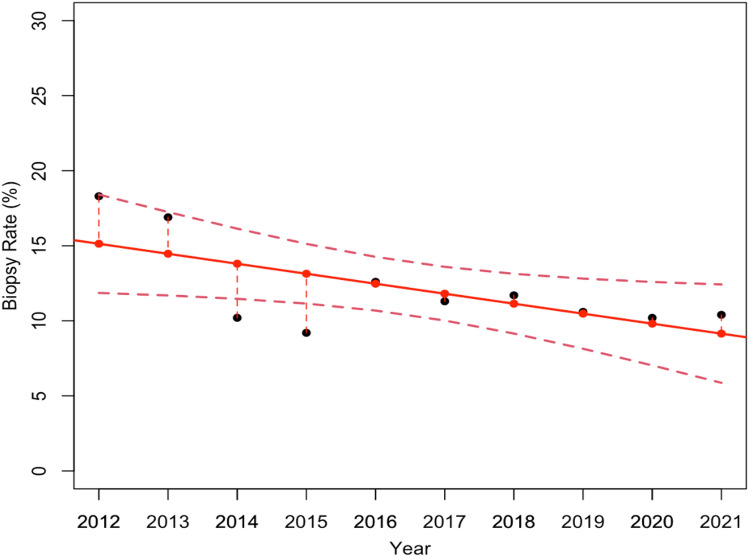
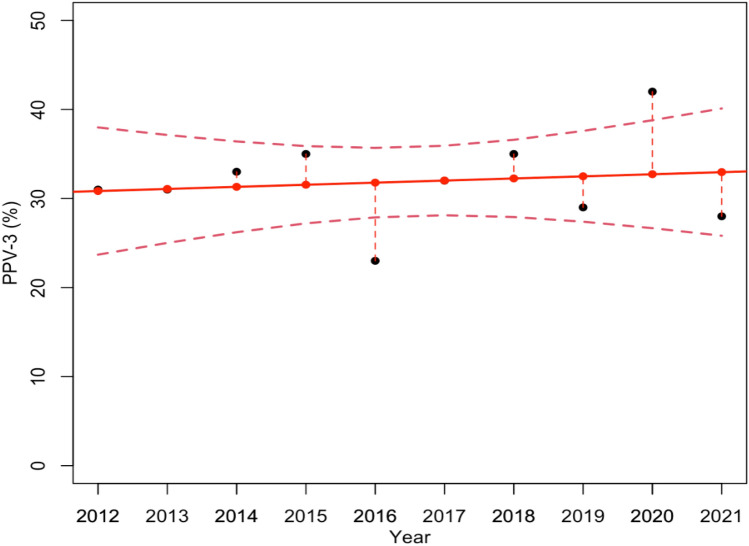
The first analysis evaluated how the overall Biopsy Rate had changed over the 10 year period. For this purpose, Table 1 shows the number of mammograms performed, the overall number of biopsies required, the number of detected B5 tumors, in addition to the estimated Biopsy Rate and PPV-3 for each year from 2012 to 2021.
Table 1.
Detailed overview of the total mammograms performed, biopsies required, and detected B5 tumors from 2012 to 2021. For each year, the Biopsy Rate and the PPV-3 are also reported
| Year | Mammograms | Biopsies | B5 tumors | Biopsy rate (%) | PPV-3 (%) |
|---|---|---|---|---|---|
| 2012 | 3247 | 594 | 278 | 18.3 | 46.8 |
| 2013 | 2731 | 462 | 254 | 16.9 | 55.0 |
| 2014 | 5665 | 576 | 343 | 10.2 | 59.5 |
| 2015 | 6968 | 642 | 350 | 9.2 | 54.5 |
| 2016 | 7373 | 927 | 327 | 12.6 | 35.3 |
| 2017 | 7314 | 828 | 376 | 11.3 | 45.4 |
| 2018 | 8556 | 1001 | 435 | 11.7 | 43.5 |
| 2019 | 9751 | 1034 | 436 | 10.6 | 42.2 |
| 2020 | 8497 | 866 | 338 | 10.2 | 39.0 |
| 2021 | 9282 | 965 | 357 | 10.4 | 37.0 |
Thus, starting from the data collected in Table 1, we estimated the first linear regression model (Fig. 1), which describes the overall Biopsy Rate variations over the 10 year period. Specifically, Fig. 1 shows an overall reduction in biopsies carried out during this period. The statistical test confirmed that the estimated model was statistically significant, with a p-value of 0.04.
Fig. 1.
Linear regression model, which estimates how the overall Biopsy Rate changed over the 10 year screening. The model resulted statistically significant with a p-value equal to 0.04
The overall Biopsy Rate investigated so far considers both CBs and VABBs. Nonetheless, since VABBs are the biopsies generally used for in-depth analysis of mammogram detected lesions, our second goal was to independently investigate both the Biopsy Rate and the PPV-3 related to the CBs and VABBs. Thus, Tables 2 and 3 reported the number of mammograms performed, the individual number of biopsies required, the number of detected B5 tumors, in addition to the estimated Biopsy Rate and PPV-3, for each individual year from 2012 to 2021, for CBs and VABBs, respectively. To demonstrate that the two samples considered were homogeneous, we computed the average age of the patients who underwent the two different procedures beforehand: the average age was 54 years (54.20±3.52) for the patients who received VABB 55 years (55.30±1.10) for the patients who underwent CB. Consequently, from data collected in both Tables 2 and 3, we estimated two linear regression models which describe the Biopsy Rate variations over the 10 year screenings for CB (Fig. 2) and VABB (Fig. 3), respectively. As a result, even though Fig. 2 shows an overall reduction in CBs required over the years, the associated linear regression model proved not to be statistically significant with a p-value equal to 0.11. On the other hand, Fig. 3 shows a relevant reduction of VABBs performed during this period, particularly after the systematic introduction of DBT in 2017, confirming that the estimated model was statistically significant with a p-value of less than 0.005.
Table 2.
Detailed overview of the total number of mammograms performed, CBs required, and detected B5 tumors from 2012 to 2021. For each year, the Biopsy Rate and the PPV-3 are also reported
| Year | Mammograms | CBs | B5 tumors | CB Biopsy Rate (%) | PPV-3 (%) |
|---|---|---|---|---|---|
| 2012 | 3247 | 444 | 232 | 13.7 | 52.3 |
| 2013 | 2731 | 348 | 219 | 12.7 | 62.9 |
| 2014 | 5665 | 473 | 309 | 8.3 | 65.3 |
| 2015 | 6968 | 501 | 300 | 7.2 | 59.9 |
| 2016 | 7373 | 733 | 282 | 9.9 | 38.5 |
| 2017 | 7314 | 710 | 338 | 9.7 | 47.6 |
| 2018 | 8556 | 818 | 371 | 9.6 | 45.4 |
| 2019 | 9751 | 845 | 381 | 8.7 | 45.1 |
| 2020 | 8497 | 761 | 294 | 9.0 | 38.6 |
| 2021 | 9282 | 852 | 323 | 9.2 | 37.9 |
Table 3.
Detailed overview of the total number of mammograms performed, stereotactic VABBs required, and detected B5 tumors from 2012 to 2021. For each year, the Biopsy Rate and the PPV-3 are also reported
| Year | Mammograms | VABBs | B5 tumors | VABB Biopsy Rate (%) | PPV-3 (%) |
|---|---|---|---|---|---|
| 2012 | 3247 | 150 | 46 | 4.6 | 30.7 |
| 2013 | 2731 | 114 | 35 | 4.2 | 30.7 |
| 2014 | 5665 | 103 | 34 | 1.8 | 33.0 |
| 2015 | 6968 | 142 | 50 | 2.0 | 35.2 |
| 2016 | 7373 | 194 | 45 | 2.6 | 23.2 |
| 2017 | 7314 | 118 | 38 | 1.6 | 32.2 |
| 2018 | 8556 | 182 | 64 | 2.1 | 35.2 |
| 2019 | 9751 | 189 | 55 | 1.9 | 29.1 |
| 2020 | 8497 | 105 | 44 | 1.2 | 41.9 |
| 2021 | 9282 | 113 | 34 | 1.2 | 30.1 |
Fig. 2.
Linear regression model estimates how the Biopsy Rate related to CBs changed over the 10 year period. The model resulted not statistically significant, with a p-value equal to 0.11
Fig. 3.
Linear regression model estimates how the Biopsy Rate related to VABBs changed over the 10 year period. The model resulted statistically significant with a p-value less than 0.005
The previously discussed results suggest that the systematic introduction of DBT in 2017 allowed an effective reduction in the number of biopsies required by radiologists, particularly with reference to VABBs. Nonetheless, this reduction could also be the consequence of a decrease in the number of B5 tumors diagnosed, leading to fewer biopsies. To investigate how the rate of B5 tumor diagnosis changed over the 10 year screening, we estimated a further linear regression model which describes the variations of the PPV-3 in relation to VABBs over time (Fig. 4). As shown in Fig. 4, the PPV-3 related to VABBs, namely, the overall percentage rate of B5 tumor diagnosed VABBs required, does not undergo significant variations over time. As a matter of fact, the estimated model proved not to be statistically significant with a p-value equal to 0.69, demonstrating that the decrease in the number of VABBs required was not the consequence of a reduction of B5 tumors diagnosed. Furthermore, this reduction is more evident in the data referring to VABBs, on the basis of the two periods of interest: from 2012 to 2015 with the acquisition of only FFDMs, and from 2017 to 2021 with the systematic use of DBT (Table 4).
Fig. 4.
Linear regression model estimates how the PPV-3 related to VABBs changed over the 10 year screening. The model resulted not statistically significant, with a p-value equal to 0.69
Table 4.
Detailed overview of the total number of mammograms performed, stereotactic VABBs required, and detected B5 tumors according to on the basis of the two periods of interest: from 2012 to 2015 and from 2017 to 2021. Both the Biopsy Rate and the PPV-3 are reported for each period
| Period | Mammograms | VABBs | B5 tumors | VABBs Biopsy Rate (%) | PPV-3 (%) |
|---|---|---|---|---|---|
| 2012–2015 | 18,611 | 509 | 115 | 2.7 | 22.6 |
| 2017–2021 | 43,400 | 707 | 235 | 1.6 | 33.2 |
Finally, with the purpose of verifying a significant association between the observed experimental results and the operators’ performances, we compared those of three operators from our institute who evaluated mammograms and performed biopsies in the last six years of the study. In particular, Table 5 reports the percentage PPV-3 value of each operator, from 2016 to 2021. The performances shown in Table 5 proved to be comparable, and the Wilcoxon statistical test demonstrated no statistically significant differences among them: Op1-Op2, p-value 0.69; Op1-Op3, p-value 0.81; Op2- Op3, p-value 0.94.
Table 5.
Overview of the B5 lesions found per number of biopsies performed and percentage PPV-3 values referring to three operators of our institute from 2016 to 2021
| Year | Operator 1 | Operator 2 | Operator 3 | |||
|---|---|---|---|---|---|---|
| B5 lesions / biopsies | PPV – 3 (%) | B5 lesions / biopsies | PPV – 3 (%) | B5 lesions / biopsies | PPV – 3 (%) | |
| 2016 | 24/123 | 20 | 3/17 | 18 | 18/54 | 33 |
| 2017 | 26/76 | 34 | 6/14 | 43 | 8/31 | 26 |
| 2018 | 40/118 | 34 | 5/16 | 31 | 20/50 | 40 |
| 2019 | 35/121 | 29 | 9/26 | 35 | 11/41 | 27 |
| 2020 | 24/63 | 38 | 11/33 | 33 | 9/20 | 45 |
| 2021 | 18/49 | 37 | 5/24 | 21 | 7/38 | 18 |
Discussion
The exclusive and systematic use of the DBT in breast cancer diagnostics improved the diagnosis accuracy, in terms of sensitivity and specificity, without significantly increasing the amount of radiation that patients undergo. Specifically, DBT has proved to be extremely sensitive as it is able to highlight small masses and distortions better than 2D FFDM, and particularly allows better detection of benign lesions from images without the need for biopsies.
Several state-of-the-art studies have already compared the FFDM with the DBT in terms of sensitivity and specificity, as well as in terms of Benign Biopsy Rate and radiologists’ performances. In this study, conversely, we compared the accuracy of the two diagnostic techniques in terms of malignant (B5) Biopsy Rate and PPV-3, particularly focusing on data related to the VABB, which is the biopsy technique generally used for in-depth analysis of mammogram detected lesions. Thus, after collecting 69,384 mammograms and 7894 biopsies performed from 2012 to 2021 on female patients afferent to the Breast Unit of the Istituto Tumori “Giovanni Paolo II” of Bari, we estimated three linear regression models which describe the overall Biopsy Rate, CBs Biopsy Rate, and VABBs Biopsy Rate variations, over the 10 year period of activity. Even though we expected the stereotactic biopsy results to be the most significant, we intentionally also reported data related to the Core Biopsies, comparing each one over the 10 year period and considering the integration of CBs which were performed on the same period.
Finally, we compared the performances of three breast radiologists at our institute to verify their individual performances in detecting neoplasms.
As a result, the first linear regression model highlighted an overall reduction of the total number of biopsies carried out during the 10 years and proved to be statistically significant with a p-value equal to 0.04. This result was also confirmed by the estimated linear regression model for analyzing the VABB Biopsy Rate variations, on the contrary, the experimental results show no significant reduction in the Core Biopsy Rate. Although the number of cases referring to the VABB procedure is significantly lower than that of the CBs, we feel that the result which emerged might well explain the significant reduction observed in the overall sample.
As a matter of fact, there was a relevant reduction in VABBs carried out during this period, particularly after the systematic introduction of DBT in 2017 when the VABB Biopsy Rate decreased from 2.7 to 1.6%: and the estimated model resulted statistically significant with a p-value of less than 0.005. Besides, we demonstrated that the decrease in the number of VABBs was not as the consequence of a reduction in B5 tumors diagnosed, by means of a further linear regression model which proved not to be statistically significant (p-value equal to 0.69).
Our analysis demonstrates that the systematic introduction of DBT in our institute reduced the overall number of biopsies required after the first mammographic screening without compromising the diagnostic accuracy. On the basis of this, patients avoid further unnecessary examinations and costs (related to breast cancer diagnosis) are reduced. In the light of these encouraging results, in a future study, we will go on to analyze data collected across a multicenter study to confirm the significant accuracy of DBT compared to 2D FFDM in terms of Malignant Biopsy Rate and PPV-3.
It should be emphasized that our study refers to the experience of “Giovanni Paolo II” Cancer Institute alone, which has been the regional oncological reference hub for breast pathology for the last decade. Nevertheless, our findings may not be representative of other realities.
Our results supported the literature, showing that DBT simultaneously improves breast cancer detection by reducing false positive recalls with fewer biopsies performed.
A limitation could be the radiation dose which, on average, is about 30% higher in the DBT plus SM (Synthesized Mammography) protocol compared to the FFDM, data which, however, are considered acceptable and in line with the European guidelines for quality assurance in mammography screening. Additionally, the systematic use of tomosynthesis could mean a longer reading time and decreased productivity. However, in our experience, an increase in mammographic performance was observed over time for the same number of operators, with the sole exception of the first part of the COVID period: some retrospective studies in the literature have also shown that radiologists using AI tools for the simultaneous reading of DBT or a combination of several techniques could reduce the reading time by maintaining a level that is not lower or obtaining better performances in breast cancer diagnosis [2, 6, 9–11, 36] as already documented on other neoplasms [4, 20, 21, 38, 43]. A further limitation of DBT is overdiagnosis, defined as the detection of multiple low-grade lesions and small tumors, which carries economic implications that need to be considered.
Future studies to explore breast cancer growth and access to long-term clinical follow-up data are needed to fully understand the complex question of identifying and treating small and slow-growing breast cancers associated with DBT screening [23].
Conclusions
This work highlights the significant impact of the systematic introduction of DBT on the results of biopsy investigations, particularly stereotaxic: the technique improves overall sensitivity and specificity in diagnosing malignant neoplastic pathology in line with what is documented in the literature. The greater specificity would also significantly reduce the number of unnecessary stereotaxic biopsy investigations with a consequent reduction in costs.
Acknowledgements
Thanks to Debbie Copely, native English speaker, for her invaluable work of linguistic support. The retrospective observational study was approved by the Scientific Board of “Istituto Tumori Giovanni Paolo II”, Bari, Italy.
Author contributions
Conceptualization, RS, GG, EN, LF, and DLF; methodology, RS, AF, GC, and DLF; software, RS, SB, AF; validation, RS, SB, AF, and DLF; formal analysis: RS, SB, and AF, investigation, RS, SB, FA, ML and DLF, resources, RM, FAZ and DLF, data curation, RS, SB, AF; writing—original draft preparation, RS, SB, AF, GG, LF, and DLF; writing—review and editing: RS, SB, FA, GC, A.D., MD, AF, GG, ML, RM, AR, FAZ, EN, LF, and DLF. The authors affiliated to Istituto Tumori “Giovanni Paolo II”, IRCCS, Bari, Italy, are responsible for the views expressed in this article, which do not necessarily represent the ones of the Institute.
Funding
This work was supported by funding from the Italian Ministry of Health “Ricerca corrente 2023”.
Data availability
The raw data supporting the conclusion of this article will be made available by the corresponding author without undue reservation.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniele La Forgia, Rahel Signorile and Samantha Bove have contributed equally to this work.
Contributor Information
Daniele La Forgia, Email: d.laforgia@oncologico.bari.it.
Rahel Signorile, Email: r.signorile@oncologico.bari.it.
Samantha Bove, Email: s.bove@oncologico.bari.it.
Francesca Arezzo, Email: f.arezzo@oncologico.bari.it.
Gennaro Cormio, Email: gennaro.cormio@uniba.it.
Antonella Daniele, Email: antonella.daniele@oncologico.bari.it.
Miriam Dellino, Email: miriamdellino@hotmail.it.
Annarita Fanizzi, Email: a.fanizzi@oncologico.bari.it.
Gianluca Gatta, Email: ggatta@sirm.org.
Miria Lafranceschina, Email: m.lafranceschina@oncologico.bari.it.
Raffaella Massafra, Email: r.massafra@oncologico.bari.it.
Alessandro Rizzo, Email: a.rizzo@oncologico.bari.it.
Francesco Alfredo Zito, Email: a.zito@oncologico.bari.it.
Emanuele Neri, Email: emanuele.neri@unipi.it.
Lorenzo Faggioni, Email: lorenzofaggioni79@gmail.com.
References
- 1.Bahl M, Mercaldo S, Dang PA, et al. Breast cancer screening with digital breast tomosynthesis: are initial benefits sustained? Radiology. 2020;295:529–539. doi: 10.1148/radiol.2020191030. [DOI] [PubMed] [Google Scholar]
- 2.Basile TMA, Fanizzi A, Losurdo L, et al. Hough transform for clustered microcalcifications detection in full-field digital mammograms. In: Tescher AG, et al., editors. Applications of Digital Image Processing XL. San Diego: SPIE; 2017. p. 41. [Google Scholar]
- 3.Bernardi D, Macaskill P, Pellegrini M, et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol. 2016;17:1105–1113. doi: 10.1016/S1470-2045(16)30101-2. [DOI] [PubMed] [Google Scholar]
- 4.Chiti G, Grazzini G, Flammia F, et al. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): a radiomic model to predict tumor grade. Radiol Med. 2022;127:928–938. doi: 10.1007/s11547-022-01529-x. [DOI] [PubMed] [Google Scholar]
- 5.Chong A, Weinstein SP, McDonald ES, Conant EF. Digital breast tomosynthesis: concepts and clinical practice. Radiology. 2019;292:1–14. doi: 10.1148/radiol.2019180760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comes MC, Fanizzi A, Bove S, et al. Early prediction of neoadjuvant chemotherapy response by exploiting a transfer learning approach on breast DCE-MRIs. Sci Rep. 2021;11:14123. doi: 10.1038/s41598-021-93592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Commission E, Consumers D-G for H and (2013) European guidelines for quality assurance in breast cancer screening and diagnosis : fourth edition, supplements. Publications Office
- 8.Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast mri vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746. doi: 10.1001/jama.2020.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conant EF, Toledano AY, Periaswamy S, et al. Improving accuracy and efficiency with concurrent use of artificial intelligence for digital breast tomosynthesis. Radiol Artif Intell. 2019;1:e180096. doi: 10.1148/ryai.2019180096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanizzi A, Pomarico D, Paradiso A, et al. Predicting of sentinel lymph node status in breast cancer patients with clinically negative nodes: a validation study. Cancers (Basel) 2021;13:352. doi: 10.3390/cancers13020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fausto A, Bernini M, La Forgia D, et al. Six-year prospective evaluation of second-look US with volume navigation for MRI-detected additional breast lesions. Eur Radiol. 2019;29:1799–1808. doi: 10.1007/s00330-018-5765-8. [DOI] [PubMed] [Google Scholar]
- 12.Freer PE, Riegert J, Eisenmenger L, et al. Clinical implementation of synthesized mammography with digital breast tomosynthesis in a routine clinical practice. Breast Cancer Res Treat. 2017;166:501–509. doi: 10.1007/s10549-017-4431-1. [DOI] [PubMed] [Google Scholar]
- 13.Fusco R, Setola SV, Raiano N, et al. Analysis of a monocentric computed tomography dosimetric database using a radiation dose index monitoring software: dose levels and alerts before and after the implementation of the adaptive statistical iterative reconstruction on CT images. Radiol Med. 2022;127:733–742. doi: 10.1007/s11547-022-01481-w. [DOI] [PubMed] [Google Scholar]
- 14.Gatta G, Cappabianca S, La Forgia D, et al. Second-generation 3D automated breast ultrasonography (Prone ABUS) for dense breast cancer screening integrated to mammography: effectiveness, performance and detection rates. J Pers Med. 2021;11:875. doi: 10.3390/jpm11090875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gennaro G, Bernardi D, Houssami N. Radiation dose with digital breast tomosynthesis compared to digital mammography: per-view analysis. Eur Radiol. 2018;28:573–581. doi: 10.1007/s00330-017-5024-4. [DOI] [PubMed] [Google Scholar]
- 16.Giampietro RR, Cabral MVG, Lima SAM, et al. Accuracy and effectiveness of mammography versus mammography and tomosynthesis for population-based breast cancer screening: a systematic review and meta-analysis. Sci Rep. 2020;10:7991. doi: 10.1038/s41598-020-64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022;72:524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert FJ, Tucker L, Young KC. Digital breast tomosynthesis (DBT): a review of the evidence for use as a screening tool. Clin Radiol. 2016;71:141–150. doi: 10.1016/j.crad.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Girometti R, Linda A, Conte P, et al. Multireader comparison of contrast-enhanced mammography versus the combination of digital mammography and digital breast tomosynthesis in the preoperative assessment of breast cancer. Radiol Med. 2021;126:1407–1414. doi: 10.1007/s11547-021-01400-5. [DOI] [PubMed] [Google Scholar]
- 20.Granata V, Fusco R, De Muzio F, et al. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol Med. 2022;127:763–772. doi: 10.1007/s11547-022-01501-9. [DOI] [PubMed] [Google Scholar]
- 21.Granata V, Fusco R, De Muzio F, et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol Med. 2022;127:461–470. doi: 10.1007/s11547-022-01477-6. [DOI] [PubMed] [Google Scholar]
- 22.Heywang-Köbrunner SH, Jänsch A, Hacker A, et al. Digital breast tomosynthesis (DBT) plus synthesised two-dimensional mammography (s2D) in breast cancer screening is associated with higher cancer detection and lower recalls compared to digital mammography (DM) alone: results of a systematic review and meta-analysis. Eur Radiol. 2022;32:2301–2312. doi: 10.1007/s00330-021-08308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofvind S, Hovda T, Holen ÅS, et al. Digital breast tomosynthesis and synthetic 2D mammography versus digital mammography: evaluation in a population-based screening program. Radiology. 2018;287:787–794. doi: 10.1148/radiol.2018171361. [DOI] [PubMed] [Google Scholar]
- 24.Honig EL, Mullen LA, Amir T, et al. Factors impacting false positive recall in screening mammography. Acad Radiol. 2019;26:1505–1512. doi: 10.1016/j.acra.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Hooley RJ. Breast density legislation and clinical evidence. Radiol Clin North Am. 2017;55:513–526. doi: 10.1016/j.rcl.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Horvat JV, Keating DM, Rodrigues-Duarte H, et al. Calcifications at digital breast tomosynthesis: imaging features and biopsy techniques. Radiographics. 2019;39:307–318. doi: 10.1148/rg.2019180124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houssami N, Lee CI. The impact of legislation mandating breast density notification – review of the evidence. The Breast. 2018;42:102–112. doi: 10.1016/j.breast.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim G, Mercaldo S, Bahl M. Impact of digital breast tomosynthesis (DBT) on finding types leading to true-positive and false-positive examinations. Clin Imaging. 2021;71:155–159. doi: 10.1016/j.clinimag.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Morris JM (2018) U.S. Food and Drug Administration [letter]. https://www.accessdata.fda.gov/cdrh_docs/pdf8/p080003s001a.pdf. Published May 16, 2013. Accessed June 7, 2018
- 30.Mundinger A. 3D Supine automated ultrasound (SAUS, ABUS, ABVS) for supplemental screening women with dense breasts. J Breast Health. 2016;12:52–55. doi: 10.5152/tjbh.2016.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortenzia O, Rossi R, Bertolini M, et al. Physical characterisation of four different commercial digital breast tomosynthesis systems. Radiat Prot Dosimetry. 2018;181:277–289. doi: 10.1093/rpd/ncy024. [DOI] [PubMed] [Google Scholar]
- 32.Park SH, Kim YS, Choi J. Dosimetric analysis of the effects of a temporary tissue expander on the radiotherapy technique. Radiol Med. 2021;126:437–444. doi: 10.1007/s11547-020-01297-6. [DOI] [PubMed] [Google Scholar]
- 33.Pattacini P, Nitrosi A, Giorgi Rossi P, et al. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the reggio emilia tomosynthesis randomized trial. Radiology. 2018;288:375–385. doi: 10.1148/radiol.2018172119. [DOI] [PubMed] [Google Scholar]
- 34.Perry N, Broeders M, de Wolf C et al (2006) European guidelines for quality assurance in breast cancer screening and diagnosis [DOI] [PubMed]
- 35.Perry N, Broeders M, de Wolf C, et al. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—summary document. Ann Oncol. 2008;19:614–622. doi: 10.1093/annonc/mdm481. [DOI] [PubMed] [Google Scholar]
- 36.Pinto MC, Rodriguez-Ruiz A, Pedersen K, et al. Impact of artificial intelligence decision support using deep learning on breast cancer screening interpretation with single-view wide-angle digital breast tomosynthesis. Radiology. 2021;300:529–536. doi: 10.1148/radiol.2021204432. [DOI] [PubMed] [Google Scholar]
- 37.Sardu C, Gatta G, Pieretti G, et al. Pre-menopausal breast fat density might predict MACE during 10 years of follow-up. JACC Cardiovasc Imag. 2021;14:426–438. doi: 10.1016/j.jcmg.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Scapicchio C, Gabelloni M, Barucci A, et al. A deep look into radiomics. Radiol Med. 2021;126:1296–1311. doi: 10.1007/s11547-021-01389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma N, McMahon M, Haigh I, et al. The potential impact of digital breast tomosynthesis on the benign biopsy rate in women recalled within the UK breast screening programme. Radiology. 2019;291:310–317. doi: 10.1148/radiol.2019180809. [DOI] [PubMed] [Google Scholar]
- 40.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 41.Sprague BL, Coley RY, Kerlikowske K, et al. Assessment of radiologist performance in breast cancer screening using digital breast tomosynthesis vs digital mammography. JAMA Netw Open. 2020;3:e201759. doi: 10.1001/jamanetworkopen.2020.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 43.Vicini S, Bortolotto C, Rengo M, et al. A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: focus on the three most common cancers. Radiol Med. 2022;127:819–836. doi: 10.1007/s11547-022-01512-6. [DOI] [PubMed] [Google Scholar]
- 44.Zackrisson S, Lång K, Rosso A, et al. One-view breast tomosynthesis versus two-view mammography in the malmö breast tomosynthesis screening trial (MBTST): a prospective, population-based, diagnostic accuracy study. Lancet Oncol. 2018;19:1493–1503. doi: 10.1016/S1470-2045(18)30521-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the corresponding author without undue reservation.