Abstract
Cigarette smoking is the most important avoidable cardiovascular risk factor. It causes endothelial dysfunction and atherosclerosis and increases the risk of its severe clinical complications like coronary artery disease, myocardial infarction, stroke, and peripheral artery disease. Several next-generation tobacco and nicotine products have been developed to decrease some of the deleterious effects of regular tobacco smoking. This review article summarizes recent findings about the impact of cigarette smoking and next-generation tobacco and nicotine products on endothelial dysfunction. Both cigarette smoking and next-generation tobacco products lead to impaired endothelial function. Molecular mechanisms of endothelial dysfunction like oxidative stress, reduced nitric oxide availability, inflammation, increased monocyte adhesion, and cytotoxic effects of cigarette smoke and next-generation tobacco and nicotine products are highlighted. The potential impact of short- and long-term exposure to next-generation tobacco and nicotine products on the development of endothelial dysfunction and its clinical implications for cardiovascular diseases are discussed.
Keywords: Cardiovascular diseases, Endothelial dysfunction, Cigarette smoking, Next-generation tobacco and nicotine products
Introduction
Cardiovascular diseases are the major causes of death [102]. Tobacco smoking is the most important avoidable risk factor of cardiovascular diseases [103]. In 2019, more than 1 billion people were smokers consuming more than 7 trillion cigarette-equivalents of tobacco [30]. The prevalence of smoking has reduced by 27–38% in males and females since 1990, but due to the increase in global population, the total number of smokers has even further increased [30]. Therefore, smoking tobacco accounted for 7.7 million deaths and 200 million disability-adjusted life-years, and was the leading risk factor for death among males (20% of male deaths) in 2019 [30]. Cigarette smoking is a well-known risk factor of atherosclerosis and its life-threatening clinical complications like coronary artery disease, myocardial infarction, stroke, and peripheral artery disease [14, 29, 30, 80]. An important initial step in the development of atherosclerosis is endothelial dysfunction [62, 79].
In an attempt to decrease the deleterious effects of classical cigarette smoking, next-generation tobacco and nicotine products have been developed [69, 73, 92]. Next-generation tobacco and nicotine products include electronic (e) cigarettes and Heat-Not-Burn Tobacco products. Despite a partial reduction of deleterious components of classical cigarette smoke, also, these novel e-cigarettes, Heat-Not-Burn Tobacco products, and water pipe smoking promote endothelial dysfunction and cardiovascular diseases [69, 90, 104]. We would like to focus in this review first on the different components of cigarette smoke and next-generation tobacco products. Next, the impact of cigarette smoke and next-generation tobacco and nicotine products on endothelial dysfunction and cardiovascular diseases and its potential clinical implications will be discussed.
Cigarette smoke and next-generation tobacco products
Cigarette smoke is an aerosol containing more than 4.700 components [37, 91] with reactive oxygen species (ROS) and carbon monoxide (CO) as important pathogenic constituents [77]. Further well-known substances found in cigarette smoke are nicotine, polycyclic aromatic hydrocarbons, and cadmium as well as other metals and substances like benzene, formaldehyde, or tar [17, 19, 21, 42, 48, 75]. Cigarette smoke is often subdivided into a particulate and vapor phase. The particulate phase is defined by the cigarette filters as they have an impact on components larger than one micrometer, whereas all components of the vapor phase are not being affected by filters [58]. Biologically and clinically relevant components of the gas phase include CO, acetaldehyde, formaldehyde, acrolein, nitric oxide (NO), and carbon dioxide. An estimate of 1015 radicals per puff could be detected within the gas phase. Due to the reaction of NO and ROS, both found in the gas phase, there is a possible increase of the radical count even after the average ROS lifetime of less than one second. These remarkable long-living radicals are still spin trapped from gas-phase smoke after more than 5 min [76]. Main components of the particulate phase are tar, defined as all particulate matter that is collected on a Cambridge filter pad other than water and nicotine, and nicotine itself [75]. Investigations using Cambridge filter pads showed a radical concentration of 1017/g within the particulate phase [77]. Another commonly used classification of cigarette emissions is the subdivision between mainstream and sidestream smoke. While mainstream smoke is directly inhaled during smoking, sidestream smoke originates from the glowing tip of conventional cigarettes with an up to 100-fold higher concentration of toxic substances. When investigating the health impact of second-hand smoking, sidestream smoke is of major relevance as 85 % of passively inhaled smoke consists of sidestream cigarette smoke [53, 81].
The first designs of alternative tobacco products were developed in the 1960s. The urge to develop potentially less harmful tobacco products further increased in the 1980s with the evaluation of the Cancer Prevention Study I & II (CPS-I & CPS-II) showing a direct link between smoking and carcinogenesis [74, 98]. Even while these new tobacco products were not launched to the market, e.g. British American Tobacco (BAT) developed a heating tobacco product already in 1962 [22, 82]. Since 2007, electronic tobacco products (like e-cigarettes) have been available on the market [45]. Today, a variety of different electronic noncombustible tobacco products are commercially available. They are summarized using the term “next-generation tobacco products” (NGP’s) [43]. Among the large numbers of noncombustible NGP’s, two main groups can be divided: e-cigarettes and Heat-Not-Burn Tobacco products (HnB-TP’s). E-cigarettes, often referred as vaporizers, can be refilled with nicotine-containing liquids in different flavors. In HnB-TP’s, common tobacco is electronically heated to 250-350 °C, rather than burned at temperatures of up to 900 °C in conventional cigarettes [9, 74].
In 2016, the first HnB-TP, referred to as Tobacco Heating System 2.2 (THS 2.2), was available for purchase. As conventional combustive cigarettes, HnB-TP’s such as IQOS® (produced by Philip Morris International Inc.), use tobacco sticks with a tobacco part and a filter through which smoke is inhaled. The tobacco part is made of 70 % tobacco, water, glycine as humectant and aerosol promoting ingredient, flavorings and binders. Using these ingredients, a thin tobacco sheet is produced and rolled for better heating properties. The filter piece consists of a polymer portion needed to cool the aerosol and a cellulose acetate mouthpiece to imitate conventional cigarettes [52, 74]. The heating process is regulated by the holder and terminates automatically, for example in the IQOS® device after 6 min or 14 puffs, to prevent pyrolysis.
The smoking habit of any tobacco product is mainly dependent on the nicotine delivery as nicotine is the addictive substance [45]. Analysis of mainstream smoke of reference cigarettes and the HnB-TP IQOS® showed no difference between these products [7]. Furthermore, comparison of the nicotine blood concentrations have shown no significant differences in nicotine peak concentration and metabolization between IQOS® and conventional cigarettes [10]. Nicotine reduction was not the main aim for the development of NGP’s, but the reduction of toxic and carcinogenic substances. Different studies showed a reduction of tar by 35-50 % (depending on the experimental settings) in mainstream smoke of HnB-TP’s and a 2-fold reduction of CO as the main component of the vapor phase [7, 61]. The mainstream smoke concentration of highly carcinogenic tobacco-specific nitrosamines (such as N-nitrosonornicotine, N′-nitro-soanatabine, N-nitrosoanabasin, and nicotine-derived nitrosamine-ketone) was reduced 8- to 22-fold compared to conventional cigarettes [7, 59, 61, 85]. Furthermore, the concentration of carbonyl compounds (such as formaldehyde, acetaldehyde, acrolein, and crotonaldehyde) is reduced by 80–97 % in HnB-TP’s [74]. The formation of ROS as mediators of oxidative stress was also reduced in the vapor and the particulate phase of HnB-TP’s. However, despite an 80 % reduction in H2O2 mainstream smoke concentration, the consumption of one pack IQOS tobacco sticks per day increases the ROS intake by more than 4 times compared to inhaling urban air (reference cities were New York, USA & Seoul, Korea) [83].
Impact of cigarette smoke and next-generation tobacco and nicotine products on endothelial dysfunction and cardiovascular diseases
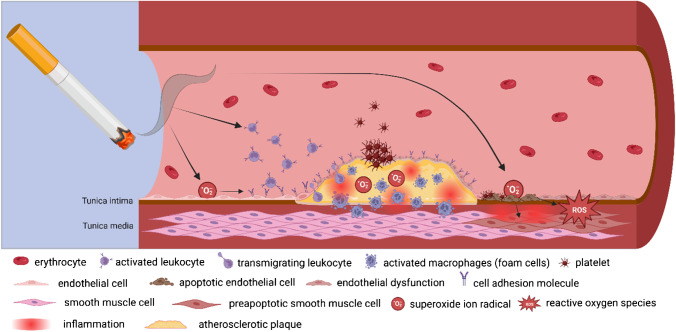
Molecular mechanisms of endothelial dysfunction involve oxidative stress, reduced NO availability, inflammation, increased monocyte adhesion [4, 24, 39, 68], and cytotoxic effects of cigarette smoke and next-generation tobacco and nicotine products (Fig. 1). Although nicotine is mainly responsible for the addiction to cigarette smoking, the oxidative smoke fraction is responsible for the oxidative stress-induced development of endothelial dysfunction and atherosclerosis [17, 18, 34, 77]. However, the in vivo concentration of ROS is not only elevated by inhaled and pulmonary absorbed components but also due to several mechanisms leading to increased endogenous ROS production. Major sources of ROS like nicotinamide adenine dinucleotide phosphate (NADPH) oxidases or xanthine oxidase can be induced by cigarette smoke [51]. α,β-unsaturated ketones and a number of saturated aldehydes as well as α,β-unsaturated aldehydes, such as acrolein and crotonaldehyde, can be found in cigarette smoke and are known to induce NADPH oxidase isoforms [44] leading to increased generation and release of superoxide anions. ROS can also be found in cigarette smoke and react with NO released by endothelial nitric oxide synthase 3 (eNOS) in endothelial cells forming peroxynitrite. The oxidation of (6R)-5,6,7,8-tetrahydro-L-biopterin, a cofactor of the eNOS [27, 31], leads to uncoupling of the eNOS enzymes regularly acting as dimers [26, 57]. When functioning as a monomer, superoxide anion is being released as an electron is transferred to O2 during oxidation, therefore increasing the ROS concentration within the endothelium [35]. Circulating blood cells are affected by an increased ROS concentration due to smoking even before endothelial cells. Leukocytes release ROS, when mice are exposed to cigarette smoke [95]. Monocytes are known to express cellular adhesion molecules, when they are activated by cigarette smoke [49]. A transmigration of activated leukocytes (such as monocytes) is furthermore supported by an increased endothelial expression of intercellular adhesion molecule 1 (ICAM1), endothelial leukocyte adhesion molecule 1 (E-selectin), and vascular cell adhesion molecule 1 (VCAM1) due to cigarette smoke components interacting with the endothelium and a cigarette smoke-dependent activation of the pro-inflammatory transcription factor nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) [12, 88]. These changes shift the endothelial phenotype from the physiological anti-thrombotic state to a pro-thrombotic and pro-inflammatory state [11]. Thrombus formation is further supported by the increased number of thrombocytes in smokers [23]. In addition, next-generation tobacco products can promote thrombosis [3]. Ultimately, smoking can alter the stability of preexisting atherosclerotic plaques by activating matrix metalloproteinases and increasing the risk of plaque rupture [17, 72] leading to acute coronary syndrome [66].
Fig. 1.
Impact of smoking on endothelial function. Smoking affects the vasculature in different ways. An increased level of superoxide ions could induce endothelial dysfunction leading to endothelial apoptosis. Cigarette smoke components are known to activate leukocytes and to induce the expression of cellular adhesion molecules in endothelial cells. This supports the adhesion and transmigration of leucocytes into the subendothelial space thus promoting atherosclerotic plaque formation and rupture leading to platelet-induced thrombus formation. Figure created with BioRender.com
In previous own studies, we analyzed the impact of cigarette smoke extract and next-generation tobacco and nicotine products on parameters of endothelial function [33, 34]. Cigarette smoking extract from reference cigarettes reduced endothelial cell viability in a dose-dependent manner. On the molecular level, cigarette smoking extract activated the nuclear factor erythroid 2-related factor 2 (NRF2) and its target genes heme oxygenase (decycling) 1 (HMOX1) or NAD(P)H quinone dehydrogenase 1 (NQO1) [33]. This supports a transient cellular adaption to cigarette smoke-induced oxidative stress. The atheroprotective activation of the Akt/eNOS pathway and improved wound healing in response to high laminar flow in human endothelial cells were inhibited by cigarette smoke extract [33]. Furthermore, cigarette smoke extract induced pro-inflammatory endothelial adhesion molecules and the adhesion of monocytes to endothelial cells under pro-atherosclerotic low flow conditions [33]. In a follow-up study, we analyzed parameters of endothelial function in response to aqueous smoke extracts of a heated tobacco product (HTP), an electronic cigarette (e-cig), a conventional reference cigarette (3R4F), and nicotine under different flow conditions [34]. All nicotine products activated anti-oxidative or pro-inflammatory responses in endothelial cells [34]. Next-generation nicotine product effects were typically lower compared to classical cigarette smoke extract. Furthermore, cigarette smoke extract impaired endothelial wound healing and induced a pro-inflammatory phenotype in comparison to next-generation tobacco and nicotine products [34]. More recently, we could show that cigarette smoking extract, aqueous smoke extracts of a heated tobacco product, an electronic cigarette, a conventional cigarette (3R4F), and pure nicotine activated anti-oxidative and pro-inflammatory processes in human monocytes [32]. Next-generation tobacco and nicotine products mediated lower responses relative to controls than monocytes exposed to cigarette smoke extract [32]. These in vitro data suggest a slightly reduced potential of next-generation tobacco and nicotine products to induce endothelial dysfunction in comparison to classical cigarette smoking. The activation of NRF2 and the upregulation of cytochrome p450 in response to cigarette smoke extract, but not to electronic cigarette aerosol extract in human coronary endothelial cells supports this concept [96]. Recently, a novel Nrf2-OSGIN1&2-HSP70 axis has been described that regulates endothelial adhesion and elevates GDF15 and HSP70 as novel biomarkers of plaque erosion in patients who smoke [84]. These recent studies shed new light into the molecular mechanisms of endothelial dysfunction in response to cigarette smoke extract and next-generation tobacco and nicotine products.
Several other experimental and clinical studies analyzed the impact of cigarette smoke extract and next-generation tobacco and nicotine products on endothelial function. Cigarette smoke extract mediates cytotoxic effects on human endothelial cells by reducing cell viability and inducing markers of apoptosis like cleaved caspase-3 and necrosis [63]. Cigarette smoke extract and its major cytotoxic component acrolein increased oxidative stress and reduced endothelial nitric oxide expression and activity [17, 40]. Heat-not-burn cigarette smoke extract decreased mitochondrial metabolic activity in human vascular endothelial cells [41]. Endothelial nitric oxide synthase activity was reduced by nicotine- and tar-free cigarette smoke extract of commercial devices like IQOS and hi-lite, but not Ploom S and glo [41]. Flavoured tobacco products are a major reason for the increasing popularity of next-generation tobacco products. However, even low concentrations of selected flavours (e.g. vanillin, menthol, cinnamaldehyde, eugenol, and acetylpyridine) can induce inflammation and impair the endothelial nitric oxide as markers of endothelial dysfunction [25]. The impact of selected components of cigarette smoke and next-generation tobacco/nicotine products on endothelial function is shown in Table 1. Potential effects of cigarette smoke and next-generation tobacco/nicotine products on the vascular wall are summarized in Table 2.
Table 1.
Impact of selected components of cigarette smoke and next-generation tobacco/nicotine products on endothelial function (modified after [28, 74])
| Substance | Unit | HnB-tobacco sticks [64] | Conventional cigarettes [16] | Reduction (%) | Impact on endothelial function |
|---|---|---|---|---|---|
| Nicotine | mg/tobacco sample* | 1.1 | 1.07–2.70 | - | Promotes endothelial dysfunction and release of catecholamines [20] and causes hemodynamic changes (e.g. alteration of heart rate and blood pressure, vasoconstriction) [5, 8, 36] |
| Acetaldehyde | μg/tobacco sample | 179.4–183.5 | 930–1540 | 80.7–88.5 | Inhalation of acetaldehyde gases at smoke-relevant concentrations impairs flow-mediated dilation (FMD) by 50 % [71] |
| Acrolein | μg/tobacco sample | 8.9–9.9 | 89.2–154.1 | 90.0–94.2 | Promotes endothelial dysfunction, oxidative stress, dyslipidemia, and platelet activation [13, 20, 55, 89, 93, 100] |
| Formaldehyde | μg/tobacco sample | 4.7–5.3 | 29.3–130.3 | 84.0–96.4 | Induces endothelial dysfunction [47] |
| Crotonaldehyde | μg/tobacco sample | <3.0 | 32.7–70.8 | 90.8–95.8 | Induces vascular injury via DNA interstrand crosslinks, glutathione perturbation, mitogen–activated protein kinase, and Wnt and ErbB signaling pathways [105] and at higher concentrations tension oscillations (spasms) and irreversibly impaired contractility [46] |
| Benzene | μg/tobacco sample | 0.5–0.6 | 49.7–98.3 | 99.0–99.5 | Increases low-density lipoprotein, decreases circulating angiogenic cells, and increases cardiovascular risk scores [1] |
| 1,3 Butadiene | μg/tobacco sample | 0.2 | 77.0–116.7 | 99.7–99.8 | Promotes oxidative stress and atherosclerosis [78] |
*Tobacco samples are defined as one tobacco stick of HnB tobacco products and one conventional cigarette. The studies used the Health Canada Intense (HCI) protocol
Table 2.
Potential effects of cigarette smoke and next-generation tobacco/nicotine products on the vascular wall
| Effects on vascular wall | Cigarette smoke | Next-generation tobacco/nicotine products |
|---|---|---|
| Oxidative stress | Induces oxidative stress | Induces lower level of oxidative stress |
| Reduced NO availability | Reduces NO availability | Less impact on NO availability |
| Inflammation | Activates and promotes inflammation | Activates and induces lower levels of inflammation |
| Increased monocyte adhesion | Increases monocyte adhesion to endothelium | Reduced impact on monocyte adhesion |
| Cytotoxic effects | Causes cytotoxic effects on endothelial cells | Cytotoxic effects may be milder |
| Endothelial phenotype | Shift of endothelial phenotype to a pro-thrombotic and pro-inflammatory state | Could affect endothelial phenotype |
| Thrombus formation | Supports thrombus formation | Could support thrombus formation |
| Plaque stability | Alters stability of preexisting atherosclerotic plaques, increased risk of plaque rupture | Could affect plaque stability |
Clinical implications
The impact of next-generation tobacco and nicotine products on endothelial function in clinical studies is still not well-understood. First studies analyzed the effects of short-term exposure to e-cigarettes. Vaping e-cigarettes did not induce changes in heart rate, systolic and diastolic blood pressure, endothelial function (measured by flow-mediated dilation), and arterial stiffness (determined by cardio-ankle vascular index) in a 2-h clinical study in young, healthy, tobacco product naïve participants [15]. However, in animal models aerosol from a single “heat-not-burn” product (IQOS) exposure impaired endothelial function (flow-mediated dilation) to the same extend as by cigarette smoke [70]. This is supported by a recent meta-analysis indicating that acute inhalation of e-cigarettes leads to impaired endothelial function [65]. In populations who cannot give up smoking, “heat-not-burn” products can reduce biomarkers of vascular inflammation, oxidative stress, and endothelial dysfunction [6]. Recent studies suggest that no single constituent of smoke is responsible for the acute impairment of endothelial function [71]. Instead, acute endothelial dysfunction by inhaled products is caused by vagus nerve signaling initiated by airway irritation [71]. In addition, chronic vaping and smoking lead to impaired flow-mediated dilation by inhibition of endothelial NO release [67]. Finally, vaping increases microvascular endothelial permeability and affects the balance of pro- and anti-oxidative processes [67]. RAGE could be in this context a novel mediator of e-cigarette-mediated endothelial dysfunction [67].
New tobacco products were developed as less harmful alternatives to cigarette smoking. They were also considered potential supporting strategy in smoking cessation. Quitting smoking will remain the most effective way to reduce the negative health impact of cigarette smoke and to improve endothelial function. Long-term studies have shown that the cardiovascular risk (including prevalence of coronary heart disease, heart failure, and mortality) of former smokers is equal to the cardiovascular risk of never-smokers after >15 years of smoking cessation [2, 97]. Current therapies of nicotine addiction include behavioral or/and medical treatment [87]. Studies investigating the effectiveness of behavioral interventions identified giving brief advice (duration < 1min) [94], counseling (in groups or individually) [56], contingency management [38], or text messaging [38, 101] as possible methods to treat patients. A first-line medical therapy, known to support acute withdrawal, treat cravings as well as to reduce the relapse risk, is a combination of varenicline with different short-acting nicotine patches [99]. In this context, the concept of using new tobacco products to support smoking cessation is still controversially discussed [86]. While the prevalence of classical cigarette smoking has been slightly reduced in the last decades, the use of next-generation tobacco products has increased to a similar degree. In the recent Population Assessment of Tobacco and Health Study, increasing rates of discontinuing cigarette smoking and smokeless tobacco use were accompanied by decreasing rates of discontinuing electronic nicotine delivery systems use among youth in the USA [50].
A recent meta-analysis suggest among patients who attempt to quit smoking, e-cigarettes might be more efficacious than conventional nicotine replacement or behavioral smoking cessation therapies [60]. However, there is currently little evidence supporting effective vaping cessation interventions and no evidence for dual use cessation interventions [54].
In summary, several experimental and clinical data suggest specific deleterious effects of cigarette smoke and next-generation tobacco and nicotine products on endothelial function. Both cigarette smoking and next-generation tobacco products lead to impaired endothelial function. Additional experimental and clinical studies will lead to a better understanding of the underlying molecular mechanisms of the regulation of endothelial function in response to single, short-term, and long-term exposure to cigarette smoke and next-generation tobacco and nicotine products.
Author contribution
J.K and H.M. wrote the main manuscript text, J.K. prepared the figure, and J.K., P.D.-N., and H.M. designed the tables. All authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by research grants of the Carus Promotionskolleg of the Faculty of Medicine Carl Gustav Carus of the TUD Dresden University of Technology (to J.K.), German Academic Exchange Service (DAAD) and the Government of Ghana (to P.D.-N.), Deutsche Forschungsgemeinschaft (DFG) (Grants MO 1695/4-1 and 5-1; to H.M., IRTG 2251; to C.B. and H.M., Grant 47081312 to C.B.), German Centre for Cardiovascular Research (DZHK) (Grant 81X2800207 to H.M.) and by funding of the Excellence Initiative by the German Federal and State Governments (Institutional Strategy, measure ‘support the best’, 3-25 2, Grant F-03661-553-41B-1250000; to H.M.).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Human and animal ethics
Not applicable.
Consent for publication
Authors give consent for the publication of identifiable details, which can include images and details within the text (“Material”) to be published in the above journal and article.
Competing interests
The authors declare no competing interests.
Footnotes
This article is published as part of the Special Issue: Impact of life style and behavioral risk factors on endothelial function and vascular biology.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abplanalp W, DeJarnett N, Riggs DW, Conklin DJ, McCracken JP, Srivastava S, Xie Z, Rai S, Bhatnagar A, O'Toole TE. Benzene exposure is associated with cardiovascular disease risk. PLoS One. 2017;12:e0183602. doi: 10.1371/journal.pone.0183602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed AA, Patel K, Nyaku MA, Kheirbek RE, Bittner V, Fonarow GC, Filippatos GS, Morgan CJ, Aban IB, Mujib M, Desai RV, Allman RM, White M, Deedwania P, Howard G, Bonow RO, Fletcher RD, Aronow WS, Ahmed A. Risk of heart failure and death after prolonged smoking cessation: role of amount and duration of prior smoking. Circ Heart Fail. 2015;8:694–701. doi: 10.1161/CIRCHEARTFAILURE.114.001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alarabi AB, Lozano PA, Khasawneh FT, Alshbool FZ. The effect of emerging tobacco related products and their toxic constituents on thrombosis. Life Sci. 2022;290:120255. doi: 10.1016/j.lfs.2021.120255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amponsah-Offeh M, Diaba-Nuhoho P, Speier S, Morawietz H. Oxidative stress, antioxidants and hypertension. Antioxidants (Basel) 2023;12:281. doi: 10.3390/antiox12020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoniewicz L, Brynedal A, Hedman L, Lundback M, Bosson JA. Acute effects of electronic cigarette inhalation on the vasculature and the conducting airways. Cardiovasc Toxicol. 2019;19:441–450. doi: 10.1007/s12012-019-09516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Begic E, Aziri B, Omeragic E, Medjedovic E, Iglica A, Stanetic B, Kovacevic-Preradovic T, Zivanovic Z, Begic A, Jankovic S, Mlaco N, Mladenovic Z, Badnjevic A (2023) Heat-not-burn tobacco products and cardiovascular risk reduction: a systematic review of randomized controlled trials. Technol Health Care. 10.3233/THC-220677 [DOI] [PubMed]
- 7.Bekki K, Inaba Y, Uchiyama S, Kunugita N. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH. 2017;39:201–207. doi: 10.7888/juoeh.39.201. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL, Kuyt F, Jacob P., 3rd Influence of nicotine on cardiovascular and hormonal effects of cigarette smoking. Clin Pharmacol Ther. 1984;36:74–81. doi: 10.1038/clpt.1984.142. [DOI] [PubMed] [Google Scholar]
- 9.Bhatnagar A, Whitsel LP, Blaha MJ, Huffman MD, Krishan-Sarin S, Maa J, Rigotti N, Robertson RM, Warner JJ. New and emerging tobacco products and the nicotine endgame: the role of robust regulation and comprehensive tobacco control and prevention: a presidential advisory from the American Heart Association. Circulation. 2019;139:e937–e958. doi: 10.1161/CIR.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 10.Brossard P, Weitkunat R, Poux V, Lama N, Haziza C, Picavet P, Baker G, Ludicke F. Nicotine pharmacokinetic profiles of the Tobacco Heating System 2.2, cigarettes and nicotine gum in Japanese smokers. Regul Toxicol Pharmacol. 2017;89:193–199. doi: 10.1016/j.yrtph.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Cacciola RR, Guarino F, Polosa R. Relevance of endothelial-haemostatic dysfunction in cigarette smoking. Curr Med Chem. 2007;14:1887–1892. doi: 10.2174/092986707781058832. [DOI] [PubMed] [Google Scholar]
- 12.Chen HW, Lii CK, Ku HJ, Wang TS. Cigarette smoke extract induces expression of cell adhesion molecules in HUVEC via actin filament reorganization. Environ Mol Mutagen. 2009;50:96–104. doi: 10.1002/em.20441. [DOI] [PubMed] [Google Scholar]
- 13.Conklin DJ, Barski OA, Lesgards JF, Juvan P, Rezen T, Rozman D, Prough RA, Vladykovskaya E, Liu S, Srivastava S, Bhatnagar A. Acrolein consumption induces systemic dyslipidemia and lipoprotein modification. Toxicol Appl Pharmacol. 2010;243:1–12. doi: 10.1016/j.taap.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooke JP. New insights into tobacco-induced vascular disease: clinical ramifications. Methodist Debakey Cardiovasc J. 2015;11:156–159. doi: 10.14797/mdcj-11-3-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossio R, Cerra ZA, Tanaka H. Vascular effects of a single bout of electronic cigarette use. Clin Exp Pharmacol Physiol. 2020;47:3–6. doi: 10.1111/1440-1681.13180. [DOI] [PubMed] [Google Scholar]
- 16.Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10:219–230. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 18.Csordas A, Wick G, Laufer G, Bernhard D. An evaluation of the clinical evidence on the role of inflammation and oxidative stress in smoking-mediated cardiovascular disease. Biomark Insights. 2008;3:127–139. doi: 10.4137/bmi.s480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darrall KG, Figgins JA, Brown RD, Phillips GF. Determination of benzene and associated volatile compounds in mainstream cigarette smoke. Analyst. 1998;123:1095–1101. doi: 10.1039/a708664d. [DOI] [PubMed] [Google Scholar]
- 20.DeJarnett N, Conklin DJ, Riggs DW, Myers JA, O'Toole TE, Hamzeh I, Wagner S, Chugh A, Ramos KS, Srivastava S, Higdon D, Tollerud DJ, DeFilippis A, Becher C, Wyatt B, McCracken J, Abplanalp W, Rai SN, Ciszewski T, Xie Z, Yeager R, Prabhu SD, Bhatnagar A. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014;3:e000934. doi: 10.1161/JAHA.114.000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding YS, Ashley DL, Watson CH. Determination of 10 carcinogenic polycyclic aromatic hydrocarbons in mainstream cigarette smoke. J Agric Food Chem. 2007;55:5966–5973. doi: 10.1021/jf070649o. [DOI] [PubMed] [Google Scholar]
- 22.Dutra LM, Grana R, Glantz SA. Philip Morris research on precursors to the modern e-cigarette since 1990. Tob Control. 2017;26:e97–e105. doi: 10.1136/tobaccocontrol-2016-053406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst E. Haemorheological consequences of chronic cigarette smoking. J Cardiovasc Risk. 1995;2:435–439. doi: 10.1177/174182679500200508. [DOI] [PubMed] [Google Scholar]
- 24.Evans PC, Davidson SM, Wojta J, Back M, Bollini S, Brittan M, Catapano AL, Chaudhry B, Cluitmans M, Gnecchi M, Guzik TJ, Hoefer I, Madonna R, Monteiro JP, Morawietz H, Osto E, Padro T, Sluimer JC, Tocchetti CG, Van der Heiden K, Vilahur G, Waltenberger J, Weber C. From novel discovery tools and biomarkers to precision medicine-basic cardiovascular science highlights of 2021/22. Cardiovasc Res. 2022;118:2754–2767. doi: 10.1093/cvr/cvac114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ, Bhatnagar A, Hamburg NM. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol. 2018;38:1607–1615. doi: 10.1161/ATVBAHA.118.311156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 27.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(829-837):837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried ND, Gardner JD. Heat-not-burn tobacco products: an emerging threat to cardiovascular health. Am J Physiol Heart Circ Physiol. 2020;319:H1234–H1239. doi: 10.1152/ajpheart.00708.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.G.B.D. Mortality Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.G.B.D. Tobacco Collaborators Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397:2337–2360. doi: 10.1016/S0140-6736(21)01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghimire K, Altmann HM, Straub AC, Isenberg JS. Nitric oxide: what’s new to NO? Am J Physiol Cell Physiol. 2017;312:C254–C262. doi: 10.1152/ajpcell.00315.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giebe S, Brux M, Hofmann A, Lowe F, Breheny D, Morawietz H, Brunssen C (2023) Comparative study of the effects of cigarette smoke versus next-generation tobacco and nicotine product extracts on inflammatory biomarkers of human monocytes. Pflugers Arch. 10.1007/s00424-023-02809-9 [DOI] [PMC free article] [PubMed]
- 33.Giebe S, Cockcroft N, Hewitt K, Brux M, Hofmann A, Morawietz H, Brunssen C. Cigarette smoke extract counteracts atheroprotective effects of high laminar flow on endothelial function. Redox Biol. 2017;12:776–786. doi: 10.1016/j.redox.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giebe S, Hofmann A, Brux M, Lowe F, Breheny D, Morawietz H, Brunssen C. Comparative study of the effects of cigarette smoke versus next generation tobacco and nicotine product extracts on endothelial function. Redox Biol. 2021;47:102150. doi: 10.1016/j.redox.2021.102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goncalves DA, Jasiulionis MG, Melo FHM. The role of the BH4 cofactor in nitric oxide synthase activity and cancer progression: two sides of the same coin. Int J Mol Sci. 2021;22:9546. doi: 10.3390/ijms22179546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gourlay SG, Benowitz NL. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin Pharmacol Ther. 1997;62:453–463. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- 37.Green CR, Rodgman A. The tobacco chemists’ research conference: a half century forum for advances in analytical methodology of tobacco and its products. Recent Adv Tob Sci. 1996;22:131–304. [Google Scholar]
- 38.Hartmann-Boyce J, Livingstone-Banks J, Ordonez-Mena JM, Fanshawe TR, Lindson N, Freeman SC, Sutton AJ, Theodoulou A, Aveyard P. Behavioural interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2021;1:CD013229. doi: 10.1002/14651858.CD013229.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofmann A, Brunssen C, Wolk S, Reeps C, Morawietz H. Soluble LOX-1: a novel biomarker in patients with coronary artery disease, stroke, and acute aortic dissection? J Am Heart Assoc. 2020;9:e013803. doi: 10.1161/JAHA.119.013803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horinouchi T, Mazaki Y, Terada K, Miwa S. Cigarette smoke extract and its cytotoxic factor acrolein inhibit nitric oxide production in human vascular endothelial cells. Biol Pharm Bull. 2020;43:1804–1809. doi: 10.1248/bpb.b20-00522. [DOI] [PubMed] [Google Scholar]
- 41.Horinouchi T, Miwa S. Comparison of cytotoxicity of cigarette smoke extract derived from heat-not-burn and combustion cigarettes in human vascular endothelial cells. J Pharmacol Sci. 2021;147:223–233. doi: 10.1016/j.jphs.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Houlgate PR, Dhingra KS, Nash SJ, Evans WH. Determination of formaldehyde and acetaldehyde in mainstream cigarette smoke by high-performance liquid chromatography. Analyst. 1989;114:355–360. doi: 10.1039/an9891400355. [DOI] [PubMed] [Google Scholar]
- 43.Jacob M. Looking back and ahead: the Food and Drug Administration’s regulation of the tobacco industry and next-generation products. Adv Dent Res. 2019;30:22–25. doi: 10.1177/0022034519872472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–1036. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- 45.Jenssen BP, Boykan R. Electronic cigarettes and youth in the United States: a call to action (at the local, national and global levels) Children (Basel) 2019;6:30. doi: 10.3390/children6020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin L, Jagatheesan G, Lynch J, Guo L, Conklin DJ. Crotonaldehyde-induced vascular relaxation and toxicity: role of endothelium and transient receptor potential ankyrin-1 (TRPA1) Toxicol Appl Pharmacol. 2020;398:115012. doi: 10.1016/j.taap.2020.115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin L, Lynch J, Richardson A, Lorkiewicz P, Srivastava S, Theis W, Shirk G, Hand A, Bhatnagar A, Srivastava S, Conklin DJ. Electronic cigarette solvents, pulmonary irritation, and endothelial dysfunction: role of acetaldehyde and formaldehyde. Am J Physiol Heart Circ Physiol. 2021;320:H1510–H1525. doi: 10.1152/ajpheart.00878.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalcher K, Kern W, Pietsch R. Cadmium and lead in the smoke of a filter cigarette. Sci Total Environ. 1993;128:21–35. doi: 10.1016/0048-9697(93)90177-8. [DOI] [PubMed] [Google Scholar]
- 49.Kalra VK, Ying Y, Deemer K, Natarajan R, Nadler JL, Coates TD. Mechanism of cigarette smoke condensate induced adhesion of human monocytes to cultured endothelial cells. J Cell Physiol. 1994;160:154–162. doi: 10.1002/jcp.1041600118. [DOI] [PubMed] [Google Scholar]
- 50.Kasza KA, Tang Z, Xiao H, Marshall D, Stanton C, Gross A, Jackson K, Kelley D, Schroeder M, Vivar J, Hyland A (2023) National longitudinal tobacco product discontinuation rates among US youth from the PATH Study: 2013-2019 (waves 1-5). Tob Control: tc-2022-057729. 10.1136/tc-2022-057729 [DOI] [PMC free article] [PubMed]
- 51.Kayyali US, Budhiraja R, Pennella CM, Cooray S, Lanzillo JJ, Chalkley R, Hassoun PM. Upregulation of xanthine oxidase by tobacco smoke condensate in pulmonary endothelial cells. Toxicol Appl Pharm. 2003;188:59–68. doi: 10.1016/S0041-008x(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 52.Kopa PN, Pawliczak R (2020) IQOS - a heat-not-burn (HnB) tobacco product - chemical composition and possible impact on oxidative stress and inflammatory response. A systematic review. Toxicol Mech Method 30:81–87. 10.1080/15376516.2019.1669245 [DOI] [PubMed]
- 53.Kritz H, Schmid P, Sinzinger H. Passive smoking and cardiovascular risk. Arch Intern Med. 1995;155:1942–1948. doi: 10.1001/archinte.1995.00430180034005. [DOI] [PubMed] [Google Scholar]
- 54.Kundu A, Kouzoukas E, Zawertailo L, Fougere C, Dragonetti R, Selby P, Schwartz R. Scoping review of guidance on cessation interventions for electronic cigarettes and dual electronic and combustible cigarettes use. CMAJ Open. 2023;11:E336–E344. doi: 10.9778/cmajo.20210325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuntic M, Oelze M, Steven S, Kroller-Schon S, Stamm P, Kalinovic S, Frenis K, Vujacic-Mirski K, Bayo Jimenez MT, Kvandova M, Filippou K, Al Zuabi A, Bruckl V, Hahad O, Daub S, Varveri F, Gori T, Huesmann R, Hoffmann T, Schmidt FP, Keaney JF, Daiber A, Munzel T. Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2) Eur Heart J. 2020;41:2472–2483. doi: 10.1093/eurheartj/ehz772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2017;3:CD001292. doi: 10.1002/14651858.CD001292.pub3. [DOI] [PubMed] [Google Scholar]
- 57.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI200314172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee CK, Doolittle DJ, Burger GT, Hayes AW. Comparative genotoxicity testing of mainstream whole smoke from cigarettes which burn or heat tobacco. Mutat Res. 1990;242:37–45. doi: 10.1016/0165-1218(90)90097-l. [DOI] [PubMed] [Google Scholar]
- 59.Leigh NJ, Palumbo MN, Marino AM, O'Connor RJ, Goniewicz ML. Tobacco-specific nitrosamines (TSNA) in heated tobacco product IQOS. Tob Control. 2018;27:s37–s38. doi: 10.1136/tobaccocontrol-2018-054318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levett JY, Filion KB, Reynier P, Prell C, Eisenberg MJ. Efficacy and safety of E-cigarette use for smoking cessation: a systematic review and meta-analysis of randomized controlled trials. Am J Med. 2023;S0002-9343(23):00295. doi: 10.1016/j.amjmed.2023.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Li X, Luo Y, Jiang X, Zhang H, Zhu F, Hu S, Hou H, Hu Q, Pang Y. Chemical analysis and simulated pyrolysis of tobacco heating system 2.2 compared to conventional cigarettes. Nicotine Tob Res. 2019;21:111–118. doi: 10.1093/ntr/nty005. [DOI] [PubMed] [Google Scholar]
- 62.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 63.Makwana O, Flockton H, Smith GA, Watters GP, Nisar R, Fields W. Mechanisms of whole smoke conditioned media induced cytotoxicity to human aortic endothelial cells. Toxicol In Vitro. 2019;58:239–244. doi: 10.1016/j.tiv.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Mallock N, Boss L, Burk R, Danziger M, Welsch T, Hahn H, Trieu HL, Hahn J, Pieper E, Henkler-Stephani F, Hutzler C, Luch A. Levels of selected analytes in the emissions of “heat not burn” tobacco products that are relevant to assess human health risks. Arch Toxicol. 2018;92:2145–2149. doi: 10.1007/s00204-018-2215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng XC, Guo XX, Peng ZY, Wang C, Liu R. Acute effects of electronic cigarettes on vascular endothelial function: a systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol. 2023;30:425–435. doi: 10.1093/eurjpc/zwac248. [DOI] [PubMed] [Google Scholar]
- 66.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 67.Mohammadi L, Han DD, Xu F, Huang A, Derakhshandeh R, Rao P, Whitlatch A, Cheng J, Keith RJ, Hamburg NM, Ganz P, Hellman J, Schick SF, Springer ML. Chronic E-cigarette use impairs endothelial function on the physiological and cellular levels. Arterioscler Thromb Vasc Biol. 2022;42:1333–1350. doi: 10.1161/ATVBAHA.121.317749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muller G, Morawietz H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal. 2009;11:1711–1731. doi: 10.1089/ARS.2008.2403. [DOI] [PubMed] [Google Scholar]
- 69.Munzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J. 2020;41:4057–4070. doi: 10.1093/eurheartj/ehaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nabavizadeh P, Liu J, Havel CM, Ibrahim S, Derakhshandeh R, Jacob Iii P, Springer ML. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob Control. 2018;27:s13–s19. doi: 10.1136/tobaccocontrol-2018-054325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nabavizadeh P, Liu J, Rao P, Ibrahim S, Han DD, Derakhshandeh R, Qiu H, Wang X, Glantz SA, Schick SF, Springer ML. Impairment of endothelial function by cigarette smoke is not caused by a specific smoke constituent, but by vagal input from the airway. Arterioscler Thromb Vasc Biol. 2022;42:1324–1332. doi: 10.1161/ATVBAHA.122.318051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newby AC. Metalloproteinases and vulnerable atherosclerotic plaques. Trends Cardiovasc Med. 2007;17:253–258. doi: 10.1016/j.tcm.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogden MW, Marano KM, Jones BA, Stiles MF. Switching from usual brand cigarettes to a tobacco-heating cigarette or snus: Part 1 Study design and methodology. Biomarkers. 2015;20:382–390. doi: 10.3109/1354750X.2015.1094133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pieper E, Mallock N, Henkler-Stephani F, Luch A. “Heat not burn” tobacco devices as new tobacco industry products: health risks. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61:1422–1428. doi: 10.1007/s00103-018-2823-y. [DOI] [PubMed] [Google Scholar]
- 75.Pillsbury HC, Brigth CC, O'Connor KJ, Irish FW. Tar and nicotine in cigarette smoke. J Assoc Off Anal Chem. 1969;52:458–462. doi: 10.1093/jaoac/52.3.458. [DOI] [Google Scholar]
- 76.Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ Health Perspect. 1983;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 78.Pulliero A, Godschalk R, Andreassi MG, Curfs D, Van Schooten FJ, Izzotti A. Environmental carcinogens and mutational pathways in atherosclerosis. Int J Hyg Environ Health. 2015;218:293–312. doi: 10.1016/j.ijheh.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 79.Punch E, Klein J, Diaba-Nuhoho P, Morawietz H, Garelnabi M. Effects of PCSK9 targeting: alleviating oxidation, inflammation, and atherosclerosis. J Am Heart Assoc. 2022;11:e023328. doi: 10.1161/JAHA.121.023328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol. 2007;5:276–292. doi: 10.2174/157016107782023406. [DOI] [PubMed] [Google Scholar]
- 81.Raupach T, Schafer K, Konstantinides S, Andreas S. Secondhand smoke as an acute threat for the cardiovascular system: a change in paradigm. Eur Heart J. 2006;27:386–392. doi: 10.1093/eurheartj/ehi601. [DOI] [PubMed] [Google Scholar]
- 82.Risi S. On the origins of the electronic cigarette: British American Tobacco’s Project Ariel (1962-1967) Am J Public Health. 2017;107:1060–1067. doi: 10.2105/AJPH.2017.303806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salman R, Talih S, El-Hage R, Haddad C, Karaoghlanian N, El-Hellani A, Saliba NA, Shihadeh A. Free-base and total nicotine, reactive oxygen species, and carbonyl emissions from IQOS, a heated tobacco product. Nicotine Tob Res. 2019;21:1285–1288. doi: 10.1093/ntr/nty235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Satta S, Beal R, Smith R, Luo X, Ferris GR, Langford-Smith A, Teasdale J, Ajime TT, Serre J, Hazell G, Newby GS, Johnson JL, Kurinna S, Humphries MJ, Gayan-Ramirez G, Libby P, Degens H, Yu B, Johnson T et al (2023) A Nrf2-OSGIN1&2-HSP70 axis mediates cigarette smoke-induced endothelial detachment: implications for plaque erosion. Cardiovasc Res. 10.1093/cvr/cvad022 [DOI] [PMC free article] [PubMed]
- 85.Schaller JP, Pijnenburg JPM, Ajithkumar A, Tricker AR. Evaluation of the Tobacco Heating System 2.2. Part 3: influence of the tobacco blend on the formation of harmful and potentially harmful constituents of the Tobacco Heating System 2.2 aerosol. Regul Toxicol Pharmacol. 2016;81(Suppl 2):S48–S58. doi: 10.1016/j.yrtph.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 86.Selamoglu M, Erbas B, Kasiviswanathan K, Barton C. General practitioners’ knowledge, attitudes, beliefs and practices surrounding the prescription of e-cigarettes for smoking cessation: a mixed-methods systematic review. BMC Public Health. 2022;22:2415. doi: 10.1186/s12889-022-14696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Selby P, Zawertailo L. Tobacco Addiction. N Engl J Med. 2022;387:345–354. doi: 10.1056/NEJMcp2032393. [DOI] [PubMed] [Google Scholar]
- 88.Shen Y, Rattan V, Sultana C, Kalra VK. Cigarette smoke condensate-induced adhesion molecule expression and transendothelial migration of monocytes. Am J Physiol. 1996;270:H1624–H1633. doi: 10.1152/ajpheart.1996.270.5.H1624. [DOI] [PubMed] [Google Scholar]
- 89.Sithu SD, Srivastava S, Siddiqui MA, Vladykovskaya E, Riggs DW, Conklin DJ, Haberzettl P, O'Toole TE, Bhatnagar A, D'Souza SE. Exposure to acrolein by inhalation causes platelet activation. Toxicol Appl Pharmacol. 2010;248:100–110. doi: 10.1016/j.taap.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skotsimara G, Antonopoulos AS, Oikonomou E, Siasos G, Ioakeimidis N, Tsalamandris S, Charalambous G, Galiatsatos N, Vlachopoulos C, Tousoulis D. Cardiovascular effects of electronic cigarettes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26:1219–1228. doi: 10.1177/2047487319832975. [DOI] [PubMed] [Google Scholar]
- 91.Smith CJ, Fischer TH. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2001;158:257–267. doi: 10.1016/s0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 92.Smith MR, Clark B, Ludicke F, Schaller JP, Vanscheeuwijck P, Hoeng J, Peitsch MC. Evaluation of the Tobacco Heating System 2.2. Part 1: Description of the system and the scientific assessment program. Regul Toxicol Pharmacol. 2016;81(Suppl 2):S17–S26. doi: 10.1016/j.yrtph.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Srivastava S, Sithu SD, Vladykovskaya E, Haberzettl P, Hoetker DJ, Siddiqui MA, Conklin DJ, D'Souza SE, Bhatnagar A. Oral exposure to acrolein exacerbates atherosclerosis in apoE-null mice. Atherosclerosis. 2011;215:301–308. doi: 10.1016/j.atherosclerosis.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;2013:CD000165. doi: 10.1002/14651858.CD000165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Talukder MA, Johnson WM, Varadharaj S, Lian J, Kearns PN, El-Mahdy MA, Liu X, Zweier JL. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am J Physiol Heart Circ Physiol. 2011;300:H388–H396. doi: 10.1152/ajpheart.00868.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Teasdale JE, Newby AC, Timpson NJ, Munafo MR, White SJ. Cigarette smoke but not electronic cigarette aerosol activates a stress response in human coronary artery endothelial cells in culture. Drug Alcohol Depend. 2016;163:256–260. doi: 10.1016/j.drugalcdep.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.US Department of Health and Human Services (2004) The 2004 United States surgeon general’s report: the health consequences of smoking. N S W Public Health Bull 15:107 [PubMed]
- 98.Thun MJ, Heath CW., Jr Changes in mortality from smoking in two American Cancer Society prospective studies since 1959. Prev Med. 1997;26:422–426. doi: 10.1006/pmed.1997.0182. [DOI] [PubMed] [Google Scholar]
- 99.U.S. Preventive Services Task Force. Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, Donahue K, Doubeni CA, Epling JW, Jr, Kubik M, Ogedegbe G, Pbert L, Silverstein M, Simon MA, Tseng CW, Wong JB. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:265–279. doi: 10.1001/jama.2020.25019. [DOI] [PubMed] [Google Scholar]
- 100.Wheat LA, Haberzettl P, Hellmann J, Baba SP, Bertke M, Lee J, McCracken J, O'Toole TE, Bhatnagar A, Conklin DJ. Acrolein inhalation prevents vascular endothelial growth factor-induced mobilization of Flk-1+/Sca-1+ cells in mice. Arterioscler Thromb Vasc Biol. 2011;31:1598–1606. doi: 10.1161/ATVBAHA.111.227124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y, Dobson R. Mobile phone text messaging and app-based interventions for smoking cessation. Cochrane Database Syst Rev. 2019;10:CD006611. doi: 10.1002/14651858.CD006611.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.World Health Organization . Global Health Estimates 2020: deaths by cause, age, sex, by country and by region, 2000-2019. Geneva: World Health Organization; 2020. [Google Scholar]
- 103.World Health Organization . WHO report on the global tobacco epidemic 2021: addressing new and emerging products. Geneva: World Health Organization; 2021. pp. 1–212. [Google Scholar]
- 104.Wu JC, Rhee JW, Sallam K. Electronic Cigarettes: Where There Is Smoke There Is Disease. J Am Coll Cardiol. 2019;74:3121–3123. doi: 10.1016/j.jacc.2019.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie MZ, Liu JL, Gao QZ, Bo DY, Wang L, Zhou XC, Zhao MM, Zhang YC, Zhang YJ, Zhao GA, Jiao LY. Proteomics-based evaluation of the mechanism underlying vascular injury via DNA interstrand crosslinks, glutathione perturbation, mitogen-activated protein kinase, and Wnt and ErbB signaling pathways induced by crotonaldehyde. Clin Proteomics. 2022;19:33. doi: 10.1186/s12014-022-09369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.