Abstract
Background:
Phosphorylated neurofilament heavy, a marker of neuroaxonal damage, is increased in horses with equine neuroaxonal dystrophy. However, the temporal dynamics of this biomarker during the post-natal risk period are not understood.
Objective:
To measure serum and cerebrospinal fluid phosphorylated neurofilament heavy concentrations in juvenile foals across the post-natal window of susceptibility for equine neuroaxonal dystrophy.
Study design:
Case-control in vivo experimental study.
Methods:
Concentrations of phosphorylated neurofilament heavy were measured using frozen serum and cerebrospinal fluid collected from 13 foals raised in a vitamin E deficient environment from 1 to 6 months of age. Four of these foals were produced by equine neuroaxonal dystrophy-affected dams, developed clinical signs consistent with equine neuroaxonal dystrophy and had a diagnosis confirmed by histopathology. The remaining 9 foals, produced by healthy mares, were vitamin E depleted and remained clinically healthy. An additional cohort of foals, produced by healthy mares, were supplemented with vitamin E (α-tocopherol; α-TOH) from birth and sampled similarly.
Results:
Serum α-TOH concentrations were significantly higher in vitamin E supplemented healthy foals. Serum phosphorylated neurofilament heavy concentrations did not differ significantly between groups at any time point. Cerebrospinal fluid phosphorylated neurofilament heavy concentrations increased with age in healthy vitamin E depleted foals (p<0.001); an effect that was not observed in healthy vitamin E supplemented foals.
Main limitations:
A genetically susceptible cohort supplemented with vitamin E was not available for comparison.
Conclusion:
We demonstrate that vitamin E depletion may elevate cerebrospinal fluid phosphorylated neurofilament heavy in otherwise healthy juvenile foals by six months of age. We highlight an important cofactor to consider when interpreting cerebrospinal fluid phosphorylated neurofilament heavy concentrations in juvenile horses.
Keywords: horse, neuroaxonal degeneration, juvenile, biomarker, alpha-tocopherol, neurofilament
Introduction
Equine neuroaxonal dystrophy (eNAD) is a neurodegenerative disease of juvenile horses, resulting in damage to spinal sensory tracts.1 It manifests clinically as proprioceptive ataxia localised to the spinal cord and is currently the second most common diagnosis for equine sensory ataxia.2 It was first identified in foals from New York state in the late 1970s and early 1980s and has long been associated with vitamin E deficiency.3,4 The disease bears many similar features to that of ataxia with vitamin E deficiency of humans (OMIM #277460). Whilst the genetic background differs, it appears that the molecular drivers of neuroaxonal degeneration are largely conserved.5–7 The role of vitamin E in protection from clinical disease in genetically susceptible juvenile humans and horses has been well demonstrated.4,8 Early studies in foals found that supplementation with vitamin E, in the form of α-tocopherol, significantly reduced the prevalence of eNAD in animals with a high suspicion of genetic susceptibility.4 The impact of vitamin E depletion on the neurological health of horses not genetically susceptible to eNAD has been less well characterised. While it is presumed that both susceptibility and deficiency are required for neuroaxonal degeneration, this has yet to be examined in the context of eNAD in vivo.
Currently, the genetic etiology of eNAD is unknown and a definitive diagnosis requires histologic examination of the spinal cord and brainstem.1 Histopathologic features consistent with eNAD include axonal swelling and spheroids in the cuneate and gracile spinocerebellar tracts.1 Recent advances have been made towards the antemortem diagnosis of the condition using biomarkers of axonal degeneration and metabolites of α-tocopherol.9,10 Phosphorylated neurofilament heavy (pNfH) has shown promise as a diagnostic aid for animals suspected of eNAD.9 Phosphorylated neurofilament heavy is a cytoskeletal protein of the axon and, with axonal damage, it is released into the cerebrospinal fluid and serum. Use of pNfH as a diagnostic tool has previously been demonstrated in children with encephalitis/encephalopathy with reversible splenial lesions11, bacterial meningitis12, hypoxic ischaemic encephalopathy13, children with cardiac lesions14,15 and pre-term infants16. The use of this marker has not been evaluated in individuals with ataxia with vitamin E deficiency, nor in the context of vitamin E deficiency in children. Additionally, given the invasive nature of cerebrospinal fluid (CSF) collection in children, a relevant animal model of juvenile neurodegeneration that can be repeatedly sampled has tremendous translational benefits.
Age is an important interacting factor to consider when assessing the pNfH concentration, especially in CSF. In normal individuals, neurofilament concentrations will increase with age, either from reduced CSF turnover or low-level age-related axon degeneration.17 Similar results were identified in healthy horses by Edwards et al. (2021), with age weakly correlated to serum pNfH and moderately correlated to CSF pNfH.9 However, in neurologically abnormal horses, age was inversely correlated to CSF pNfH concentrations.9 Since many of these neurologically abnormal horses included horses affected with eNAD, the question arose if vitamin E depletion alone, without concurrent apparent neurological disease, could lead to elevations in CSF pNfH concentrations early in life.
Vitamin E inadequacy is a pervasive health issue amongst people and animals.18 Inadequacy encompasses both absolute deficiencies, as well as concentration or intakes that do not meet individual nutritional needs. There is evidence that vitamin E inadequacy, as measured by circulating α-tocopherol concentrations, may be increasing in prevalence amongst humans.19 The ability to study both naturally occurring neurological disease and vitamin E deficiency in a relevant animal model has strong translational benefits. Therefore, the aim of this study was two-fold. Firstly, to evaluate serum and CSF pNfH concentrations over time for the first six months of life in a cohort of eNAD susceptible and non-susceptible foals. Secondly, we aimed to evaluate the impact of vitamin E depletion on serum and CSF pNfH concentrations in otherwise healthy foals throughout post-natal development. We hypothesised that foals with a genetic propensity for eNAD will have increased serum and CSF pNfH concentrations when compared to neurologically normal foals. We further hypothesised that vitamin E depletion will be associated with increased serum and CSF pNfH concentrations in otherwise neurologically healthy foals.
Materials and Methods
Study design
Case-control study. Samples from foals previously described by Finno et al. (2015) were available for analysis in this study.20 Briefly, 13 American Quarter Horse foals, born in 2010 (n=4), 2011 (n=7) and 2012 (n=2), were maintained in vitamin E deplete conditions from birth to one year of age. Dietary vitamin E depletion was induced by feeding only hay and a vitamin/mineral concentrate that did not contain vitamin E to the dams and foals.20 Foals did not have access to grass pasture. This diet met all the Nutritional Research Council recommendations for growing foals21 other than vitamin E intake.
Four of these foals (n=1 male, n=3 female) were produced by mares with a high suspicion of eNAD as the mares had neurological deficits and three of the four dams had previously produced necropsy-confirmed eNAD affected foals. These four foals developed clinical signs consistent with eNAD by six months of age. The remaining 9 vitamin E depleted foals, produced by healthy mares, remained neurologically appropriate (n=5 males, n=4 females). For the first year of life, serum and CSF samples were collected every 1-2 months. Cerebrospinal fluid was collected from atlantooccipital cisterna by needle centesis under general anaesthesia. All CSF samples underwent routine fluid analysis (Table S1). Foals that developed clinical signs typical of eNAD (symmetrical proprioceptive deficits) were humanely euthanised to confirm the diagnosis of eNAD by histopathology as described by Aleman et al. (2011).22 Presence of bilateral, symmetric, neuronal degeneration and vacuolation, with neuronal loss and axonal spheroids within the nucleus gracilis, nucleus cuneatus medialis, and nucleus cuneatus lateralis constituted histologic criteria for diagnosis of eNAD.22 Given that age at sampling varied across the 3 years, post-natal days 30, 60 and 180 were selected for further analysis in order to optimise sample sizes across time points. Samples (serum and CSF) were centrifuged within one hour of collection and the recovered supernatant stored at −80°C until analysis.
Since both the original eNAD and healthy foals were raised in vitamin E deplete conditions20, CSF and serum samples from an additional cohort of five foals orally supplemented with 10 IU/kg of water dispersible RRR-α-tocopherol (Elevate-WD, Kentucky Equine Research) from birth were collected at post-natal day 30, 60 and 180. Samples were collected from three American Quarter Horse foals born in 2020 (n=1 male, n=2 females) and two male Connemara cross foals born in 2021. These foals were produced by neurologically healthy mares. All foals were examined monthly for the first six months and thereafter every three months for the first year of life for evidence of neurological disease.
Sample size for the vitamin E deplete groups were predetermined as this study used banked samples.20 The prospective cohort sample size was determined by the maximum number of available foals raised at the same facility and born over a similar time frame as the Vitamin E deplete cohorts.
Serum Alpha-tocopherol Analysis
Serum alpha-tocopherol concentrations were analysed by high-performance liquid chromatography with fluorescence detection as previously described.20
ELISA Analysis
All samples were analysed in 2020-2021 using a validated sandwich ELISA for pNfH (EnCor Biotechnology Inc) on paired serum and CSF samples from all animals according to Edwards et al. (2020).9 Due to the highly conserved lysine-serine-proline repeats found in neurofilaments, immunoreactivity is similarly highly conserved, as demonstrated in earlier equine studies using this ELISA and a similar ELISA.23–26 Briefly, paired serum and CSF samples from each animal were analysed on the same plate. The lower and upper limit of detection for this assay is 0.07 ng/ml and 10 ng/ml, respectively. Samples for serum were diluted to 1:2.5 and CSF to 1:5, to fall within the detection range. A standard curve using pNfH derived from lyophilised bovine spinal cord and blank sample diluent was run on every plate. Optical density (OD) was measured using a solid-state plate reader (Byonoy) at 450nm. Samples were analysed in duplicate, with the mean sample optical density used to derive the sample value, provided that the sample OD coefficient of variation was ≤15%. Coefficient of variation was determined by ratio of standard deviation (SD) to the mean (χ) value (). Quantitative values were determined by linear regression fitting of the standard curve. Where values were below the limit of detection of 0.07 ng/mL, they were imputed to 0.07 ng/mL for analysis (9). These values, where applicable, will be given as not detectable (ND).
Data analysis
Data were analysed for normality using Shapiro-Wilk test. Normally distributed data were evaluated by 2-way repeated measures ANOVA. Non-normally distributed data were analysed by repeated measures ANOVA using the rank aligned transformation method.27 Values for serum pNfH were not significantly different for depleted foals, regardless of presence of eNAD, therefore this group was also evaluated as pooled deficient group. Turkey’s test was used for post-hoc multiple comparisons with Holm correction for multiple comparisons. A p-value of <0.05 was considered statistically significant. Analysis was performed with RStudio (RStudio, Inc.). Figures were generated with Prism version 9.2.0 (GraphPad Software Inc.)
Results
Serum Alpha-tocopherol
Serum alpha-tocopherol concentrations were higher in vitamin E supplemented healthy foals at all time points compared to all other time points from healthy vitamin E depleted foals and eNAD foals (Figure 1). A significant group x time interaction was detected (P<0.0001), with group attributing 69.72% of the total variance (P<0.0001).
Figure 1:

Serum vitamin E (alpha-tocopherol) concentrations in juvenile horses. Alpha-tocopherol concentrations were significantly higher in healthy supplemented foals and all other foals at all time points. Healthy vitamin E depleted foals and eNAD foals were not significantly different from one another at any timepoint. Note differing Y-axes between groups. The purple line denotes the lower threshold for normal serum alpha-tocopherol concentrations in normal horses as defined by Finno et al. (2015).20 ***p<0.001, ****p<0.0001.
Phosphorylated neurofilament heavy ELISA performance
Mean intraassay coefficient of variation plate standard OD was 9.89% (range 4.81%-14.0%). Mean intraassay coefficient of variation (CV) of OD for serum and CSF samples were 10.4% (range 7.8%-15.9%) and 8.12% (7.45%-9.47%), respectively.
Serum phosphorylated neurofilament heavy concentrations
There was no significant effect of group or age (30, 60 and 180 days) on serum pNfH concentrations (Table S2). All healthy supplemented foals had serum pNfHconcentrations below the limit of detection at all time points. When all samples derived from vitamin E depleted foals were pooled and compared to vitamin E supplemented foals, there was no significant effect of vitamin E supplementation on serum pNfH concentrations (P=0.14).
Cerebrospinal fluid phosphorylated neurofilament heavy concentrations
Post-natal day 30, day 60 and day 180 CSF pNfH concentrations were analysed. A significant effect of group (p=0.004), age (p=0.006) and group x age interaction (p=0.02) were detected; however, post-hoc analyses did not identify any significant pairwise differences (Figure 2). To examine the effect of vitamin E depletion on healthy foals with ageing, the effect of time was analysed within the healthy vitamin E depleted and healthy vitamin E supplemented groups. Cerebrospinal fluid pNfH concentrations significantly increased in healthy vitamin E depleted foals with age (p=0.0009; Figure 2). Post-hoc testing identified significant increases in CSF pNfH concentrations in healthy vitamin E depleted foals at day 180 when compared to day 30 (P=0.01) and day 60 (P=0.01). This increase in CSF pNfH concentrations was not observed in healthy vitamin E supplemented foals (Figure 2). Foals with eNAD demonstrated a decrease in CSF pNfH concentrations at day 60, followed by an increase at day 180; an effect that was not significant (Figure 2).
Figure 2:
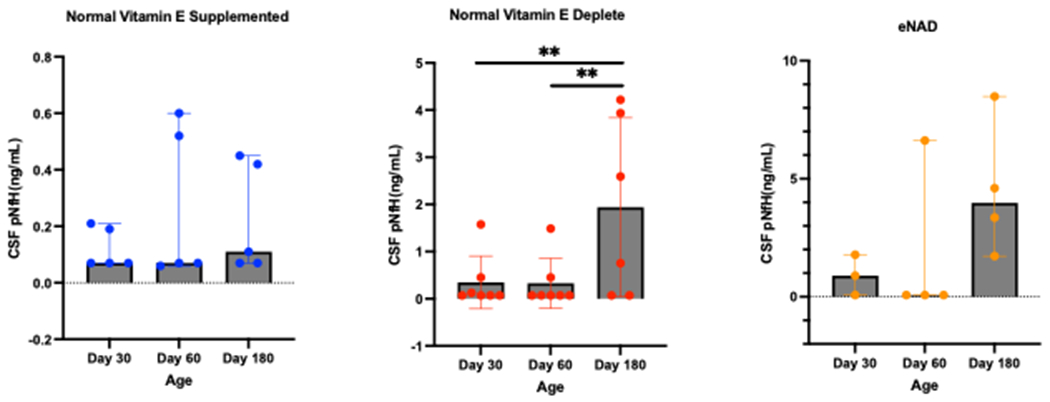
Cerebrospinal fluid (CSF) concentration of phosphorylated neurofilament-heavy (pNfH) in healthy vitamin E supplemented juvenile horses, healthy vitamin E depleted juvenile horses and juvenile horses with necropsy confirmed equine neuroaxonal dystrophy. Note differing Y-axes between groups. Repeated measures ANOVA, following rank aligned transformation, identified a significant effect of group (p=0.004), age (p=0.006) and group x age interaction (p=0.022); however, post-hoc analyses did not identify any significant pairwise comparisons. Post-hoc testing identified significant increases in CSF pNfH concentrations in healthy vitamin E depleted foals at day 180 when compared to day 30 and day 60. **p<0.01.
Discussion
This study demonstrates a distinct temporal association between vitamin E depletion and the axonal damage specific marker pNfH in juvenile horses. By the age of six months, CSF pNfH concentrations in foals definitively diagnosed with eNAD diverge from both healthy vitamin E depleted foals and healthy vitamin E supplemented foals. Importantly, we also demonstrate that vitamin E depleted foals, without apparent neurological disease, also have elevations in CSF pNfH during the critical window for post-natal development. Thus, subclinical axonal degeneration may occur with vitamin E deficiency early in life.
Neurofilaments as markers of axonal damage are susceptible to several drivers.17 Previous work in horses demonstrated the same positive association between age and pNfH that has been demonstrated in ageing humans.17,25 Vitamin E depleted foals included in the current study were also included in Edwards et al. (2021); however, only the samples derived at 4 days of age were included.9 The current study confirms that foals raised in vitamin E deficient conditions have an accelerated accrual of pNfH in cerebrospinal fluid, with detection as early as two months and in the majority of foals by six months of age, particularly those that progressed to clinical eNAD.
It is of note that, in this study, foals that developed the neurological consequence of vitamin E deficiency (eNAD) and those that did not both had elevations in CSF. A similar pattern of elevation has been documented in children with bacterial meningitis, regardless of whether they develop long-term neurological deficits or have apparent resolution.12 In both cases, the magnitude of increase is higher and sustained in those individuals with neurological sequelae. Importantly, it indicates that, even without detectable neurological deficits, there may be neuroaxonal damage/degeneration associated with vitamin E depletion. This is a novel finding during post-natal development and indicates that, regardless of susceptibility or long-term consequence, adequate vitamin E concentrations are required in the first months of life to prevent neuroaxonal damage. Speculatively for eNAD, this may indicate that animals that go onto develop clinical disease have a reduced capacity for damage, enabling the progression to clinical disease. This has been suggested previously, where administration of α-tocopherol in genetically susceptible herds reduced the prevalence of clinical disease.4 Additionally, diagnostic use of this assay in juvenile horses may need to be tempered with the concurrent measurement of serum alpha-tocopherol concentrations. Despite the accelerated accrual, this data does not indicate that an age-specific reference range need be developed for CSF pNfH when applied to aid in the diagnosis of eNAD.9 This novel finding is limited by normal foals having a short follow up period (one year) and no histologic investigation of neural tissue. It is possible that apparently neurologically normal foals could have developed clinical disease and/or histologic lesions at a later age. Further investigation in the current study was not possible as it utilised stored samples.
Many samples failed to reach the limit of detection, especially serum samples. Since neurofilaments are robust to handling conditions, including repeated freeze-thaw, and long storage times, it is likely that this failure is due to the sensitivity of the assay rather than sample quality.28 In serum, however, either higher levels of fibrin products in circulation or activation of remaining fibrinogen on thawing may directly interfere with antibody-antigen binding.29,30 Therefore, interference by the sample matrix cannot be completely ruled out. More sensitive platforms, such as single molecule analysis (Simoa), would allow for enhanced detection of pNfH in serum.17 More accurate assessment of serum concentrations may improve the diagnostic capability of pNfH for eNAD in juvenile horses. While foals with eNAD had higher serum concentrations at 180 days, this study did not have sufficient power to demonstrate a difference from the other groups. Data from the current study indicates that serum is currently not a suitable matrix for diagnostic use in juvenile horses.
This study controls for the effect of vitamin E through the inclusion of a supplemented group of foals. A weakness, however, is that it does not include eNAD susceptible foals that were also supplemented with α-tocopherol from birth. Given that the putative genetic variant(s) for this condition are yet to be determined in horses, purposeful production of such foals is challenging.2 The foals in the original study were all from mothers exhibiting clinical traits consistent with eNAD that had previously produced foals with confirmed eNAD. These same mares were too old to safely carry prospective pregnancies or had been euthanised at the time pNfH concentrations were evaluated. Thus, advanced reproductive techniques such as intracytoplasmic sperm injection, were not feasible. Additionally, as all progeny with eNAD required histologic diagnosis, there were no younger animals to replace the original breeding population. Therefore, it was not possible to assess pNfH concentrations over time in susceptible foals without the induction of vitamin E depletion. Previous evidence suggests that the clinical phenotype may be prevented4, however, whether axonal degeneration occurs despite supplementation remains unknown. Additionally, findings from this study are primarily restricted to foals of the Quarter Horse breed. Additional studies including additional breeds is needed to address the breed specific effects on the current results.
Data from the current study indicates that subclinical axonal degeneration is associated with vitamin E deficiency in juvenile horses. Degeneration was more pronounced in foals that subsequently developed clinical signs consistent with eNAD, and significantly diverged from foals that were supplemented with alpha-tocopherol by six months of age. These findings help to refine the use of this biomarker in horses, but also has translational implications for human health given the shared molecular mechanisms of vitamin E depletion dependent neurodegeneration.
Supplementary Material
Table S1: Cerebrospinal fluid (CSF) fluid analysis summary. Values are median (range). TNCC=total nucleated cell count, RBC=red blood cell. NA=not assessed.
Table S2: Serum phosphorylated neurofilament heavy concentrations in juvenile horses by age at measurement. All values are median (range). Values are in ng/mL. ND=below the limit of detection for the assay.
Acknowledgements
We acknowledge the technical staff at the University of California, Davis Center for Equine Health for their care of the animals and assistance with sample collection.
Sources of funding
Supported by the University of California, Davis Center for Equine Health with funds provided by the State of California parimutuel fund. Graduate support was provided by private donation. Support for CJF was provided by the National Institutes of Health (NIH) (K01OD015134 and L40 TR001136).
Footnotes
Authors’ declarations of interests
No competing interests have been declared.
Ethical animal research
All procedures were performed in accordance with the institutional animal care and use committee at the University of California (Protocol #21343).
Informed consent
Not applicable
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Finno CJ, Valberg SJ, Shivers J, D’Almeida E, Armién AG. Evidence of the Primary Afferent Tracts Undergoing Neurodegeneration in Horses with Equine Degenerative Myeloencephalopathy Based on Calretinin Immunohistochemical Localization. Veterinary Pathology. 2016;53(1):77–86. 10.1177/0300985815598787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns EN, Finno CJ. Equine degenerative myeloencephalopathy: prevalence, impact, and management. Veterinary Medicine (Auckland, N.Z.), 2018;9:63–67. 10.2147/VMRR.S148542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayhew IG, deLahunta A, Whitlock RH, Geary JC. Equine degenerative myeloencephalopathy. Journal of the American Veterinary Medical Association, 1977;170(2):195–201. [PubMed] [Google Scholar]
- 4.Beech J. Neuroaxonal dystrophy of the accessory cuneate nucleus in horses. Veterinary Pathology, 1984;21(4):384–393. 10.1177/030098588402100404 [DOI] [PubMed] [Google Scholar]
- 5.Finno CJ, Peterson J, Kang M, Park S, Bordbari MH, Durbin-Johnson B, Settles M, Perez-Flores MC, Lee JH, Yamoah EN. Single-Cell RNA-seq Reveals Profound Alterations in Mechanosensitive Dorsal Root Ganglion Neurons with Vitamin E Deficiency. iScience, 2019;21:720–735. 10.1016/j.isci.2019.10.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finno CJ, Bordbari MH, Gianino G, Ming-Whitfield B, Burns E, Merkel J, Britton M, Durbin-Johnson B, Sloma EA, McMackin M, Cortopassi G, Rivas V, Barro M, Tran CK, Gennity I, Habib H. Xu L, Puschner B, Miller AD. An innate immune response and altered nuclear receptor activation defines the spinal cord transcriptome during alpha-tocopherol deficiency in Ttpa-null mice. Free Radical Biology & Medicine, 2018;120:289–302. 10.1016/j.freeradbiomed.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finno CJ, Bordbari MH, Valberg SJ, Lee D, Herron J, Hines K, Monsour T, Scott E, Bannasch DL, Mickelson J, Xu L. Transcriptome profiling of equine vitamin E deficient neuroaxonal dystrophy identifies upregulation of liver X receptor target genes. Free Radical Biology & Medicine, 2016;101:261–271. 10.1016/j.freeradbiomed.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohlschütter A, Finckh B, Nickel M, Bley A, Hübner C. First Recognized Patient with Genetic Vitamin E Deficiency Stable after 36 Years of Controlled Supplement Therapy. Neuro-degenerative diseases, 2020;20(1):35–38. 10.1159/000508080 [DOI] [PubMed] [Google Scholar]
- 9.Edwards LA, Donnelly CG, Reed SM, Valberg S, Chigerwe M, Johnson AL, Finno CJ. Serum and cerebrospinal fluid phosphorylated neurofilament heavy protein concentrations in equine neurodegenerative diseases. Equine Veterinary Journal, 2022;54(2):290–298. 10.1111/evj.13452. [DOI] [PubMed] [Google Scholar]
- 10.Hales EN, Habib H, Favro G, Katzman S, Sakai RR, Marquardt S, Bordbari MH, Ming-Whitfield B, Peterson J, Dahlgren AR, Rivas V, Ramirez CA, Peng S, Donnelly CG, Dizmang BS, Kallenberg A, Grahn R, Miller AD, Woolard K, Moeller B, Puschner B, Finno CJ. Increased α-tocopherol metabolism in horses with equine neuroaxonal dystrophy. Journal of Veterinary Internal Medicine, 2021;35(5):2473–2485. 10.1111/jvim.16233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motobayashi M, Fukuyama T, Okuno-Yuguchi J, Tsukahara K, Nagaharu S, Hagimoto R, Kinoshita T, Nakazawa Y, Inaba Y. Subclinical Neuroaxonal Damage in Patients with Clinically Mild Encephalitis/Encephalopathy With a Reversible Splenial Lesion. Pediatric Neurology, 2017;74:e3–e4. 10.1016/j.pediatrneurol.2017.05.026 [DOI] [PubMed] [Google Scholar]
- 12.Matsushige T, Ichiyama T, Kajimoto M, Okuda M, Fukunaga S, Furukawa S. Serial cerebrospinal fluid neurofilament concentrations in bacterial meningitis. Journal of the Neurological Sciences, 2009;280(1-2):59–61. 10.1016/j.jns.2009.01.021 [DOI] [PubMed] [Google Scholar]
- 13.Douglas-Escobar M, Yang C, Bennett J, Shuster J, Theriaque D, Leibovici A, Kays D, Zheng T, Rossignol C, Shaw G, Weiss MD. A pilot study of novel biomarkers in neonates with hypoxic-ischemic encephalopathy. Pediatric Research, 2010;68(6):531–536. 10.1203/PDR.0b013e3181f85a03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee T, Chikkabyrappa SM, Reformina D, Mastrippolito A, Chakravarti SB, Mosca RS, Shaw G, Malhotra SP. Ubiquitin C-Terminal Hydrolase 1 and Phosphorylated Axonal Neurofilament Heavy Chain in Infants Undergoing Cardiac Surgery: Preliminary Assessment as Potential Biomarkers of Brain Injury. World Journal for Pediatric & Congenital Heart Surgery, 2018;9(4):412–418. 10.1177/2150135118762390 [DOI] [PubMed] [Google Scholar]
- 15.McPhillips L, Kholwadwala D, Sison CP, Gruber D, Ojamaa K. A Novel Brain Injury Biomarker Correlates with Cyanosis in Infants with Congenital Heart Disease. Pediatric Cardiology, 2019;40(3):546–553. 10.1007/s00246-018-2023-4 [DOI] [PubMed] [Google Scholar]
- 16.Goeral K, Hauck A, Atkinson A, Wagner MB, Pimpel B, Fuiko R, Klebermass-Schrehof K, Leppert D, Kuhle J, Berger A, Olischar M, Wellmann S. Early life serum neurofilament dynamics predict neurodevelopmental outcome of preterm infants. Journal of Neurology, 2021;268(7):2570–2577. 10.1007/s00415-021-10429-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J. Neurofilaments as biomarkers in neurological disorders. Nature reviews. Neurology, 2018;14(10):577–589. 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 18.Traber MG. Vitamin E: necessary nutrient for neural development and cognitive function. The Proceedings of the Nutrition Society, 2021;80(3):319–326. 10.1017/S0029665121000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Péter S, Friedel A, Roos FF, Wyss A, Eggersdorfer M, Hoffmann K, Weber P. A Systematic Review of Global Alpha-Tocopherol Status as Assessed by Nutritional Intake Levels and Blood Serum Concentrations. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition, 2015;85(5-6):261–281. 10.1024/0300-9831/a000281 [DOI] [PubMed] [Google Scholar]
- 20.Finno CJ, Estell KE, Katzman S, Winfield L, Rendahl A, Textor J, Bannasch DL, Puschner B. Blood and Cerebrospinal Fluid α-Tocopherol and Selenium Concentrations in Neonatal Foals with Neuroaxonal Dystrophy. Journal of Veterinary Internal Medicine, 2015;29(6):1667–1675. 10.1111/jvim.13618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council (US) Nutrient requirements of horses. Washington, D.C: National Academies Press. 2007. [Google Scholar]
- 22.Aleman M, Finno CJ, Higgins RJ, Puschner B, Gericota B, Gohil K, LeCouteur RA, Madigan JE. Evaluation of epidemiological, clinical, and pathological features of neuroaxonal dystrophy in Quarter Horses. Journal of the American Veterinary Medical Association, 2011;239(6):823–833. 10.2460/javma.239.6.823 [DOI] [PubMed] [Google Scholar]
- 23.Lee VM, Otvos L Jr, Carden MJ, Hollosi M, Dietzschold B, Lazzarini RA. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proceedings of the National Academy of Sciences of the United States of America, 1988;85(6):1998–2002. 10.1073/pnas.85.6.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ringger NC, Giguère S, Morresey PR, Yang C, Shaw G. Biomarkers of brain injury in foals with hypoxic-ischemic encephalopathy. Journal of Veterinary Internal Medicine, 2011;25(1):132–137. 10.1111/j.1939-1676.2010.0645.x [DOI] [PubMed] [Google Scholar]
- 25.Morales Gómez AM, Zhu S, Palmer S, Olsen E, Ness SL, Divers TJ, Bischoff K, Mohammed HO. Analysis of neurofilament concentration in healthy adult horses and utility in the diagnosis of equine protozoal myeloencephalitis and equine motor neuron disease. Research in Veterinary Science, 2019;125:1–6. 10.1016/j.rvsc.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 26.Intan-Shameha AR, Divers TJ, Morrow JK, Graves A, Olsen E, Johnson AL, Mohammed HO. Phosphorylated neurofilament H (pNF-H) as a potential diagnostic marker for neurological disorders in horses. Research in Veterinary Science, 2017;114:401–405. 10.1016/j.rvsc.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 27.Wobbrock JO, Findlater L, Gergle D, Higgins JJ. The aligned rank transform for nonparametric factorial analyses using only anova procedures. In: Proceedings of the SIGCHI conference on human factors in computing systems 2011;pp. 143–146. [Google Scholar]
- 28.Altmann P, Leutmezer F, Zach H, Wurm R, Stattmann M, Ponleitner M, Petzold A, Zetterberg H, Berger T, Rommer P, Bsteh G. Serum neurofilament light chain withstands delayed freezing and repeated thawing. Scientific reports, 2020;10(1):19982. 10.1038/s41598-020-77098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton MH, Morris DD, Crowe N, Collatos C, Prasse KW. Hemostatic indices in healthy foals from birth to one month of age. Journal of Veterinary Diagnostic Investigation: Official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc 1995;7(3):380–385. 10.1177/104063879500700314 [DOI] [PubMed] [Google Scholar]
- 30.Tate J, Ward G. Interferences in immunoassay. The Clinical biochemist. Reviews, 2004;25(2):105–120. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Cerebrospinal fluid (CSF) fluid analysis summary. Values are median (range). TNCC=total nucleated cell count, RBC=red blood cell. NA=not assessed.
Table S2: Serum phosphorylated neurofilament heavy concentrations in juvenile horses by age at measurement. All values are median (range). Values are in ng/mL. ND=below the limit of detection for the assay.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


