Abstract
INTRODUCTION:
Biomarkers for prediction of cognitive decline in patients with amnestic mild cognitive impairment and amnestic mild dementia are needed for both clinical practice and clinical trials.
METHODS:
We evaluated the ability of tau-PET, cortical atrophy on MRI, baseline cognition, APOE-status, plasma and cerebrospinal fluid levels of phosphorylated tau-217, neurofilament light, and amyloid-β42/40 (individually and in combination) to predict cognitive decline over two years in BioFINDER-2 and ADNI.
RESULTS:
Baseline tau-PET and a composite baseline cognitive score were the strongest independent predictors of cognitive decline. Cortical thickness and neurofilament light provided some additional information. Using a predictive algorithm to enrich patient selection in a theoretical clinical trial led to a significantly lower required sample size.
DISCUSSION:
Models including baseline tau-PET and cognition consistently provided the best prediction of change in cognitive function over 2 years in patients with amnestic mild cognitive impairment or mild dementia.
Keywords: AD, Tau, PET, blood biomarkers, cognition
Introduction
Amyloid β (Aβ) plaques and tau tangles are the pathologies that define Alzheimer’s disease (AD).[1] Cerebrospinal fluid (CSF) Aβ was shown to be associated with an increased risk of progression from mild cognitive impairment (MCI) to AD dementia already 20 years ago,[1-3] and the first PET tracer for Aβ was developed shortly thereafter.[4] More recently, methods to accurately measure Aβ in plasma have been developed.[1, 5-7] Over the past several years, new methods have been developed to visualize tau pathology in vivo using tau positron emission tomography (PET) imaging[8] and to determine levels of phosphorylated tau (p-tau) in CSF[9, 10] and plasma.[1, 11-16] The rapid development of tau-PET tracers has led to the recent approval, by the United States Food and Drug Administration, of [18F]flortaucipir as a diagnostic agent in AD dementia.[17] Tau-PET has been shown to reliably detect the tau aggregates formed in AD,[18, 19] and shows strong associations with both cognitive decline [20-24] and neurodegeneration. [25, 26] Levels of p-tau in CSF and plasma have been shown to begin increasing at the asymptomatic (preclinical) stage of AD in response to very early Aβ pathology. [27-30] Higher baseline concentrations of p-tau have also been shown to accurately predict progression to AD dementia in both cognitively unimpaired (CU) individuals and in patients with MCI.[12, 31-33] Neurofilament Light (NfL), a more general marker of neurodegeneration, has been reported to be increased in AD [34], and to be associated with conversion from MCI to AD dementia.[32]
As mentioned above, we and others have recently shown that blood-based biomarkers of Aβ (A), tau (T) and neurodegeneration (N) can predict both future cognitive decline and conversion to AD dementia.[11-16, 31-33] Further, tau-PET has also been shown to be an important predictor of cognitive decline in AD.[26, 35, 36] However, there is a clear lack of head-to-head comparisons of these type of promising fluid and imaging biomarkers, and there is also an urgent need to determine optimal biomarker combinations for prediction of cognitive decline in patients with MCI or mild dementia over a clinically relevant time span such as 24 months. This information is of great importance both in clinical settings to establish the risk of cognitive decline in symtomatic patients at a subject level, and in the settings of clinical trials directed against symptomatic AD where follow-up time typically ranges between 18 and 24 months [37-39].
We therefore aimed to determine the ability of different blood and CSF, as well as imaging ATN biomarkers associated with AD, to independently predict cognitive decline in patients with objective memory impairment. To this end, we analysed the ability of i) the most relevant plasma and CSF biomarkers (i.e. p-tau217, neurofilament light (NfL) and the ratio of Aβ42 to Aβ40 [Aβ42/40]), ii) tau-PET ([18F]RO948 standardized uptake value ratios (SUVRs)) in three different regions-of-interest (ROIs)), iii) baseline cognition, iv) MRI (cortical thickness in an “AD-signature” temporal-ROI[40]), and v) the main genetic risk variant for sporadic AD (the APOE ε4 allele), to predict longitudinal cognitive performance over two years in patients presenting with amnestic mild cognitive impairment (MCI) or amnestic mild dementia. We included patients with amnestic MCI or mild amnestic dementia without requiring them to already have evidence of Aβ pathology (defined by CSF or PET) to be able to identify which markers best predict cognitive decline indepedent of Aβ-status. This is a relevant situation in clinical practice, where most patients with amnestic memory impairment have an unknown Aβ-status. In a sensitivity analysis we restricted the participants to only include Aβ-positive participants to mimic a clinical trial setting. Based on the main results presented in this study, including all participants, we have developed a prototype of an on-line prognostic tool that can be used to predict cognitive decline over 24 months, either to provide indvidualized prognostic information in clinic practice or when recruiting suitable participants to clinical trials. Importantly, the main results were replicated in an independent cohort (ADNI).
Methods
Participants
We included participants from the Swedish BioFINDER-2 study (n=118; May 2017 – March 2021; www.biofinder.se). The inclusion criteria for the present study were 1) either amnestic MCI (n=90) or early amnestic dementia (n=28) and 2) a baseline Mini-Mental State Examination (MMSE) score of ≥ 22 points. 3) A complete dataset for all studied biomarkers. In addition to presenting with amnestic memory problems patients with MCI either fulfilled established DSM-5 clinical criteria for mild neurocognitive disorder, or met the DSM-5 criteria for major neurocognitive disorder possibly due to AD.
BioFINDER-2 participants underwent a medical history and neurological examination, brain MRI, blood and CSF sampling, [18F]RO948 tau-PET and repeated neuropsychological testing after 1, 2 and 3 years. Only participants with cognitive follow-up data extending over ≥2 years were included in the analysis, but results from all available time points were used for cognitive slope calculation. At baseline, participants also underwent a cognitive battery including trailmaking test-A & B (TMT-A, TMT-B), animal fluency (AF) and the wordlist delayed recall part of the ADAS-cog. These tests were used to calculate a baseline cognitive composite score by computing z-scores based on the mean and standard deviation of cognitively unimpaired (CU) participants (n=465) from the BioFINDER2 study. The baseline cognitive composite was calculated as -ADAS-cogz-score + -TMT-Bz-score + AFz-score. These three cognitive tests were used in combination since they were shown to be the best predictors of conversion to AD dementia.[33] For a sensitivity analysis using a modified Preclinical Alzheimer Cognitive Composite (PACC) as an outcome we calculated a modified PACC z-scores using the formula: MMSE z-score + 2 x (-ADAS-cogz-score) + -TMT-Az-score + AFz-score. TMT-A was used instead of TMT-B in the longitudinal analysis to minimize the loss of AD participants at follow-up visits. Written informed consent was obtained from all participants prior to entering the study and the study was approved by the regional review board for human research ethics at Lund University. Details on ADNI participants are provided in the supplement.
Image acquisition, processing and biofluid biomarker collection and processing
Image acquisition and processing as well as biofluid biomarker handling are described in detail in the supplementary information. In an initial analysis we found that Braak III/IV was the best predictor (highest t-value) of cognitive decline of these three meta-ROIs, and consequently only the Braak III/IV (temporal ROI) region was used in further analyses, to avoid multiple dependent tau-PET predictors (Supplementary Table 1).
Statistics
Linear regression modelling was used to predict change in cognition with each biomarker measured separately as the main predictor and in combination. Age, sex, education and baseline MMSE were included as covariates. Change in MMSE (slope) was calculated for each individual as the slope of a linear regression based on all available follow-up visits. Only individuals who had all available biomarker measurements were included in order to ensure direct comparability of model results. Models were evaluated using t-values, R2 and change in Akaike Information Criterion (AIC) values. A model with an AIC value more than two points lower than another model can be considered significantly different.[32] To find the most parsimonious model that could predict cognitive decline we performed an initial selection using the R package MuMIn, which tests all possible variable combinations and then ranks the models according to their AIC.[41] As a complementary model, stepwise removal of the variable with the highest p-value from the full model was performed and the model with the lowest AIC was considered as the optimal model with the best tradeoff between model fit and complexity. The parsimonious model selected was the model with the fewest predictors within 2 AIC points from the optimal model. Note that before starting the analyses, to limit the number of biomarkers studied and minimize the risk of random false-positive findings, we selected biomarkers shown in previous studies to be associated to cognitive decline in AD. To avoid collinearity due to dependent predictors (such as for example pTau measured in CSF and plasma), and since in clinical practice or clinical trial settings often blood, but not CSF, is sampled, we performed the analysis of plasma and CSF biomarkers separately.
Finally, we performed a simulated clinical trial power analysis in which the ability of each measure to increase trial power was determined when used for inclusion screening. First, the number of trial participants needed to achieve 80% power to detect a reduction in cognitive change was calculated for each group without any additional inclusion criteria (“unenriched scenario”). Next, the same calculation was performed when assuming that only individuals in the 25%, 30%, 35% etc. top percentile of risk for cognitive decline as predicted by the parsimonius model would be included in the trial (“enriched scenario”). The percent difference in number of trial participants needed between the unenriched and enriched scenarios was then reported along with p-values based on the proportion of 1000 boostrap trials in which the enriched scenario required fewer participants than the unenriched scenario. All statistical tests were two-tailed with a significance level of 0.05. All analyses were performed using the R programming language (v 4.0).
Results
Participants and biomarkers
118 participants presenting with memory impairment (either amnestic MCI or amnestic mild AD dementia) from the Swedish BioFINDER-2 study were included in the study. The mean age was 71.0 ± 8.6 years, mean education duration was 12.8 ± 4.4 years, 48% were females, and mean baseline MMSE was 26.4 ± 2.4 (range 22-30). Participant demographics for the included cohort are provided in Table 1. MMSE scores were obtained at baseline and at annual follow-up for up to three years. Cognitive decline was computed as the change (slope) in MMSE score per year and correlated to ten biomarkers: plasma and CSF p-tau217, plasma and CSF NfL, plasma and CSF Aβ42/40 ratio, tau-PET SUVR in a temporal ROI (corresponding to Braak imaging stages III-IV), APOE ε4 status, cortical thickness in “AD-signature” cortex and the cognitive baseline composite score.
Table 1.
Demographic information
| Participants | |
|---|---|
| n | 118 |
| Sex (F/M) | 57/61 |
| Age (years ± SD) | 71.0 ± 8.6 |
| Education (years ± SD) | 12.8 ± 4.4 |
| Baseline MMSE (mean ± SD) | 26.4 ± 2.4 |
| MMSE slope (mean ± SD) | −1.40 ± 1.92 |
| mPACC slope (mean ± SD) § | −1.30 ± 2.67 |
| Cognitive baseline z-score (mean ± SD) | −6.3 ± 3.2 |
| [18F]RO948 temporal SUVR (mean ± SD) | 1.47 ± 0.47 |
| Plasma p-tau217 (ng/ml; mean ± SD) | 3.97 ± 5.02 |
| Plasma NfL (ng/ml; mean ± SD) | 21.2 ± 24.4 |
| Plasma Aβ42/40 ratio (mean ± SD) | 0.21 ± 0.04 |
| CSF p-tau217 (ng/ml; mean ± SD) | 278 ± 286 |
| CSF NfL (ng/ml; mean ± SD) | 234 ± 182 |
| CSF Aβ42/40 ratio (mean ± SD) | 0.07 ± 0.03 |
| Aβ positive (%) | 77/118 (65) |
CSF = cerebrospinal fluid; F = female; M = male; MMSE = minimental state exam; SD = standard deviation; SUVR = standardized uptake value ratio.
modified PACC measurement used for a sensitivity analysis (n=103).
Prediction of cognitive decline by individual biomarkers
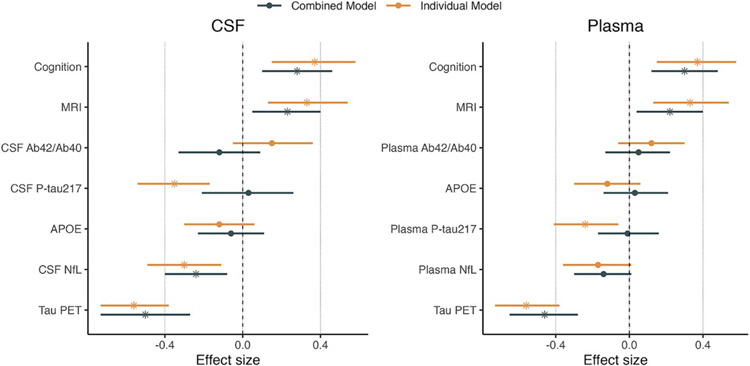
We found that baseline tau-PET SUVR in the temporal ROI showed the highest t-values, R2 and lowest AIC values when each biomarker was used individually to predict change in cognition (t = −6.26, R2 = 0.33, ΔAIC −33 compared to a model including only the covariates; Figure 1 and Supplementary Tables 2 and 3). Baseline cognition, cortical atrophy, CSF NfL and plasma and CSF p-tau217 also individually significantly predicted cognitive decline (Figure 1, Supplementary Tables 2 and 3). As expected, models including all biomarkers provided better model fit (higher R2-values). They also provided better ΔAIC-values as compared to the best single predictor tau-PET (Supplementary Tables 2 and 3).
Figure 1. Prediction of cognitive decline using biomarkers individually or in combination.
The figure shows the effect sizes for each biomarker in predicting future cognitive decline either alone (orange bars, on top) or in a combined model (black bars, below). Significant biomarkers are represented with a star. The model using cerebrospinal fluid (CSF) biomarkers is shown in the left panel and the model using plasma biomarkers in the right panel. Bars represent 95% Confidence Intervals.
Prediction of cognitive decline by biomarker combinations
We next determined the most parsimonious model that could provide a non-inferior prediction of future cognitive decline compared to the full models combining all predictors. We therefore sequentially removed one biomarker at a time from the full model in a stepwise fashion (the biomarker with the highest p-value in the model was removed) and refit the model. Data showing the change in AIC and R2 upon biomarker removal are presented in Table 2. The combination of tau-PET, baseline cognition, cortical atrophy and NfL provided the most parsimonious models (with the fewest number of predictors, and AIC within <2 from the model with the lowest AIC), where tau-PET and baseline cogntion were the strongest predictors (Table 2). We next confirmed this model using an automated data-driven model selection (MuMin) to evaluate the ability of all possible biomarker combinations to predict cognitive decline. Again we found that for both plasma and CSF analyses, the combination of tau-PET, cortical atrophy, NfL and baseline cognition provided the lowest AICs.
Table 2.
Selection of the most parsimonious model for predicting cognitive decline in patients with amnestic MCI and amnestic mild dementia
| Model | Plasma p- tau217 |
APOE ε4 status |
Plasma Aβ ratio |
Plasma NfL |
MR AD cortex |
Baseline cognition |
Tau-PET | R2 | p-value | AIC |
|---|---|---|---|---|---|---|---|---|---|---|
| Full plasma model | −0.11 (0.91) | 0.38 (0.71) | 0.54 (0.59) | −1.85 (0.07) | 2.43 (0.02) | 3.27 (0.001) | −4.89 (<0.0001) | 0.44 | <0.0001 | 108 |
| plasma model −1 | − | 0.37 (0.71) | 0.54 (0.59) | −1.87 (0.06) | 2.48 (0.01) | 3.28 (0.001) | −5.30 (<0.0001) | 0.44 | <0.0001 | 106 |
| plasma model −2 | − | − | 0.41 (0.68) | −1.99 (0.05) | 2.47 (0.02) | 3.27 (0.001) | −5.34 (<0.0001) | 0.44 | <0.0001 | 104 |
| plasma model −3 | − | − | − | −2.02 (0.046) | 2.48 (0.01) | 3.29 (0.001) | −5.49 (<0.0001) | 0.44 | <0.0001 | 103 |
| plasma model −4 | − | − | − | − | 2.36 (0.02) | 3.19 (0.002) | −5.59 (<0.0001) | 0.42 | <0.0001 | 105 |
| plasma model −5 | − | − | − | − | − | 3.18 (0.002) | −6.08 (<0.0001) | 0.39 | <0.0001 | 109 |
| plasma model −6 | − | − | − | − | − | − | −6.26 (<0.0001) | 0.33 | <0.0001 | 117 |
| Model | CSF p- tau217 |
APOE ε4 status |
CSF Aβ ratio |
MR AD cortex |
CSF NfL |
Baseline cognition |
Tau-PET | R2 | p-value | AIC |
| Full CSF model | 0.22 (0.82) | −0.67 (0.51) | −1.16 (0.25) | 2.56 (0.01) | −2.90 (0.005) | 3.13 (0.002) | −4.27 (<0.0001) | 0.47 | <0.0001 | 102 |
| CSF model −1 | − | −0.69 (0.49) | −1.32 (0.19) | 2.59 (0.01) | −2.91 (0.004) | 3.13 (0.002) | −5.26 (<0.0001) | 0.47 | <0.0001 | 100 |
| CSF model −2 | − | − | −1.13 (0.26) | 2.66 (0.009) | −2.84 (0.005) | 3.17 (0.002) | −5.32 (<0.0001) | 0.47 | <0.0001 | 98.8 |
| CSF model −3 | − | − | − | 2.70 (0.008) | −2.90 (0.005) | 3.04 (0.003) | −5.29 (<0.0001) | 0.46 | <0.0001 | 98.2 |
| CSF model −4 | − | − | − | − | −2.58 (0.01) | 3.04 (0.003) | −5.85 (<0.0001) | 0.42 | <0.0001 | 104 |
| CSF model −5 | − | − | − | − | − | 3.18 (0.002) | −6.08 (<0.0001) | 0.39 | <0.0001 | 109 |
| CSF model −6 | − | − | − | − | − | − | −6.26 (<0.0001) | 0.33 | <0.0001 | 117 |
Results from the stepwise regression model. The variable with the highest p-value was removed from the model and the R2 and AIC of the new model assessed. For both plasma and CSF analyses the lowest AIC was achieved with models containing [18F]RO948 (tau-PET), Neurofilament Light (NfL), and baseline cognitive data (data highlighted with light orange). All models included sex, education, age, and baseline MMSE as covariates. Tau-PET = [18F]RO948 Standardized Uptake Ratio values in a temporal ROI; MR AD cortex = Cortical thickness in “AD signature cortex” (see methods for details); Aβ ratio = Aβ42/40 ratio. Biomarker values indicate t-values (p-values).
In a sensitivity analysis, when restricting the analysis to only amnestic MCI participants (n=90), we found similar results (Supplementary Table 4). Further, when including only participants that were Aβ-positive (Supplementary Table 5) to mimic the scenario of a clinical trial, tau-PET and baseline cognition were significant predictors, while NfL and cortical atrophy were no longer significant predictors. Finally, when using change in modified PACC over time as the cognitive outcome (instead of change in MMSE), we again found that tau-PET and baseline cognition were the strongest predictors of cognitive decline in the parsimonious models, but with minor contributions from plasma NfL and plasma p-tau217 (Supplementary Table 6). In another sensitivity analysis we found no added value of plasma or CSF glial fibrillary acidic protein (GFAP) or CSF levels of the synaptic marker Neurogranin in the BioFINDER cohort (data not shown).
To validate our findings we included 50 participants from the ADNI cohort, having a complete set of biomarkers for: age, sex, education, baseline and longitudinal MMSE, baseline cognition, tau-PET ([18F]flortaucipir), APOE ε4-status, Aβ-status, plasma NfL, plasma p-tau181 and cortical thickness. We found that tau-PET and baseline cognition were again the best predictors for longitudinal cognitive decline, but plasma NfL and cortical atrophy did not contribute to the model (Supplementary Table 7).
Enrichment for clinical trials using biomarkers
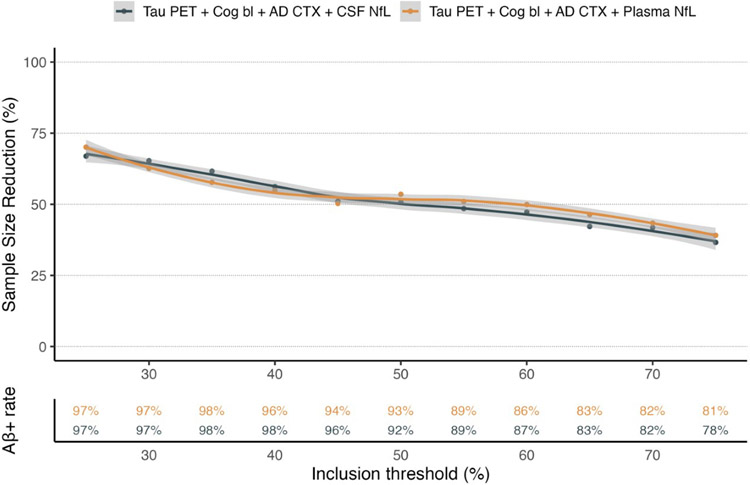
With the aim of studying the importance of screening biomarker data for clinical trial design we next calculated the impact of biomarker enrichment on group sizes needed to achieve a preset statistical power of 80%. We found that using the parsimonious models defined in BioFINDER-2, i.e. tau-PET, baseline cognition, NfL and cortical thickness, to enrich for higher risk of cognitive decline resulted in significant reduction in group sizes with preserved statistical power (Figure 2 and Supplementary Figure 1; full details provided in Supplementary Table 8). These results are independent of the assumed treatment effect. There were no statistically significant differences in the reduced group sizes between using CSF or plasma biomarkers (Supplementary Figure 1). The results indicated that restricting inclusion to the 50% of participants with the highest predicted risk for cognitive decline resulted in a need for ~52% fewer participants compared to having no enrichment strategy (Figure 2). Biomarker enrichment further resulted in removal of Aβ negative individuals even if Aβ biomarkers were not used in the parsimonious selection model. For example, in an unselected population, 65% of amnestic individuals were Aβ-positive, compared to ~80% using a 75% cut off (i.e. including the 75% with the most pathological values, and excluding the 25% with most normal values), 92-93% using a 50% cut off and 97% using a 25% cut off. In a sensitivity analysis, a more simple model only containing the most important predictors (that is: tau-PET and cognition) performed similarly to the full models for study enrichment (Supplementary Figure 2).
Figure 2. Enrichment for clinical trials using the parsimonious model biomarkers.
The top panel of the graph shows the effect on group size needed to include in a clinical trial to retain statistical power when enriching for pathological values for the tau-PET, Baseline cognition, cortical thickness and NfL biomarkers (cerebrospinal fluid (CSF) NfL in black, plasma NfL in orange). The bottom panel shows the rate of Aβ positivity with the different inclusion thresholds (CSF in black, plasma in orange).
Generation of a prediction algorithm for future cognitive decline
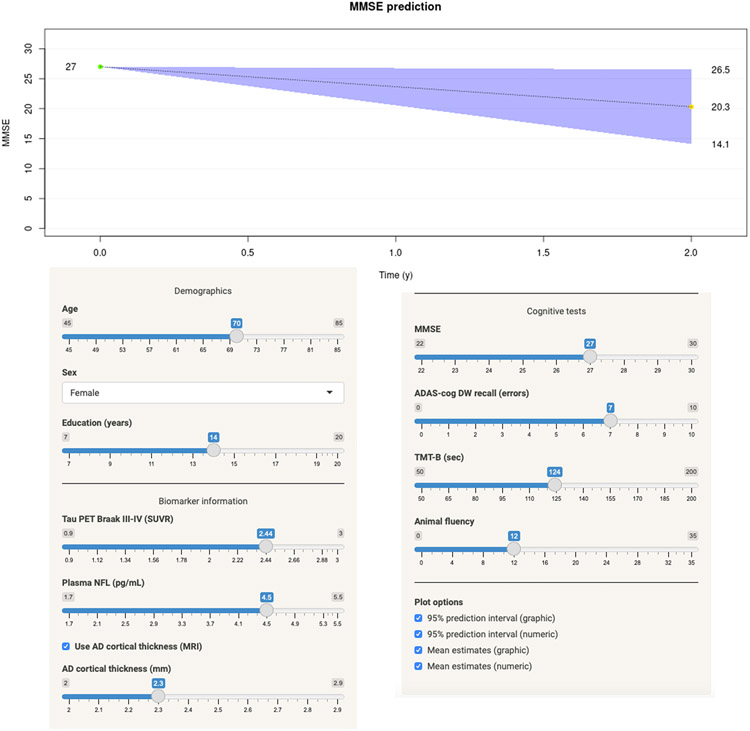
To simplify the use of the data provided herein we have generated a web-based application for calculating the risk for cognitive decline over a two-year period, based on the full BioFINDER2 dataset. The web-application is available at: https://brainapps.shinyapps.io/PredictMMSE/ (Figure 3).
Figure 3. Prediction algorithm for cognitive decline.
Example of the implementation of the regression models at https://brainapps.shinyapps.io/PredictMMSE/. At this web-site it is possible to enter basic demographic data (age, sex and education), biomarker data (tau-PET temporal ROI SUVR and plasma NfL (pg/ml)) as well as raw cognitive test scores (MMSE, ADAS delayed recall, TMT-B and animal fluency). The example shows the predicted individual change in cognition for a 70-year old female who has 14 years of education, a pathological tau-PET (2.44 SUVR), a plasma NfL of 4.5 pg/ml, and a cortical thickness of 2.3 mm (please note that entering cortical thickness is optional). She has a baseline MMSE score of 27, scores seven errors on a ten-word delayed recall test, completes the Trail-Making Test B in 124 seconds and names twelve animals in 1 min.
Discussion
Plasma[12, 33], CSF [42, 43] and imaging biomarkers [35, 36, 42-48] of A, T and N have previously been used individually or in combination to predict cognitive decline and conversion to AD dementia. However, a comprehensive direct head-to-head comparison of the relative contributions of plasma, CSF and tau-PET biomarkers to the prediction of cognitive decline is lacking. To address this gap and to allow direct comparisons of the relative contribution of the different biomarkers to the prediction we used a dataset where data for all studied biomarkers were available in all participants. In short we found that models consisting of tau-PET and baseline cognition, were most strongly and consistently associated with subsequent cogntive decline in heterogenous populations of patients with amnestic MCI or mild amnestic dementia. Further, there were more modest and more variable contributions of NfL and cortical thickness, but neither plasma (or CSF) p-tau, plasma (or CSF) Aβ42/Aβ40, or APOE4 genotype were included in the main models.
The present results are in line with a recent study showing that tau-PET is superior to Aβ-PET and MRI when predicting subsequent cognitive change in AD,[35] but in that large multicenter cohort neither plasma biomarkers, CSF biomarkers, nor baseline cognition were studied.
The present finding that NfL and cortical atrophy provides modest, but independent, information compared to tau-PET alone might be expected, considering that NfL and cortical atrophy reflects ongoing axonal degeneration and substance loss of the brain, which is clearly different to the tau aggregates detected with tau-PET imaging.[1] Previous studies have suggested a role for structural cortical volumetric or thickness MRI measures[43, 45, 48, 49] in prediction of cognitive decline in AD. Cross-sectionally we and others have reported that temporal cortical atrophy on MRI and tau-PET are associated with cognitive performance.[42, 44, 46, 47, 50] However, neither NfL, nor cortical atrophy were significant predictors in the ADNI validation cohort, possibly reflecting the smaller sample size and potentially also the lower number of early AD dementia participants in the ADNI dataset.
The main explanation why plasma (or CSF) p-tau was not selected in the main parsimonious models is likely because this biomarker, similar to Aβ42/Aβ40 and Aβ-PET, becomes abnormal much earlier than tau-PET, in the case of Aβ42/Aβ40 likely already 10-30 years before onset of objective memory impairment.[1, 11, 15] Tau-PET is more closely related to neurodegeneration and cognitive decline during the symptomatic stages of AD[1, 35], likely explaining why we found that plasma (and CSF) p-tau does not seem to contribute with independent information beyond tau-PET in patients with MCI and mild dementia. CSF p-tau 217 was pathological in 60% of participants at baseline (including all AD-dementia participants). In comparison, 38% of participants had a pathological RO948 PET at baseline. That said, plasma p-tau might be more useful during preclinical stages of AD, where it can predict future increase in tau-PET uptake.[51]
Further, baseline cognition (here evaluated using composites of memory and executive function) showed an association with future cognitive decline even after adjusting for baseline MMSE, but performed significantly inferior compared to the parsimonious model or tau-PET when used alone (Table 2, Supplementary tables 2 and 3). Still, including baseline cognition added independent information to the parsimonious models on top of tau-PET, cortical thickness and NfL data, showing the value of brief cognitive testing in the clinic when predicting subsequent cognitive decline. Similarily, we have previously found that the same three cognitive tests together with plasma p-tau can predict conversion to AD dementia in patients with SCD or MCI, but again tau-PET was not available in that study.[33]
Conducting clinical studies is very expensive since large populations need to be included to have a sufficient number of progressors over a relatively short time interval to show an effect of the treatment. In an unselected population, only a minority of patients with MCI or mild dementia will progress significantly over a two-year period. Optimizing the trial design by selecting patients that are more likely to progress is therefore of great importance for reducing the number of required participants. We consequently aimed to see whether preselecting study participants for a theoretical clinical trial based on their baseline biomarker levels could reduce the number of required participants without compromising statistical power. Restricting the study population using the parsimonious model to more pathological biomarker values resulted in a significant reduction in sample sizes needed (Figure 2; Supplementary Table 8 and Supplementary Figure 1). Importantly for the use of these biomarkers in selection of participants for future clinical studies of AD, selection based on the parsimonious model including only tau-PET, baseline cognition, cortical atrophy, and NfL also selected for Aβ positivity, thereby decreasing the need for performing both Aβ- and tau-PET in the selection process.
In light of the recently published Phase II study of donanemab in early AD[38] where cognitive decline continued despite the clearance of Aβ plaques as assessed by Aβ-PET, it may be argued that including patients based on tau-positivity, as assessed by tau-PET, may be too late since the treatment may not be able to halt disease progression. The parsimonius model presented in this article may therefore prove more suitable for anti-tau trials, acting at a later stage of the disease progression.
From a clinical perspective, knowing the likelihood that a patient in the clinic will remain stable or is likely to significantly deteriorate over a relevant time interval is of great importance. “How quickly does my memory deteriorate?” was recently listed as the most important question to be answered by both AD patients and caregivers in a survey study[52], followed by other questions related to cognition. Being able to address these questions is therefore of large interest to meet the concerns of the patients affected by the disease. By knowing the tau-PET status, the baseline performance on a few brief cognitive screening tests, NfL, a prediction can now be made using an easy-to-use on-line tool developed using the results of the present study (https://brainapps.shinyapps.io/PredictMMSE/).
There are limitations of this study. First, the follow-up period is rather short, although it is within the range of many therapeutical trials in symptomatic AD (ClinicalTrials.gov: Clarity AD, EMERGE, GRADUATE 1&2, and ENGAGE, and [37-39]), and represents a foreseeable time perspective from a clinical point of view. Second, the number of participants is relatively low, especially for the number of AD dementia participants, and the majority are of European descent. We cannot exclude that there may be small predictive effects seen with the non-significant biomarkers if the sample size was increased, and the results would benefit from being replicated in additional large independent cohorts. Likewise, we cannot exclude that other biomarkers may show better predictive abilities with a more diverse ethnic background. Third, MMSE may not be the optimal readout for longitudinal cognition in all settings, even though it often performs well to detect decline in populations with patients with MCI or mild dementia and it is often included in clinical trials as a secondary outcome. A sensitivity analysis using a modified PACC, designed to be an earlier marker of cognitive decline, as the cognitive outcome resulted in a similar outcome compared to using longitudinal MMSE. Fourth, we aimed at making a comprehensive comparative study of biomarkers for cognitive decline in early AD, but still important biomarkers, such as for example FDG PET, were not available in the dataset and have not been included in the present study.
Conclusions
We found that tau-PET, baseline cognition, cortical thickness, p-tau217-levels in blood and CSF as well as CSF NfL, can all individually predict future cognitive decline in patients with amnestic MCI or mild dementia. However, models including tau-PET and baseline cognition consistently provided the best prediction of cognitive decline in this heterogenous patient population, implying that tau-PET might be an important addition to the diagnostic work-up in situations when prognostic information is of importance. We further found that selecting a study population based on these biomarkers can result in clearly reduced number of participants needed in clinical trials, e.g. anti-tau trials, with cognitive decline as primary outcome.
Supplementary Material
Acknowledgements
We wish to thank the participants of the study. The precursor of [18F]RO948 was kindly provided by Hoffman La Roche.
Funding
Work at the authors’ research center was supported by the Swedish Research Council (2016-00906), the Knut and Alice Wallenberg foundation (2017-0383), the Marianne and Marcus Wallenberg foundation (2015.0125), the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation (AF-939932; AF-939981), the Swedish Brain Foundation (FO2019-0326), The Parkinson foundation of Sweden (1280/20), the Skåne University Hospital Foundation (2020-O000028), Regionalt Forskningsstöd (2020-0314) and the Swedish federal government under the ALF agreement (2018-Projekt0279). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), and the UK Dementia Research Institute at UCL. KB is supported by the Swedish Research Council (#2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), and the National Institute of Health (NIH), USA, (grant #1R01AG068398-01).
ADNI funding: Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Disclosures
KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, all unrelated to the work presented in this paper.
HZ has served at scientific advisory boards and/or as a consultant for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, CogRx and Red Abbey Labs, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
GK and EB are both full time employees of F. Hoffmann-La Roche Ltd, Basel, Switzerland.
SP has served on scientific advisory boards and/or given lectures in symposia sponsored by Roche, Biogen, and Geras Solutions.
OH has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Alzpath, Biogen, Cerveau and Roche.
The other coauthors report no disclosures.
Data Sharing
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation and decisions by the Swedish Ethical Review Authority, which should be regulated in a material transfer agreement.
References
- [1].Hansson O Biomarkers for neurodegenerative diseases. Nat Med. 2021;27:954–63. [DOI] [PubMed] [Google Scholar]
- [2].Andreasen N, Minthon L, Vanmechelen E, Vanderstichele H, Davidsson P, Winblad B, et al. Cerebrospinal fluid tau and Abeta42 as predictors of development of Alzheimer's disease in patients with mild cognitive impairment. Neurosci Lett. 1999;273:5–8. [DOI] [PubMed] [Google Scholar]
- [3].Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of beta-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. [DOI] [PubMed] [Google Scholar]
- [4].Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. [DOI] [PubMed] [Google Scholar]
- [5].Janelidze S, Teunissen CE, Zetterberg H, Allue JA, Sarasa L, Eichenlaub U, et al. Head-to-Head Comparison of 8 Plasma Amyloid-beta 42/40 Assays in Alzheimer Disease. JAMA Neurol. 2021;78:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature. 2018;554:249–54. [DOI] [PubMed] [Google Scholar]
- [7].Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13:841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leuzy A, Chiotis K, Lemoine L, Gillberg PG, Almkvist O, Rodriguez-Vieitez E, et al. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol Psychiatry. 2019;24:1112–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Janelidze S, Stomrud E, Smith R, Palmqvist S, Mattsson N, Airey DC, et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer's disease. Nat Commun. 2020;11:1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leuzy A, Janelidze S, Mattsson-Carlgren N, Palmqvist S, Jacobs D, Cicognola C, et al. Comparing the Clinical Utility and Diagnostic Performance of CSF P-Tau181, P-Tau217, and P-Tau231 Assays. Neurology. 2021;97:e1681–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Janelidze S, Berron D, Smith R, Strandberg O, Proctor NK, Dage JL, et al. Associations of Plasma Phospho-Tau217 Levels With Tau Positron Emission Tomography in Early Alzheimer Disease. JAMA Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, et al. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26:379–86. [DOI] [PubMed] [Google Scholar]
- [13].Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422–33. [DOI] [PubMed] [Google Scholar]
- [14].Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14:989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020;324:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26:387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jie C, Treyer V, Schibli R, Mu L. Tauvid: The First FDA-Approved PET Tracer for Imaging Tau Pathology in Alzheimer's Disease. Pharmaceuticals (Basel). 2021;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fleisher AS, Pontecorvo MJ, Devous MD Sr., Lu M, Arora AK, Truocchio SP, et al. Positron Emission Tomography Imaging With [18F]flortaucipir and Postmortem Assessment of Alzheimer Disease Neuropathologic Changes. JAMA Neurol. 2020;77:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith R, Wibom M, Pawlik D, Englund E, Hansson O. Correlation of In Vivo [18F]Flortaucipir With Postmortem Alzheimer Disease Tau Pathology. JAMA Neurol. 2019;76:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ossenkoppele R, Smith R, Ohlsson T, Strandberg O, Mattsson N, Insel PS, et al. Associations between tau, Abeta, and cortical thickness with cognition in Alzheimer disease. Neurology. 2019;92:e601–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pontecorvo MJ, Devous MD Sr., Navitsky M, Lu M, Salloway S, Schaerf FW, et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140:748–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Scholl M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 2016;89:971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, Hassenstab JJ. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology. 2018;91:e859–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pereira JB, Harrison TM, La Joie R, Baker SL, Jagust WJ. Spatial patterns of tau deposition are associated with amyloid, ApoE, sex, and cognitive decline in older adults. Eur J Nucl Med Mol Imaging. 2020;47:2155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, et al. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol. 2019;85:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Palmqvist S, Insel PS, Stomrud E, Janelidze S, Zetterberg H, Brix B, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. EMBO Mol Med. 2019;11:e11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Suarez-Calvet M, Karikari TK, Ashton NJ, Lantero Rodriguez J, Mila-Aloma M, Gispert JD, et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer's continuum when only subtle changes in Abeta pathology are detected. EMBO Mol Med. 2020:e12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mattsson-Carlgren N, Andersson E, Janelidze S, Ossenkoppele R, Insel P, Strandberg O, et al. Abeta deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer's disease. Sci Adv. 2020;6:eaaz2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mattsson-Carlgren N, Janelidze S, Bateman RJ, Smith R, Stomrud E, Serrano GE, et al. Soluble P-tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau. EMBO Mol Med. 2021;13:e14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Karikari TK, Benedet AL, Ashton NJ, Lantero Rodriguez J, Snellman A, Suarez-Calvet M, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer's Disease Neuroimaging Initiative. Mol Psychiatry. 2020. [DOI] [PubMed] [Google Scholar]
- [32].Cullen NC, Leuzy A, Palmqvist S, Janelidze S, Stomrud E, Pesini P, et al. Individualized prognosis of cognitive decline and dementia in mild cognitive impairment based on plasma biomarker combinations. Nature Aging. 2021;1:114–23. [DOI] [PubMed] [Google Scholar]
- [33].Palmqvist S, Tideman P, Cullen N, Zetterberg H, Blennow K, Dage JL, et al. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nature Medicine. 2021. [DOI] [PubMed] [Google Scholar]
- [34].Ashton NJ, Janelidze S, Al Khleifat A, Leuzy A, van der Ende EL, Karikari TK, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12:3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ossenkoppele R, Smith R, Mattsson-Carlgren N, Groot C, Leuzy A, Strandberg O, et al. Accuracy of Tau Positron Emission Tomography as a Prognostic Marker in Preclinical and Prodromal Alzheimer Disease: A Head-to-Head Comparison Against Amyloid Positron Emission Tomography and Magnetic Resonance Imaging. JAMA Neurol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pontecorvo MJ, Devous MD, Kennedy I, Navitsky M, Lu M, Galante N, et al. A multicentre longitudinal study of flortaucipir (18F) in normal ageing, mild cognitive impairment and Alzheimer's disease dementia. Brain. 2019;142:1723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer's Disease. N Engl J Med. 2018;378:321–30. [DOI] [PubMed] [Google Scholar]
- [38].Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021;384:1691–704. [DOI] [PubMed] [Google Scholar]
- [39].Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Abeta protofibril antibody. Alzheimers Res Ther. 2021;13:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jack CR Jr., Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Burnham KP, Anderson DR. Multimodel inference - understanding AIC and BIC in model selection. Sociol Method Res. 2004;33:261–304. [Google Scholar]
- [42].Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and Abeta imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med. 2016;8:338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, et al. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology. 2016;87:375–83. [DOI] [PubMed] [Google Scholar]
- [45].Jack CR Jr., Barnes J, Bernstein MA, Borowski BJ, Brewer J, Clegg S, et al. Magnetic resonance imaging in Alzheimer's Disease Neuroimaging Initiative 2. Alzheimers Dement. 2015;11:740–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mattsson N, Insel PS, Donohue M, Jogi J, Ossenkoppele R, Olsson T, et al. Predicting diagnosis and cognition with (18)F-AV-1451 tau PET and structural MRI in Alzheimer's disease. Alzheimers Dement. 2019;15:570–80. [DOI] [PubMed] [Google Scholar]
- [48].Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, et al. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol Aging. 2010;31:1401–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bauer CM, Cabral HJ, Killiany RJ. Multimodal Discrimination between Normal Aging, Mild Cognitive Impairment and Alzheimer's Disease and Prediction of Cognitive Decline. Diagnostics (Basel). 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ossenkoppele R, Schonhaut DR, Scholl M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139:1551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Leuzy A, Smith R, Cullen NC, Strandberg O, Vogel JW, Binette AP, et al. Biomarker-Based Prediction of Longitudinal Tau Positron Emission Tomography in Alzheimer Disease. JAMA Neurol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mank A, van Maurik IS, Bakker ED, van de Glind EMM, Jonsson L, Kramberger MG, et al. Identifying relevant outcomes in the progression of Alzheimer's disease; what do patients and care partners want to know about prognosis? Alzheimers Dement (N Y). 2021;7:e12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation and decisions by the Swedish Ethical Review Authority, which should be regulated in a material transfer agreement.