Abstract
This randomized controlled trial (NCT03475316) examined the relative efficacy of 6 months of social ballroom dancing and treadmill walking on a composite executive function score, generated from digit symbol substitution test, flanker interference, and walking while talking tasks. Brain activation during functional magnetic resonance imaging (fMRI) versions of these executive function tasks were secondary outcomes. Twenty-five dementia-at-risk older adults (memory impairment screen score of ≥3 to ≤6 and/or an Alzheimer’s disease-8 Dementia Screening Interview of ≥1) were randomized in June 2019 to March 2020—16 completed the intervention before study termination due to the COVID-19 (eight in each group). Composite executive function scores improved post-intervention in both groups, but there was no evidence for between-group differences. Social dancing, however, generated greater improvements on digit symbol substitution test than treadmill walking. No intervention-related differences were observed in brain activation—although less hippocampal atrophy (tertiary) was observed following social dancing than treadmill walking. These preliminary findings are promising but need to be confirmed in future large-scale and sufficiently powered randomized controlled trials.
Keywords: exercise, dance, hippocampus, hippocampal volume
There is currently no cure for Alzheimer’s disease (AD) and related dementias. Thus, identifying safe and efficacious interventions to reduce the risk of AD is important—particularly in dementia-at-risk older adults—defined here with a memory impairment score between 3 and 6 (Lipton et al., 2003) and/or an AD-8 Dementia Screening Interview score of ≥1 (Galvin et al., 2005, 2006). We have previously found that this group of at-risk older adults is quite prevalent (27%) in our community and is 2.4 times more likely to develop cognitive impairment or dementia (Ayers et al., 2020). Some studies suggest that traditional aerobic exercise (e.g., treadmill walking) has modest benefits on cognitive functions in aging—particularly on executive functions (EFs) such as planning, reasoning, problem-solving, organization, attention allocation, and selecting, or inhibiting, appropriate actions (Colcombe & Kramer, 2003; Kramer & Colcombe, 2018; Smith et al., 2010). Yet, the results of randomized controlled trials (RCTs) of traditional exercise to prevent cognitive decline have been mixed (Demurtas et al., 2020; National Academies of Sciences, 2017). EFs decline in aging and are impaired in mild cognitive impairment, AD, and related dementias (Kirova et al., 2015). Benefits of aerobic exercise on EFs are typically attributed to neuroplasticity (adaptive structural and/or functional brain changes) in the hippocampus and prefrontal cortex (Colcombe et al., 2004; Erickson et al., 2007; Stern et al., 2019). Yet, long-term adherence to traditional exercise programs is low, particularly among older adults (CDC, 2017; Dishman et al., 1985; Elsawy & Higgins, 2010; Singh, 2002). Identifying safe and effective activities that are aerobically demanding, but also socially, and cognitively stimulating, may be key to generating substantial and sustainable cognitive and brain benefits.
Social ballroom dancing is an aerobic, social, and cognitive activity. Previous research suggests that regular participation in social dancing reduces the risk for dementia and improves balance (Verghese, 2006; Verghese et al., 2003). Further evidence suggests that dancing improves mobility and cognition (Coubard et al., 2011; Doi et al., 2017; Fausto et al., 2021; Ferguson-Stegall et al., 2017; Fraser et al., 2014; Hackney et al., 2015; Hamacher et al., 2015; Kattenstroth et al., 2013; Pichierri, Coppe et al., 2012; Pichierri, Murer, & de Bruin, 2012; Rehfeld et al., 2018; Wu et al., 2010)—including performance on dual-task walking, which necessitates a subdomain of EF called attention allocation (Hackney et al., 2015; Pichierri, Coppe, et al., 2012; Pichierri, Murer, & de Bruin, 2012). Recent systematic reviews further suggest that dancing improves global cognition and EF (Hewston et al., 2021; Liu et al., 2021; Meng et al., 2020). A key weakness of existing studies is that dancing is typically contrasted with relatively inactive controls such as stretching/toning, health education, or usual care—making it difficult to determine if dancing is more beneficial than traditional aerobic activities such as walking or running. The few older adult studies that have contrasted dancing with other aerobic activities have observed neuroplasticity changes, but no cognitive benefits (Burzynska et al., 2017; Rehfeld et al., 2018).
The current single-blind RCT (clinicaltrials.gov: NCT03475316) contrasted 6 months of social ballroom dancing with 6 months of treadmill walking in dementia-at-risk older adults—which was operationalized as a score of ≥3 and ≤6 on the memory impairment screen (MIS; Lipton et al., 2003) and/or a score of ≥1 on the AD-8 dementia screening interview (Galvin et al., 2005, 2006). The first aim was to provide a preliminary examination of the relative efficacy of social dancing and treadmill walking on EF. The primary outcome was a (z-standardized) composite EF score generated from digit symbol substitution test (DSST), flanker interference, and walking while talking (WWT) tasks. The second aim was to provide a preliminary examination of intervention-related functional neuroplasticity. Thus, the secondary outcomes were functional activation/deactivation patterns during fMRI-adapted version of these EF tasks (Blumen et al., 2014; Habeck et al., 2016; Stern et al., 2014). Intervention-related changes in individual EF tasks and structural neuroplasticity (white matter hyperintensities, white matter integrity, hippocampal volume, and cortical thickness) were also explored. We hypothesized that the aerobic, social, and cognitive demands of social ballroom dancing would lead to greater intervention-related changes in EF and neuroplasticity than treadmill walking.
Methods
Study Design
The trial (clinicaltrials.gov: NCT03475316) design and procedures have been previously described (Blumen et al., 2020). In brief, this single-blind RCT contrasted 6 months (90 min, twice weekly) of social ballroom dancing with 6 months (90 min, twice weekly) of treadmill walking. Trial procedures were approved by institutional review board at Albert Einstein College of Medicine. Participants provided written consent, and procedures were completed in accordance with the Declaration of Helsinki. The inclusion criteria were as follows: age ≥65 years, MIS score of ≥3 and ≤6 (Lipton et al., 2003), and/or an AD-8 dementia screening interview score of ≥1 (Galvin et al., 2005, 2006), willingness to complete MRI, English speaking, and a plan to be in the area for the next ≥1 year. The exclusion criteria were as follows: dementia (previous diagnosis or screen as described below), serious chronic or acute illness, mobility limitation that would prevent participation in the intervention, neurodegenerative disease, and current or recent (past 6 months) participation in other exercise intervention trials or dancing programs.
Screening and Recruitment
Potential participants were identified from Bronx county population lists, outpatient clinic populations at Montefiore-Einstein, and previous studies at Albert Einstein College of Medicine. They were screened over the phone for dementia and additional eligibility criteria using the MIS (Lipton et al., 2003), the AD-8 (Galvin et al., 2005, 2006), and a medical history questionnaire. The MIS has a sensitivity of 85% and a specificity of 86% for detecting dementia (Cordell et al., 2013; Holsinger et al., 2007; Lipton et al., 2003; Milne et al., 2008; Verghese, Noone et al., 2012). The AD-8 has a sensitivity of 74% and a specificity of 84% for detecting dementia (Galvin et al., 2005, 2006). A phone-based MRI safety screen for MRI contraindications (e.g., pacemaker) was also administered. Further screening for dementia (using the Montreal Cognitive Assessment [MOCA]; Nasreddine et al., 2005; Smith, Gildeh, & Holmes, 2007) and MRI safety was completed during the baseline visit (prior to randomization). Participants also completed a Physical Activity Readiness Questionnaire (Bredin et al., 2013) that was reviewed by a board-certified Rehabilitation Medicine clinician (A.A.) along with all other available medical information to ensure fitness and safety to participate in physical exercise.
Randomization and Blinding
The statistician ( C.W.; not involved in testing or interventions) generated a blocked randomization sequence stratified by age (65–80 years; >80 years) and sex, using SAS PROC PLAN (version 9.4) to randomly assign participants (1:1) to either social dancing or treadmill walking. Research staff who were blinded to group assignment (Day & Altman, 2000) completed all outcome assessments, and those who administered intervention sessions were instructed not to discuss the details of the interventions to participants across groups.
Social Dancing
Each 90-min dance session took place twice a week for 6 months—a dose that exceeds current exercise recommendations, systematic reviews, and RCTs (CDC, 2017; Doi et al., 2017; Hwang & Braun, 2015)—and consisted of warm-up (10 min stretching to music), low-intensity dances (30 min; e.g., foxtrot, waltz), a break (10 min), moderate or higher intensity dances (30 min; e.g., salsa, east coast swing), and cooldown (10 min). Under the supervision of experienced instructors (in teaching older adults), participants practiced dancing in groups of two to six (http://www.rbcares.org/). The duration and frequency of dance sessions were chosen in light of previous studies of the cognitive and neuroplasticity benefits of dancing interventions (Burzynska et al., 2017; Hewston et al., 2021; Liu et al., 2021; Meng et al., 2020; Wu et al., 2021; Zhu et al., 2020). Low and moderate (or higher) intensity dances were determined based on discussion with dance instructors combined with prior literature on energy expenditure during different ballroom dances (Lankford et al., 2014). Research staff trained by a board-certified Rehabilitation Medicine specialist (A.A.) monitored heart rate and blood pressure prior to warm-up, during the break, and after cool down. If systolic blood pressure was >150 mmHg, participants were not allowed to start or continue a session. Physical exertion was monitored with the subjective Borg scale (Borg, 1970). Duration began with 10 min of warm-up and 30 min of low-intensity dances and proceeded to moderate-intensity dances only if systolic blood pressure remained ≤150 mmHg and perceived exertion on the Borg scale ≤14 (somewhat hard or light).
Treadmill Walking
Each 90-min treadmill walking session took place twice a week for 6 months—and consisted of warm-up (10 min stretching to music), low-intensity walking (30 min), break (10 min), moderate or higher intensity walking (30 min), and cooldown (10 min). The treadmill walking sessions were done at separate times and locations in our center to avoid interactions with the dance group. Research staff ensured that walking speed began at 1.0 mile per hour, was increased to a moderate (conversational) pace, and did not exceed 3.0 miles per hour. Duration began with 10 min of warm-up and 30 min of low-intensity walking and proceeded to moderate-intensity walking only if systolic blood pressure remained ≤150 mmHg and perceived exertion on the Borg scale was ≤14 (somewhat hard or light). A pace of 2–3 miles per hour was considered moderate to higher intensity walking and is consistent with physical activity guidelines for moderate-intensity exercise (Manson et al., 1999; Services, 2018). Heart rate, blood pressure, and physical exertion were monitored as described above. Treadmill sessions were completed in groups of up to three, and music from the same playlists used during dancing was played during treadmill walking sessions.
The Primary Outcome
The primary outcome was a composite EF score generated from standardized (z scored) performance on DSST (Wechsler, 1997), flanker interference (Eriksen & Eriksen, 1974; Fan et al., 2002), and WWT (Verghese et al., 2002) tasks. The specific administration procedures of each EF task are described in Supplementary Material S1 (available online). We selected three EF tests because a single test would not capture all facets of the EF domain. The DSST is a standardized neuropsychological assessment that involves both EFs (e.g., planning, strategizing; Jaeger, 2018 and processing speed; Baudouin et al., 2009)—and lower scores are associated with aging (Hoyer et al., 2004), dementia (Rapp & Reischies, 2005), and poorer physical functions (Williamson et al., 2009). The flanker interference task is a computerized task that engages an EF called interference resolution and is sensitive to aerobic exercise (Colcombe et al., 2004). The WWT task is a divided attention task (Verghese et al., 2002) that predicts falls, frailty, disability, mortality, and dementia (Ceïde et al., 2018; Verghese et al., 2002, 2012) and assesses a person’s ability to allocate attention to competing task demands.
The Secondary Outcome
The secondary outcome was functional neuroplasticity during fMRI-adapted DSST (Habeck et al., 2016; Stern et al., 2014), flanker interference, and imagined WWT tasks (Blumen et al., 2014). The MRI acquisition parameters and the specific administration procedures of each fMRI-adapted EF task are described in Supplementary Material S2 (available online). All fMRI tasks were preprocessed and analyzed at the individual level using Statistical Parametric Mapping 12 (SPM12). Group-level patterns of activation and deactivation were then derived with ordinal trend covariance analyses (OrTCVA; http://www.nitrc.org/projects/gcva_pca; Habeck & Stern, 2007; Habeck et al., 2005). Subject-specific factor scores (SSFS; the extent to which participants expressed a derived pattern) were entered into linear mixed-effects models.
Safety and Feasibility
Safety and feasibility metrics assessed included (a) recruitment sources; (b) session completion rates; (c) retention rates; and (d) acceptability (poststudy questionnaire), and safety was assessed by adverse events reported throughout the study period.
Additional/Tertiary Study Measures
Additional/tertiary study measures aimed to socially, physically, cognitively, and emotionally characterize participants and identify additional confounders and outcomes for upcoming studies. These included the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (Stewart et al., 2001), gait speed (in centimeter per second) during STW, balance (unipedal stance time [in seconds]), and the Geriatric Depression Scale (GDS; Yesavage et al., 1982). Additional neuroplasticity measures (see Supplementary Material S2 [available online] for MRI parameters) were as follows: white matter integrity (mean fractional anisotropy of 18 white matter tracts (using TRActs Constrained by UnderLying Anatomy; Yendiki et al., 2011, 2014), white matter hyperintensities (using Lesion Segmentation Toolbox; Schmidt et al., 2012, 2019), overall hippocampal and subfield volume (using FreeSurfer, version 6.0 https://surfer.nmr.mgh.harvard.edu/; Iglesias et al., 2015), and cortical thickness (also using FreeSurfer, version 6.0 https://surfer.nmr.mgh.harvard.edu/; Dale et al., 1999; Fischl et al., 1999, 2004).
Statistical Approach
Linear mixed-effects models were used with the intention-to-treat principle to evaluate the primary, secondary, and additional study outcomes as a function of intervention arm (social dancing vs. treadmill walking) and time (pre–post). Of primary interest was the interaction or the difference in pre–post change between social dancing and treadmill walking arms. Estimates, standard errors (SEs), and 95% confidence intervals (CIs) are reported in the text and/or in tables. We expected a correlation of .80 between repeated composite EF scores. With 16 participants in each intervention arm, and assuming a 20% dropout rate at postintervention, we could detect a difference of .0075 SD per month in EF slope, using baseline/preintervention, 2 months, 4 months, and 6 months/post-intervention between groups, with 80% power, using a two-sided tests with an alpha level of .05. The sample size of 16 per group is within the number recommended for neuroimaging pilot and feasibility studies (Hupé, 2015; Mumford, 2012; Smith, Jenkinson, et al., 2007) and will be used to guide study design and modeling strategy in future (large scale) dance and treadmill interventions.
Results
Participants
Twenty-five participants were randomized between June 2019 and March 2020. Most participants (69% in social dancing arm, 58% in treadmill walking arm) were recruited from previous studies at Albert Einstein College of Medicine. Randomized participants had a mean age of 76.45 years (SD = 5.79) and a mean MOCA score of 22.84 (SD = 3.85). Sixty-six percent were women, 48% White/European American, 24% Black/African American, and 20% Hispanic/Latino. See Table 1 for sample characteristics as a function of study arm. Sixteen participants (eight in each study arm) fully completed the intervention prior to study termination, which was terminated early due to the COVID-19 pandemic. Study flow and additional inclusion/exclusion details are summarized in Figure 1.
Table 1.
Baseline Characteristics of Dementia-at-Risk Older Adults Randomized Into Social Ballroom Dancing and Treadmill Walking Arms
| Characteristics | Social dancing | Treadmill walking |
|---|---|---|
|
| ||
| Total randomized | 13 | 12 |
| Gender | ||
| Male | 5 (38%) | 6 (50%) |
| Female | 8 (62%) | 6 (50%) |
| Ethnicity | ||
| Hispanic or Latino | 2 (15%) | 3 (25%) |
| Not Hispanic or Latino | 11 (85%) | 9 (75%) |
| Race | ||
| American Indian/Alaska Native | 1 (8%) | 0 (0%) |
| Black or African American | 2 (15%) | 4 (33%) |
| White | 7 (54%) | 5 (42%) |
| More than one race | 1 (8%) | 1 (8%) |
| Unknown or not reported | 2 (15%) | 2 (17%) |
| Age (years) | ||
| Mean | 77.40 | 75.43 |
| Median | 77.08 | 73.95 |
| SD | 5.82 | 5.84 |
| Education (years) | ||
| Mean | 16.62 | 14.67 |
| Median | 16.00 | 14.50 |
| SD | 4.61 | 4.23 |
| MOCA score | ||
| Mean | 22.54 | 23.17 |
| Median | 24.00 | 24.50 |
| SD | 3.93 | 3.90 |
| AD-8 score | ||
| Mean | 1.62 | .92 |
| Median | 2.00 | 1.00 |
| SD | 1.33 | .67 |
| MIS score | ||
| Mean | 7.23 | 6.67 |
| Median | 8.00 | 6.50 |
| SD | 1.30 | 1.16 |
| Digit symbol substitution test (correct items) | ||
| Mean | 40.62 | 44.62 |
| Median | 32.00 | 45.00 |
| SD | 13.21 | 16.16 |
| Flanker interference task (response time; ms) | ||
| Mean | 82.12 | 98.01 |
| Median | 90.04 | 73.52 |
| SD | 60.34 | 72.61 |
| Walking while talking (velocity; cm/s) | ||
| Mean | 64.05 | 68.23 |
| Median | 66.10 | 62.50 |
| SD | 24.07 | 29.08 |
Note. AD-8 = Alzheimer’s disease-8; MOCA = Montreal Cognitive Assessment; MIS = memory impairment screen.
Figure 1 —
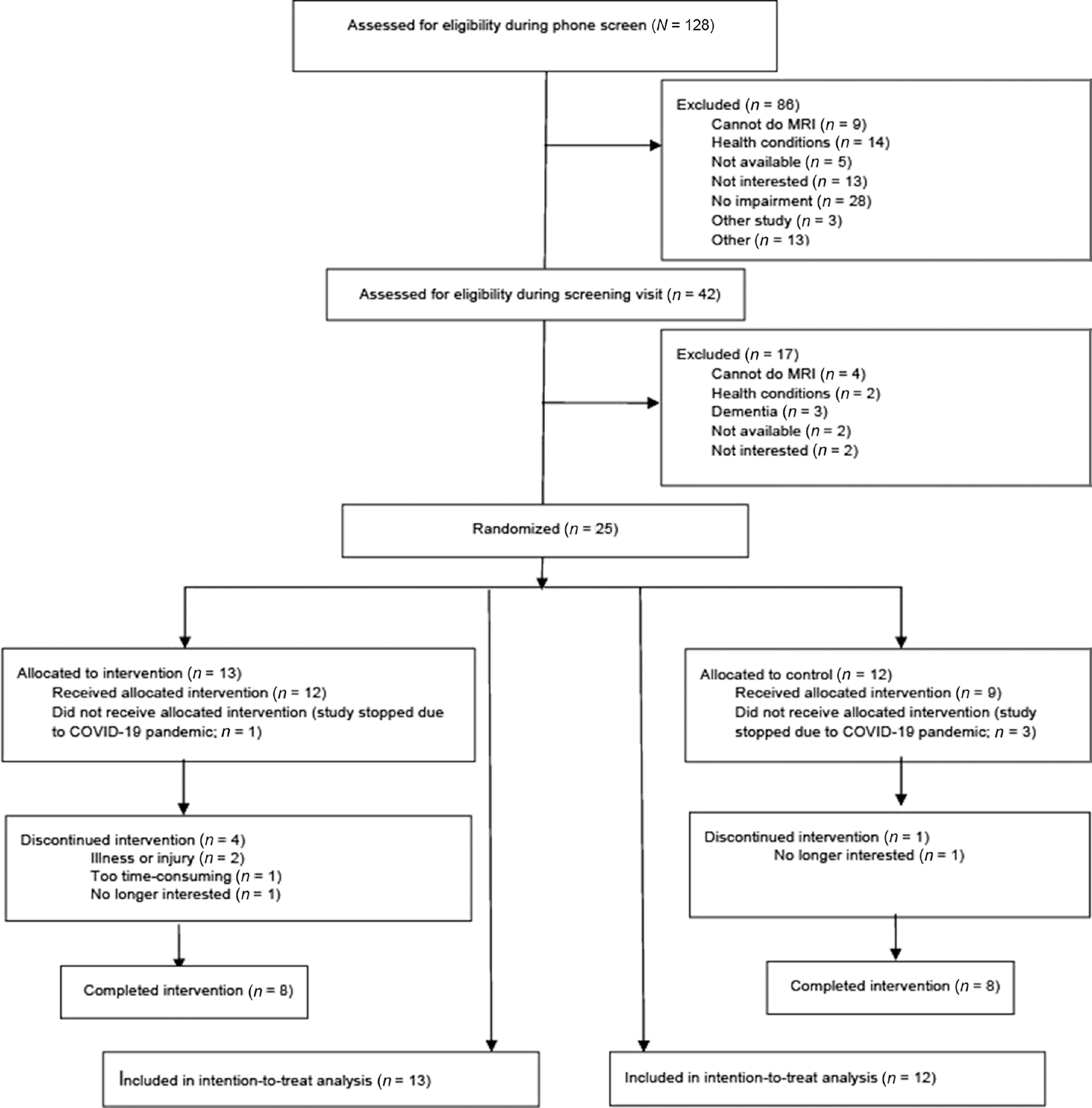
CONSORT (Consolidated Standards of Reporting Trail) flowchart of study participants.
Primary Outcome
Pre–post changes in the EF composite score and individual tests are summarized in Table 2. The pre–post changes in z-standardized composite EF scores significantly improved in both social dancing (1.16) and treadmill walking (0.99) arms. The pre–post change in the composite EF score was 0.17 greater in the social dancing arm than in the treadmill walking arm, but the between-group difference was not statistically significant, SE = 0.57, 95% CI [−1.14, 1.49]; p = .77. When individual EF tests were examined separately as raw scores, the pre–post change in DSST performance was 6.57 (correct items) greater in the social dancing arm than in the treadmill walking arm (SE = 2.77, 95% CI [0.46, 12.67]; p = .02). The pre–post change in flanker interference response time or WWT velocity were not different in the social dancing (−22.4 ms and 5.6 cm/s) and the treadmill walking arm (−49.31 ms and −4.73 cm/s)—SE = 28.83, 95% CI [−37.32, 91.14]; p = .37 and SE = 6.90, 95% CI [−5.20, 26.02]; p = .17.
Table 2.
Intervention-Related (Social Dancing vs. Treadmill Walking) Changes in the EF Composite Score (Primary Outcome) and Individual EF Tasks
| Intervention-related change: Social dancing vs. treadmill walking |
Pre-post change: Social dancing |
Pre-post change: Treadmill walking |
|
|---|---|---|---|
| Outcomes | Estimate; SE [95% CI] | Estimate; SE [95% CI] | Estimate; SE [95% CI] |
|
| |||
| Primary outcome | |||
| Executive function (EF; composite score) | 0.17; 0.57 [−1.14, 1.49] | 1.16; 0.42 [0.20, 2.12]* | 0.99; 0.39 [0.08, 1.89]* |
| Individual EF tasks | |||
| Walking while talking (velocity; cm/s) | 10.41; 6.90 [−5.20, 26.02] | 5.68; 5.53 [−6.84, 18.20] | −4.73; 4.12 [−14.06, 4.60] |
| Flanker interference (response time; ms) | 26.91; 28.83 [−37.32, 91.94] | −22.40; 15.60 [−57.18, 12.38] | −49.31; 24.23 [−103.30, 4.69] |
| Digit symbol substitution (correct items) | 6.57; 2.77 [0.46, 12.67]* | 11.00; 1.89 [6.84, 15.17]* | 4.44; 2.02 [−0.02, 8.89] |
Note. Pre-post changes for each intervention arm are also included. CI = confidence interval; EF = executive function.
Estimate statistically significant at p < .05.
Secondary Outcomes
Fifteen randomized participants completed the baseline MRI (nine in the social dancing arm and six in the treadmill walking arm). Five participants did not complete baseline MRI due to MRI contraindications. An additional five participants completed (or partially completed) the baseline MRI but had unusable data due to the inability to complete the tasks/session or corrupted data files. Twelve participants (seven in the social dancing arm and five in the treadmill walking arm) completed the postintervention MRI. Pre–post changes in neuroplasticity are summarized in Table 3. The OrTCVA analyses of the DSST task did not reveal a significant activation/deactivation pattern during digit-symbol decisions relative to rest (p > .05). The subject-specific factor scores were entered into linear mixed-effects models to generate estimates that could guide future studies, but were not different between study arms (SE = 202.69, 95% CI [−231.12, 563.44]; p = .41). The OrTCVA analyses of the flanker interference task revealed a significant activation/deactivation pattern during incongruous relative to congruous trials (p < .0001). The pre–post change in the factors score in the social dancing arm, however, was not significantly different from the treadmill walking arm (SE = 28.47, 95% CI [−31.20, 80.40]; p = .39). The OrTCVA analyses of the imagery of WWT task revealed a significant activation/deactivation pattern during imagery of WWT relative to talking or single-task walking (p < .0001). The pre–post change in the factors score in the social dancing arm, however, was not different from the treadmill walking arm (SE = 51.20, 95% CI [−42.14, 158.58], p = .21).
Table 3.
Intervention-Related Changes in Secondary (Functional Neuroplasticity) and Additional Outcome Measures
| Intervention-related change: Social dancing vs. treadmill walking |
|
|---|---|
| Outcomes | Estimate; SE [95% CI] |
|
| |
| Secondary outcomes (functional neuroplasticity) | |
| Imagery of walking while talking | 58.22; 51.20 [−42.14, 158.58] |
| Flanker interference | 24.60; 28.47 [−31.20, 80.40] |
| Digit symbol substitution | 166.16; 202.70 [−231.12, 563.44] |
| Additional outcomes (structural neuroplasticity) | |
| White matter lesion volume | −0.50; 0.82 [−2.11, 1.10] |
| White matter lesion number | −2.31; 4.45 [−11.05, 6.42] |
| Hippocampal volume (left hemisphere) | 34.16; 51.96 [−67.67, 135.99] |
| Hippocampal volume (right hemisphere) | 272.76; 109.42 [58.29, 487.22]* |
| Additional outcomes (mood; physical function) | |
| Geriatric depression score | 1.08; 1.11 [−1.30, 3.46] |
| Unipedal stance | 0.57; 5.91 [−13.40, 14.55] |
| Normal pace walking (velocity) | 7.27; 8.57 [−11.60, 26.14] |
| CHAMPS all physical activity | 0.33; 4.54 [−9.57, 10.22] |
| CHAMPS moderate physical activity | 0.77; 2.70 [−5.10, 6.64] |
Note. CHAMPS = Community Healthy Activities Model Program for Seniors; CI = confidence interval.
Estimate statistically significant with a p < .05.
Safety and Feasibility
The mean number of classes attended was 27.69 (SE = 5.11) in the social dancing arm and 32.98 (SE = 5.72) in the treadmill walking arm (p = .57). Dropout rates were 30.8% (n = 4) in the social dancing arm and 8.3% (n = 1) in the treadmill walking arm, t(23) = 1.40, p = .17. Two of the social dancing participants (15.4%) dropped out due to illness or injury (unrelated to participation in the intervention). In the social dancing group, one participant (7.69%) dropped out because the intervention was too time-consuming, while another participant (7.69%) dropped out due to lack of interest. Finally, one treadmill participant (8.3%) dropped out due to lack of interest. A total of four participants (one in the social dancing arm and three in the treadmill walking arm) did not receive the allocated interventions because the study was stopped due to the COVID-19 pandemic.
Participants who completed the intervention (eight in the social dancing group and eight in the treadmill walking group) completed a postintervention questionnaire. The average response to the postintervention question “How much did you enjoy participating in the study?” (on a scale from 1 = not at all to 5 = very much) was 4.86—and did not significantly differ between intervention arms (M social dancing = 4.75; M treadmill walking = 5.00; p = .29). The average response to how likely they were to recommend the study to a friend (using the same scale) also did not differ between intervention arms (M social dancing = 5.00; M treadmill walking = 4.83; p = .26). Finally, when questioned about the frequency of the intervention sessions (1 = “too often,” 2 = “too rarely,” 3 = “often enough”) and the length of the intervention (1 = “too long,” 2 = “too short,” 3 = “just right”), most participants (in both study arms) reported that twice a week was “often enough” (85.71%) and 6 months was “just right” (64.29%). No participant reported that intervention sessions took place “too often,” and only 7.14% reported that the length of the intervention was “too long.”
Only one report of a serious adverse event unrelated to the study occurred (hospitalization due to high blood pressure). The most common mild adverse event was falls (social dancing = 4; treadmill walking = 1)—but no falls occurred during or immediately following the intervention sessions. One treadmill participant reported shortness of breath twice while exercising. Two treadmill participants reported a musculoskeletal injury (one possibly study-related). Finally, two participants (one in each study arm) had high blood pressure recorded during their session.
Additional/Tertiary Measures
Pre–post changes in additional measures are summarized in Table 3. Pre–post changes in (a) overall and moderate physical activities on the CHAMPS, (b) STW speed, (c) unipedal stance time, and (d) depressive symptoms on the GDS—were not different in the social dancing arm and the treadmill walking arm (p > .05; see Table 3). Pre–post changes in overall white matter hyperintensity volume or count and white matter integrity of 18 different tracts were also not different in the two intervention arms (p > .05). Pre–post changes in right (but not left) hemisphere hippocampal volume, however, was significantly higher in the social dancing arm (0.07% decrease) than in the treadmill walking arm (9.51% decrease), SE = 109.42, 95% CI [58.29, 487.22]; p = .01 (see Table 3), indicating reduced right hippocampal atrophy following social dancing than treadmill walking. This difference was primarily attributed to hippocampal subfields: CA1, molecular layer, granular cell layer (of dentate gyrus), CA3, CA4, and fimbria (see Supplementary Table S1 [available online]). Pre–post increases in cortical thickness in the treadmill walking arm—not in the social dancing arm—were also observed in pars triangularis, pars orbitalis, and rostral anterior cingulate (see Supplementary Table S2 [available online]).
Discussion
This preliminary RCT examined the relative efficacy of social dancing and treadmill walking on EF and neuroplasticity in dementia-at-risk older adults. Our preliminary results suggest that social dancing is safe and feasible—yet may not generate greater benefits than treadmill walking on EF or neuroplasticity in general. On select measures of EF (DSST) and neuroplasticity (hippocampal atrophy), however, differences differed as a function of intervention arm.
Pre–post improvements in composite EF performance were observed in both intervention arms (see Table 2). This finding is consistent with previous observations of EF benefits following traditional aerobic exercise (Colcombe & Kramer, 2003; Kramer & Colcombe, 2018; Smith et al., 2010) and dancing (Hackney et al., 2015; Pichierri, Coppe, et al., 2012; Pichierri, Murer, & de Bruin, 2012). Pre–post changes in our composite measure of EF, however, did not significantly differ between social dancing and treadmill walking arms. Pre–post change in functional activation/deactivation patterns during parallel fMRI tasks also did not significantly differ between study arms. There are several potential reasons for these outcomes including the small sample size, the length and frequency of the intervention, and the matched intensity of exercises in the two study arms. The length and frequency of the intervention (90-min sessions twice a week for 6 months), however, went above and beyond current exercise recommendations (150 min per week), systematic reviews, and RCTs—which challenges the suggestion that this intervention was not long or frequent enough. Postintervention reports from study participants also suggest that the intervention length and frequency of sessions were appropriate. It is also possible that the added social and cognitive demands of social dancing relative to treadmill walking does not confer added benefits on EF or functional neuroplasticity when the intensity is matched—and/or that the complexity of learning dance steps reduces the cardiovascular demands of dancing, at least initially (Rodrigues-Krause et al., 2018). Future studies that systematically vary the intensity of exercises are needed to confirm this suggestion and to determine whether low- or high-intensity dancing lead to smaller or greater benefits on EF and functional neuroplasticity. The differential benefits observed on select measures of EF and structural neuroplasticity, however, speak to the potential of social dancing.
Pre–post change in DSST was significantly greater in the social dancing arm than the treadmill walking arm. This preliminary finding is encouraging and supports our hypothesis that social dancing may be more beneficial than traditional aerobic activities, but needs to be confirmed in future (large-scale) RCTs. This preliminary finding was not accompanied with a relatively greater increase (or lesser decrease) in cortical thickness in prefrontal cortex regions among those in the social dancing arm than in the treadmill walking arm. Yet, consistent with previous traditional exercise trials (Colcombe et al., 2004; Erickson et al., 2007; Stern et al., 2019), increases in cortical thickness in some prefrontal (pars triangularis; pars orbitalis) and cingulate (rostral anterior cingulate) regions were observed in the treadmill walking arm. Significantly less pre–post right hippocampal atrophy, however, was observed in the social dancing arm than the treadmill walking arm. These preliminary findings are encouraging because hippocampal atrophy is associated with, and predicts the conversion from, healthy aging and mild cognitive impairment to AD (Apostolova et al., 2006; Chételat et al., 2008; Jack et al., 2000). Left and right hippocampal changes have been observed following traditional aerobic exercise (Firth et al., 2018; Ji et al., 2021; Li et al., 2017), but not social dancing. A recent study, for example, found increases in self-reported physical activity, but no hippocampal volume changes, following a 4-month dancing program (contrasted with wait-list control) that was implemented in older adults with Latino/Hispanic backgrounds (Guzman et al., 2021). It is possible social dancing led to less right hippocampal atrophy than treadmill walking in our study because it involves spatial (allocentric or flexible) navigation (Lithfous et al., 2013), lasted for an extended (6 months) period of time, and focused on dementia-at-risk older adults.
Strengths and Limitations
A key strength of this RCT is that we directly contrasted a socially, cognitively, and aerobically demanding activity (social dancing) with a traditional aerobic activity (treadmill walking). We designed a 6-month single-blind RCT that matched the length, duration, and frequency of study arms and minimized the effect of confounders. We examined the relative efficacy of these activities on EF and neuroplasticity, as well as their safety and feasibility. Our social ballroom dancing and treadmill walking programs were found to be safe, feasible, and enjoyable (e.g., no study-related adverse events reported and participants were likely to recommend the program to a friend)—and completion rate and dropout rates were comparable to exercise interventions of similar length even when the study was performed within COVID-19 restrictions (e.g., Stern et al., 2019; Tak et al., 2012). A key weakness of this RCT is the small sample size, which was further affected by early study termination due to the COVID-19 pandemic. Therefore, our findings should be interpreted with caution. The primary goal of the pilot study was to evaluate feasibility and provide preliminary data for future large-scale hypothesis-driven study, not hypothesis testing. Therefore, our sample size was estimated based on the pragmatics of recruitment and the necessities for examining feasibility (Leon et al., 2011). Thus, large-scale RCTs are clearly needed to confirm these preliminary findings. Larger and more systematic examinations of the relative efficacy of social dancing and treadmill walking on different cognitive functions are also needed. The EF (DSST) task that benefitted more from social dancing than treadmill walking in this pilot is far from process pure but has been linked to processing speed, attention, working memory, associative learning, verbal abilities, visuo-spatial functions, and multisensory integration (Beres & Baron, 1981; Hoyer et al., 2004; Jaeger, 2018; Laux & Lane, 1985; Lezak, 1995; Maxwell, 1960; Thornton & Carmody, 2012). Future implementations of these physical activity programs should also identify, query, and provide additional support (when needed) for those at increased risk for low adherence and dropout, due to low socioeconomic status, obesity/overweight, low physical activity, low self-efficacy, or loneliness (Jancey et al., 2007).
Conclusion
The current RCT provides preliminary evidence for that social dancing is safe and feasible for dementia-at-risk older adults. Although EF improved postintervention in both study arms, there was no evidence for between-group differences in EF or functional neuroplasticity. Social dancing, however, generated greater improvements on DSST than treadmill walking. No intervention-related differences were observed in brain activation—although less hippocampal atrophy (tertiary) was observed following social dancing than treadmill walking. The current RCT also provides initial evidence for that social dancing can improve DSST and reduce hippocampal atrophy to a greater extent than treadmill walking. Future, large-scale and sufficiently powered clinical trials, however, are needed to confirm these preliminary findings—and the results of this study should therefore be interpreted with caution.
Supplementary Material
Acknowledgments
This research was funded by National Institute of Health/National Institute on Aging grant R21AG057586 and Dance for Cognitive Enhancement (dancealz.org).
References
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, & Thompson PM (2006). Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Archives of Neurology, 63(5), 693–699. 10.1001/archneur.63.5.693 [DOI] [PubMed] [Google Scholar]
- Ayers E, Blumen HM, & Verghese J (2020). A strategic and cost efficient method for recruiting older adults at high risk for dementia. Alzheimer’s & Dementia, 16(Suppl. 10), Article e038151. 10.1002/alz.038151 [DOI] [Google Scholar]
- Baudouin A, Clarys D, Vanneste S, & Isingrini M (2009). Executive functioning and processing speed in age-related differences in memory: Contribution of a coding task. Brain and Cognition, 71(3), 240–245. 10.1016/j.bandc.2009.08.007 [DOI] [PubMed] [Google Scholar]
- Beres CA, & Baron A (1981). Improved digit symbol substitution by older women as a result of extended practice. Journal of Gerontology, 36(5), 591–597. 10.1093/geronj/36.5.591 [DOI] [PubMed] [Google Scholar]
- Blumen HM, Ayers E, Wang C, Ambrose AF, & Verghese J (2020). A social dancing pilot intervention for older adults at high risk for Alzheimer’s disease and related dementias. Neurodegenerative Disease Management, 10(4), 183–194. 10.2217/nmt-2020-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumen HM, Holtzer R, Brown LL, Gazes Y, & Verghese J (2014). Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Human Brain Mapping, 35(8), 4090–4104. 10.1002/hbm.22461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G (1970). Perceived exertion as an indicator of somatic stress. Scandinavian Journal of Rehabilitation Medicine, 2(2), 92–98. [PubMed] [Google Scholar]
- Bredin SS, Gledhill N, Jamnik VK, & Warburton DER (2013). PAR-Q+ and ePARmed-X+: New risk stratification and physical activity clearance strategy for physicians and patients alike. Canadian Family Physician, 59(3), 273–277. [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Jiao Y, Knecht AM, Fanning J, Awick EA, Chen T, … Kramer AF (2017). White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Frontiers in Aging Neuroscience, 9, Article 59. 10.3389/fnagi.2017.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2017). Data, trends, and maps. https://www.cdc.gov/nccdphp/dnpao/data-trends-maps/index.html
- Ceïde ME, Ayers EI, Lipton R, & Verghese J (2018). Walking while talking and risk of incident dementia. The American Journal of Geriatric Psychiatry, 26(5), 580–588. 10.1016/j.jagp.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chételat G, Fouquet M, Kalpouzos G, Denghien I, De la Sayette V, Viader F, … Desgranges B (2008). Three-dimensional surface mapping of hippocampal atrophy progression from MCI to AD and over normal aging as assessed using voxel-based morphometry. Neuropsychologia, 46(6), 1721–1731. 10.1016/j.neuropsychologia.2007.11.037 [DOI] [PubMed] [Google Scholar]
- Colcombe S, & Kramer AF (2003). Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science, 14(2), 125–130. 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, … Elavsky S (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences, 101(9), 3316–3321. 10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell CB, Borson S, Boustani M, Chodosh J, Reuben D, Verghese J, … Fried LB (2013). Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the medicare annual wellness visit in a primary care setting. Alzheimers Dement, 9(2), 141–150. 10.1016/j.jalz.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Coubard OA, Duretz S, Lefebvre V, Lapalus P, & Ferrufino L (2011). Practice of contemporary dance improves cognitive flexibility in aging. Frontiers in Aging Neuroscience, 3, Article 13. 10.3389/fnagi.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Day SJ, & Altman DG (2000). Blinding in clinical trials and other studies. BMJ, 321(7259), 504. 10.1136/bmj.321.7259.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demurtas J, Schoene D, Torbahn G, Marengoni A, Grande G, Zou L, … Veronese N (2020). Physical activity and exercise in mild cognitive impairment and dementia: An umbrella review of intervention and observational studies. Journal of the American Medical Directors Association, 21(10), 1415–1422. 10.1016/j.jamda.2020.08.031 [DOI] [PubMed] [Google Scholar]
- Dishman RK, Sallis JF, & Orenstein DR (1985). The determinants of physical activity and exercise. Public Health Reports, 100(2), 158–171. 10.2307/20056432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Verghese J, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, … Shimada H (2017). Effects of cognitive leisure activity on cognition in mild cognitive impairment: Results of a randomized controlled trial. Journal of the American Medical Directors Association, 18(8), 686–691. 10.1016/j.jamda.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Elsawy B, & Higgins K (2010). Physical activity guidelines for older adults. American Family Physician, 81(1), 55–59. [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, … Kramer AF (2007). Training-induced functional activation changes in dual-task processing: An fMRI study. Cerebral Cortex, 17(1), 192–204. 10.1093/cercor/bhj137 [DOI] [PubMed] [Google Scholar]
- Eriksen B, & Eriksen C (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. 10.3758/BF03203267 [DOI] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–347. 10.1162/089892902317361886 [DOI] [PubMed] [Google Scholar]
- Fausto BA, Azimipour S, Charles L, Yarborough C, Grullon K, Hokett E, … Gluck MA (2021). Cardio-dance exercise to improve cognition and mood in older African Americans: A propensity-matched cohort study. Journal of Applied Gerontology, 41(2), 496–505. 10.1177/07334648211010580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Stegall L, Vang M, Wolfe AS, & Thomsen KM (2017). A 9-week Jaques-Dalcroze eurhythmics intervention improves single and dual-task gait speed in community-dwelling older people. Journal of Physical Activity and Health, 14(9), 740–744. 10.1123/jpah.2017-0416 [DOI] [PubMed] [Google Scholar]
- Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, & Ward PB (2018). Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage, 166, 230–238. 10.1016/j.neuroimage.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, & Dale AM (1999). Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207. 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, … Dale AM (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Fraser SA, Elliott V, de Bruin ED, Bherer L, & Dumoulin C (2014). The effects of combining videogame dancing and pelvic floor training to improve dual-task gait and cognition in women with mixed-urinary incontinence. Games for Health: Research, Development, and Clinical Applications, 3(3), 172–178. 10.1089/g4h.2013.0095 [DOI] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, … Morris JC (2005). The AD8: A brief informant interview to detect dementia. Neurology, 65(4), 559–564. 10.1212/01.wnl.0000172958.95282.2a [DOI] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Xiong C, & Morris JC (2006). Validity and reliability of the AD8 informant interview in dementia. Neurology, 67(11), 1942–1948. 10.1212/01.wnl.0000247042.15547.eb [DOI] [PubMed] [Google Scholar]
- Guzman J, Aguinaga S, Balbim GM, Lamar M, Marques IG, & Marquez DX (2021). The effects of the BAILAMOS dance program on hippocampal volume in older Latinos: A randomized controlled pilot study. Translational Behavioral Medicine, 11(10), 1857–1862. 10.1093/tbm/ibab009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Gazes Y, Razlighi Q, Steffener J, Brickman A, Barulli D, … Stern Y (2016). The reference ability neural network study: Life-time stability of reference-ability neural networks derived from task maps of young adults. NeuroImage, 125, 693–704. 10.1016/j.neuroimage.2015.10.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, & Stern Y (2005). An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-to-sample task. Cognitive Brain Research, 23(2–3), 207–220. 10.1016/j.cogbrainres.2004.10.010 [DOI] [PubMed] [Google Scholar]
- Habeck C, & Stern Y (2007). Neural network approaches and their reproducibility in the study of verbal working memory and Alzheimer’s disease. Clinical Neuroscience Research, 6(6), 381–390. 10.1016/j.cnr.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Hall CD, Echt KV, & Wolf SL (2015). Multimodal exercise benefits mobility in older adults with visual impairment: A preliminary study. Journal of Aging and Physical Activity, 23(4), 630–639. 10.1123/japa.2014-0008 [DOI] [PubMed] [Google Scholar]
- Hamacher D, Hamacher D, Rehfeld K, Hokelmann A, & Schega L (2015). The effect of a six-month dancing program on motor-cognitive dual-task performance in older adults. Journal of Aging and Physical Activity, 23(4), 647–652. 10.1123/japa.2014-0067 [DOI] [PubMed] [Google Scholar]
- Hewston P, Kennedy CC, Borhan S, Merom D, Santaguida P, Ioannidis G, … Papaioannou A (2021). Effects of dance on cognitive function in older adults: A systematic review and meta-analysis. Age Ageing, 50(4), 1084–1092. 10.1093/ageing/afaa270 [DOI] [PubMed] [Google Scholar]
- Holsinger T, Deveau J, Boustani M, & Williams JW Jr. (2007). Does this patient have dementia? JAMA, 297(21), 2391–2404. 10.1001/jama.297.21.2391 [DOI] [PubMed] [Google Scholar]
- Hoyer WJ, Stawski RS, Wasylyshyn C, & Verhaeghen P (2004). Adult age and digit symbol substitution performance: A meta-analysis. Psychology and Aging, 19(1), 211–214. 10.1037/0882-7974.19.1.211 [DOI] [PubMed] [Google Scholar]
- Hupé J-M (2015). Statistical inferences under the Null hypothesis: Common mistakes and pitfalls in neuroimaging studies. Frontiers in Neuroscience, 9, Article 18. 10.3389/fnins.2015.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang PW-N, & Braun KL (2015). The effectiveness of dance interventions to improve older adults’ health: A systematic literature review. Alternative Therapies in Health and Medicine, 21(5), Article 64. [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, … Wald LL (2015). A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage, 115, 117–137. 10.1016/j.neuroimage.2015.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O’brien P, Smith GE, Ivnik RJ, … Kokmen EJN (2000). Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology, 55(4), 484–490. 10.1212/WNL.55.4.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J (2018). Digit symbol substitution test: The case for sensitivity over specificity in neuropsychological testing. Journal of Clinical Psychopharmacology, 38(5), 513–519. 10.1097/JCP.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancey J, Lee A, Howat P, Clarke A, Wang K, & Shilton T (2007). Reducing attrition in physical activity programs for older adults. Journal of Aging and Physical Activity, 15(2), 152–165. http://hdl.handle.net/20.500.11937/25434 [DOI] [PubMed] [Google Scholar]
- Ji L, Steffens DC, & Wang L (2021). Effects of physical exercise on the aging brain across imaging modalities: A meta-analysis of neuroimaging studies in randomized controlled trials. International Journal of Geriatric Psychiatry, 36(8), 1148–1157. 10.1002/gps.5510 [DOI] [PubMed] [Google Scholar]
- Kattenstroth J-C, Kalisch T, Holt S, Tegenthoff M, & Dinse HR (2013). Six months of dance intervention enhances postural, sensorimotor, and cognitive performance in elderly without affecting cardiorespiratory functions. Frontiers in Aging Neuroscience, 5, Article 5. 10.3389/fnagi.2013.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirova A-M, Bays RB, & Lagalwar S (2015). Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. BioMed Research International, 2015, Article 748212. 10.1155/2015/748212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, & Colcombe S (2018). Fitness effects on the cognitive function of older adults: A meta-analytic study—Revisited. Perspectives on Psychological Science, 13(2), 213–217. 10.1177/1745691617707316 [DOI] [PubMed] [Google Scholar]
- Lankford DE, Bennion TW, King J, Hessing N, Lee L, & Heil DP (2014). The energy expenditure of recreational ballroom dance. International Journal of Exercise Science, 7(3), 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux LF, & Lane DM (1985). Information processing components of substitution test performance. Intelligence, 9(2), 111–136. 10.1016/0160-2896(85)90012-1 [DOI] [Google Scholar]
- Leon AC, Davis LL, & Kraemer HC (2011). The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research, 45(5), 626–629. 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD (1995). Neuropsychological assessment (3rd ed.). Oxford University Press. [Google Scholar]
- Li M. y., Huang M. m., Li S. z., Tao J, Zheng G. h., & Chen L. d. (2017). The effects of aerobic exercise on the structure and function of DMN-related brain regions: A systematic review. International Journal of Neuroscience, 127(7), 634–649. 10.1080/00207454.2016.1212855 [DOI] [PubMed] [Google Scholar]
- Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, … Buschke H (2003). Screening for dementia by telephone using the memory impairment screen. Journal of the American Geriatrics Society, 51(10), 1382–1390. 10.1046/j.1532-5415.2003.51455.x [DOI] [PubMed] [Google Scholar]
- Lithfous S, Dufour A, & Després O (2013). Spatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: Insights from imaging and behavioral studies. Ageing Research Reviews, 12(1), 201–213. 10.1016/j.arr.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Liu C, Su M, Jiao Y, Ji Y, & Zhu S (2021). Effects of dance interventions on cognition, psycho-behavioral symptoms, motor functions, and quality of life in older adult patients with mild cognitive impairment: A meta-analysis and systematic review. Frontiers in Aging Neuroscience, 13, Article 706609. 10.3389/fnagi.2021.706609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, … Hennekens CH (1999). A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. New England Journal of Medicine, 341(9), 650–658. 10.1056/NEJM199908263410904 [DOI] [PubMed] [Google Scholar]
- Maxwell A (1960). Obtaining factor scores on the Wechsler adult intelligence scale. Journal of Mental Science, 106(444), 1060–1062. 10.1192/bjp.106.444.1060 [DOI] [PubMed] [Google Scholar]
- Meng X, Li G, Jia Y, Liu Y, Shang B, Liu P, … Chen, L. (2020). Effects of dance intervention on global cognition, executive function and memory of older adults: A meta-analysis and systematic review. Aging Clinical and Experimental Research, 32(1), 7–19. 10.1007/s40520-019-01159-w [DOI] [PubMed] [Google Scholar]
- Milne A, Culverwell A, Guss R, Tuppen J, & Whelton R (2008). Screening for dementia in primary care: A review of the use, efficacy and quality of measures. International Psychogeriatrics, 20(5), 911–926. 10.1017/S1041610208007394 [DOI] [PubMed] [Google Scholar]
- Mumford JA (2012). A power calculation guide for fMRI studies. Social Cognitive and Affective Neuroscience, 7(6), 738–742. 10.1093/scan/nss059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2017). Preventing cognitive decline and dementia: A way forward. [PubMed]
- Pichierri G, Coppe A, Lorenzetti S, Murer K, & de Bruin ED (2012). The effect of a cognitive-motor intervention on voluntary step execution under single and dual task conditions in older adults: A randomized controlled pilot study. Clinical Interventions in Aging, 7, 175–184. 10.2147/CIA.S32558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichierri G, Murer K, & de Bruin ED (2012). A cognitive-motor intervention using a dance video game to enhance foot placement accuracy and gait under dual task conditions in older adults: A randomized controlled trial. BMC Geriatrics, 12(1), Article 74. 10.1186/1471-2318-12-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp MA, & Reischies FM (2005). Attention and executive control predict Alzheimer Disease in late life: Results from the berlin aging study (BASE). The American Journal of Geriatric Psychiatry, 13(2), 134–141. 10.1097/00019442-200502000-00007 [DOI] [PubMed] [Google Scholar]
- Rehfeld K, Luders A, Hokelmann A, Lessmann V, Kaufmann J, Brigadski T, … Muller NG (2018). Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PLoS One, 13(7), Article e0196636. 10.1371/journal.pone.0196636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Krause J, Farinha JB, Ramis TR, Boeno FP, dos Santos GC, Krause M, & Reischak-Oliveira A (2018). Cardiorespiratory responses of a dance session designed for older women: A cross sectional study. Experimental Gerontology, 110, 139–145. 10.1016/j.exger.2018.06.003 [DOI] [PubMed] [Google Scholar]
- Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, … Mühlau M (2012). An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. NeuroImage, 59(4), 3774–3783. 10.1016/j.neuroimage.2011.11.032 [DOI] [PubMed] [Google Scholar]
- Schmidt P, Pongratz V, Küster P, Meier D, Wuerfel J, Lukas C, … Mühlau M (2019). Automated segmentation of changes in FLAIR-hyperintense white matter lesions in multiple sclerosis on serial magnetic resonance imaging. NeuroImage: Clinical, 23, Article 101849. 10.1016/j.nicl.2019.101849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Services U (2018). Physical activity guidelines for Americans (2nd ed.). US Government Printing Office. [Google Scholar]
- Singh MAF (2002). Exercise comes of age: Rationale and recommendations for a geriatric exercise prescription. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 57(5), M262–M282. 10.1093/gerona/57.5.M262 [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, … Sherwood A (2010). Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine, 72(3), 239–252. 10.1097/PSY.0b013e3181d14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Beckmann C, Miller K, & Woolrich M (2007). Meaningful design and contrast estimability in fMRI. Neuroimage, 34(1), 127–136. 10.1016/j.neuroimage.2006.09.019 [DOI] [PubMed] [Google Scholar]
- Smith T, Gildeh N, & Holmes C (2007). The montreal cognitive assessment: Validity and utility in a memory clinic setting. The Canadian Journal of Psychiatry, 52(5), 329–332. 10.1177/070674370705200508 [DOI] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Steffener J, Barulli D, Gazes Y, Razlighi Q, … Salthouse T (2014). The reference ability neural network study: Motivation, design, and initial feasibility analyses. NeuroImage, 103, 139–151. 10.1016/j.neuroimage.2014.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, MacKay-Brandt A, Lee S, McKinley P, McIntyre K, Razlighi Q, … Sloan RP (2019). Effect of aerobic exercise on cognition in younger adults: A randomized clinical trial. Neurology, 92(9), e905–e916. 10.1212/WNL.0000000000007003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, & Ritter PL (2001). CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Medicine & Science in Sports & Exercise, 33(7), 1126–1141. 10.1097/00005768-200107000-00010 [DOI] [PubMed] [Google Scholar]
- Tak EC, van Uffelen JG, Paw MJCA, van Mechelen W, & Hopman-Rock M (2012). Adherence to exercise programs and determinants of maintenance in older adults with mild cognitive impairment. Journal of Aging and Physical Activity, 20(1), 32–46. 10.1123/japa.20.1.32 [DOI] [PubMed] [Google Scholar]
- Thornton KE, & Carmody DP (2012). Symbol digit and the quantitative EEG. Journal of Neurotherapy, 16(3), 210–222. 10.1080/10874208.2012.705762 [DOI] [Google Scholar]
- Verghese J (2006). Cognitive and mobility profile of older social dancers. Journal of the American Geriatrics Society, 54(8), 1241–1244. 10.1111/j.1532-5415.2006.00808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Buschke H, Viola L, Katz M, Hall C, Kuslansky G, & Lipton R (2002). Validity of divided attention tasks in predicting falls in older individuals: A preliminary study. Journal of the American Geriatrics Society, 50(9), 1572–1576. http://www.ncbi.nlm.nih.gov/pubmed/12383157 [DOI] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, & Wang C (2012). Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. Journal of the American Geriatrics Society, 60(10), 1901–1905. 10.1111/j.1532-5415.2012.04145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, … Buschke H (2003). Leisure activities and the risk of dementia in the elderly. New England Journal of Medicine, 348(25), 2508–2516. 10.1056/NEJMoa022252 [DOI] [PubMed] [Google Scholar]
- Verghese J, Noone ML, Johnson B, Ambrose AF, Wang C, Buschke H, … Mathuranath PS (2012). Picture-based memory impairment screen for dementia. Journal of the American Geriatrics Society, 60(11), 2116–2120. 10.1111/j.1532-5415.2012.04191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997). Wechsler adult intelligence scale – III. Psychological Corporation. [Google Scholar]
- Williamson JD, Espeland M, Kritchevsky SB, Newman AB, King AC, Pahor M, … Investigators LS (2009). Changes in cognitive function in a randomized trial of physical activity: Results of the lifestyle interventions and independence for elders pilot study. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 64(6), 688–694. 10.1093/gerona/glp014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VX, Chi Y, Lee JK, Goh HS, Chen DYM, Haugan G, … Klainin-Yobas P (2021). The effect of dance interventions on cognition, neuroplasticity, physical function, depression, and quality of life for older adults with mild cognitive impairment: A systematic review and meta-analysis. International Journal of Nursing Studies, 122, Article 104025. 10.1016/j.ijnurstu.2021.104025 [DOI] [PubMed] [Google Scholar]
- Wu W-L, Wei T-S, Chen S-K, Chang J-J, Guo L-Y, & Lin H-T (2010). The effect of Chinese Yuanji-dance on dynamic balance and the associated attentional demands in elderly adults. Journal of Sports Science & Medicine, 9(1), 119–126. https://www.ncbi.nlm.nih.gov/pubmed/24149395 [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, & Fischl B (2014). Spurious group differences due to head motion in a diffusion MRI study. NeuroImage, 88, 79–90. 10.1016/j.neuroimage.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, … Fischl B (2011). Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics, 5, Article 23. 10.3389/fninf.2011.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. http://www.ncbi.nlm.nih.gov/pubmed/7183759 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhong Q, Ji J, Ma J, Wu H, Gao Y, … Wang T (2020). Effects of aerobic dance on cognition in older adults with mild cognitive impairment: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 74(2), 679–690. 10.3233/JAD-190681 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


