Visual Abstract
Key Words: atrial natriuretic peptide, heart failure, palmitoylation, Rab3, exocytosis
Highlights
-
•
zDHHC9 regulates cardiomyocyte Rab3a activity and ANP release through palmitoylation of Rab3gap1.
-
•
Rab3a localizes to atrial granules in vivo, and transgenic overexpression of zDHHC9 in cardiomyocytes results in dysregulated atrial ANP, Rab3a, and Rab3gap1 levels, atrial granule morphology, and circulating ANP levels.
-
•
Roles identified for zDHHC9, Rab3gap1, and Rab3a in molecular regulation of ANP secretion provide novel targets for therapeutic enhancement of natriuretic peptide signaling in heart failure and cardiovascular disease.
Summary
Production and release of natriuretic peptides by the stressed heart reduce cardiac workload by promoting vasodilation, natriuresis, and diuresis, which has been leveraged in the recent development of novel heart-failure pharmacotherapies, yet the mechanisms regulating cardiomyocyte exocytosis and natriuretic peptide release remain ill defined. We found that the Golgi S-acyltransferase zDHHC9 palmitoylates Rab3gap1 resulting in its spatial segregation from Rab3a, elevation of Rab3a-GTP levels, formation of Rab3a-positive peripheral vesicles, and impairment of exocytosis that limits atrial natriuretic peptide release. This novel pathway potentially can be exploited for targeting natriuretic peptide signaling in the treatment of heart failure.
Palmitoylation or S-acylation, the reversible covalent attachment of saturated fatty acids to protein cysteines, regulates protein trafficking and targeting to membrane microdomains and, consequently, can modulate protein stability, interactions, and cellular functions.1,2 Palmitoylation occurs by the activity of 23 zinc finger and Asp-His-His-Cys (zDHHC) S-acyltransferases, multipass transmembrane enzymes that transfer fatty acids (typically palmitate) to protein substrates on the cytoplasmic face of the organelle or plasma membrane where the zDHHC is localized.1,2 Although palmitoylation has established functions in anterograde trafficking of membrane proteins through the secretory pathway3,4 and synaptic vesicle fusion and neurotransmitter release,5, 6, 7, 8, 9 regulatory roles in exocytosis and hormone secretion in non-neuronal cells are not well studied.
A majority of zDHHC enzymes localize to the Golgi apparatus,1 where they are ideally positioned to regulate membrane protein trafficking as well as secretory pathway machinery.10 zDHHC9 is a Golgi-localized enzyme, originally characterized as the S-acyltransferase with specificity for modification of H-Ras and N-Ras.11,12 Subsequently, zDHHC9 has been found to have indispensable functions in neurons, including regulation of dendrite outgrowth, synapse formation, and GLUT1 glucose transporter trafficking,8,13 and to promote tumorigenesis in multiple types of cancer.12, 13, 14 Several mutations in ZDHHC9 have been identified in patients with X-linked intellectual disability (XLID),15, 16, 17, 18 underscoring critical functions of zDHHC9 in the brain. However, although zDHHC9 is robustly expressed in the heart11 and reportedly downregulated in myocardium of patients with heart failure,19,20 its functions in cardiac myocytes have not been interrogated.
The heart secretes hormones into the circulation to elicit adaptive physiological responses in target organs including the kidney, vasculature, adipose tissue, and skeletal muscle.21,22 Natriuretic peptides, including atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), are secreted by the heart and activate natriuretic peptide receptors that contain an intracellular guanylate cyclase domain to evoke elevation of intracellular cGMP levels,21,23 thereby inducing vasodilation, natriuresis, and diuresis and lowering blood pressure, volume, and cardiac workload. Cardiomyocytes ramp up synthesis and secretion of natriuretic peptides in response to mechanical stretch, hypertension, or cardiac injury, enabling the use of their serum levels as clinical biomarkers of heart failure and severity of cardiac disease.21,24 Natriuretic peptide signaling has emerged as an attractive pharmacologic target, with the neprilysin inhibitor, sacubitril, being prescribed in combination with an angiotensin receptor blocker (valsartan) to treat both chronic and acute decompensated heart failure with greater efficacy than standard-of-care angiotensin converting enzyme inhibitor therapy.25,26
Despite the clinical significance and identification of some proteins responsible for generation of secretory granules/dense core vesicles (DCVs)27 and SNAREs that mediate fusion of ANP-containing DCVs with the sarcolemma,28 molecular mechanisms modulating ANP processing, trafficking, and exocytosis in cardiomyocytes remain ill-defined. Rab GTPases orchestrate organelle and vesicle membrane remodeling, trafficking, and fusion to coordinate transport through the endomembrane system,29 including regulation of trafficking of β-adrenergic receptors30,31 and ion channels32 in cardiomyocytes. A number of Rab proteins are abundantly expressed in the heart, including Rab3,30,33,34 a key regulator of exocytosis35, 36, 37 that promotes regulated secretion of neuropeptides and neurotrophins by DCVs in neurons,36,37 yet no studies, to our knowledge, have established a definitive role for Rab proteins in cardiomyocyte secretion.
Rab protein activity is regulated by Rab guanine nucleotide exchange factors (Rab GEFs) that aide in GTP-loading, and Rab GTPase activating proteins (Rab GAPs) that accelerate GTP hydrolysis to inactivate Rab proteins.29,38 Rab3 proteins (Rab3a/b/c/d) associate with vesicle membranes via geranylgeranyl lipid modifications on their C-terminus, and, once activated, Rab3-GTP interacts with effectors to aide in docking and priming of DCVs for fusion with the plasma membrane.37,39, 40, 41 Rab3 GTPase activating protein 1 (Rab3gap1) hydrolyzes GTP on Rab3a to enable its detachment from DCVs, thereby promoting successive rounds of exocytosis and secretion of DCV contents into the extracellular space.38, 39, 40 Indeed, GTP hydrolysis and nucleotide cycling on Rab3a are required for complete docking, fusion pore opening, and exocytosis.36,39,40,42, 43, 44, 45 Consistent with this, loss of Rab3gap1 results in defective calcium-stimulated glutamate release by cerebrocortical neurons despite elevated levels of Rab3a-GTP in the brain.45 Mutations in the RAB3GAP1 or RAB3GAP2 genes encoding the catalytic and noncatalytic subunits, respectively, of the Rab3gap enzyme, cause Warburg Micro syndrome, a rare neuromuscular disorder characterized by severe developmental defects of the brain, eye, and genitalia.46,47 Notably, genome-wide association studies (GWAS) identified a single nucleotide polymorphism in the RAB3GAP1 locus as the most significantly associated with cardiac sudden death,48 suggesting roles for Rab3gap1 in cardiac pathophysiology.
Here, we identified novel functions for the S-acyl transferase, zDHHC9, in regulation of the secretory pathway in cardiomyocytes through palmitoylation of Rab3gap1 and modulation of Rab3a-mediated exocytosis. We generated transgenic mice overexpressing zDHHC9 in cardiomyocytes that develop dilated cardiomyopathy with advanced age that is preceded by enhanced palmitoylation and retention of Rab3gap1 at the Golgi, elevated GTP-loading of Rab3a, and an increase in atrial ANP protein levels but reduction in circulating ANP levels. Mechanistically, zDHHC9 overexpression elicits expansion of Rab3a-positive vesicles but impairs ANP secretion in cardiomyocytes, whereas knockdown of zDHHC9 enhances phenylephrine-induced ANP secretion, suggesting zDHHC9-mediated regulation of Rab3gap1 disrupts Rab3a nucleotide cycling to perturb DCV exocytosis. These studies establish zDHHC9 as a regulator of cardiac homeostasis and endocrine signaling and suggest novel roles for zDHHC9, Rab3gap1, and Rab3a in ANP secretion.
Methods
Animal models
Cardiomyocyte-specific zDHHC9 transgenic mice were generated on the FVB/N genetic background using the bigenic tet-off α-myosin heavy chain (MHC) promoter expression system.49 Mouse Zdhhc9 cDNA (Horizon Discovery, # MMM1013-202767122) was cloned into the modified α-MHC promoter expression plasmid using the SalI and HindIII restriction endonuclease sites followed by NotI digestion and gel purification of the α-MHC promoter-Zdhhc9 cDNA fragment for oocyte injection, as described previously.50,51 Zdhhc9 transgenic mice were bred to transgenic mice expressing the tetracycline transactivator (tTA), driven by the same modified α-MHC promoter to generate double transgenic (DTg) mice that overexpress zDHHC9 protein in cardiomyocytes and control single transgenic or nontransgenic genotypes. Transgenic mice were fed normal lab chow (without tetracycline or doxycycline) to elicit constitutive α-MHC promoter-driven expression of the Zdhhc9 transgene in DTg mice, which starts on approximately postnatal day 2 in cardiomyocytes.52 Echocardiography was performed on anesthetized mice at the indicated ages to assess left ventricular structure and function, as described previously.50,53,54 All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Michigan (IACUC protocol #PRO00009098) and were in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health).
Plasmids, viral, and protein production
The indicated cDNAs were subcloned into pacAd5-CMV-K-N-pA shuttle vector (Cell Biolabs) using the In-Fusion HD cloning kit (Takara) for generation of recombinant adenovirus using the RAPAd CMV Adenoviral Expression System (Cell Biolabs) at the University of Michigan Viral Vector Core, with the exception of the zDHHC9 adenoviruses, which were similarly generated using the AdEasy Adenoviral Vector System (Agilent) by subcloning into the pShuttle-CMV vector according to the manufacturer’s protocols. Briefly, shuttle plasmids containing the desired cDNAs were digested with PacI restriction endonuclease and cotransfected with the PacI-digested pacAd5 9.2-100 backbone vector for adenoviral generation in HEK 293T cells in the case of the RAPAd system (Cell Biolabs), or digested with PME1 restriction endonuclease for cotransformation with the linearized (PacI-digested) pAdEasy-1 vector into BJ5183 E. Coli cells for recombination followed by DNA purification and PacI-digestion of the recombinant adenoviral plasmid for transfection in AD-293 cells (Agilent) for production of recombinant adenovirus with the AdEasy system (Agilent). Adenoviral constructs were generated to express mouse Zdhhc9 cDNA (Horizon Discovery, # MMM1013-202767122), mouse Rab3gap1 cDNA (Horizon Discovery, #MMM1013-202770447), and GFP-Rab3a (Addgene #49542).55 For the Rab3gap1 adenovirus, an N-terminal myc tag was added by multiple-insert cloning of the myc coding sequence PCR amplified from myc-BioID2-MCS (Addgene #74223)56 and the Rab3gap1 cDNA into the pacAd5-CMV-KN-pA shuttle vector according to the manufacturer's protocol (Takara). A mutation (a262g) encoding an arginine to lysine change at amino acid residue 88 was found by DNA sequencing of the Rab3gap1 cDNA and corrected by site-directed mutagenesis with the QuickChange II XL kit (Agilent) using the following primers: Rab3gap1 g262a for 5′-CCTCCAATTCTTCATCCTTTCTTTCTTTATCAGGAGACTCTTG-3′, Rab3gap1 g262a and Rev 5′-CAAGAGTCTCCTGATAAAGAAAGAAAGGATGAAGAATTGGAGG-3′. The transferase-deficient DHHS mutant of Zdhhc9 and GAP-deficient Rab3gap1 R728A mutant were similarly generated from the pShuttle-CMV or pacAd5-CMV-KN-pA shuttle plasmids containing wildtype mouse Zdhhc9 or Rab3gap1 cDNAs, respectively, with the following primers: Zdhhc9 DHHS For 5′-CCCACCCAGGGGCTGTGATGGTCGAAG-3′, Zdhhc9 DHHS Rev 5′-CTTCGACCATCACAGCCCCTGGGTGGG-3′; Rab3gap1 R728A For 5′-CTGGGATCTTCATAGCGGCACTCAGCTCCCC-3′, Rab3gap1 R728A Rev 5′-GGGGAGCTGAGTGCCGCTATGAAGATCCCCAG-3′. The control β-galactosidase (βGal) adenovirus was described previously.57
Glutathione S-transferase (GST) fusion proteins were generated as described previously.54 Briefly, to produce the GST fusion protein containing the Rab3-GTP binding domain of Rim1 used for purification of active Rab3a, a fragment of rat Rim1 cDNA encoding amino acids 1-200 was subcloned from pCMV-HA-Rim158 (gift of Dr. Ronald Holz, University of Michigan) into the pGEX-4T-1 vector (GE Healthcare) and transformed into BL21 E. Coli (Thermo Fisher, # C600003). GST or GST-Rim1 (1-200) protein expression was induced in BL21 cultures by isopropyl-β-D-thiogalactoside (IPTG, Sigma #I6758) and bacteria were spun down and protein extracted in lysis buffer (50 mM Tris pH 7.5, 1% triton X-100, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT) supplemented with protease inhibitors (1 mM AEBSF, 10 μg/mL aprotinin, 10 μg/mL leupeptin). Resuspended bacteria were centrifuged at 14,000 revolutions per minute (rpm) for 30 minutes at 4 °C, and the resultant supernatant containing recombinant GST proteins was then aliquoted and stored at –80 °C until further use in the GST pull-down assays as described below. DNA sequences of all constructs were fully verified by Sanger sequencing (Genewiz).
Western blotting
Cardiac tissue or cells were homogenized with beads (Next Advance Bullet Blender) or a D1000 Handheld Homogenizer (Benchmark) in RIPA buffer (50 mM Tris•HCl pH 7.4, 1% triton X-100, 1% sodium deoxycholate, 1 mM EDTA, 0.1% SDS) with protease inhibitors (1 mM AEBSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin) and clarified by centrifugation at 12,000 rpm for 10 minutes at 4 °C. For detection of zDHHC9 in vivo, cardiac tissue was homogenized and sonicated (3 times for 10 seconds at 30% duty cycle and power 3 Branson Sonifier 450) in triton buffer (50 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, 1% triton X-100) with protease inhibitors, clarified by centrifugation at 12,000 rpm for 10 minutes at 4 °C. Triton-insoluble pellets were then resuspended and sonicated in urea lysis buffer (50 mM Tris, pH 7.6, 6M urea, 10 mM Na4P2O7•10H2O, 10% glycerol, 2% SDS) with protease inhibitors, centrifuged at 12,000 rpm for 10 minutes at 4 °C, and the clarified lysate used for immunoblotting. Western blotting was performed by standard SDS-PAGE and immunoblotting as described,54 with the following primary antibodies diluted in 5% dry milk in Tris-buffered saline with 0.1% tween-20 (TBST): Rab3gap1 (Proteintech, 21663-1-AP, 1:1,000), zDHHC9 (St John's Laboratory, STJ92709, 1:500), GFP (Novus Biologicals, NB600-597), Rab3a (Synaptic Systems, 107111, 1:1,000), Gapdh (Fitzgerald, 10R-G109A, 1:50,000), ANP (Millipore, AB5490,1:500), myc (Millipore, 05-419, 1:1,000), GFP (Novus, NB600-597, 1:1,000), β-actin (Abcam, ab8227, 1:1,000), H-Ras (Santa Cruz, sc-29, 1:500), N-Ras (Santa Cruz, sc-31, 1:500), Gα12 (Santa Cruz, sc-409, 1:500), PI4K2α (gift from Pietro de Camilli, Yale University, 1:1,000),59 Jph2 (gift from Long-Sheng Song, University of Iowa, 1:1,000),60,61 Rab27b (Synaptic Systems, 168103, 1:1,000), and α-tubulin (Santa Cruz, sc-5286, 1:500). Immunoblots were then incubated with the appropriate IRDye secondary antibody (LiCor Biosciences) diluted 1:10,000 in 5% dry milk with 0.02% SDS and developed on an Odyssey CLx imager (LiCor Biosciences).
Immunocytochemistry and immunohistochemistry
Cells were washed in PBS, fixed in 4% paraformaldehyde (Electron Microscopy Sciences) diluted in PBS for 15 minutes at room temperature, and blocked in ICC buffer (PBS, 5% goat serum, 1% BSA, 1% glycine, 0.2% triton X-100) for 1 hour at room temperature. Cells were then immunostained with primary antibodies diluted in ICC buffer overnight at 4 °C followed by the appropriate Alexa Fluor conjugated secondary antibodies (Invitrogen) diluted 1:1000 in ICC buffer for 2 hours at room temperature and mounting with ProLong Gold Antifade Mountant with DAPI (Invitrogen). Images were taken on Zeiss LSM 880 Airyscan confocal microscope equipped with Plan-Apochromat 63X objective (numerical aperture [NA] 1.4), C-Apochromat 40X objective (NA 1.2), or Plan-Apochromat 20X objective (NA 0.8). Images were acquired from fixed samples at room temperature using the Zen imaging software (Zeiss), followed by processing and quantification of fluorescence intensity and colocalization (Pearson’s coefficient) using ImageJ (NIH). The following primary antibodies were used for immunocytochemistry: ANP (Millipore, AB5490, 1:100), GM130 (BD Biosciences, 610822, 1:100), myc (Millipore, 05-419, 1:200), zDHHC9 (St John's Laboratory, STJ92709, 1:200), α-actinin (Sigma, A78118, 1:1,000), and Integrin-β1 (Millipore, MAB1997, 1:200).
Cryosectioning and immunohistochemistry (IHC) were performed as described previously.62 Briefly, atria were excised from mice and fixed in 4% paraformaldehyde for 4 hours, after which atria were incubated in 30% sucrose overnight. The tissue was embedded in O.C.T (Tissue-Tek), frozen at –80 °C, and then cryosectioned. Antigen retrieval was performed by incubating cryosections in 100 mM glycine at room temperature for 10 minutes, followed by 0.05% SDS at 50 °C for 30 minutes. Cryosections were blocked in IHC buffer (PBS, 5% goat serum, 1% BSA, 1% glycine, and 0.2% Triton X-100) at room temperature for 1 hour and then incubated with anti-ANP (1:100 in IHC buffer) at 4 °C overnight. Cryosections were then washed and incubated with Alexa Fluor 488 conjugated goat anti-rabbit secondary antibody (1:500 in IHC buffer) and wheat germ agglutinin (Invitrogen, 0.5 μg/mL) for 1 hour at room temperature. After, sections were mounted with ProLong Gold Antifade Mountant with DAPI and imaged on Zeiss LSM 880 confocal microscope as described above.
Acyl resin-assisted capture and s-acyl proteomics
S-acylated or palmitoylated proteins were purified from ventricles or cultured cells using Acyl Resin-Assisted Capture (Acyl-RAC) as described previously.63 Cell or tissue extracts were generated in RIPA buffer, as previously described, and diluted with 100 mM HEPES pH 7.4 with 1 mM EDTA and SDS to a final concentration of 2.5% for blocking of free thiols by incubation with 0.3% S-methyl methanethiosulfonate (TCI, #M1382) for 20 minutes at 42 °C. Samples were then acetone precipitated, resolubilized in 100 mM HEPES pH 7.4, 1 mM EDTA, 1% SDS and diluted to equal concentrations, and 450 μL thiol-blocked lysate was incubated with 200 μL 100 mM HEPES pH 7.4, 1 mM EDTA, 50 μL thiopropyl sepharose 6B (Sigma, #T8387), and 300 μL of 1M hydroxylamine pH 7.4 or 150 mM Tris•HCl pH 7.4 (negative control) for 3 hours at room temperature. Thiopropyl sepharose with bound (previously S-acylated) proteins was then washed 3 times with 100 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.3% SDS and eluted by boiling in 5X Laemmli buffer for SDS-PAGE. Palmitoylation levels of target proteins were quantified by normalizing to input levels. For proteomics, proteins eluted from thiopropyl sepharose were separated by SDS-PAGE and stained with the ProteoSilver Silver Stain Kit (Sigma, #PROTSIL) according to the manufacturer's protocol, and the region from ∼90-200 KDa was excised and subjected to trypsin digestion and nano-LC-MS/MS at the University of Michigan Proteomics Resource Facility.
Primary cardiomyocyte isolation and cell culture
Rat neonatal ventricular cardiomyocytes (RNCMs) were isolated from P1-P3 Sprague-Dawley rat pups using the Neonatal Cardiomyocyte Isolation System (Worthington Biochemical), as described previously.57 RNCMs were cultured in Medium 199 (M199, Corning), supplemented with 20% bovine growth serum (BGS) (Hyclone #SH3054103) and 1% penicillin-streptomycin (Hyclone #SV30010). Cells were allowed to attach for 24 hours and transduced with adenovirus for 6 hours in serum-free Dulbecco's Modified Eagle Medium (DMEM) (Corning) and then given fresh DMEM media containing 2% BGS. RNCMs were transfected with Zdhhc9 siRNA (ON-TARGETplus, Horizon Discovery #L-058018-01-0005) or control siRNA (Santa Cruz, sc-37007) with Lipofectamine RNAiMax Reagent (Invitrogen, #56532), according to the manufacturer's instructions.
Adult mouse ventricular cardiomyocytes (AMVMs) were isolated from adult mouse hearts using the Langendorff-free method64 and cultured in M199, supplemented with 5% BGS, 1% insulin, transferrin, selenium (ITS, Sigma, I3146), 10 mM butanedione monoxime (BDM) (Sigma, B0753), and 1% chemically defined lipid concentrate (CD lipid, Thermo Fisher, 11905-031). Cells were allowed to attach for 4 hours and transduced with adenovirus for 6 hours in serum-free DMEM media and then given fresh 5% BGS-containing M199 media.
Secretion assays, Rab3-GTP pull-down assays, and coimmunoprecipitation
For secretion assays, 48 hours after adenovirus transduction or siRNA transfection, cardiomyocytes were washed with 1x PBS and cultured in serum-free DMEM medium for 24 hours. For zDHHC9 knockdown studies, cells were cultured with 10 μM phenylephrine (PE) (Sigma, P6126) or equivalent volume of 1x PBS as a control in serum-free DMEM medium for 24 hours. Culture medium was harvested, spun down at 500 × g at 4 °C for 5 minutes to pellet cell debris, and concentrated in Amicon Ultra centrifugal filters (Millipore, UFC8010) by centrifugation at 5,000 × g for 30 min at 4 °C, after which Laemmli buffer was added to 40 μL of concentrated medium, boiled at 95 oC for 5 minutes, and subjected to SDS-PAGE and immunoblotting. Intracellular lysates were harvested for Western blotting as described above.
For GST pull-down assays, 10 μg of GST as a control or the GST-Rim1 (1-200) fusion protein was incubated with glutathione sepharose 4B (GE Healthcare, #17-0756-05) for 1 hour at 4 °C. The beads were spun down at 2000 rpm, and supernatant was carefully removed. The beads were washed 5 times in washing buffer (50 mM Tris pH 7.5, 0.5% triton X-100, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT) with 1 μg/mL aprotinin, 1 μg/mL leupeptin, and 0.1 mM AEBSF. Mouse heart, RNCM, or AD-293 cell extracts in Mg2+ lysis buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Ipegal CA-630, 10 mM MgCl2, 1 mM EDTA, 2% glycerol; Millipore, #20-168) with protease inhibitors (1 mM AEBSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin) were incubated with GST fusion protein-bound glutathione sepharose beads in lysis buffer overnight at 4 °C, followed by 4 washes in wash buffer and elution from glutathione sepharose for SDS-PAGE by boiling in Laemmli buffer. Rab3a-GTP levels were quantified and normalized to Gapdh protein levels.
For coimmunoprecipitation (co-IP) assays, AD-293 cells (Agilent) were cotransfected with pacAd5-myc-Rab3gap1 and pacAd5-GFP-Rab3a-Q81L plasmids along with the empty pShuttle-CMV vector, pShuttle-Zdhhc9, or the pShuttle-Zdhhc9-DHHS plasmids described here, using PEI MAX (Transfection Grade Linear Polyethylenimine Hydrochloride, Polysciences) according to the manufacturer’s protocol. The pacAd5-GFP-Rab3a-Q81L plasmid was generated by subcloning GFP-Rab3a-Q81L (Addgene #49582) cDNA into the pacAd5-CMV-K-N-pA vector (Cell Biolabs), as described above. After 48 hours, AD-293 cells were lysed in NP40 lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40) with protease inhibitors (1 mM AEBSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin), clarified by centrifugation at 12,000 rpm for 10 minutes at 4°C, and immunoprecipitated with anti-GFP Magnetic Beads (MBL International, D153-11) or anti-myc antibody (Millipore, 05-419) coupled to Protein G Dynabeads (Invitrogen, 10003D) overnight at 4 °C. Immunoprecipitates were then washed 5 times in wash buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% NP-40), and bound proteins eluted for SDS-PAGE by boiling in Laemmli buffer at 95 °C for 5 minutes.
Click chemistry
AD-293 cells in DMEM with 2% BGS were transfected in 6-well plates using PEI MAX (Polysciences), trypsinized, and replated onto individual 6-cm plates the next day, and on the following day were metabolically labeled for 5 to 6 hours in serum-free DMEM with 1% fatty acid-free BSA (Sigma, A6003), with or without 100 μM palmitic acid alkyne (alk-14, Cayman Chemicals, #13266). Cells were then harvested in lysis buffer (25 mM Tris buffer pH 7.4, 150 mM NaCl, 2% SDS, 1% NP-40) with protease inhibitors (1 mM AEBSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin) and Pierce Universal Nuclease (Thermo Scientific, 88701), protein extracts clarified by centrifuging at 14,000 rpm for 15 minutes at 4°C, and lysates diluted in IP wash buffer (25 mM Tris buffer pH 7.4, 150 mM NaCl, 0.1% SDS, 0.2% NP-40) with protease inhibitors for immunoprecipitation overnight at 4 oC, with anti-c-myc magnetic beads (Thermo Scientific, 88842). Immunoprecipitates were washed twice with IP wash buffer and resuspended in 38.2 μL IP wash buffer followed by addition of 7.2 μL 5X click chemistry reaction mixture, containing for each reaction: 2.4 μL of 10mM TBTA (PrimeTech, in DMSO), 2 μL of 40 mM CuSO4 (Sigma, 61230), 2 μL of 40 mM TCEP HCl (Thermo Scientific, PG82089), and 0.8 μL of 100μM IRDye 800CW Azide (LiCor Biosciences, 929-60000, in DMSO). Click chemistry reactions were then incubated with rotation in the dark at room temperature for 30 minutes, followed by addition of 8 μL of 5x Laemmli buffer and boiling at 95 oC for 5 minutes to elute immunoprecipitate-click reaction complexes for SDS-PAGE and immunoblotting as described above.
Quantitative real-time polymerase chain reaction (PCR)
RNA was isolated from atria or ventricles using TRIzol reagent (Invitrogen, 15596026), cDNA synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, 4368814), and qPCR performed on a QuantStudio 7 Flex (Applied Biosystems) with Power SYBR Green Master Mix (Applied Biosystems, 4367659) and gene-specific primers in a total reaction volume of 15 μL. The primer sequences were as follows: Nppa For 5′-TTCTTCCTCGTCTTGGCCTTT-3′, Rev 5′-GACCTCATCTTCTACCGGCATCT-3′; Golga7 For 5′-GCTTCCGTGTAACTTCAA-3′, Rev 5′-CTGCTTCATTCATTCCTTAT-3′; Gapdh For 5′- TGCCCCCATGTTTGTGATG-3′; Rev 5′-TGTGGTCATGAGCCCTTCC-3′. The delta-delta Ct method was used to determine the relative target gene expression and normalized to Gapdh levels.
ANP ELISA assay
Blood samples were collected from 5- to 7-week-old tTA and DTgZdhhc9 mice by cardiac puncture and placed on ice for 30 minutes. Samples were centrifuged at 1,000 x g for 10 minutes, and the resultant supernatant was retrieved as the serum sample and subjected to commercially available mouse ANP ELISA kit (Raybiotech, EIAM-ANP-1) according to the manufacturer’s instructions.
Transmission electron microscopy and immunogold labeling
Transmission electron microscopy was performed to evaluate cardiomyocyte ultrastructure in atrial tissue, as we have done previously.50,51,57 Briefly, atria were fixed in 3% paraformaldehyde/3% glutaraldehyde in 0.1 M sodium cacodylate buffer, followed by embedding in epoxy resin, sectioning, and counterstaining. For immunogold labeling, atrial samples were fixed in 3% paraformaldehyde/3% glutaraldehyde, processed, and mounted on nickel grids blocked in 2% fish-skin gelatin and 5% BSA (Electron Microscopy Sciences) for 30 minutes, and incubated with Rab3a antibody (LifeSpan BioSciences, LS-B14834, 1:5) in 2% fish-skin gelatin and 2% BSA overnight. Grids were washed in 0.5% BSA in 0.1X PBS and then incubated with colloidal gold-conjugated donkey antimouse (ultra-small, Electron Microscopy Sciences, 1:10) in 2% fish-skin gelatin and 2% BSA solution for 1.5 hours at room temperature. Grids were washed, followed by antibody fixation in 0.5% glutaraldehyde and contrast enhancement with uranyl acetate. Imaging was performed on a Jeol JEM-1400 Plus transmission electron microscope at 80kV, using a 2k CMOS camera at the University of Michigan Microscopy Core.
Statistics
All statistical analyses were performed using GraphPad Prism version 9.3.1. The Shapiro-Wilk’s test was performed to test normality of data. Normally distributed data from 2 groups were tested for statistical significance, using Student’s t-test with post hoc testing using Welch’s correction. Normally distributed data from multiple groups were tested for statistical significance, using 1-way analysis of variance (ANOVA) with post hoc testing, using Tukey’s test. Survival analysis was performed by the Kaplan-Meier method. Pearson’s coefficient values for quantification of colocalization were generated using ImageJ (NIH) and Student’s t-test or 1-way ANOVA was used to determine statistical significance among groups. Data are presented as mean ± standard deviation (SD). A value of P < 0.05 was considered statistically significant.
Results
Cardiomyocyte-specific overexpression of zDHHC9 causes dilated cardiomyopathy
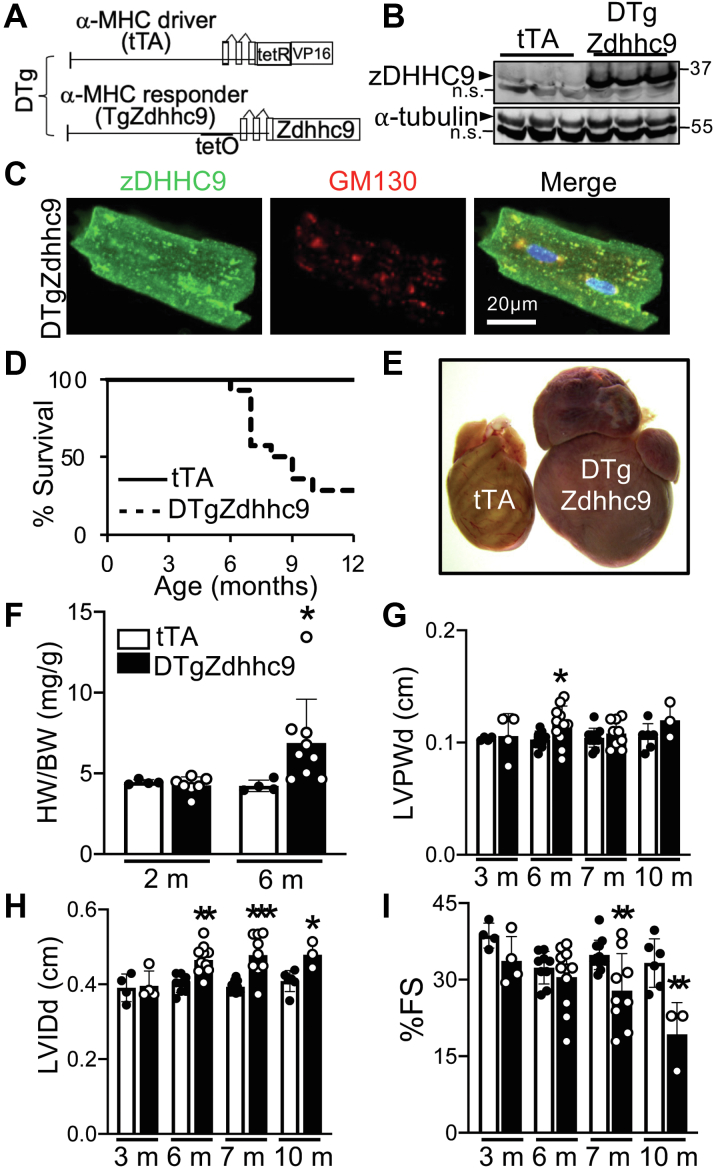
The Golgi-localized S-acyl transferase, zDHHC9, has been shown to palmitoylate H-Ras and N-Ras to induce their trafficking to sites of action at the plasma membrane.11,12 In the heart, activation of Ras has been implicated in cardiac hypertrophy and remodeling.65,66 To study functions of palmitoylation in vivo, we generated mice with cardiomyocyte-specific overexpression of zDHHC9 using the α-myosin heavy chain (α-MHC) promoter-driven bigenic tet-off transgene system49 (Figure 1A) in which zDHHC9 expression is induced in hearts of double-transgenic (DTg) mice containing both the tetracycline transactivator (tTA) and Zdhhc9 cDNA transgenes (Figure 1B, Supplemental Figure 1A). Cardiomyocytes from DTgZdhhc9 mice exhibit localization of zDHHC9 protein predominantly at the Golgi apparatus, similar to tTA controls, although DTg myocytes do display some zDHHC9 protein that extends beyond the cis-Golgi marker, GM130 (Figure 1C, Supplemental Figure 1B). Expression of Golga7 mRNA that encodes the zDHHC9 cofactor, GPC16 (Golga7), was not altered in hearts overexpressing zDHHC9 (Supplemental Figure 1C). Overexpression of zDHHC9 in cardiac myocytes resulted in lethality at approximately 8 months of age (Figure 1D), caused by dilated cardiomyopathy (Figures 1E to 1I). Gross morphology revealed substantial enlargement and dilation of atria and ventricles (Figure 1E) and increased heart weight-to-body weight ratios (HW-BW) in DTgZdhhc9 mice at 6 months of age (Figure 1F). Echocardiographic analyses revealed a transient increase in left ventricular wall thickness at 6 months of age (Figure 1G, Supplemental Table 1) and, more prominently, significant left ventricular dilation (Figure 1H) that were followed by impaired systolic function and progressive cardiomyopathy in DTgZdhhc9 mice by 7 months of age (Figures 1H and 1I, Supplemental Table 1). Administration of doxycycline to transgenic mice until weaning to elicit transgene expression exclusively in the adult heart resulted in a nearly indistinguishable dilated cardiomyopathy phenotype (data not shown), suggesting cardiac maladaptation in response to enhanced zDHHC9 expression occurs because of its functions in the adult heart. Thus, enhanced cardiomyocyte zDHHC9 activity promotes dilated cardiomyopathy and cardiac decompensation.
Figure 1.
Overexpression of zDHHC9 in Mouse Cardiomyocytes Results in Cardiomyopathy
(A) Schematic of bigenic tet-off system used to overexpress zDHHC9 in the heart. (B) Immunoblots showing expression of zDHHC9 in DTgZdhhc9 hearts compared with tTA controls. n.s. = nonspecific band. (C) Immunostaining for zDHHC9 (green) and GM130 (Golgi marker, red) in adult cardiomyocytes isolated from DTgZdhhc9 mice. (D) Kaplan-Meier survival curve showing mortality in transgenic mice with cardiomyocyte-specific expression of zDHHC9. n = 10 for tTA, n = 14 for DTgZdhhc9. (E) Gross cardiac morphology of tTA control compared with DTgZdhhc9 littermate that died at 6 months of age. (F) Heart weight to body weight ratios at 2 months and 6 months of age. n = 4-9. (G to I) Echocardiographic measurement of (G) left ventricular (LV) posterior-wall thickness (LVPWd) and (H) LV internal diameter (LVIDd) during diastole and (I) % fractional shortening (FS) at the indicated age. m = months. n = 3-13. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 compared with tTA controls, Student's t-test with Welch’s correction. Data are presented as mean ± SD.
Identification of Rab3gap1 as a novel substrate of zDHHC9
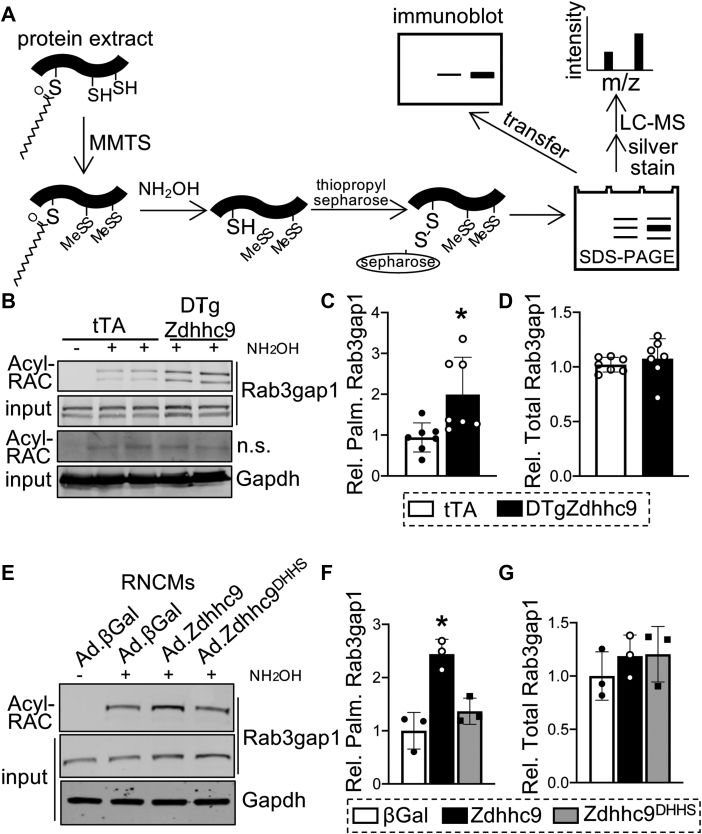
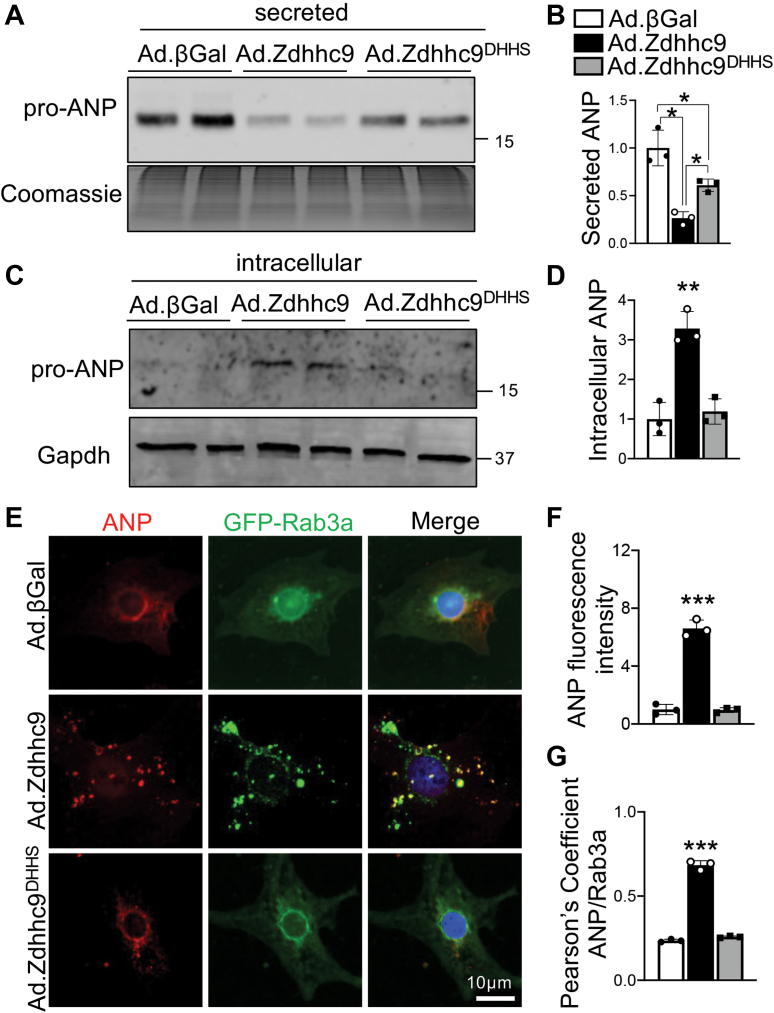
To delineate whether zDHHC9 modulates Ras protein palmitoylation in the heart, we affinity purified palmitoylated proteins from DTgZdhhc9 and control tTA hearts by Acyl Resin-Assisted Capture (Acyl-RAC),63 followed by SDS-PAGE and immunoblotting (Figure 2A). Surprisingly, palmitoylation of H-Ras and N-Ras were unchanged in DTgZdhhc9 hearts relative to tTA controls (Supplemental Figures 2A and 2B), indicating palmitoylation of Ras proteins is not responsible for adverse cardiac remodeling in DTgZdhhc9 mice. Likewise, a survey of known palmitoylated proteins, including Gα12, junctophillin-2 (Jph2), and phosphatidylinositol 4-kinase type 2-alpha (Pi4k2α), revealed no increase in palmitoylation in DTgZdhhc9 hearts compared with tTA controls (Supplemental Figure 2C). Next, we sought to identify novel cardiac substrates of zDHHC9 by employing an unbiased proteomic approach. Palmitoylated proteins were purified from cardiac lysates of tTA and DTgZdhhc9 mice at 2 months of age by Acyl-RAC, followed by SDS-PAGE, silver staining, and trypsin digestion for mass spectrometry sequencing (Figure 2A, Supplemental Figure 3). The regions spanning ∼90-200 kDa from the silver-stained gel showed multiple bands with enhanced signal in DTgZdhhc9 compared with tTA, and this region was excised for sequencing by nano-LC-MS/MS (Supplemental Figure 3A). Rab3 GTPase-activating protein catalytic subunit (Rab3gap1), which was previously identified by S-acyl proteomics in mouse brain,67 was among the proteins with the greatest elevation in palmitoylation in DTgZdhhc9 hearts compared with tTA controls as detected by mass spectrometry (Supplemental Figure 3B). Acyl-RAC followed by immunoblotting revealed a significant increase in palmitoylation of Rab3gap1 in DTgZdhhc9 hearts compared with tTA controls (Figures 2B to 2D), confirming induction of Rab3gap1 palmitoylation by zDHHC9 in the heart. Further, we isolated rat neonatal cardiomyocytes (RNCMs) and transduced them with adenoviruses to express wildtype zDHHC9 (Ad.Zdhhc9), an enzymatically dead mutant zDHHC9 (Ad.Zdhhc9DHHS), or β-galactosidase (Ad.βGal), as a control. zDHHC9 protein levels were increased approximately 4-fold by adenoviral transduction of Ad.Zdhhc9 and Ad.Zdhhc9DHHS compared with Ad.βGal-transduced control myocytes (Supplemental Figures 4A and 4B). Similar to transgenic hearts, zDHHC9 overexpressing RNCMs exhibited enhanced palmitoylation of Rab3gap1 compared with βGal controls and RNCMs expressing the transferase-deficient zDHHC9 mutant (Figures 2E to 2G).
Figure 2.
zDHHC9 Activity Enhances Palmitoylation of Rab3gap1 in the Heart
(A) Schematic showing procedure for Acyl resin-assisted capture (Acyl-RAC) to purify cysteine palmitoylated proteins for Western blotting or mass spectrometry (LC-MS). MMTS = methyl methanethiosulfonate. Adapted from Forrester et al. 2011. (B) Rab3gap1 palmitoylation in zDHHC9 transgenic hearts at 2 months of age. Acyl-RAC followed by immunoblotting to detect palmitoylated Rab3gap1 in DTgZdhhc9 hearts compared with tTA controls. n.s. = nonspecific band. (C,D) Quantification of (C) palmitoylated and (D) total Rab3gap1. ∗P < 0.05, compared with controls, Student's t-test with Welch’s correction. (E) Acyl-RAC followed by immunoblotting to detect palmitoylated Rab3gap1 in rat neonatal cardiomyocytes (RNCMs) transduced with adenoviruses to express wildtype zDHHC9 (Ad.Zdhhc9), enzymatically dead mutant zDHHC9 (Ad.Zdhhc9DHHS), or β-galactosidase (Ad.βGal) as a control and quantification of (F) palmitoylated and (G) total Rab3gap1. ∗P < 0.05, 1-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. Data are presented as mean ± SD.
To further confirm that zDHHC9 acyltransferase activity mediates palmitoylation of Rab3gap1, AD-293 cells were transfected with myc-Rab3gap1 and either zDHHC9, the transferase-deficient zDHHC9DHHS mutant, or GST as a control, and metabolically labeled with palmitic acid alkyne (alk-14). Following metabolic labeling with the clickable bioorthogonal palmitate analogue, alk-14, cells were harvested, and Rab3gap1 was immunoprecipitated from lysates, followed by a Cu(I)-catalyzed azide-alkyne cycloaddition click chemistry reaction to conjugate palmitoylated Rab3gap1 to IR800 for detection (Supplemental Figure 5A). zDHHC9-overexpressing AD-293 cells showed elevated palmitoylation of myc-Rab3gap1 compared with the controls (Supplemental Figure 5B), confirming that zDHHC9 S-acyltransferase activity promotes palmitoylation of Rab3gap1. Collectively, these data identify Rab3gap1 as a novel substrate of zDHHC9.
zDHHC9-mediated palmitoylation regulates Rab3gap1 localization and Rab3a activity in cardiomyocytes
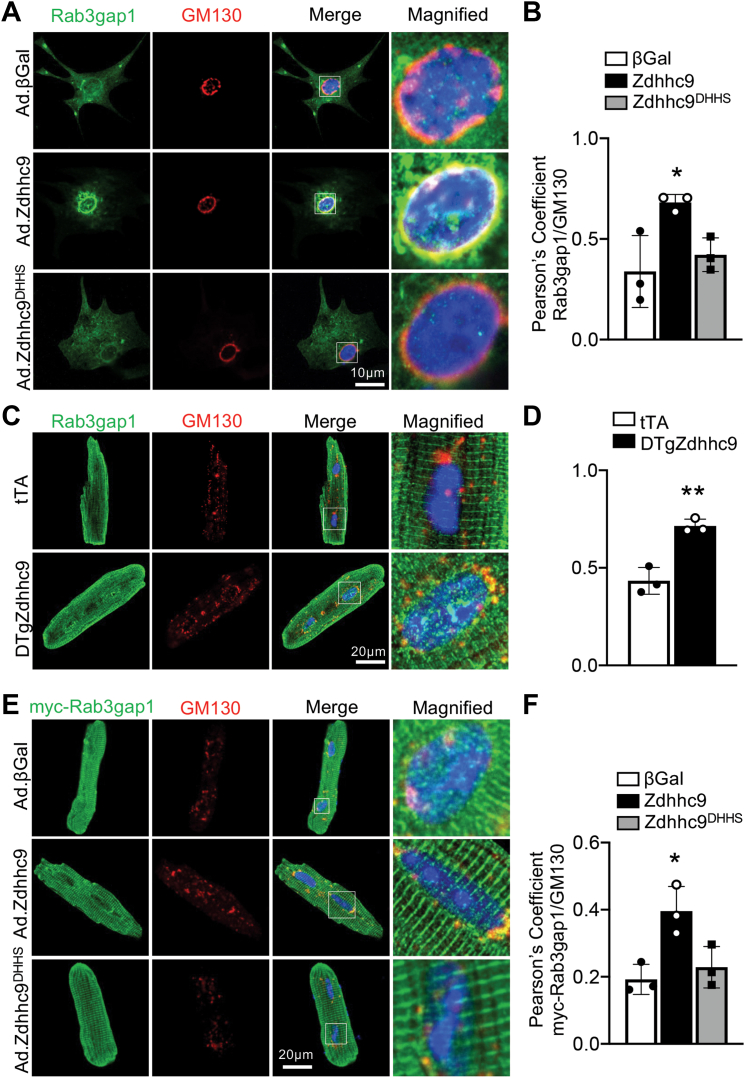
To determine whether subcellular localization of Rab3gap1 is regulated by zDHHC9-mediated palmitoylation, we transduced RNCMs with Ad.βGal, Ad.Zdhhc9, or Ad.Zdhhc9DHHS and performed coimmunostaining for endogenous Rab3gap1 and GM130, a Golgi-specific marker. Interestingly, Ad.Zdhhc9 RNCMs showed Golgi and vesicular localization of Rab3gap1, whereas Ad.βGal and Ad.Zdhhc9DHHS RNCMs showed a more diffuse expression pattern of Rab3gap1, predominantly in the cytoplasm (Figures 3A and 3B). Likewise, immunostaining of adult cardiomyocytes isolated from DTgZdhhc9 hearts showed increased colocalization of endogenous Rab3gap1 with GM130 in comparison with tTA cardiomyocytes (Figures 3C and 3D). Next, we cotransduced Ad.βGal, Ad.Zdhhc9, or Ad.Zdhhc9DHHS in adult mouse ventricular myocytes (AMVMs) with adenovirus encoding myc-tagged Rab3gap1 (Ad.Rab3gap1) to elucidate impacts of zDHHC9 activity on Rab3gap1 localization. Immunofluorescent images showed a striated pattern for Rab3gap1 in all groups, similar to the localization pattern of endogenous Rab3gap1 in Figure 3C, but also a significant distribution at the Golgi specifically in zDHHC9-overexpressing adult cardiomyocytes, as evidenced by the increased colocalization of Rab3gap1 with GM130 in Ad.Zdhhc9 myocytes compared with Ad.βGal and Ad.Zdhhc9DHHS myocytes (Figures 3E and 3F). Surprisingly, immunocytochemistry for endogenous and adenovirally overexpressed Rab3gap1 showed significant expression in a striated pattern that localized mostly in between Z-discs (Supplemental Figures 6A and 6C) and also exhibited partial colocalization with integrin-β1 (Supplemental Figure 6B and 6D), suggesting Rab3gap1 may also function at the M-line or costamere, respectively, in addition to its association with the Golgi and DCVs. Most notably, however, zDHHC9 activity promoted localization of Rab3gap1 at the Golgi (Figures 3A to 3F). These findings suggest zDHHC9-regulated palmitoylation of Rab3gap1 retains it at the Golgi or delays its movement to the cell periphery, which could spatially restrict access of Rab3gap1 to its substrate, Rab3-GTP, that physically associates with exocytic vesicles that dock and fuse to the plasma membrane.36,39,40
Figure 3.
zDHHC9 Regulates Rab3gap1 Localization in Cardiomyocytes
(A) Representative images of RNCMs transduced with adenoviruses for wildtype zDHHC9 (Ad.Zdhhc9), enzymatically dead zDHHC9 (Zdhhc9DHHS), or βGal control and immunostained for endogenous Rab3gap1 (green) and GM130 (Golgi marker, red). (B) Quantification of Pearson’s correlation coefficients for colocalization of Rab3gap1 and GM130 in RNCMs. ∗P < 0.05, 1-way ANOVA with Tukey’s multiple comparisons test. (C) Representative images of adult cardiomyocytes from tTA and DTgZdhhc9 hearts, immunostained for endogenous Rab3gap1 (green) and GM130 (Golgi marker, red). (D) Quantification of Pearson’s correlation coefficients for colocalization of Rab3gap1 and GM130 in cardiomyocytes. ∗∗P < 0.01, compared with tTA controls, Student's t-test with Welch’s correction. (E) Representative images of adult mouse ventricular myocytes (AMVMs) transduced with adenovirus to express myc-tagged wildtype Rab3gap1 and wildtype zDHHC9 (Ad.Zdhhc9), enzymatically dead zDHHC9 (Zdhhc9DHHS), or βGal control followed by immunostaining for myc (Rab3gap1, green) and GM130 (Golgi marker, red). (F) Quantification of Pearson’s correlation coefficients for colocalization of myc-Rab3gap1 and GM130 in AMVMs. ∗P < 0.05, 1-way ANOVA with Tukey’s multiple comparisons test. n = 3 independent experiments with 60 to 90 cells analyzed for each experiment. Nuclei were stained blue with DAPI in A, C, and E. Data are presented as mean ± SD. Abbreviations as in Figures 1 and 2.
Rab3gap1 catalyzes hydrolysis of GTP on Rab3 family proteins to terminate their activity, thereby enabling dissociation of Rab3 from secretory vesicles and release of vesicle contents by exocytosis.38, 39, 40 As such, loss of Rab3gap1 results in increased Rab3a-GTP levels in mouse brain but blunted glutamate release by cerebrocortical neurons.45 To elucidate whether zDHHC9-regulated palmitoylation of Rab3gap1 modulates Rab3a activity in cardiomyocytes, we performed pull-down assays using a GST fusion protein containing amino acids 1-200 of the Rab3 effector protein, Rim1, that specifically binds active, GTP-bound Rab3A (GST-Rim11-200)45,68 (Supplemental Figure 7A). Intriguingly, Rab3a-GTP levels were increased in DTgZdhhc9 hearts compared with tTA control hearts (Figures 4A and 4B), although total Rab3a levels were unchanged (Figures 4A and 4C). To further investigate Rab3a activity in vitro, we cotransduced RNCMs with Ad.βGal, Ad.Zdhhc9, or Ad.Zdhhc9DHHS and adenovirus-expressing GFP-tagged Rab3a (Ad.GFP-Rab3a) and subjected cell lysates to Rab3a-GTP pulldown assays. Overexpression of zDHHC9 in cardiomyocytes resulted in elevated Rab3a-GTP levels in comparison with βGal controls and cardiomyocytes expressing the transferase-deficient zDHHC9DHHS mutant (Figures 4D and 4E) and also appeared to stabilize Rab3a, resulting in greater total Rab3a protein expression (Figures 4D and 4F). Likewise, overexpression of zDHHC9 in HEK293AD cells augmented Rab3a-GTP levels relative to cells transfected with Zdhhc9DHHS or empty vector plasmids (Supplemental Figures 7B to 7D), indicating that zDHHC9 S-acyltransferase activity promotes GTP-loading on Rab3a.
Figure 4.
zDHHC9 Promotes Rab3a GTP Loading and Impairs Interaction of Rab3gap1 With Rab3a-GTP
(A-C) Rab3a-GTP levels in zDHHC9 transgenic hearts. (A) Rab3a-GTP was affinity purified from cardiac lysates with GST-Rim1(1-200) followed by immunoblotting. Quantification of (B) Rab3a-GTP and (C) total Rab3a levels. ∗P < 0.05 compared with tTA controls, Student's t-test with Welch’s correction. (D to F) Rab3a-GTP levels in RNCMs transduced with adenovirus to express GFP-Rab3a and wildtype zDHHC9 (Ad.Zdhhc9), enzymatically dead zDHHC9 (Zdhhc9DHHS), or βGal control. (D) Rab3a-GTP was affinity purified from the indicated RNCM lysates with GST-Rim1(1-200) followed by immunoblotting. Quantification of (E) Rab3a-GTP and (F) total Rab3a levels. ∗∗P < 0.01, ∗∗∗P < 0.001, 1-way ANOVA with Tukey’s multiple comparisons test. (G to J) Interaction of Rab3a-GTP with Rab3gap1. AD-293 cells were cotransfected with the GFP-Rab3a-Q81L mutant, myc-tagged Rab3gap1, and plasmids encoding empty vector (pShuttle), zDHHC9, or the zDHHC9DHHS transferase-dead mutant. (G) Immunoprecipitation of Rab3aQ81L with anti-GFP or (H) Rab3gap1 with anti-myc followed by immunoblotting with the indicated antibodies. (I) Quantification of relative levels of Rab3gap1 coimmunoprecipitated with Rab3Q81L (anti-GFP), and (J) relative levels of Rab3Q81L coimmunoprecipitated with Rab3gap1 (anti-myc) as in G and H, respectively. n = 3 independent experiments. ∗P < 0.05, ∗∗P < 0.01 1-way ANOVA with Tukey’s multiple comparisons test. Data are presented as mean ± SD. Abbreviations as in Figures 1 and 2.
zDHHC9-mediated palmitoylation of Rab3gap1 restricts it at the Golgi apparatus (Figure 3), where it may be unable to access and inactivate Rab3a that is associated with secretory vesicles actively participating in exocytosis and regulated hormone secretion. To test this, we performed coimmunoprecipitation studies in which HEK293AD cells were cotransfected with the constitutively GTP-bound GFP-Rab3aQ81L mutant (Rab3gap1 substrate), myc-tagged Rab3gap1, and plasmids encoding empty vector, zDHHC9, or the zDHHC9DHHS transferase-dead mutant. Strikingly, overexpression of zDHHC9, but not the enzymatically dead mutant, significantly impaired the interaction between Rab3aQ81L and Rab3gap1 (Figures 4G to 4J), suggesting that zDHHC9 spatially segregates Rab3gap1 at the Golgi and inhibits its ability to access and inactivate Rab3a on secretory vesicles.
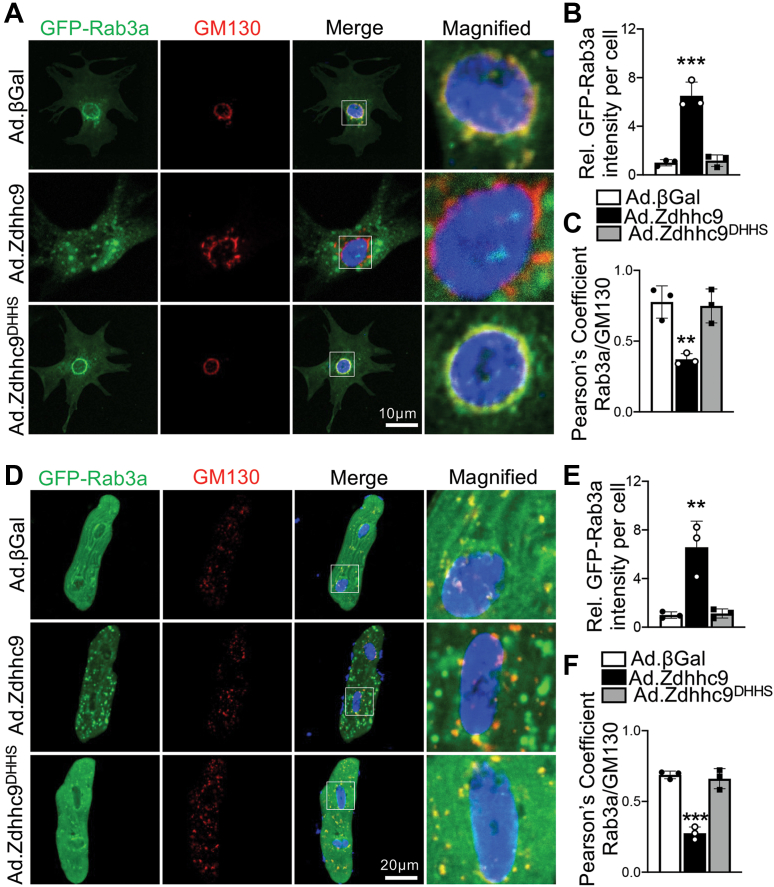
To further assess impacts of zDHHC9 activity on Rab3a function in cardiomyocytes, we used confocal microscopy to visualize GFP-Rab3a in RNCMs cotransduced with Ad.βGal, Ad.Zdhhc9, or Ad.Zdhhc9DHHS. Surprisingly, Rab3a was expressed in large puncta in vesicular pattern at the cell periphery in zDHHC9-overexpressing myocytes, whereas Rab3a was found predominantly at the Golgi apparatus in βGal control and zDHHC9DHHS-expressing cardiomyocytes (Figures 5A to 5C). Similarly, AMVMs overexpressing zDHHC9 exhibited dramatic expansion and localization of Rab3a-positive vesicles at the cardiomyocyte periphery in contrast to zDHHC9DHHS and βGal controls with Rab3a expression almost exclusively colocalizing with perinuclear and intermyofibrillar Golgi membranes (Figures 5D to 5F). zDHHC9 overexpression did not increase palmitoylation of Rab3a (Supplemental Figure 8), indicating the remarkable effects of zDHHC9 on Rab3a GTP-loading and vesicular expansion occur indirectly, likely downstream of Rab3gap1 palmitoylation. Moreover, palmitoylation of Rab27b, which also regulates exocytosis,29 was unchanged in DTgZdhhc9 hearts (Supplemental Figure 8). Taken together, these results suggest that zDHHC9 activity enhances Rab3a-GTP levels by impinging upon Rab3gap1-regulated GTP hydrolysis on Rab3a and promotes expansion and movement of Rab3a-positive secretory vesicles away from the Golgi and toward the sarcolemma.
Figure 5.
zDHHC9 Overexpression Enhances Accumulation of Rab3a-Positive Vesicles at the Cardiomyocyte Periphery
(A) Representative images of RNCMs transduced with adenovirus to express GFP-Rab3a and wildtype zDHHC9 (Ad.Zdhhc9), enzymatically dead zDHHC9 (Zdhhc9DHHS), or βGal control and immunostained for the Golgi marker GM130 (red). Quantification of (B) relative GFP-Rab3a fluorescence intensity and (C) Pearson’s correlation coefficients for colocalization of GFP-Rab3a with GM130 in RNCMs. (D) Representative images AMVMs transduced with adenovirus to express GFP-Rab3a and wildtype zDHHC9 (Ad.Zdhhc9), enzymatically dead zDHHC9 (Zdhhc9DHHS), or βGal control and immunostained for the Golgi marker GM130 (red). Quantification of (E) relative GFP-Rab3a fluorescence intensity and (F) Pearson’s correlation coefficients for colocalization of GFP-Rab3a with GM130 in AMVMs. Nuclei were stained with DAPI (blue) in A and D. ∗∗P < 0.01, ∗∗∗P < 0.001, 1-way ANOVA with Tukey’s multiple comparisons test. n = 3 independent experiments with 60 to 90 cells analyzed for each experiment. Data are presented as mean ± SD. Abbreviations as in Figures 1 and 2.
zDHHC9-mediated palmitoylation and Rab3a GTPase control atrial natriuretic peptide release
Rab3 proteins have well-characterized functions in regulated exocytosis and secretion of neuropeptides and neurotransmitters.35, 36, 37 Cardiomyocytes also function as endocrine cells, most notably through regulated secretion of natriuretic peptides, ANP and BNP, which is induced in cardiovascular disease and elicits cardioprotection through vasodilatory, natriuretic, and diuretic actions.21,23,24 Nonetheless, the Rab proteins functionally involved in secretion of natriuretic peptides in cardiomyocytes remain unknown. To determine whether zDHHC9-induced regulation of Rab3a modulates secretion of ANP by cardiomyocytes, we transduced RNCMs with Ad.βGal, Ad.Zdhhc9, or Ad.Zdhhc9DHHS and collected the culture supernatant and harvested intracellular protein lysates to immunoblot for secreted vs intracellular ANP, respectively. Western blot analyses revealed overexpression of zDHHC9 in cardiomyocytes substantially reduced secretion of ANP in an S-acyltransferase-dependent manner (Figures 6A and 6B). In line with this observation, we observed increased intracellular expression of ANP in zDHHC9 overexpressing cardiomyocytes compared with controls (Figures 6C and 6D), suggesting zDHHC9 activity impairs ANP secretion, resulting in its intracellular accumulation in cardiomyocytes. These data in zDHHC9 overexpressing cardiomyocytes are consistent with Rab3gap1 loss-of-function phenotype in which GTP-loading on Rab3a is increased but secretion is impaired.42,45
Figure 6.
zDHHC9 Overexpression Impairs Atrial Natriuretic Peptide Secretion in Cardiomyocytes
(A) Immunoblotting and (B) quantification of atrial natriuretic peptide (ANP) in culture media from RNCMs transduced with adenoviruses to express wildtype zDHHC9 (Ad.Zdhhc9), enzymatically dead zDHHC9 (Zdhhc9DHHS), or βGal control. The membrane was Coomassie stained as a loading control. ∗P < 0.05, 1-way ANOVA with Tukey’s multiple comparisons test. (C) Immunoblotting and (D) quantification of intracellular ANP in cell lysates from RNCMs transduced with the indicated adenoviruses. Gapdh was used as a loading control. ∗∗ P<0.01, 1-way ANOVA with Tukey’s multiple comparisons test. (E) Representative images of RNCMs transduced with adenovirus to express GFP-Rab3a and wildtype zDHHC9 (Ad.Zdhhc9), enzymatically dead zDHHC9 (Zdhhc9DHHS), or βGal control and immunostained for endogenous ANP (red). Nuclei were stained with DAPI (blue). (F-G) Quantification of (F) relative ANP fluorescent intensity and (G) Pearson’s correlation coefficients for colocalization of GFP-Rab3a with ANP. ∗∗∗P < 0.001, 1-way ANOVA with Tukey’s multiple comparisons test. n = 3 independent experiments with 60 to 90 cells analyzed for each experiment. Data are presented as mean ± SD. Abbreviations as in Figures 1 and 2.
We next sought to determine whether Rab3a is associated with ANP-containing secretory vesicles in cardiomyocytes. We cotransduced RNCMs with Ad.GFP-Rab3a and Ad.βGal, Ad.Zdhhc9, or Ad.Zdhhc9DHHS and immunostained for endogenous ANP. As expected, ANP was predominantly localized in a perinuclear pattern consistent with the Golgi apparatus in RNCMs69 expressing βGal or the transferase-dead zDHHC9 mutant (Figure 6E). Remarkably, in zDHHC9-overexpressing cardiomyocytes, ANP was localized in a punctate pattern that largely colocalized with Rab3a-positive vesicles (Figures 6E to 6G), suggesting that zDHHC9 promotes loading of ANP in Rab3a-containing secretory vesicles.
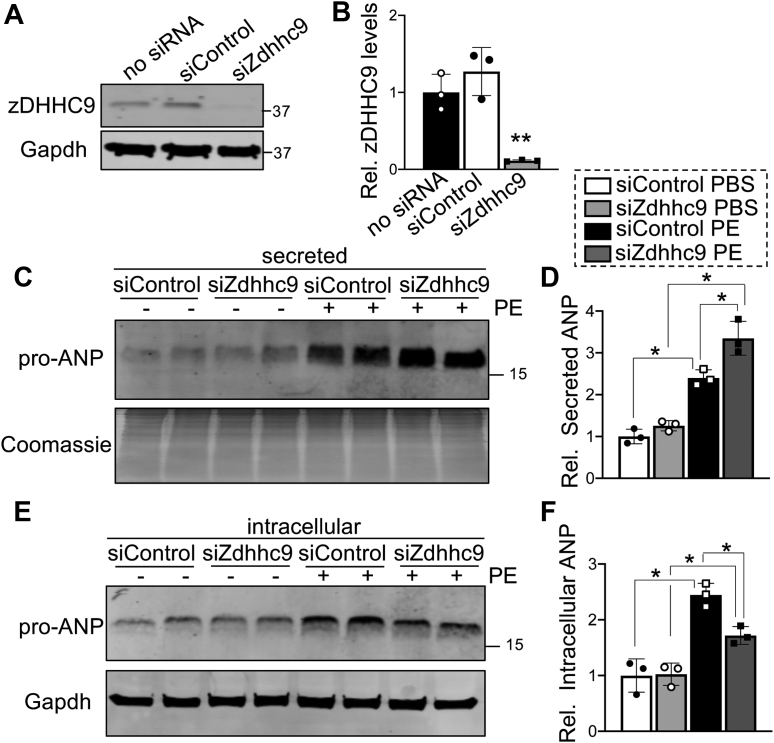
To further investigate regulation of cardiomyocyte ANP secretion by zDHHC9, we used siRNA-mediated knockdown of Zdhhc9 in RNCMs (Figures 7A and 7B) to evaluate the necessity of zDHHC9 activity for proper stimulated ANP secretion. Cardiomyocytes respond to pathologic stimuli including mechanical stretch and agonists that activate Gαq-coupled receptors, such as PE, endothelin-1, and angiotensin-II, by inducing de novo biosynthesis, processing, transport, and secretion of ANP.21,23,27 As expected, PE treatment resulted in elevation of both intracellular and secreted ANP in control conditions (Figures 7C to 7F). Notably, secretion assays revealed that knockdown of zDHHC9 augmented the PE-induced secretion of ANP by cardiomyocytes (Figures 7C and 7D). Moreover, depletion of zDHHC9 attenuated the accumulation of intracellular ANP in cardiomyocytes after PE treatment (Figures 7E and 7F), likely caused by more efficient exocytosis and release of ANP (Figures 7C and 7D). These data suggest that zDHHC9 deficiency enhances secretion of ANP from cardiomyocytes in response to pathologic stimulation.
Figure 7.
zDHHC9 Depletion Enhances Phenylephrine-induced Atrial Natriuretic Peptide Secretion
(A) Immunoblotting and (B) quantification of zDHHC9 protein expression in RNCMs transfected with control siRNA (siControl) or siRNA targeting Zdhhc9 (siZdhhc9). ∗∗P < 0.01, 1-way ANOVA with Tukey’s multiple comparisons test. (C) Immunoblotting and (D) quantification of atrial natriuretic peptide (ANP) levels in culture media from RNCMs transfected with siControl or siZdhhc9 and treated with PBS or phenylephrine (PE). The membrane was Coomassie stained as a loading control. (E) Immunoblotting and (F) quantification of intracellular ANP in cell lysates from RNCMs transfected with siControl or siZdhhc9 with or without PE treatment. Gapdh was used as a loading control. ∗P < 0.05, 1-way ANOVA with Tukey’s multiple comparisons test. Data are presented as mean ± SD. Abbreviations as in Figures 1 and 2.
To examine if the effect of zDHHC9 knockdown on PE-induced ANP secretion in cardiomyocytes is caused by modulation of Rab3gap1 palmitoylation, we treated RNCMs with PE or PBS as a control with or without siRNA-mediated knockdown of zDHHC9 expression and subjected cell lysates to Acyl-RAC followed by immunoblotting, to determine Rab3gap1 palmitoylation levels. Western blotting analyses revealed Rab3gap1 palmitoylation levels were unchanged under basal conditions, but although PE treatment increased Rab3gap1 palmitoylation in control siRNA RNCMs, this PE-induced increase in Rab3gap1 palmitoylation was significantly reduced by knockdown of zDHHC9 (Figures 8A and 8B), without significant changes to total Rab3gap1 levels (Figures 8A and 8C). These data indicate that zDHHC9 is responsible for PE-induced Rab3gap1 palmitoylation and suggest that zDHHC9 activity may be upregulated in response to PE.
Figure 8.
Knockdown of zDHHC9 Inhibits Phenylephrine-Induced Rab3gap1 Palmitoylation and Rab3a GTP Loading
(A) Acyl-RAC followed by immunoblotting to detect palmitoylated Rab3gap1 in lysates of RNCMs transfected with siControl or siZdhhc9 and treated with PBS or phenylephrine (PE). Quantification of (B) palmitoylated and (C) total Rab3gap1 protein. n = 3 independent experiments. ∗∗P < 0.01, 1-way ANOVA with Tukey’s multiple comparisons test. (D) Immunoblotting and quantification of (E) Rab3a-GTP and (F) total Rab3a levels in siControl- or siZdhhc9-transfected RNCMs with adenoviral expression of GFP-Rab3a and treatment with PBS or PE. n = 3 independent experiments. ∗P < 0.05, 1-way ANOVA with Tukey’s multiple comparisons test. (G) Representative images of siControl- and siZdhhc9-transfected RNCMs with adenoviral expression of GFP-Rab3a and treatment with PBS or PE followed by immunostaining for endogenous ANP (red). Nuclei were stained with DAPI (blue). (H) Quantification of GFP-Rab3a and (I) ANP relative fluorescence intensity. ∗P < 0.05, 1-way ANOVA with Tukey’s multiple comparisons test. n = 3 independent experiments with 60 to 90 cells analyzed for each experiment. Data are presented as mean ± SD. Abbreviations as in Figures 1 and 2.
To further probe this mechanism, we evaluated Rab3a activation in response to PE. PE treatment led to substantial elevation of total Rab3a and Rab3a-GTP levels in control RNCMs, which were both significantly attenuated by depletion of zDHHC9 (Figures 8D to 8F). Immunofluorescent imaging revealed that PE treatment elicited an increase in GFP-Rab3a puncta at the cell periphery, in conjunction with elevated ANP expression in a punctate pattern that predominantly colocalized with Rab3a (Figure 8G). Knockdown of zDHHC9 did not prevent formation of peripheral Rab3a vesicles or ANP colocalization with Rab3a puncta in myocytes in response to PE stimulation but did reduce the number and intensity of Rab3a-positive and ANP-containing vesicles in response to PE (Figures 8G to 8I), perhaps caused by more rapid flux through the secretory pathway or efficient exocytosis that promotes PE-stimulated ANP release (Figures 7C and 7D). These observations indicate zDHHC9 depletion inhibits Rab3gap1 palmitoylation in response to pathologic stimulation (Figures 8A and 8B) and could thereby promote more effective secretion of ANP by Rab3a-positive vesicles owing to more effective nucleotide cycling on Rab3a, in contrast to zDHHC9 overexpression that impairs Rab3a nucleotide cycling and ANP release.
To investigate the effects of Rab3gap1 GAP activity on ANP release, we generated adenovirus to express a GAP-deficient mutant of Rab3gap1 (Ad.Rab3gap1R728A), in which the essential arginine residue (R728) in the GAP domain is mutated to alanine to impair GAP activity.70 We transduced RNCMs with Ad.Rab3gap1, Ad.Rab3gap1R728A, or Ad.βGal, and evaluated localization of Rab3gap1, Rab3a vesicle formation, Rab3a GTP-loading, and ANP release. Localization of wildtype Rab3gap1 and Rab3gap1R728A in cardiomyocytes was similar, with no significant differences in association with the Golgi (Figure 9A and 9C), and expression of the Rab3gap1 GAP mutant did not significantly alter Rab3a localization or vesicle formation, with all groups showing predominantly Golgi localization of GFP-Rab3a (Figures 9B and 9D). RNCMs expressing wildtype Rab3gap1 showed substantially reduced levels of Rab3a-GTP compared with βGal controls, as expected, whereas expression of the Rab3gap1R728A mutant resulted in a relative restoration of Rab3a-GTP levels, albeit not to the same extent as βGal controls (Figures 9E to 9G). Notably, expression of wildtype Rab3gap1 resulted in a profound increase in ANP secretion by RNCMs that was largely mitigated in RNCMs expressing the Rab3gap1R728A mutant (Figures 9H and 9J), whereas intracellular ANP levels were unaltered by expression of wildtype or mutant Rab3gap1 (Figures 9I and 9K). These results affirm the role of Rab3gap1 enzymatic activity and regulation of Rab3a GTPase activity in the molecular control of ANP secretion by cardiomyocytes.
Figure 9.
Atrial Natriuretic Peptide Secretion From Cardiomyocytes is Dependent on Rab3gap1 GAP Activity
(A) Representative images of RNCMs transduced with adenoviruses for myc-tagged wildtype Rab3gap1 (Rab3gap1), a myc-tagged-GAP -deficient Rab3gap1 mutant (Rab3gap1R728A), or β-galactosidase control (βGal) and immunostained for myc (Rab3gap1, green) and GM130 (Golgi marker, red). (B) Representative images of RNCMs transduced with adenoviruses for GFP-Rab3a and either Rab3gap1, Rab3gap1R728A or βGal and immunostained for GM130 (Golgi marker, red). (C) Pearson’s correlation coefficients for colocalization of Rab3gap1 and GM130 in the indicated groups of RNCMs. n = 3 independent experiments with 60 to 90 cells analyzed for each experiment. (D) Quantification of GFP-Rab3a relative fluorescence intensity. n = 3 independent experiments with 60 to 90 cells analyzed for each experiment. (E) Immunoblotting and quantification of (F) Rab3a-GTP and (G) total Rab3a levels in RNCMs transduced with adenoviruses expressing βGal, Rab3gap1, or Rab3gap1R728A. Immunoblotting of (H) secreted and (I) intracellular ANP in RNCMs transduced with adenoviruses expressing βGal, Rab3gap1, or Rab3gap1R728A. Coomassie staining or immunoblotting for Gapdh were used as loading controls in H and I, respectively. Quantification of (J) secreted and (K) intracellular ANP levels in the indicated groups of RNCMs as shown in H and I, respectively. n = 3 independent experiments. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001, 1-way ANOVA with Tukey’s multiple comparisons test. Data are presented as mean ± SD. Abbreviations as in Figures 1 and 2.
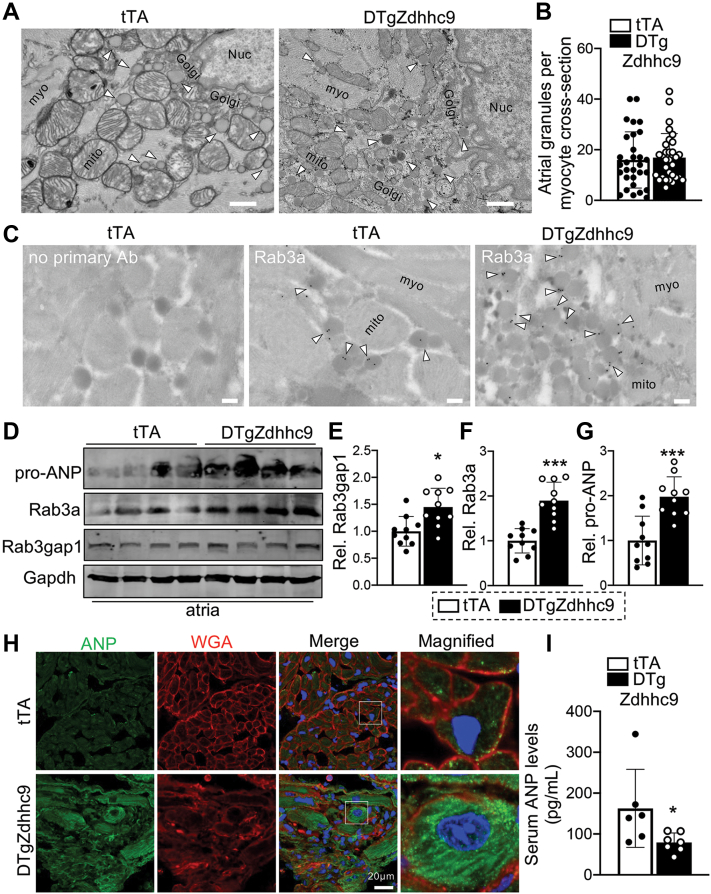
Atria are the predominant sites of ANP synthesis and secretion in vivo,21 so we examined atria in zDHHC9 transgenic mice at 2 months of age, before the development of cardiac hypertrophy and left ventricular dysfunction (Figure 1). We used transmission electron microscopy (TEM) to assess atrial ultrastructure. Analyses of low magnification TEM images of DTgZdhhc9 atria revealed evidence of atrial dysmorphology, including excessive collagen deposition and disruption of myofilament and mitochondrial organization compared with tTA controls (Supplemental Figure 9A). Although we did not observe differences in the overall number of secretory granules in tTA and DTgZdhhc9 atrial myocytes, the lumen of granules in DTgZdhhc9 atrial cardiomyocytes were more electron dense in comparison with tTA controls (Figures 10A and 10B), indicative of greater ANP content that is highly electron dense and is by far the most abundant component stored in atrial granules.21,27 To confirm that Rab3a is recruited to atrial granules in vivo, we used immunogold-labeling TEM with an anti-Rab3a antibody to evaluate Rab3a subcellular localization. TEM imaging demonstrated that Rab3a was primarily localized to the outer surface of atrial granules in both tTA and DTgZdhhc9 atria (Figure 10C), suggesting a role in atrial granule exocytosis and ANP secretion in vivo. Further, immunoblotting analyses demonstrated abundant expression of ANP, Rab3a, and Rab3gap1 in atria, consistent with a functional role in atrial granule exocytosis, and protein levels were all induced in DTgZdhhc9 atria in comparison with tTA controls (Figures 10D to 10G), suggesting dysfunction of this pathway in zDHHC9-overexpressing atria. ANP (Nppa) mRNA levels were also upregulated in DTgZdhhc9 atria (Supplemental Figure 9B), and immunohistochemistry in DTgZdhhc9 cryosections compared with tTA controls revealed intracellular accumulation of ANP in a vesicular pattern in zDHHC9-overexpressing atrial myocytes in vivo (Figure 10H). To elucidate whether intracellular accumulation of ANP resulted in a defect in ANP release, we subjected serum samples from tTA and DTgZdhhc9 mice to ANP ELISA assays, revealing a significant reduction in serum ANP levels in DTgZdhhc9 mice compared with tTA controls (Figure 10I), indicating a defect in ANP secretion by zDHHC9-overexpressing atria.
Figure 10.
Dysregulation of Rab3a, Rab3gap1, ANP, and Atrial Granule Morphology in zDHHC9 Overexpressing Hearts
(A) Transmission electron microscopy images showing enhanced electron density of atrial granule core in zDHHC9-overexpressing hearts at 2 months of age: myofibrils (myo), mitochondria (mito), nucleus (Nuc) and Golgi apparatus (Golgi). Arrowheads indicate atrial granules. Scale bar = 600 nm. (B) Quantification of number of secretory granules per atrial myocyte cross-section in tTA and DTgZdhhc9 mice; 30 cells from 2 mice per genotype were quantified. (C) Rab3a subcellular localization at atrial granules. Transmission electron microscopy of tTA and DTgZdhhc9 atria with immunogold labeling of anti-Rab3a. Arrowheads indicate labeling of Rab3a at the periphery of secretory granules. There was minimal labeling on myofibrils (myo) and mitochondria (mito). Scale bar = 200 nm. (D) Immunoblotting and quantification of (E) Rab3gap1, (F) Rab3a, and (G) ANP protein levels in tTA and DTgZdhhc9 atria at 2 months of age. n =10 per genotype. (H) Representative images of immunostaining for ANP (green) and wheat germ agglutinin (WGA, red) staining to demarcate the cell membrane in cryosections from tTA and DTgZdhhc9 atria at 2 months of age. (F) Concentration of ANP (pg/mL) in serum from tTA and DTgZdhhc9 mice at 5 to 7 weeks of age. n =6 to 7. ∗P < 0.05, ∗∗∗P < 0.001 compared with tTA controls, Student's t-test with Welch’s correction. Data are presented as mean ± SD. Abbreviations as in Figures 1 and 2.
Discussion
Palmitoylation is a reversible post-translational lipid modification capable of regulating the structure and activity of membrane proteins and the targeting of soluble proteins to cellular membrane domains, thereby influencing protein localization, activity, interaction with cofactors, or stability.1,2,71 Importantly, palmitoylation is emerging as a targetable modification to impede maladaptive membrane-localized signaling.12,72,73 Specified substrate recruitment by zDHHC substrate interaction domains74,75 and the solving of zDHHC enzyme crystal structures76 suggest translational potential of targeting zDHHC enzymes or substrate palmitoylation sites as novel therapeutics.
zDHHC9 is perhaps the most well-studied S-acyltransferase because of the strong association between mutations in ZDHHC9, an X-linked gene, and cognitive impairment and epilepsy in male subjects.15, 16, 17, 18 Consistent with this, deletion of Zdhhc9 in mice results in modest but significant neurologic deficits, including hypotonia, increased seizure-like activity, and deficits in spatial learning and neuronal dendrite outgrowth.8,77 zDHHC9 has more recently been found to play critical roles in tumor growth, as loss of zDHHC9 reduces colony formation and improves survival in models of glioblastoma and leukemia.12,13 In neuronal cells, zDHHC9 has been shown to catalyze palmitoylation of the small GTPases H-Ras and TC10 as well as the glucose transporter, GLUT1.8,12,78 zDHHC9 is also expressed in extraneuronal tissues, including the heart,11 but its cardiac functions have not been evaluated. Moreover, relatively little is known about the functions of palmitoylation and zDHHC enzymes more generally in the heart beyond their role in ion channel trafficking and regulation.2
In vitro, zDHHC9 requires interaction with the Golgi membrane protein, GCP16 (Golga7), to form a functional S-acyltransferase complex,11 and other studies have used coexpression of zDHHC9 and GCP16 to investigate functional effects of zDHHC9-mediated palmitoylation.8,13 We observed significant S-acyltransferase activity and induction of Rab3gap1 palmitoylation with overexpression of zDHHC9 alone in cardiomyocytes, both in vitro and in vivo, as well as in metabolic labeling studies in HEK cells. Thus, overexpression of zDHHC9 alone appears to be either sufficient to impart increased zDHHC9 S-acyltransferase activity in vivo, or Golga7 protein may be stabilized and upregulated by zDHHC9 overexpression to evoke increased zDHHC9 transferase activity.
Here, we found zDHHC9-mediated palmitoylation regulates exocytosis and hormone secretion in cardiomyocytes. To interrogate functions of zDHHC9 in the heart, we generated a cardiomyocyte-specific overexpression transgenic mouse model. Surprisingly, H-Ras and N-Ras palmitoylation were unaltered by zDHHC9 overexpression, although mice overexpressing zDHHC9 develop dilated cardiomyopathy and have increased mortality, suggesting pivotal functions for zDHHC9-regulated palmitoylation in the heart. We undertook an unbiased proteomic approach to identify cardiac substrates of zDHHC9 that contribute to its role in cardiac physiology. S-acyl proteomics and follow-up experiments identified increased palmitoylation of Rab3gap1 in zDHHC9 overexpressing hearts before the onset of cardiomyopathy, suggesting roles for zDHHC9 in regulating cardiomyocyte secretory pathway activity and exocytosis.
Rab3gap1 accelerates hydrolysis of GTP on Rab3a to GDP, thereby promoting Rab3a dissociation from exocytic DCVs, DCV fusion with the plasma membrane, and release of DCV contents into the extracellular space.39,40,42 Rab3gap1 expression is highly enriched in heart and skeletal muscle,38 suggesting critical functions in myocytes. Mutations in RAP3GAP1 not only cause Warburg Micro syndrome, a multisystem autosomal recessive disorder characterized by severe congenital defects of the brain and eye,46 but a recent GWAS study also found an SNP in RAB3GAP1 to be associated with a significant increase in cardiac sudden death caused by ischemic heart disease.48 In this study, we identified, for the first time, that Rab3gap1 GAP activity has fundamental roles in regulation of ANP release by cardiomyocytes.
Rab3a has been extensively studied in neurotransmitter release and regulated exocytosis in neurons and chromaffin cells.37,39 58 However, despite expression of Rab3 and its regulators and effectors in the heart,33,34,38,79 its roles in cardiomyocyte biology remain elusive. Established functions of Rab proteins in cardiomyocytes are largely limited to regulation of ion-channel trafficking32 and mitophagy.80,81 Intriguingly, Rab3a interacts with VAMP-2,82,83 a v-SNARE involved in ANP secretion.28 Here, we uncovered novel roles for Rab3a in cardiomyocyte exocytosis and natriuretic peptide secretion that are regulated by zDHHC9 S-acyltransferase activity.
zDHHC9 induces Rab3gap1 palmitoylation in cardiomyocytes in vitro and in vivo, enhances Rab3gap1 localization at the Golgi apparatus, and promotes Rab3 GTP-loading and Rab3a-positive secretory vesicles at the cardiomyocyte periphery in comparison with control cardiomyocytes, in which a majority of Rab3a protein is located at the Golgi. These data suggest zDHHC9-mediated palmitoylation of Rab3gap1 may serve as a Golgi retention signal or delay its transit to the plasma membrane, thereby impairing Rab3gap1-dependent GTP hydrolysis and inactivation of Rab3a at the sarcolemma. Rab3gap1 palmitoylation was also enhanced in a zDHHC9-dependent manner in response to phenylephrine, suggesting zDHHC9 levels or activity may be induced in response to cardiac stress to modulate cardiomyocyte exocytosis and ANP release.
Cycling of Rab3a between the GTP- and GDP-bound forms is necessary for sustained exocytosis and secretion of neurohormones and peptides, as this nucleotide cycling is essential for the competency of exocytic vesicles to dock and fuse with the plasma membrane.39,40,42, 43, 44 For example, secretion of brain-derived neurotrophic factor (BDNF) by astrocytes requires Rab3gap1-mediated GTP : GDP exchange on Rab3a such that knock-in of a mutant huntingtin protein that binds Rab3a-GTP and prevents its interaction with Rab3gap1 results in elevated Rab3a-GTP levels but impairment of BDNF secretion.42 Similarly, Rab3gap1 gene-deleted mice exhibit enhanced GTP-loading of Rab3a in the brain but impaired neuronal glutamate release.45 Thus, spatial segregation of Rab3gap1 at the Golgi from Rab3a at docked, cargo-containing DCVs at the plasma membrane by zDHHC9-mediated palmitoylation could function similarly to loss of Rab3gap1 and impinge on Rab3-mediated exocytosis and secretion. Moreover, dysregulation of Rab3 activity in neurons could also contribute to the molecular pathogenesis of XLID in patients with mutations in ZDHHC9 and neurologic deficits in Warburg Micro syndrome caused by RAB3GAP1 mutations.
The best-studied hormones released by cardiac myocytes are natriuretic peptides, yet the Rab proteins controlling their regulated secretion have not been identified.21,28,84 Although Rab6,85 Rab12,86 and other Rab proteins87 have been found to be associated with secretory granules of atrial cardiomyocytes, no functional roles for these or other Rab proteins in modulation of natriuretic peptide secretion have been established. Given their upregulation in response to cardiac stress, such as mechanical stretch or activation of Gαq-coupled receptors, and their adaptive endocrine functions, the synthesis, trafficking, and secretion of natriuretic peptides in cardiomyocytes is tightly controlled.21,84 ANP and other natriuretic peptides are synthesized as preprohormones that are processed by signal peptidases to generate prohormones that traverse the endoplasmic reticulum and Golgi apparatus and are packaged into DCVs.21,27,84 proANP packaged into atrial granules/DCVs is cosecretionally cleaved by the transmembrane serine protease, corin, on the extracellular surface of cardiomyocytes, to generate the active ANP hormone that potently activates natriuretic peptide receptors and production of intracellular cGMP.21,27,84 However, despite the abundance of knowledge regarding transcriptional regulation and proteolytic processing of natriuretic peptides, detailed regulatory mechanisms governing their flux through the secretory pathway and secretion via exocytosis, including the Rab proteins involved in this process, are lacking. Interestingly, a mutant Rim1 protein that specifically binds Rab3a-GTP colocalizes with GFP-tagged ANP in chromaffin cells,58 suggesting a role for Rab3a in ANP trafficking and secretion.
To probe for impacts of zDHHC9 on the secretory pathway, we investigated ANP secretion and, surprisingly, found that overexpression of zDHHC9 results in intracellular retention and reduced secretion of ANP in cardiomyocytes, whereas knockdown of zDHHC9 increases ANP secretion in response to the hypertrophic agonist PE. zDHHC9 expression or treatment with PE both promoted the movement of Rab3a from the Golgi to post-Golgi vesicles at the cardiomyocyte periphery and increased steady-state levels of Rab3a-GTP. However, zDHHC9 overexpression uniquely resulted in accumulation of intracellular ANP and a deficit in its secretion rather than the robust release of ANP observed in response to PE. Moreover, the lumen of atrial granules appeared more electron dense in atria of zDHHC9 transgenic mice, concomitant with reduced serum ANP levels compared with controls, suggesting defective ANP secretion by zDHHC9-overexpressing atria. These data support a physiological role for Rab3a in natriuretic peptide secretion in cardiomyocytes and support a mechanism of defective Rab3a nucleotide cycling and exocytosis in zDHHC9 overexpressing cardiomyocytes that renders DCVs incapable of proper release of ANP.
zDHHC9 functions in regulating Rab3a-mediated exocytosis and natriuretic peptide secretion are likely not the sole underlying cause of the dilated cardiomyopathy observed in cardiac-specific zDHHC9 transgenic mice, although chronic impairment of natriuretic peptide secretion could promote chronic hypertension that hastens cardiac dysfunction and decompensation. Notably, knockdown of zDHHC9 enhanced secretion of ANP in response to the hypertrophic agonist phenylephrine, raising the possibility that inhibiting zDHHC9 could be harnessed as a therapeutic strategy to promote secretion of natriuretic peptides in the diseased heart. Indeed, a number of legacy and contemporary heart-failure therapeutics activate natriuretic peptide receptors or production of their downstream second messengers (ie, cGMP), including synthetic natriuretic peptide analogues,88,89 guanylate cyclase activators,90,91 and the neprilysin inhibitor, sacubitril, which inhibits the breakdown of natriuretic peptides and is becoming widely used for the treatment of heart failure in combination with the angiotensin receptor blocker valsartan.25,26
Study Limitations
As the name suggests, ANP is predominantly synthesized and secreted by atrial cardiomyocytes, although in response to pathologic stimulation ventricular cardiomyocytes also generate and secrete ANP.21,28,84 It is not clear if zDHHC9 functions in a generic Rab3a-regulated branch of anterograde trafficking through the secretory pathway or if it has a more specific role in controlling exocytosis of ANP-containing DCVs in atrial cardiomyocytes in vivo. A majority of studies of ANP secretion here were performed in neonatal rat ventricular myocytes that possess a constitutive secretory pathway for natriuretic peptide release in comparison with the more regulated and inducible secretion of natriuretic peptides that are stored prepackaged in secretory granules of adult atrial cardiomyocytes, which accounts for the majority of ANP release in the context of cardiovascular disease.21,23,27,84,92 However, we did observe Rab3a almost exclusively on atrial granules in vivo and protein levels of ANP, Rab3a, and Rab3gap1 were upregulated and DCV morphology disrupted in zDHHC9-overexpressing atria. Most importantly, DTgZdhhc9 mice had a reduction in circulating ANP levels, suggesting roles for zDHHC9, Rab3gap1, and Rab3a in regulated ANP release from atria in vivo. Future studies will interrogate the roles of zDHHC9, Rab3gap1 palmitoylation, and Rab3a in pathophysiological ANP release from atrial myocytes and consequences on blood pressure and target organ function. Regardless, studies herein shed light on novel functions of zDHHC9 in cardiomyocyte exocytosis and regulation of Rab3gap1 palmitoylation and Rab3a activity that may also contribute to neurologic diseases associated with mutations in RAB3GAP1 or ZDHHC9.
Conclusions
The small GTPase Rab3a localizes to ANP-containing secretory granules in cardiomyocytes and control of its GTPase activity by Rab3gap1 and zDHHC9 modulates exocytosis and ANP secretion. Elucidation of the precise mechanisms and molecular machinery regulating natriuretic peptide storage, trafficking, and release by secretory granules may provide novel targets to promote elevation of circulating natriuretic peptides that could be of therapeutic benefit in certain forms of heart failure.
Funding Support and Author Disclosures
This work was supported by grants from the National Institutes of Health (R00HL136695 to Dr Brody) and the American Heart Association (827440 to Dr Essandoh). All other authors have reported that they have no relationships relevant to the content of this manuscript to disclose.
Acknowledgments
The authors are grateful for microscopy and proteomics resources and technical assistance provided by the University of Michigan Medical School Microscopy Core and Proteomics Resource Facility, respectively.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
Perspectives.
Appendix
References
- 1.Chamberlain L.H., Shipston M.J. The physiology of protein S-acylation. Physiol Rev. 2015;95:341–376. doi: 10.1152/physrev.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Essandoh K., Philippe J.M., Jenkins P.M., Brody M.J. Palmitoylation: a fatty regulator of myocardial electrophysiology. Front Physiol. 2020;11:108. doi: 10.3389/fphys.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst A.M., Syed S.A., Zaki O., et al. S-palmitoylation sorts membrane cargo for anterograde transport in the Golgi. Dev Cell. 2018;47:479–493.e7. doi: 10.1016/j.devcel.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt M.F., Schlesinger M.J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979;17:813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- 5.Prescott G.R., Gorleku O.A., Greaves J., Chamberlain L.H. Palmitoylation of the synaptic vesicle fusion machinery. J Neurochem. 2009;110:1135–1149. doi: 10.1111/j.1471-4159.2009.06205.x. [DOI] [PubMed] [Google Scholar]
- 6.Salaun C., James D.J., Greaves J., Chamberlain L.H. Plasma membrane targeting of exocytic SNARE proteins. Biochim Biophys Acta. 2004;1693:81–89. doi: 10.1016/j.bbamcr.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Shimell J.J., Globa A., Sepers M.D., et al. Regulation of hippocampal excitatory synapses by the Zdhhc5 palmitoyl acyltransferase. J Cell Sci. 2021:134. doi: 10.1242/jcs.254276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimell J.J., Shah B.S., Cain S.M., et al. The X-linked intellectual disability gene zdhhc9 is essential for dendrite outgrowth and inhibitory synapse formation. Cell Rep. 2019;29:2422–2437.e8. doi: 10.1016/j.celrep.2019.10.065. [DOI] [PubMed] [Google Scholar]
- 9.Shah B.S., Shimell J.J., Bamji S.X. Regulation of dendrite morphology and excitatory synapse formation by zDHHC15. J Cell Sci. 2019;132 doi: 10.1242/jcs.230052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst A.M., Toomre D., Bogan J.S. Acylation: a new means to control traffic through the Golgi. Front Cell Dev Biol. 2019;7:109. doi: 10.3389/fcell.2019.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swarthout J.T., Lobo S., Farh L., et al. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J Biol Chem. 2005;280:31141–31148. doi: 10.1074/jbc.M504113200. [DOI] [PubMed] [Google Scholar]
- 12.Liu P., Jiao B., Zhang R., et al. Palmitoylacyltransferase Zdhhc9 inactivation mitigates leukemogenic potential of oncogenic Nras. Leukemia. 2016;30:1225–1228. doi: 10.1038/leu.2015.293. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z., Li X., Yang F., Chen C., et al. DHHC9-mediated GLUT1 S-palmitoylation promotes glioblastoma glycolysis and tumorigenesis. Nat Commun. 2021;12:5872. doi: 10.1038/s41467-021-26180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansilla F., Birkenkamp-Demtroder K., Kruhoffer M., et al. Differential expression of DHHC9 in microsatellite stable and instable human colorectal cancer subgroups. Br J Cancer. 2007;96:1896–1903. doi: 10.1038/sj.bjc.6603818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raymond F.L., Tarpey P.S., Edkins S., et al. Mutations in ZDHHC9, which encodes a palmitoyltransferase of NRAS and HRAS, cause X-linked mental retardation associated with a Marfanoid habitus. Am J Hum Genet. 2007;80:982–987. doi: 10.1086/513609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker K., Astle D.E., Scerif G., et al. Epilepsy, cognitive deficits and neuroanatomy in males with ZDHHC9 mutations. Ann Clin Transl Neurol. 2015;2:559–569. doi: 10.1002/acn3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J.Y., Lee I.G., Shin S., Kim M., Jang J.H., Park J. The first patient with sporadic X-linked intellectual disability with de novo ZDHHC9 mutation identified by targeted next-generation sequencing. Eur J Med Genet. 2017;60:499–503. doi: 10.1016/j.ejmg.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Bathelt J., Astle D., Barnes J., Raymond F.L., Baker K. Structural brain abnormalities in a single gene disorder associated with epilepsy, language impairment and intellectual disability. Neuroimage Clin. 2016;12:655–665. doi: 10.1016/j.nicl.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miles M.R., Seo J., Jiang M., et al. Global identification of S-palmitoylated proteins and detection of palmitoylating (DHHC) enzymes in heart. J Mol Cell Cardiol. 2021;155:1–9. doi: 10.1016/j.yjmcc.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Main A., Boguslavskyi A., Howie J., et al. Dynamic but discordant alterations in zDHHC5 expression and palmitoylation of its substrates in cardiac pathologies. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.1023237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetze J.P., Bruneau B.G., Ramos H.R., Ogawa T., de Bold M.K., de Bold A.J. Cardiac natriuretic peptides. Nat Rev Cardiol. 2020;17:698–717. doi: 10.1038/s41569-020-0381-0. [DOI] [PubMed] [Google Scholar]
- 22.Woodall B.P., Gresham K.S., Woodall M.A., et al. Alteration of myocardial GRK2 produces a global metabolic phenotype. JCI Insight. 2019;5 doi: 10.1172/jci.insight.123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloch K.D., Seidman J.G., Naftilan J.D., Fallon J.T., Seidman C.E. Neonatal atria and ventricles secrete atrial natriuretic factor via tissue-specific secretory pathways. Cell. 1986;47:695–702. doi: 10.1016/0092-8674(86)90512-x. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J., Pei L. Cardiac endocrinology: heart-derived hormones in physiology and disease. J Am Coll Cardiol Basic Trans Science. 2020;5:949–960. doi: 10.1016/j.jacbts.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMurray J.J., Packer M., Desai A.S., et al. Investigators PARADIGM-HF and Committees Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 26.Velazquez E.J., Morrow D.A., DeVore A.D., et al. Investigators PARADIGM-HF Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 27.Back N., Luxmi R., Powers K.G., Mains R.E., Eipper B.A. Peptidylglycine alpha-amidating monooxygenase is required for atrial secretory granule formation. Proc Natl Acad Sci USA. 2020;117:17820–17831. doi: 10.1073/pnas.2004410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferlito M., Fulton W.B., Zauher M.A., Marban E., Steenbergen C., Lowenstein C.J. VAMP-1, VAMP-2, and syntaxin-4 regulate ANP release from cardiac myocytes. J Mol Cell Cardiol. 2010;49:791–800. doi: 10.1016/j.yjmcc.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 30.Odley A., Hahn H.S., Lynch R.A., et al. Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acad Sci USA. 2004;101:7082–7087. doi: 10.1073/pnas.0308335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etzion S., Etzion Y., DeBosch B., Crawford P.A., Muslin A.J. Akt2 deficiency promotes cardiac induction of Rab4a and myocardial beta-adrenergic hypersensitivity. J Mol Cell Cardiol. 2010;49:931–940. doi: 10.1016/j.yjmcc.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balse E., Steele D.F., Abriel H., Coulombe A., Fedida D., Hatem S.N. Dynamic of ion channel expression at the plasma membrane of cardiomyocytes. Physiol Rev. 2012;92:1317–1358. doi: 10.1152/physrev.00041.2011. [DOI] [PubMed] [Google Scholar]
- 33.Wu G., Yussman M.G., Barrett T.J., et al. Increased myocardial Rab GTPase expression: a consequence and cause of cardiomyopathy. Circ Res. 2001;89:1130–1137. doi: 10.1161/hh2401.100427. [DOI] [PubMed] [Google Scholar]
- 34.Ujihara Y., Kanagawa M., Mohri S., et al. Elimination of fukutin reveals cellular and molecular pathomechanisms in muscular dystrophy-associated heart failure. Nat Commun. 2019;10:5754. doi: 10.1038/s41467-019-13623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaekura K., Julyan R., Wicksteed B.L., et al. Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J Biol Chem. 2003;278:9715–9721. doi: 10.1074/jbc.M211352200. [DOI] [PubMed] [Google Scholar]
- 36.Persoon C.M., Hoogstraaten R.I., Nassal J.P., et al. The RAB3-RIM pathway is essential for the release of neuromodulators. Neuron. 2019;104:1065–1080.e12. doi: 10.1016/j.neuron.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geppert M., Bolshakov V.Y., Siegelbaum S.A., et al. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- 38.Fukui K., Sasaki T., Imazumi K., Matsuura Y., Nakanishi H., Takai Y. Isolation and characterization of a GTPase activating protein specific for the Rab3 subfamily of small G proteins. J Biol Chem. 1997;272:4655–4658. doi: 10.1074/jbc.272.8.4655. [DOI] [PubMed] [Google Scholar]
- 39.Huang C.C., Yang D.M., Lin C.C., Kao L.S. Involvement of Rab3A in vesicle priming during exocytosis: interaction with Munc13-1 and Munc18-1. Traffic. 2011;12:1356–1370. doi: 10.1111/j.1600-0854.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 40.Lin C.C., Huang C.C., Lin K.H., et al. Visualization of Rab3A dissociation during exocytosis: a study by total internal reflection microscopy. J Cell Physiol. 2007;211:316–326. doi: 10.1002/jcp.20938. [DOI] [PubMed] [Google Scholar]
- 41.Essandoh K., Auchus R.J., Brody M.J. Cardiac decompensation and promiscuous prenylation of small GTPases in cardiomyocytes in response to local mevalonate pathway disruptiondagger. J Pathol. 2021;256:249–252. doi: 10.1002/path.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong Y., Zhao T., Li X.J., Li S. Mutant huntingtin impairs BDNF release from astrocytes by disrupting conversion of Rab3a-GTP into Rab3a-GDP. J Neurosci. 2016;36:8790–8801. doi: 10.1523/JNEUROSCI.0168-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Weering J.R., Toonen R.F., Verhage M. The role of Rab3a in secretory vesicle docking requires association/dissociation of guanidine phosphates and Munc18-1. PloS One. 2007;2:e616. doi: 10.1371/journal.pone.0000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bustos M.A., Roggero C.M., De la Iglesia P.X., Mayorga L.S., Tomes C.N. GTP-bound Rab3A exhibits consecutive positive and negative roles during human sperm dense-core granule exocytosis. J Mol Cell Biol. 2014;6:286–298. doi: 10.1093/jmcb/mju021. [DOI] [PubMed] [Google Scholar]
- 45.Sakane A., Manabe S., Ishizaki H., et al. Rab3 GTPase-activating protein regulates synaptic transmission and plasticity through the inactivation of Rab3. Proc Natl Acad Sci USA. 2006;103:10029–10034. doi: 10.1073/pnas.0600304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aligianis I.A., Johnson C.A., Gissen P., et al. Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nat Genet. 2005;37:221–223. doi: 10.1038/ng1517. [DOI] [PubMed] [Google Scholar]
- 47.Borck G., Wunram H., Steiert A., et al. A homozygous RAB3GAP2 mutation causes Warburg Micro syndrome. Hum Genet. 2011;129:45–50. doi: 10.1007/s00439-010-0896-2. [DOI] [PubMed] [Google Scholar]
- 48.Huertas-Vazquez A., Nelson C.P., et al. Novel loci associated with increased risk of sudden cardiac death in the context of coronary artery disease. PloS One. 2013;8 doi: 10.1371/journal.pone.0059905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanbe A., Gulick J., Hanks M.C., Liang Q., Osinska H., Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 50.Brody M.J., Vanhoutte D., Bakshi C.V., et al. Disruption of valosin-containing protein activity causes cardiomyopathy and reveals pleiotropic functions in cardiac homeostasis. J Biol Chem. 2019;294:8918–8929. doi: 10.1074/jbc.RA119.007585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brody M.J., Vanhoutte D., Schips T.G., et al. Defective flux of thrombospondin-4 through the secretory pathway impairs cardiomyocyte membrane stability and causes cardiomyopathy. Mol Cell Biol. 2018;38:e00114–e00118. doi: 10.1128/MCB.00114-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis J., Maillet M., Miano J.M., Molkentin J.D. Lost in transgenesis: a user's guide for genetically manipulating the mouse in cardiac research. Circ Res. 2012;111:761–777. doi: 10.1161/CIRCRESAHA.111.262717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brody M.J., Feng L., Grimes A.C., et al. LRRC10 is required to maintain cardiac function in response to pressure overload. Am J Physiol Heart Circ Physiol. 2016;310:H269–H278. doi: 10.1152/ajpheart.00717.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brody M.J., Hacker T.A., Patel J.R., et al. Ablation of the cardiac-specific gene leucine-rich repeat containing 10 (Lrrc10) results in dilated cardiomyopathy. PloS One. 2012;7 doi: 10.1371/journal.pone.0051621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang B., Hubber A., McDonough J.A., Roy C.R., Scidmore M.A., Carlyon J.A. The anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes. Cell Microbiol. 2010;12:1292–1307. doi: 10.1111/j.1462-5822.2010.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D.I., Jensen S.C., Noble K.A., et al. An improved smaller biotin ligase for BioID proximity labeling. Mol Biol Cell. 2016;27:1188–1196. doi: 10.1091/mbc.E15-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brody M.J., Schips T.G., Vanhoutte D., et al. Dissection of thrombospondin-4 domains involved in intracellular adaptive endoplasmic reticulum stress-responsive signaling. Mol Cell Biol. 2015;36:2–12. doi: 10.1128/MCB.00607-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun L., Bittner M.A., Holz R.W. Rab3a binding and secretion-enhancing domains in Rim1 are separate and unique: studies in adrenal chromaffin cells. J Biol Chem. 2001;276:12911–12917. doi: 10.1074/jbc.M011110200. [DOI] [PubMed] [Google Scholar]
- 59.Guo J., Wenk M.R., Pellegrini L., Onofri F., Benfenati F., De Camilli P. Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc Natl Acad Sci USA. 2003;100:3995–4000. doi: 10.1073/pnas.0230488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo A., Wang Y., Chen B., et al. E-C coupling structural protein junctophilin-2 encodes a stress-adaptive transcription regulator. Science. 2018:362. doi: 10.1126/science.aan3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J., Ciampa G., Zheng D., et al. Calpain-2 specifically cleaves Junctophilin-2 at the same site as Calpain-1 but with less efficacy. Biochem J. 2021;478:3539–3553. doi: 10.1042/BCJ20210629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beedle A.M., Turner A.J., Saito Y., et al. Mouse fukutin deletion impairs dystroglycan processing and recapitulates muscular dystrophy. J Clin Invest. 2012;122:3330–3342. doi: 10.1172/JCI63004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forrester M.T., Hess D.T., Thompson J.W., et al. Site-specific analysis of protein S-acylation by resin-assisted capture. J Lipid Res. 2011;52:393–398. doi: 10.1194/jlr.D011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackers-Johnson M., Li P.Y., Holmes A.P., O'Brien S.M., Pavlovic D., Foo R.S. A simplified, Langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ Res. 2016;119:909–920. doi: 10.1161/CIRCRESAHA.116.309202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Proud C.G. Ras, PI3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc Res. 2004;63:403–413. doi: 10.1016/j.cardiores.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Matsuda T., Jeong J.I., Ikeda S., et al. H-ras isoform mediates protection against pressure overload-induced cardiac dysfunction in part through activation of AKT. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collins M.O., Woodley K.T., Choudhary J.S. Global, site-specific analysis of neuronal protein S-acylation. Sci Rep. 2017;7:4683. doi: 10.1038/s41598-017-04580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., Okamoto M., Schmitz F., Hofmann K., Sudhof T.C. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 69.Nash C.A., Wei W., Irannejad R., Smrcka A.V. Golgi localized beta1-adrenergic receptors stimulate Golgi PI4P hydrolysis by PLCepsilon to regulate cardiac hypertrophy. eLife. 2019;8 doi: 10.7554/eLife.48167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clabecq A., Henry J.P., Darchen F. Biochemical characterization of Rab3-GTPase-activating protein reveals a mechanism similar to that of Ras-GAP. J Biol Chem. 2000;275:31786–31791. doi: 10.1074/jbc.M003705200. [DOI] [PubMed] [Google Scholar]
- 71.Won S.J., Martin B.R. Temporal profiling establishes a dynamic S-palmitoylation cycle. ACS Chem Biol. 2018;13:1560–1568. doi: 10.1021/acschembio.8b00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Remsberg J.R., Suciu R.M., Zambetti N.A., et al. ABHD17 regulation of plasma membrane palmitoylation and N-Ras-dependent cancer growth. Nat Chem Biol. 2021;17:856–864. doi: 10.1038/s41589-021-00785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin D.T., Conibear E. ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. eLife. 2015;4 doi: 10.7554/eLife.11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plain F., Howie J., Kennedy J., et al. Control of protein palmitoylation by regulating substrate recruitment to a zDHHC-protein acyltransferase. Commun Biol. 2020;3:411. doi: 10.1038/s42003-020-01145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lemonidis K., Gorleku O.A., Sanchez-Perez M.C., Grefen C., Chamberlain L.H. The Golgi S-acylation machinery comprises zDHHC enzymes with major differences in substrate affinity and S-acylation activity. Mol Biol Cell. 2014;25:3870–8383. doi: 10.1091/mbc.E14-06-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rana M.S., Kumar P., Lee C.J., Verardi R., Rajashankar K.R., Banerjee A. Fatty acyl recognition and transfer by an integral membrane S-acyltransferase. Science. 2018;359 doi: 10.1126/science.aao6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kouskou M., Thomson D.M., Brett R.R., et al. Disruption of the Zdhhc9 intellectual disability gene leads to behavioural abnormalities in a mouse model. Exp Neurol. 2018;308:35–46. doi: 10.1016/j.expneurol.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chai S., Cambronne X.A., Eichhorn S.W., Goodman R.H. MicroRNA-134 activity in somatostatin interneurons regulates H-Ras localization by repressing the palmitoylation enzyme, DHHC9. Proc Natl Acad Sci USA. 2013;110:17898–17903. doi: 10.1073/pnas.1317528110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Selma-Soriano E., Casillas-Serra C., Artero R., Llamusi B., Navarro J.A., Redon J. Rabphilin silencing causes dilated cardiomyopathy in a Drosophila model of nephrocyte damage. Sci Rep. 2021;11 doi: 10.1038/s41598-021-94710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saito T., Nah J., Oka S.I., et al. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J Clin Invest. 2019;129:802–819. doi: 10.1172/JCI122035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tong M., Saito T., Zhai P., et al. Alternative mitophagy protects the heart against obesity-associated cardiomyopathy. Circ Res. 2021;129:1105–1121. doi: 10.1161/CIRCRESAHA.121.319377. [DOI] [PubMed] [Google Scholar]
- 82.Martincic I., Peralta M.E., Ngsee J.K. Isolation and characterization of a dual prenylated Rab and VAMP2 receptor. J Biol Chem. 1997;272:26991–26998. doi: 10.1074/jbc.272.43.26991. [DOI] [PubMed] [Google Scholar]
- 83.Compton S.L., Behrend E.N. PRAF1: a Golgi complex transmembrane protein that interacts with viruses. Biochem Cell Biol. 2006;84:940–948. doi: 10.1139/o06-176. [DOI] [PubMed] [Google Scholar]
- 84.Glembotski C.C. Getting a G-RRP on regulated exocytosis in the heart. J Cell Biol. 2007;179:371–373. doi: 10.1083/jcb.200710021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iida H., Tanaka S., Shibata Y. Small GTP-binding protein, Rab6, is associated with secretory granules in atrial myocytes. Am J Physiol. 1997;272:C1594–C1601. doi: 10.1152/ajpcell.1997.272.5.C1594. [DOI] [PubMed] [Google Scholar]
- 86.Iida H., Wang L., Nishii K., Ookuma A., Shibata Y. Identification of rab12 as a secretory granule-associated small GTP-binding protein in atrial myocytes. Circ Res. 1996;78:343–347. doi: 10.1161/01.res.78.2.343. [DOI] [PubMed] [Google Scholar]
- 87.Muth E., Driscoll W.J., Smalstig A., Goping G., Mueller G.P. Proteomic analysis of rat atrial secretory granules: a platform for testable hypotheses. Biochim Biophys Acta. 2004;1699:263–275. doi: 10.1016/j.bbapap.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Chen H.H., Anstrom K.J., Givertz M.M., et al. NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H.H., Wan S.H., Iyer S.R., et al. First-in-human study of MANP: a novel ANP (atrial natriuretic peptide) analog in human hypertension. Hypertension. 2021;78:1859–1867. doi: 10.1161/HYPERTENSIONAHA.121.17159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Armstrong P.W., Pieske B., Anstrom K.J., VICTORIA Study Group Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 91.Armstrong P.W., Lam C.S.P., Anstrom K.J., VITALITY-HFpEF Study Group Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA. 2020;324:1512–1521. doi: 10.1001/jama.2020.15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brandenburg S., Arakel E.C., Schwappach B., Lehnart S.E. The molecular and functional identities of atrial cardiomyocytes in health and disease. Biochim Biophys Acta. 2016;1863:1882–1893. doi: 10.1016/j.bbamcr.2015.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.