Abstract
Background
Detecting high‐risk arrhythmia is important in diagnosing patients with palpitations. We compared the diagnostic accuracies of 7‐day patch‐type electrocardiographic (ECG) monitoring and 24‐h Holter monitoring for detecting significant arrhythmias in patients with palpitations.
Methods
This was a single‐center prospective trial with 58 participants who presented with palpitations, chest pain or syncope. Outcomes were defined as the detection of any one of six arrhythmias, including supraventricular tachycardia (SVT), atrial fibrillation or atrial flutter lasting more than 30 s, pauses of more than 3 s, high‐degree atrioventricular block, ventricular tachycardia (VT) >3 beats, or polymorphic VT/ventricular fibrillation. The McNemar test for paired proportions was used to compare arrhythmia detection rates.
Results
The overall arrhythmia detection rate was higher with 7‐day ECG patch monitoring than with 24‐h Holter monitoring (34.5% vs. 19.0%, p = .008). Compared with the use of 24‐h Holter monitors, the use of 7‐day ECG patch monitors was associated with higher detection of SVT (29.3% vs. 13.8%, p = .042). No serious adverse skin reactions were reported among the ECG patch‐monitored participants.
Conclusions
The results suggest that a 7‐day patch‐type continuous ECG monitor is more effective for the detection of supraventricular tachycardia than is a 24‐h Holter monitor. However, the clinical significance of device detected arrhythmia should be consolidated.
Keywords: ambulatory electrocardiography monitoring, cardiac arrhythmias, Holter monitoring, wearable electronic devices
A 7‐day patch‐type continuous electrocardiographic monitor is more effective in detecting significant arrhythmias than is a 24‐h Holter monitor.
1. INTRODUCTION
Palpitations are one of the most common symptoms in patients who present to primary care clinicians and cardiologists. 1 Although the causes are usually benign, palpitations occasionally manifest as potentially life‐threatening arrhythmias. Thus, an appropriate evaluation of palpitations is required.
Cardiac arrhythmias, including the development of new arrhythmias or significant changes in the rate of previously stable arrhythmias, are common causes of palpitations. Conventional Holter monitoring plays a significant role in the diagnosis of arrhythmias when evaluating palpitations. 2 However, it has the drawbacks of low diagnostic yield in detecting paroxysmal arrhythmias, burdensome wires that interfere with daily activities, and the inability of some patients to activate the event recorders when symptoms occur. 3 , 4
Recently, several newer generation electrocardiography (ECG) monitoring devices with advanced technologies have shown more advantages over the conventional 24‐h Holter monitoring devices in terms of convenience, efficient energy use, longer duration of monitoring, wireless data transfer, and no interruption of daily activities. 5 Researchers developed a deep neural network to diagnose cardiac arrhythmia, demonstrating its superior ability to classify the 12 rhythm classes compared with interpretations by individual cardiologists. 6 Detection rates of cardiac arrhythmias for extended durations with fully automated and highly accurate systems were studied, and the results suggested that they could aid cardiologists in the accurate detection of arrhythmias. 7 , 8
The MEMO patch version 1 (HUINNO Co., Ltd.) is the first Korean Food and Drug Administration (KFDA)‐approved, single‐lead, lightweight, 7‐day ambulatory ECG adhesive patch monitor (Figure 1). Soon after the release of version 1, version 2 was developed, tested, and approved for 14‐days of ambulatory ECG monitoring by KFDA. The device has no wires and can be re‐attached every day with disposable 3M adhesives; therefore, it does not interfere with the patient's daily activities.
FIGURE 1.
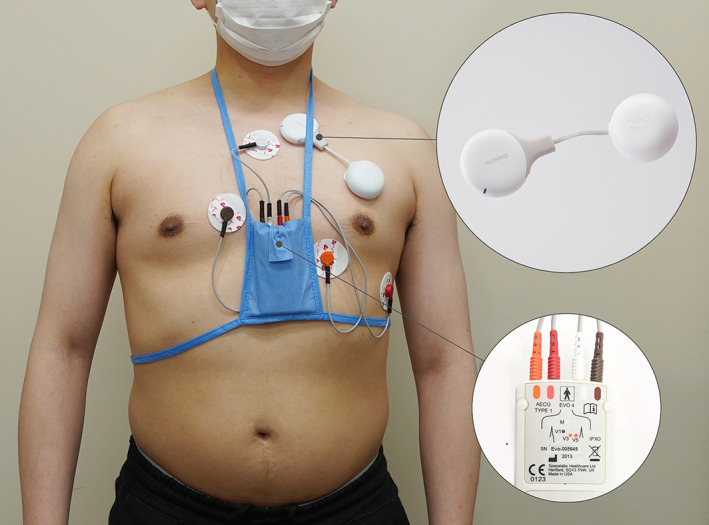
Overview of where the concomitant Holter electrocardiography and MEMO® patch is worn on the torso.
We compared the diagnostic accuracies of 7‐day adhesive patch monitoring (the MEMO patch version 1) and 24‐h Holter monitoring for detecting arrhythmias in patients with palpitations.
2. METHODS
2.1. Study design and population
The Institutional Review Board of Seoul National University Bundang Hospital approved the protocol (approval No: E‐1901‐516‐001), and all participants provided informed consent to participate before enrollment. This was a single‐center, prospective cohort study. Between March 2021 and August 2021, 70 patients who visited the family medicine clinic for symptoms of palpitations, chest pain, or syncope or were referred to the cardiology department for ambulatory ECG monitoring were screened for eligibility. Patients were enrolled if they were >19 years old, capable of providing voluntary informed consent, and able to adhere to the study protocol during the 7 days of keeping a MEMO patch for ECG monitoring. Patients were excluded if they had undergone or were scheduled to undergo direct current cardioversion or catheter or surgical ablation procedures during the monitoring period due to underlying arrhythmias, or if they had known allergic reactions to adhesive patches. Of the 70 participants screened, 60 were enrolled in this study.
2.2. Study protocol and outcomes
A three channel Holter monitor (EVO; SPACELABS Healthcare Co.) was used for the first 24 h and the MEMO patch was concomitantly fitted over the left pectoral area of each participant's chest by clinical staffs as shown in Figure 1. The participants were given a symptom diary to record the suspected symptoms of arrhythmias and the time of symptom onset and finish. The participants were also instructed to wear the MEMO patch monitor for up to 7 days. On day 7, the participants returned the MEMO patch monitor via a prepaid mail package to the laboratory. After 1 week of monitoring, the participants visited the outpatient clinic for the results of the Holter and MEMO patch monitors and completed a survey regarding overall ease of use and satisfaction.
The MEMO patch can store ECG data for up to 14 days in its internal storage. The stored ECG data can be extracted by connecting the device to a dedicated cradle with software for data download. The extracted ECG data are automatically uploaded to a cloud‐based system through the software, and the initial analysis by the artificial neural network, constructed and trained by HUINNO, is automatically triggered. The network used in this study for the seven‐class ECG classification was based on the 152‐layer convolutional neural network architecture with skip connections, which is called a residual network, and squeeze and excitation blocks. 9 The ECG recording was initially analyzed using deep learning‐based automated algorithms, 9 however, technicians manually double checked and reviewed the artificial intelligence (AI) diagnosis and if needed, revised the diagnosis after it was confirmed by cardiologists. The correction rate of the AI driven diagnosis, except for the ECG noise, included a total of three diagnoses (3/51, 5.9%), specifically, two for second‐degree (Mobitz type I) atrioventricular block (AVB) and one for intermittent 2:1 AVB.
The network was trained by cardiologist‐reviewed 18 000 lead II ECG data recordings acquired from the Seoul National University Bundang Hospital.
The Holter and MEMO data were subjected to a technical review for report generation and quality assurance. This report was then uploaded to a secure website for independent review by two investigators (J.H.L. and Y.C.). If there were any discrepancies in the interpretation of the ECG signals, then the sets of signals were sent to a senior cardiologist who decided on the final classification (I.‐Y.O.).
Outcomes were defined as the detection of any one of the six arrhythmias: (1) supraventricular tachycardia (>3 beats, not including atrial fibrillation or flutter), (2) atrial fibrillation or atrial flutter lasting more than 30 s, (3) pause of more than 3 s, (4) AVB (second‐degree AVB Mobitz type II or third‐degree AVB), (5) ventricular tachycardia >3 beats, or (6) polymorphic ventricular tachycardia or ventricular fibrillation.
2.3. Statistics
The MEMO Patch and the Holter device were used simultaneously on the same patients during the first 24 h. This study compared the detection rates of arrhythmia events between the MEMO patch and Holter device over the total monitoring time of each device. Continuous variables are presented as means and standard deviations, and categorical variables are shown as numbers with percentages. The McNemar test for paired proportions was used to compare the detection rate for arrhythmias between the Holter and MEMO patch monitors.
2.3.1. Estimation of sample size calculation
A previous study showed that the adhesive patch monitor detected 36 more arrhythmia events than did the Holter monitor, while the Holter monitor detected one event undetected by the adhesive patch monitor. 10 Another study found that 202 atrial fibrillation or flutter episodes were detected in six patients with 14‐day ECG patches, while only one atrial fibrillation episode was detected in a patient with a 24‐h Holter monitor. 11 Since our study aimed to compare 7‐day ECG patch monitors with 24‐h Holter monitors, we assumed that the odds ratio of detecting arrhythmias could be six times higher with the MEMO patch than with the 24‐h Holter monitor. A sample size of at least 57 after attrition achieved 90% power for a two‐tailed McNemar test with a type 1 error of 0.05 (G*Power software version 3.1.9.4). 12
3. RESULTS
Of the 70 participants screened, 60 were enrolled; 2 enrolled patients were lost to follow‐up. A total of 58 participants with data available from both the Holter and MEMO patch groups were included in the final analysis. The baseline characteristics of the patients are presented in Table 1. The mean age was 50.5 years and 57% (33/58) were women. Hypertension (15/58, 26%) and dyslipidemia (14/58, 24%) were common underlying medical conditions.
TABLE 1.
Baseline characteristics of participants (N = 58).
| Characteristics | |
|---|---|
| Age (year), mean (SD) | 50.03 (15.07) |
| Female, n (%) | 33 (56.9) |
| Body mass index, kg/m2, mean (SD) | 23.85 (3.72) |
| Comorbidities, n (%) | |
| Hypertension | 15 (25.9) |
| Dyslipidemia | 14 (24.1) |
| Diabetes mellitus | 4 (6.9) |
| Aortic dissection | 1 (1.7) |
| Transient ischemic attack | 1 (1.7) |
| Concurrent medication, n (%) | |
| Antihypertensive medication | 26 (44.8) |
| Lipid‐lowering medication | 17 (29.3) |
| Psycholeptic treatment | 5 (8.6) |
| Anti‐diabetic medication | 4 (6.9) |
| Sex hormone treatments | 4 (6.9) |
| Antithrombotic agents | 3 (5.2) |
| Anti‐inflammatory agents | 3 (5.2) |
Abbreviation: SD, standard deviation.
3.1. Detection rate for significant arrhythmias
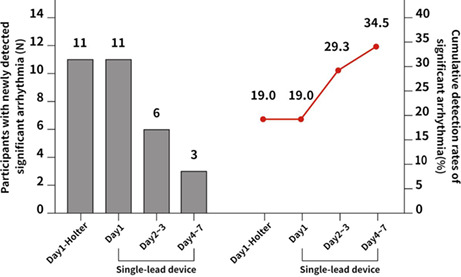
The detection rates of significant arrhythmias, defined as the outcomes, are summarized in Table 2. The cumulative detection rates of MEMO patches on days 1 and 7 were 19.0% (11/58) and 34.5% (20/58), respectively, and that of Holter monitors on day 1 was 19.0%. Of the 44 participants without arrhythmias in 24‐h Holter monitoring, nine had significant arrhythmias in 7‐day MEMO patch monitoring (Figure 2).
TABLE 2.
Detection of significant arrhythmias with a 24‐h Holter monitor versus a 7‐day MEMO patch monitor.
| MEMO patch monitor | 24‐h Holter monitor | p‐value | |
|---|---|---|---|
| Number of participants diagnosed with significant arrhythmias | 20/58 (34.5%) | 11/58 (19.0%) | .008 |
| Number of detected arrhythmias | 25 | 11 | .005 |
| Supraventricular tachycardia | 17 | 8 | .042 |
| Atrial fibrillation/atrial flutter | 6 | 2 | .143 |
| Sinus pause (≥3 s) | 1 | 1 | 1.000 |
| Second (Mobitz type II)/third‐degree AVB | 0 | 0 | ‐ |
| Ventricular tachycardia (≥3 events) | 1 | 0 | .315 |
| Polymorphic ventricular tachycardia/ventricular fibrillation | 0 | 0 | ‐ |
Abbreviation: AVB, atrioventricular block.
FIGURE 2.
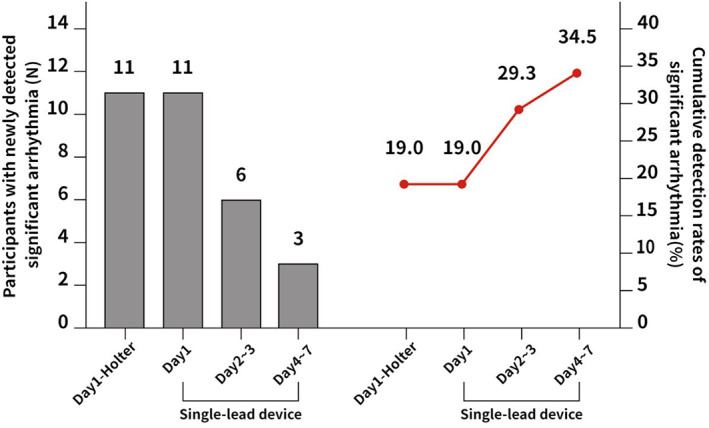
Detection and cumulative rates of significant arrhythmias using 24‐h Holter and 7‐day MEMO patch monitors.
Among the 58 participants, six atrial fibrillation/atrial flutter episodes were detected by MEMO patch monitoring, and only two were detected by Holter monitoring.
The detection rate for total significant arrhythmias was higher with MEMO patches (34.5% vs. 19.0%, p = .008), as shown in Table 2.
3.2. Detection of all arrhythmias
A total of 51 arrhythmias were detected by MEMO patch monitoring and 22 arrhythmias were detected by Holter monitoring, as shown in Table 3. Supraventricular tachycardia was the most commonly detected arrhythmia (29.3% by the MEMO patch and 13.8% by the Holter monitor, p = .042).
TABLE 3.
Detection of total arrhythmia events with a 24‐h Holter monitor versus a 7‐day MEMO patch monitor.
| MEMO patch monitor | 24‐h Holter monitor | p‐value | |
|---|---|---|---|
| Number of participants diagnosed with arrhythmias | 29/58 (50.0%) | 17/58 (29.3%) | .001 |
| Number of detected arrhythmias | 51 | 22 | <.001 |
| Supraventricular tachycardia | 17 | 8 | .042 |
| Atrial fibrillation/atrial flutter | 6 | 2 | .143 |
| Atrial premature beats | 4 | 4 | 1.000 |
| Ventricular premature beats | 5 | 5 | 1.000 |
| Tachycardia‐bradycardia syndrome | 1 | 0 | .315 |
| Sinus pause (≥3 s) | 1 | 1 | 1.000 |
| Second‐degree (Mobitz type I) AVB | 4 | 2 | .402 |
| Second‐degree (Mobitz type II) AVB | 0 | 0 | ‐ |
| Intermittent 2:1 AVB | 1 | 0 | .315 |
| Third‐degree AVB | 0 | 0 | ‐ |
| Wolff–Parkinson–White syndrome | 1 | 0 | .315 |
| Sick sinus syndrome | 1 | 0 | .315 |
| Short run of accelerated idioventricular rhythm | 1 | 0 | .315 |
| Ventricular tachycardia (≥3 events) | 1 | 0 | .315 |
| Polymorphic ventricular tachycardia/ventricular fibrillation | 0 | 0 | ‐ |
Abbreviation: AVB, atrioventricular block.
Notably, tachycardia‐bradycardia syndrome, Wolff–Parkinson–White syndrome, sick sinus syndrome, short run of accelerated idioventricular rhythm, and ventricular tachycardia were only detected using the MEMO patch. Several examples of arrhythmias detected by the MEMO patch are presented in Figure 3.
FIGURE 3.
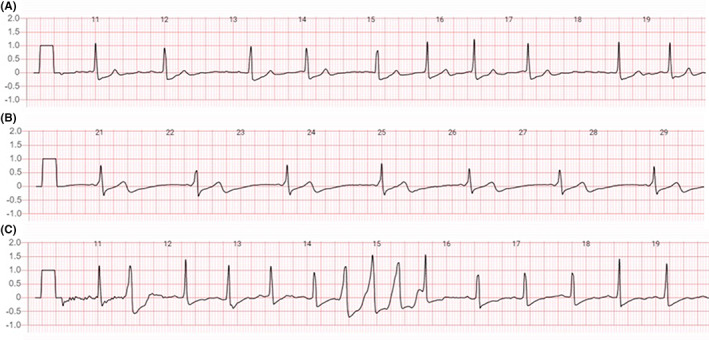
Examples of significant arrhythmias detected by a 7‐day electrocardiography monitor. (A) Electrocardiogram of a patient with asymptomatic atrial fibrillation. (B) Electrocardiogram of a patient with Wolff–Parkinson–White syndrome; (C) Electrocardiogram of a patient with ventricular tachycardia.
3.3. Adverse skin reactions
There were 99 reported adverse skin reactions among 9% (5/58) of the participants. Approximately 86% (85/99) of symptoms were self‐limited itching sensations with mild redness, which were categorized as mild reactions; 82% (81/99) of all cases occurred 24 h after adhesive patches were attached to the participants' skins. No severe reactions were observed in this study.
4. DISCUSSION
Our study demonstrated that 7 days of monitoring with a wearable ECG patch was superior to 24 h of monitoring with a Holter monitor for detecting clinically significant supraventricular tachycardia among patients with palpitations, chest pain, or syncope. However, 7 days of monitoring failed to detect other clinically significant arrhythmias including atrial fibrillation more effectively than a Holter monitor in our study participants.
Both MEMO patch monitoring and Holter monitoring showed similar arrhythmia detection rates over 24 h. Compared to 24‐h Holter monitoring, the detection rate increased 1.24‐fold during the extended 6 days of MEMO patch monitoring.
The detection rate with 14‐day Zio patch monitoring (iRhythm Technologies, Inc.) was higher than that with 24‐h Holter monitoring (96 vs. 61 events, p < .001). 10 Another study conducted by Chua et al. showed that 14 days of patch‐type ECG monitoring detected more arrhythmia events than did 24‐h Holter monitoring (66% vs. 9%, p < .001). 11 Another study also confirmed that 14‐day ECG patch monitoring showed higher arrhythmia detection rates (up to 60% [94/158]) than did 24‐h Holter monitoring (up to 19%). 13 The diagnostic yield of arrhythmias usually increases proportionally with the monitoring duration. However, several studies showed that approximately 90% of total arrhythmias can be detected if ECG monitoring periods are between 7 and 10 days. 11 , 13 In our study, 7‐days of ECG patch monitoring among generally low risk patients with symptoms could yield a low detection rate of significant arrhythmias other than supraventricular tachycardia. Thus, a longer monitoring duration of 14 days may be needed to detect significant arrhythmias including subclinical atrial fibrillation/flutter.
Holter monitors are the device of choice for cardiac monitoring to detect arrhythmias. However, because of their size, inconvenience, and relatively short monitoring period, their usefulness in long‐term ambulatory monitoring is limited. Recently, many wearable devices have been introduced for monitoring patients remotely, which are small, comfortable, and compact. Several modalities of ECG monitoring exist, including monitoring type (continuous versus event monitoring), presence of event trigger function, presence of real‐time monitoring, or duration of monitoring. 14 Compared with Holter monitors, patch‐type monitors consist of a single lead, are easier to use, and have higher adherence; however, they might suffer from low signal quality with different body types. 15 Patch‐type ECGs usually do not have functions such as event‐based recording or real‐time data monitoring. In patients with intermittent palpations, patch‐type ECG monitoring may be appropriate for symptom rhythm correlations.
Among patients treated for atrial fibrillation, 72‐h single‐lead ECG monitoring was superior to 24‐h Holter monitoring; an additional 13.6% of patients with negative results in Holter monitoring were diagnosed with paroxysmal atrial fibrillation using 72‐h single‐lead ECG monitoring. 16 Undiagnosed paroxysmal atrial fibrillation can lead to recurrent stroke or embolic events. 17 , 18 In addition, a higher atrial burden is a significant risk factor for clinical atrial fibrillation and future stroke. 19 In this regard, continuous monitoring using patch‐type ECG can be useful, especially for patients with a history of strokes or transient ischemic attacks. 20 Ambulatory patch‐type ECG monitoring may also be useful in emergency department patients with unexplained syncope. 21
Smartphone‐based arrhythmia detection using a combined approach with single‐lead ECG and photoplethysmography is a promising tool for the early detection of atrial fibrillation. 22 Due to their general availability, smartphone‐based algorithms enable patients to easily record symptomatic arrhythmias; however, short subclinical episodes may not be detected. A recent meta‐analysis showed that smartphones using only photoplethysmography‐based algorithms seemed to be biased, low‐quality monitoring devices with unrealistically high sensitivity and specificity. 23
Other types of portable devices include the external loop recorder, which has a recording time of up to 4 weeks, and requires activation by patients during the onset of symptoms. In contrast, patch‐type ECG monitors have no external leads or wires, usually have a recording time of up to 14 days, and continuously monitor the patients' signals; thus, they are more useful in conditions such as syncope. 24 Implantable loop recorders have the longest recording time of up to 3 years, activated by both an automatic algorithm and patient input; however, invasive procedures, high costs, and limited availability constrain their widespread use among patients. 24 Various devices with different diagnostic yields, cost‐effectiveness, and patient preference and convenience should be considered when choosing diagnostic methods for symptomatic patients or screening high‐risk patients.
In our study, we also found that meaningful arrhythmias, including sick sinus syndrome and Wolff–Parkinson–White syndrome, were only detected in MEMO patch monitoring. Tachycardia bradycardia syndrome and sick sinus syndrome can be categorized as sinus node dysfunction, which can cause many symptoms, including syncope, presyncope, and lightheadedness, and patients with these conditions may need permanent pacemakers and concomitant treatment for atrial fibrillation. 25 Wolff–Parkinson–White syndrome causes palpitations or syncope, and if accompanied by atrial fibrillation, it may cause ventricular fibrillation and sudden death, although that is rare. 26
This study has several limitations. The participants were recruited from one hospital, which limits the generalizability of the study findings. Moreover, there might have been false‐positive or false‐negative episodes with the MEMO patch monitoring that could not be accounted for because of the lack of validated comparable tools. However, considering the same detection rates on the first day of concomitant Holter and MEMO patch monitoring, it can be inferred that the detection rates of both devices were similar. Further studies are required to confirm the effectiveness of 7‐day continuous ECG patch monitoring in detecting meaningful arrhythmias in specific populations.
5. CONCLUSION
In this single‐center prospective trial, 7‐day patch‐type continuous ECG monitoring was more effective in detecting supraventricular tachycardia than was 24‐h Holter monitoring. However, detecting other types of significant arrhythmias with ECG patch monitoring might require a longer duration of more than 7 days. Further studies on the efficacy of detecting specific arrhythmias in certain populations are required. In addition, studying the comparative effectiveness will help choose the most appropriate options for patients in terms of monitoring periods, costs, convenience, and limitations among various types of ECG devices.
AUTHOR CONTRIBUTIONS
Ju Young Kim conceived, designed, and performed the study. Ji Hyun Lee, Youngjin Cho, and Hyejin Lee designed and performed the study and reviewed the literature. Yeongjoon Gil and Sunghoon Jung performed the artificial intelligence analytic program and were involved in the interpretation of the results. Dae In Kim, Joo Yeon Yoo, and Jeong Yeon Kwak performed the clinical study and collected clinical data. Dae In Kim, Myung Geun Shin, and Jeong Yeon Kwak performed statistical analyses. Il‐young Oh took part in the study design and was responsible for the final draft. All authors have contributed to the manuscript and approved the submitted version.
FUNDING INFORMATION
This work was mainly supported by the Korea International Cooperation Agency under the title “Prediction and management system for the patients of cardiovascular disease” between 2018 and 2021 (No. 2018‐1580). This study was also partly supported by the Korea Medical Device Development Fund from the Ministry of Science and ICT (Project number: 1711138361), Ministry of Trade Industry and Energy (Project number: RS‐2020‐KD00017), Ministry of Health & Welfare (Project number: 1711139106), and Ministry of Food and Drug Safety (Project Number: RS‐2021‐KD000011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
JuYK is employed part‐time at HUINNO Co., Ltd Seoul, Korea. YG, SJ, DK, MGS, JYY, and JK are currently employed at HUINNO Co., Ltd Seoul, Korea. The rest of the authors declare that they have no conflicts of interest.
DECLARATIONS
Approval of the research protocol: The Institutional Review Board of Seoul National University Bundang Hospital approved the protocol on February 08, 2019. Informed consent: All participants provided informed consent to participate before enrollment. Registry and the Registration No: Approval No: E‐1901‐516‐001. Animal studies: N/A.
ETHICS APPROVAL STATEMENT
The Institutional Review Board of Seoul National University Bundang Hospital approved the protocol (approval No: E‐1901‐516‐001).
PATIENT CONSENT STATEMENT
All participants provided informed consent to participate before enrollment.
ACKNOWLEDGMENTS
We would like to thank the following family physicians and research coordinators that collaborated with our study: Eunbyol Cho, Sumi Lee, Siye Gil, and Hyerim Kim, of the Seoul National University Bundang Hospital. We also thank all the patients who participated in the study.
Kim JY, Oh I‐Y, Lee H, Lee JH, Cho Y, Gil Y, et al. The efficacy of detecting arrhythmia is higher with 7‐day continuous electrocardiographic patch monitoring than with 24‐h Holter monitoring. J Arrhythmia. 2023;39:422–429. 10.1002/joa3.12865
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author (Il‐Young Oh) upon reasonable request.
REFERENCES
- 1. Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685–9. [DOI] [PubMed] [Google Scholar]
- 2. Paudel B, Paudel K. The diagnostic significance of the holter monitoring in the evaluation of palpitation. J Clin Diagn Res. 2013;7:480–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol. 2007;18:473–7. [DOI] [PubMed] [Google Scholar]
- 4. Sivakumaran S, Krahn AD, Klein GJ, Finan J, Yee R, Renner S, et al. A prospective randomized comparison of loop recorders versus Holter monitors in patients with syncope or presyncope. Am J Med. 2003;115:1–5. [DOI] [PubMed] [Google Scholar]
- 5. Yenikomshian M, Jarvis J, Patton C, Yee C, Mortimer R, Birnbaum H, et al. Cardiac arrhythmia detection outcomes among patients monitored with the Zio patch system: a systematic literature review. Curr Med Res Opin. 2019;35:1659–70. [DOI] [PubMed] [Google Scholar]
- 6. Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP, et al. Cardiologist‐level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yildirim O, Talo M, Ciaccio EJ, Tan RS, Acharya UR. Accurate deep neural network model to detect cardiac arrhythmia on more than 10,000 individual subject ECG records. Comput Methods Programs Biomed. 2020;197:105740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atal DK, Singh M. Arrhythmia classification with ECG signals based on the optimization‐enabled deep convolutional neural network. Comput Methods Programs Biomed. 2020;196:105607. [DOI] [PubMed] [Google Scholar]
- 9. Park J, Kim J‐k, Jung S, Gil Y, Choi J‐I, Son HS. ECG‐signal multi‐classification model based on squeeze‐and‐excitation residual neural networks. Appl Sci. 2020;10:6495. [Google Scholar]
- 10. Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, et al. Comparison of 24‐hour Holter monitoring with 14‐day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(95):e11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chua SK, Chen LC, Lien LM, Lo HM, Liao ZY, Chao SP, et al. Comparison of arrhythmia detection by 24‐hour Holter and 14‐day continuous electrocardiography patch monitoring. Acta Cardiol Sin. 2020;36:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang H. Sample size determination and power analysis using the G*power software. J Educ Eval Health Prof. 2021;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu CM, Chang SL, Yeh YH, Chung FP, Hu YF, Chou CC, et al. Enhanced detection of cardiac arrhythmias utilizing 14‐day continuous ECG patch monitoring. Int J Cardiol. 2021;332:78–84. [DOI] [PubMed] [Google Scholar]
- 14. Sharma AN, Baranchuk A. Ambulatory external electrocardiography monitoring: Holter, extended Holter, mobile cardiac telemetry monitoring. Card Electrophysiol Clin. 2021;13:427–38. [DOI] [PubMed] [Google Scholar]
- 15. Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, et al. 2017 ISHNE‐HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm. 2017;14:e55–96. [DOI] [PubMed] [Google Scholar]
- 16. Kwon S, Lee SR, Choi EK, Ahn HJ, Song HS, Lee YS, et al. Comparison between the 24‐hour holter test and 72‐hour single‐lead electrocardiogram monitoring with an adhesive patch‐type device for atrial fibrillation detection: prospective cohort study. J Med Internet Res. 2022;24:e37970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doijiri R, Yamagami H, Morimoto M, Iwata T, Hashimoto T, Sonoda K, et al. Paroxysmal atrial fibrillation in cryptogenic stroke patients with major‐vessel occlusion. Front Neurol. 2020;11:580572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alves M, Narciso MR, Cruz J, Rocha M, Fonseca T. Paroxysmal atrial fibrillation detection in patients with acute ischemic stroke through prolonged Holter: prospective study. Aging Clin Exp Res. 2019;31:469–74. [DOI] [PubMed] [Google Scholar]
- 19. Yang SY, Huang M, Wang AL, Ge G, Ma M, Zhi H, et al. Atrial fibrillation burden and the risk of stroke: a systematic review and dose‐response meta‐analysis. World J Clin Cases. 2022;10:939–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sposato LA, Cipriano LE, Saposnik G, Ruíz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta‐analysis. Lancet Neurol. 2015;14:377–87. [DOI] [PubMed] [Google Scholar]
- 21. Reed MJ, Grubb NR, Lang CC, Gray AJ, Simpson K, MacRaild A, et al. Diagnostic yield of an ambulatory patch monitor in patients with unexplained syncope after initial evaluation in the emergency department: the PATCH‐ED study. Emerg Med J. 2018;35:477–85. [DOI] [PubMed] [Google Scholar]
- 22. Papaccioli G, Bassi G, Lugi C, Parente E, D'Andrea A, Proietti R, et al. Smartphone and new tools for atrial fibrillation diagnosis: evidence for clinical applicability. Minerva Cardiol Angiol. 2022;70:616–27. [DOI] [PubMed] [Google Scholar]
- 23. Gill S, Bunting KV, Sartini C, Cardoso VR, Ghoreishi N, Uh HW, et al. Smartphone detection of atrial fibrillation using photoplethysmography: a systematic review and meta‐analysis. Heart. 2022;108:1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel UK, Malik P, Patel N, Patel P, Mehta N, Urhoghide E, et al. Newer diagnostic and cost‐effective ways to identify asymptomatic atrial fibrillation for the prevention of stroke. Cureus. 2021;13:e12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erickson SL. Wolff‐Parkinson‐white syndrome: a review and an update. Crit Care Nurse. 1989;9:28–35. [PubMed] [Google Scholar]
- 26. Chang W, Li G. Clinical review of sick sinus syndrome and atrial fibrillation. Herz. 2022;47:244–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Il‐Young Oh) upon reasonable request.


