Abstract
Objective
In recent years, the microneedle radiofrequency (MRF) has been widely used for skin rejuvenation, but histological studies on the immediate trauma caused by different parameters of non‐insulated RF microneedles
Methods
The skin of three pigs was treated with different needle depths, pulse widths and energy levels of non‐insulated microneedle RF. Samples were collected before, immediately, and 2 weeks after treatment. The immediate histological response of each group was assessed and quantified by hematoxylin and eosin staining, Masson staining and Victoria Blue staining.
Results
In the treatment of non‐insulated microneedle RF, different energy levels affected mainly the range of thermal damage (p = 0.044), and different needle depths affected mainly the depth of the cavity (p = 0.022). But the width of the coagulation zone width was determined by different factors. There was no significant difference in the histology of immediate damage caused by different pulse widths. Reepithelialization of the epidermis and basic wound repair can be completed within 2 weeks.
Conclusion
Non‐insulated RF microneedle therapy is an effective and safe treatment that can stimulate dermal wound healing with less thermal coagulation and a wide range of reversible thermal damage. However, it should be noted that the set needle depth may not correspond to the actual penetration depth, nor to the actual depth of histologic trauma.
Keywords: histological reaction, microneedle radiofrequency, non‐insulated, skin, thermal damage
1. INTRODUCTION
Over the past decade, microneedles radiofrequency (MRF) has been widely used to combat aging and treat a variety of skin problems. 1 , 2 Multiple arrays of microneedles are arranged on the disposable tip, and RF electricity is transmitted through the microneedles to form an RF thermocoagulation zone in the tissue and remodel collagen and elastin fibers to achieve skin reconstruction. 3 MRF perfectly combines the mechanical transdermal function of microneedles with the thermal damage of RF, which can deliver RF energy at a deeper tissue depth than traditional ablative laser or RF alone.
The needle body of MRF devices is divided into insulated and non‐insulated microneedles. The first generation of MRF output technology is insulated microneedles, which release energy only at the needle tip. By adjusting the penetration depth, the energy can be transferred to the fixed layer of the dermis. 4 Currently, the iteratively developed non‐insulated microneedles can deliver RF throughout the needle body. Each puncture can effectively stimulate each layer of the dermis, and heat the dermis tissue in a larger area, avoiding multiple punctures with RF, effectively shortening the treatment time, reducing pain, and achieving a better clinical effect.
The parameters of non‐insulated RF microneedles mainly include needle depth, RF pulse width, and RF energy. Hantash et al. 5 performed the first study on the histologic effect of RF microneedles and found that the impedance of the different skin layers varied greatly, which directly determined the range of the RF thermocoagulation zone generated according to RF and thus determined the repair time and final clinical effect. Zheng et al. 6 also performed a more detailed study using MRF and showed that the depth of treatment positively correlated with the height of the thermocoagulation zone formed. However, these two studies were based on the insulated microneedles. Compared to the non‐insulated microneedles, both have different modes of RF energy delivery, so the microdamage zone in the skin varies accordingly. What are the histological effects of different combinations of non‐insulated MRF treatment parameters, and how does increasing the needle depth, pulse width and power affect the thermal damage to the skin? These are the questions we need to answer.
In this study, pigs were chosen as the experimental model, whose skin tissue structure is most similar to that of humans. Using the new generation of non‐insulated MRF devices, we investigated the characteristics of immediate skin injury by insulated MRF in various parameters, including injury zone characteristics and quantitative statistics, which could help us better understand the histological effects of insulated MRF. Moreover, this would not only provide a sufficient theoretical basis for clinical application but also a reliable histological basis for future research and development of MRF devices.
2. METHODS AND METERIALS
Three male Bama pigs, 8–9 months old, weighing 16–18 kg. The EndyMed PRO platform system (EndyMed Medical, Caesarea, Israel) is equipped with the Intensif microneedle RF handpiece. The disposable tip is 5×5 matrices composed of 25 non‐insulated microneedles. The Ethics Committee of the Plastic Surgery Hospital of the Chinese Academy of Medical Sciences has approved the study [2022(231)]. All experiments were performed in accordance with the approved guidelines.
2.1. Study design
Seven treatment areas with different parameters were designed for the backs of the pigs, as shown in Figure 1. Methylene was used to mark each area on the skin. The size of each treatment area was set at 3×4 cm and evenly distributed on the backs of the pigs to ensure that the distance between each treatment area was large enough to reduce the influence of RF on other treatment areas. Each treatment area was treated with two passes.
FIGURE 1.
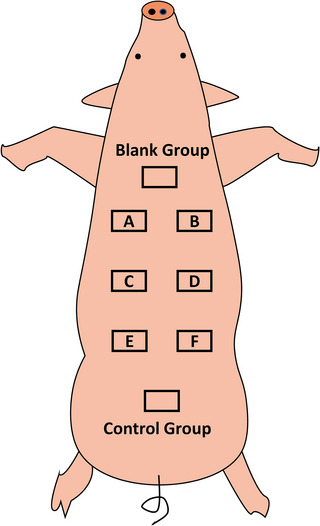
Experimental design on pigs.
The specific treatment parameters for each area are listed in Table 1. The formula for calculating the energy level of each needle is (power×pulse width)/number of needles. Group A and Group B were set to the same needle depth and power to compare histological differences at different pulse widths, while Group B and Group C were set to the same pulse width and power to compare histological differences at different needle depths (shallow penetration). Group C and Group D were set to the same needle depth and pulse width to compare the histological differences at two energy levels. To compare the histological difference at different needle depths (deep penetration), the pulse width and power were set to be the same for Group E and Group F. At the same time, the Control group was set as a simple mechanical trauma group, with the needle depth set to 2.5 mm and the energy set to 0. The empty skin group of each experimental pig was taken from the untreated back area about 5 cm above the whole treatment area.
TABLE 1.
Grouping parameters of microneedle radiofrequency (MRF).
| Group | Needle depth (mm) | Pulse width (ms) | Power (W) | Energy level (mJ/pin) |
|---|---|---|---|---|
| A | 1.5 | 80 | 12 | 38.4 |
| B | 1.5 | 110 | 12 | 52.8 |
| C | 2.5 | 110 | 12 | 52.8 |
| D | 2.5 | 110 | 18 | 79.2 |
| E | 3.5 | 140 | 18 | 100.8 |
| F | 5.0 | 140 | 18 | 100.8 |
| Control | 2.5 | 110 | 0 | 0 |
Before the procedure, pigs were given general anesthesia by intramuscular injection of 0.5 mg/kg tiletamine and zolazepam (Zoletil 50, VIRBAC, FR) with atropine (0.05 mg/kg). The intraoperative dose of Sutaide was half of the initial dose. After anesthesia, the back was shaved, gently cleaned with soap, disinfected with iodophor and alcohol, and then treated with MRF. The skin reaction of pigs was evaluated and photographed before and after the treatments. The whole skin layer and part of the subcutaneous tissue were taken from each treatment group before, immediately, and 2 weeks after the treatment. The wound was sutured with 2‐0 nylon thread, and then the whole back was disinfected with alcohol three times. After each operation, 1.6 million units of penicillin sodium were injected intramusculally for 3 days. The pigs were euthanized at the end of the experiment.
2.2. Macroscopic examination and histological analysis
After fixation in 10% buffered formalin, the samples were embedded in paraffin and sliced with a thickness of 4 µm. From each treatment area, 20–30 consecutive sections were taken to capture and confirm images of the deepest lesions in each treatment group. Hematoxylin and eosin (HE) staining were used to examine the injury morphology of the skin tissue, Masson staining was used to examine changes in collagen fibers, and Victoria Blue staining was used to observe changes in elastic fibers. The slides were scanned and analyzed using a microdigital slice scanning system (EasyScan Pro 6, Motic Ltd, CN). The depth of the cavity, the width of the coagulation zone, and the sub‐damage zone on one side were measured using HE staining. The deepest and most obvious damage was taken from each experimental pig in each treatment area for measurement.
2.3. Statistical analysis
SPSS 26.0 software was used to analyze the data results, which are expressed as mean ± SD. An unpaired t‐test was used for a two‐group comparison. Multiple comparisons were assessed with ANOVA analysis, and the LSD test was used for further comparisons. The difference was statistically significant at p < 0.05.
3. RESULTS
3.1. General injury pattern
Immediately after MRF, the injury was grossly elliptical in a flame‐like stratification divided mainly into three levels: at the top, the area of the cavity with tissue loss. It continued downward into the coagulation zone surrounded by the zone of thermal injury. Figure 2A shows Masson staining and Victoria blue staining of the tissue immediately after treatment, indicating that both collagen fibers and elastic fibers were typically damaged by heat injury.
FIGURE 2.

Histological effect immediately after microneedle radiofrequency (MRF). (A) Injury in Group E (needle depth: 3.5 mm, pulse width: 140 ms, power: 18 W, energy level: 100.8 mJ/pin). (left) Masson stain. (right) Victoria Blue stain. Scale bar = 60 µm. (B) Immediate skin response in each group. (C) Thermal damage of MRF would not destroy the structural integrity of skin appendage (Group A: needle depth of 1.5 mm, pulse width 80 ms, power 12 W, energy level 38.4 mJ/pin). Scale bar = 30 µm. (D) Typical immediate histologic response in each group in hematoxylin and eosin (HE) staining. Scale bar = 60 µm. (E) Quantitive statistics of each group. (F) Histological effect on same needle depth with RF and without RF: (left) Group C (needle depth: 2.5 mm, pulse width: 110 msec, power: 12 W, energy Level: 52.8 mJ/pin). (right) Control Group (Needle Depth: 2.5 mm, Power: 0 V). Scale bar = 60 µm.
3.2. Quantitive characteristics of injury
Figure 2B shows the immediate skin reactions after treatment with different parameters. The skin in all groups showed mild redness and swelling, among which groups E and F were accompanied by some small hemorrhagic spots. Figure 2D displayed the typical histological of groups A–F in the HE staining. The transverse comparison under different parameters can be observed. In Figure 2E, the differences in the depth of the cavity, the width of the coagulation zone, and the sub‐damage zone in groups A–F were analyzed.
To investigate the effect of different needle depths on the immediate response, we compared Group B with Group C at shallow penetration (1.5 mm vs 2.5 mm) and Group E with Group F at deep penetration (3.5 mm vs 5.0 mm). For shallow penetration, the cavity depth of Group C was significantly deeper than that of Group B (group B:112.41 ± 45.31 µm vs. Group C:288.84 ± 98.00 µm, p = 0.022). However, no significant difference in cavity depth was observed for deep penetration (Group E: 358.80 ± 87.85 µm vs. Group F: 402.06 ± 19.44 µm, p = 0.531). The change in power may have a significant effect on the width of the sub‐damage zone (Group C: 146.03 ± 126.68 µm vs. Group D: 303.57 ± 40.15 µm, p = 0.044), but had no significant contribution on either cavity depth or coagulation zone width. The different pulse width setting has no obvious effect on the coagulation zone and the sub‐damage zone. As the energy level was increased, the micro‐damage zone gradually increased. There were significant differences in the width of the coagulation zone between the different groups (p = 0.01), but there was no significant difference between the groups when compared under a single factor.
3.3. Comparison in cavity depth with control group
Instant trauma of Group C was compared with those of the Control group (Figure 2F). Under the same needle depth, the damage in the Control group was circular in shape, without an obvious coagulation zone or surrounding sub‐damage zone. The cavity depth of the Control Group was (159.54 ± 35.82) µm, which had no significant difference from the one in the C group (p = 0.103).
3.4. Changes on skin appendages
As to the influence of MRF on skin appendages, we found pictures that MRF directly acting on sebaceous glands under Group A parameters (Figure 2C). The sub‐damaged area covered part of the hair matrix and dermal papilla area, but did not damage the integrity of the hair follicle structure.
3.5. Reepithelialized process
At 2 weeks, skin redness and swelling were not observed. Scab, congestion, infection, blister and other reactions were not found in all groups. After examination of sections in each group, no obvious MRF injuries were found in the epidermis and dermis. The epidermis has been reepithelialized. Fibroblasts and neutrophils were observed between collagen fibers in the dermis, while new collagen was produced to replace the hollow coagulation area. However, the sparse distribution of dermal edema in the acute stage of inflammation led to the separation of collagen fibers and the formation of edema space. Figure 3A showed the skin color of the clinical patient returned to normal and the wound was slightly scab 4 days after MRF.
FIGURE 3.
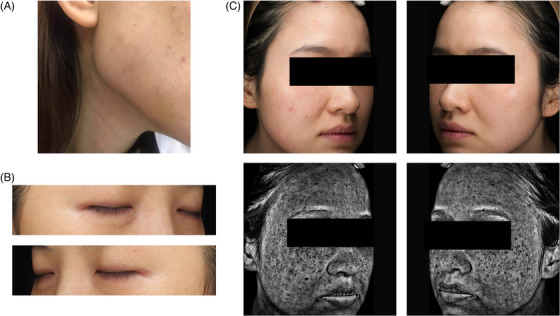
Clinical images after microneedle radiofrequency (MRF) treatment. (A) 1 week after treatment. (B) One month after RF treatment, the color of the bilateral lower eyelids remained grid‐like hyperpigmentation. (C) One month after MRF treatment, grid‐like dark hyperpigmentation was left on both cheeks. (top) Standard digital photo. (bottom) Visia photo.
4. DISCUSSION
From the schematic representation of the immediate histologic response after MRF, it is evident that the gross injury has a flame‐like, layered structure with a cavity at the tip. With the same needle depth setting, we found that there was no difference in the depth of the cavity between Group C with RF and the Control Group without RF, indicating that the cavity area was mainly caused by mechanical damage during penetration. The shape of the damage may be related to the ways, speed, penetration depth of the needles, and thickness of the skin. The outermost layer is the annular area of thermal damage caused by thermal dispersion in the dermis. It would become a strong driving force to initiate the tissue repair response and collagen regeneration. Cho et al. 7 observed similar forms of injury during RF treatment with microneedles on mouse skin. The thermal subdamaged area surrounding the cavitation and coagulation zone is the degeneration of collagen fibers caused by mass heating. In HE staining, it shows up as basophilic collagen. This thermal sub‐damaged area is key to the subsequent remodeling and regeneration of collagen.
The microdamage zone of non‐insulated MRF is mainly composed of “mechanical damage + tissue coagulation + thermal damage”. The histological characteristics of the microlesion were significantly different from those of the microlesion caused by the fractional laser (ablative CO2 laser and non‐ablative laser), as shown in Figure 4. The ablative fractional CO2 laser (Encore, Luminis, MA) in Deep FX mode is a regular wedge‐shaped gasification zone. Due to the high energy density and small spot area, the thermal damage zone is deep and narrow, while the heat dispersion range is small. However, the microdamage zone of the non‐ablative fractional laser is a regular columnar coagulation zone, and the thermal damage zone is almost absent.
FIGURE 4.

Tissue damage characteristic of non‐insulated microneedle radiofrequency (MRF), fractional ablative CO2 laser (Deep FX mode), and fractional non‐ablative laser.
The optimal mode of thermal damage to tissue is to minimize necrotizing skin damage while bringing heat into deeper layers of tissue as much as possible. Compared with CO2 laser, the cavity formed by MRF is smaller, but the repair is faster. Meanwhile, the depth and width of the thermal damage area generated by MRF are significantly greater than those caused by the above two. In our work, MRF‐induced tissue defect repair and epithelialization were completed in 2 weeks in all experimental groups, while Snast et al. 8 proved that 7–14 days following the treatment on 240 mJ of CO2 laser, although the re‐epithelialization was complete, the defect of the dermal cavity still needed further repair. MRF can directly deliver mass heating into the deep dermis through penetration. Additionally, it has a longer pulse width (about 100 ms) than fractional lasers (about 1 ms), resulting in more extensive thermal dispersion.
Greater sub‐damage zone reach, which means a wider range of heat dispersion, resulting in more extensive thermal damage and collagen remodeling and regeneration. Of course, if the energy level is too high, the volume of the microlesion will also be too large, leading to the occurrence of postoperative pinocular or grid‐like pigmentation (Figure 3B,C) in both photograph and VISIA (Canfield Scientific., Fairfield, NJ, USA) images.
As for the actual treatment depth in the tissue, on the one hand, we found that a significant part of the needle body cannot penetrate the skin, especially at a deep insertion depth. This means that the set needle depth may not be fully inserted into the skin. When comparing shallow penetration (Groups B and C: 1.5 and 2.5 mm) and deep penetration (Groups E and F: 3.5 and 5.0 mm), there were significant differences in the cavity depth. However, there were no significant differences between the deep penetration comparison. During the experiment, we also notice that when the needle depth was set at 1.5 and 2.5 mm, the needle could basically fully penetrate the pig skin, while when the needle depth was set at 3.5 and 5.0 mm, the needle body obviously could not fully penetrate the skin, as shown in the video in Supplementary 1. So if the needle penetrates the skin completely, the setting of the needle depth is proportional to the depth of the cavity. But when the needle depth is set too deep to fully penetrate the skin, there was a small difference in the actual skin penetration depth, which parallels our results. In clinical treatment, when treating soft tissue areas (e.g., cheeks) or when the needle depth was set too deep (≥3 mm), it could be seen that the needle body could not fully penetrate the skin.
On the other hand, even if the needle body can completely enter the skin, the depth of the actual micro‐damage lesion was much shallower than the set needle depth. Taking Group C as an example, the needle depth was set at 2.5 mm, but the depth of the cavity area was only (288.84 ± 98.00) µm, which was much shallower than the set needle depth.
More importantly, the heat of non‐insulated microneedles is evenly distributed throughout the needle body, which is different from that of insulated microneedles, only distributing in the tip. When the same energy level is applied to different needle depths, the energy allocated to the unit depth of microneedles piercing into the skin will be definitely different. Therefore, the actual needle depth will greatly affect the actual treatment intensity. How to ensure the standardization of the penetration depth is a problem to be solved in the development of the equipment. It is also a crucial prerequisite for further improving the MRFs.
In this study, we designed two passes of treatment in the same area, but the microlesions on the histological sections were relatively few and sparse. To some extent, it affects our measurement of microlesion. It also means that the treatment coverage density of two passes is still far from adequate. the tip head's area is 64mm2, with a total of 25 needles. Taking group C as an example, the diameter of microlesion caused by each needle was about 367 µm, and the cross‐sectional area was about 2.75 mm2. The coverage rate of a single pass was only about 4.30%, which is very safe coverage. Therefore, the treatment density can be increased clinically by increasing the treatment pass. 2‐3 passes are currently safe and feasible. However, if we choose to increase the energy level to achieve increasing the area of each microlesion, in order to improve the treatment coverage, it may lead to the occurrence of postoperative pinocular pigmentation. The latest generation of MRF should consider increasing the needle density to reduce the treatment pass and duration.
In addition, it was observed in our experiment, that when MRF was applied directly near the hair follicle with low energy, it would not damage the integrity of the hair follicle. Zheng et al. 6 also found that the structural integrity of the hair follicle was damaged when the RF microneedle depth was set at 3.5 mm, the energy level was 50.0 V and the pulse width was 50 ms, but when the energy was halved, the structural integrity of the hair follicle was only coagulated without damaging. This suggests that RF microneedles are energy dependent on hair follicles or sebaceous glands, safe for hair use at appropriate energy levels.
5. CONCLUSION
Non‐insulated RF microneedle therapy is an effective and safe treatment that can stimulate dermal wound repair with less thermal coagulation and a wide range of reversible thermal damage. However, it should be pointed out that the set needle depth may not be consistent with the actual penetration depth, and also with the actual depth of histologic trauma.
Supporting information
Supplementary 1 The microneedles cannot penetrate the pig's skin completely in Group F (needle depth: 5.0 mm, pulse width: 140 ms, power: 18 W, energy level: 100.8 mJ/pin).
ACKNOWLEDGMENTS
This study was supported by the Special Research Fund for Plastic Surgery Hospital, the Chinese Academy of Medical Sciences, and Peking Union Medical College (YS202034). The authors declare that they have no conflict of interest. EndyMed (EndyMed Medical, Caesarea, Israel) provided Intensfi Tips for this research.
Feng J, Zhang L, Qi J, Huang L. Histological damage characteristics and quantitive analysis of porcine skin with non‐insulated microneedle radiofrequency. Skin Res Technol. 2023;29:ert13396. 10.1111/srt.13396
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, Huang, upon reasonable request.
REFERENCES
- 1. Tan MG, Jo CE, Chapas A, Khetarpal S, Dover JS. Radiofrequency microneedling: a comprehensive and critical review. Dermatol Surg. 2021;47(6):755‐761. [DOI] [PubMed] [Google Scholar]
- 2. Dayan E, Chia C, Burns AJ, Theodorou S. Adjustable depth fractional radiofrequency combined with bipolar radiofrequency: a minimally invasive combination treatment for skin laxity. Aesthet Surg J. 2019;39(3):S112‐S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alessa D, Bloom JD. Microneedling options for skin rejuvenation, including non‐temperature‐controlled fractional microneedle radiofrequency treatments. Facial Plast Surg Clin North Am. 2020;28(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 4. Cohen BE, Elbuluk N. Microneedling in skin of color: A review of uses and efficacy. J Am Acad Dermatol. 2016;74(2):348‐355. [DOI] [PubMed] [Google Scholar]
- 5. Hantash BM, Renton B, Berkowitz RL, Stridde BC, Newman J. Pilot clinical study of a novel minimally invasive bipolar microneedle radiofrequency device. Lasers Surg Med. 2009;41(2):87‐95. [DOI] [PubMed] [Google Scholar]
- 6. Zheng Z, Goo B, Kim D‐Y, Kang J‐S, Cho SB. Histometric analysis of skin‐radiofrequency interaction using a fractionated microneedle delivery system. Dermatol Surg. 2014;40(2):134‐141. [DOI] [PubMed] [Google Scholar]
- 7. Cho SB, Na J, Zheng Z, Lim JM, Kang JS, Lee JH, et al. In vivo skin reactions from pulsed‐type, bipolar, alternating current radiofrequency treatment using invasive noninsulated electrodes. Skin Res Technol. 2018;24(2):318‐325. [DOI] [PubMed] [Google Scholar]
- 8. Snast I, Lapidoth M, Levi A. Clinical and histological evaluation of a dual sequential application of fractional 10,600 nm and 1570 nm lasers, compared to single applications in a porcine model. Lasers Med Sci. 2022;37(3):1983‐1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1 The microneedles cannot penetrate the pig's skin completely in Group F (needle depth: 5.0 mm, pulse width: 140 ms, power: 18 W, energy level: 100.8 mJ/pin).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Huang, upon reasonable request.


