Abstract
Flowers are critical for successful reproduction and have been a major axis of diversification among angiosperms. As the frequency and severity of droughts are increasing globally, maintaining water balance of flowers is crucial for food security and other ecosystem services that rely on flowering. Yet remarkably little is known about the hydraulic strategies of flowers. We characterized hydraulic strategies of leaves and flowers of ten species by combining anatomical observations using light and scanning electron microscopy with measurements of hydraulic physiology (minimum diffusive conductance (g min) and pressure-volume (PV) curves parameters). We predicted that flowers would exhibit higher g min and higher hydraulic capacitance than leaves, which would be associated with differences in intervessel pit traits because of their different hydraulic strategies. We found that, compared to leaves, flowers exhibited: 1) higher g min, which was associated with higher hydraulic capacitance (C T); 2) lower variation in intervessel pit traits and differences in pit membrane area and pit aperture shape; and 3) independent coordination between intervessel pit traits and other anatomical and physiological traits; 4) independent evolution of most traits in flowers and leaves, resulting in 5) large differences in the regions of multivariate trait space occupied by flowers and leaves. Furthermore, across organs intervessel pit trait variation was orthogonal to variation in other anatomical and physiological traits, suggesting that pit traits represent an independent axis of variation that have as yet been unquantified in flowers. These results suggest that flowers, employ a drought-avoidant strategy of maintaining high capacitance that compensates for their higher g min to prevent excessive declines in water potentials. This drought-avoidant strategy may have relaxed selection on intervessel pit traits and allowed them to vary independently from other anatomical and physiological traits. Furthermore, the independent evolution of floral and foliar anatomical and physiological traits highlights their modular development despite being borne from the same apical meristem.
Keywords: hydraulics, water relations, xylem, flower, drought tolerance, minimum cuticular conductance, photosynthesis, leaf
1. Introduction
Flowers play a crucial role during the reproductive phase of angiosperms, and their importance during this period has influenced angiosperm diversification and spread (Crane et al., 1995; Sprengel, 1996; Sargent and Ackerly, 2008). Producing and maintaining flowers requires the allocation of resources, such as water, carbon, and nutrients (Bazzaz et al., 1987; Reekie and Bazzaz, 1987a; Reekie and Bazzaz, 1987b; Reekie and Bazzaz, 1987c). Though these physiological costs of flowers are often assumed to be minimal in the context of the whole plant, resource limitation or stressful abiotic conditions can exacerbate the costs of producing and maintaining flowers (Lambrecht, 2013; Burkle and Runyon, 2016; Waser and Price, 2016; Bourbia et al., 2020; Harrison Day et al., 2022). The abiotic conditions that influence the physiological costs also act as agents of selection on floral traits and, in general, can be as strong an agent of selection on flowers as pollinators (Ashman and Schoen, 1994; Galen et al., 1999; Caruso, 2006; Lambrecht and Dawson, 2007; Teixido and Valladares, 2014; Lambrecht et al., 2017; Roddy et al., 2018; Caruso et al., 2019; Roddy et al., 2019; Kuppler and Kotowska, 2021a; Kuppler et al., 2021b). One of the most important resources for plant growth and reproduction is water, and the frequency and severity of droughts is increasing globally (Adams et al., 2017; Choat et al., 2018; Brodribb et al., 2020). These droughts potentially threaten food production and other ecosystem services that rely on flowering. Thus, maintaining water balance is critical to flower functioning, successful reproduction, and flower and fruit development (Galen et al., 1999; Lambrecht, 2013; Roddy et al., 2016; Zhang et al., 2018; Bourbia et al., 2020).
Like leaves, flowers are terminal structures often located in the hottest and driest parts of the plant crown, meaning they are exposed to similar evaporative environments as leaves (Blanke and Lovatt, 1993; Roddy and Dawson, 2012). In order to optimize photosynthesis in leaves, plants must prevent declines in water content, which requires coordination in the structural traits governing water flow through each component of the plant hydraulic pathway (Brodersen et al., 2014; Jupa et al., 2017; Li et al., 2019; Song et al., 2021; Fontes et al., 2022). For example, coordination between leaf vein density and stomatal density is nearly ubiquitous across studies and highlights the important roles that leaf veins and stomata play in coordinating liquid and vapor fluxes through the leaf to maintain water balance (Sack and Frole, 2006; Brodribb et al., 2007; Noblin et al., 2008; Boyce et al., 2009; Brodribb and Cochard, 2009; de Boer et al., 2012). However, over short timescales, water loss can exceed water supply, causing declines in water potentials in the xylem. Under extreme cases, excessive water loss and low water potentials can pull air into the xylem vessels from either outside the xylem or from adjacent, already embolized vessels, leading to the spread of air embolisms and xylem dysfunction (Dixon and Joly, 1895; Sperry and Tyree, 1988; Lens et al., 2011; Tyree and Zimmermann, 2013).
One of the major determinants of both the vulnerability of the xylem to embolism spread and also the efficiency of water flow through the xylem is the structure of intervessel pits and pit membranes that connect adjacent xylem conduits. Pit membranes, in particular, can be responsible for 50% or more of the total hydraulic resistance in the xylem (Wheeler et al., 2005; Choat et al., 2006; Hacke et al., 2006; Pittermann et al., 2006; Kaack et al., 2019). Comparative studies have shown that pit morphology can vary in terms of pit and pit aperture size, pit shape, and pit density, and that these pit traits can correlate with vessel diameter, vessel wall thickness, and photosynthetic rates, and vary both among species and among habitats (Schmitz et al., 2007; Lens et al., 2011; Jacobsen et al., 2016; Li et al., 2019; Zhang et al., 2021). In general, larger pit membranes are associated with higher hydraulic conductivity but are more vulnerable to embolism (Choat et al., 2005; Wheeler et al., 2005; Ellmore et al., 2006; Hacke et al., 2006; Lens et al., 2011). Furthermore, pit aperture shape can also influence embolism resistance: species with more cavitation-resistant branches exhibit narrower and more elliptical pit apertures (Lens et al., 2011; Scholz et al., 2013; Li et al., 2019). Thus, intervessel pit traits are important factors influencing both hydraulic safety and efficiency (Hacke et al., 2009; Jansen et al., 2009; Blackman et al., 2010; Li et al., 2016; Zhang et al., 2021), though they have not been systematically quantified in reproductive organs.
Compared to leaves, relatively little is known about the hydraulic traits of flowers, despite their importance to reproduction for most species (Gleason, 2018). Flowers have, at most, very few stomata (Lipayeva, 1989; Roddy et al., 2016; Zhang et al., 2018), meaning that water loss occurs primarily via diffusion across the cuticle (Roddy, 2019), and very low vein densities compared to leaves (Roddy et al., 2013; Zhang et al., 2018). As a result, minimum diffusive conductance (g min) (Kerstiens, 1996) is strongly coordinated with petal vein density and hydraulic conductance, suggesting that g min is critical to floral water balance and hydraulic conductance (Roddy et al., 2016). This has important implications for water balance during drought conditions. While leaves can close their stomata to limit water loss (Meinzer, 2002), without stomata flowers are likely unable to curtail water loss (Roddy et al., 2016; Roddy, 2019), which can cause them to lose more water during drought than leaves (Lambrecht, 2013; Bourbia et al., 2020). Thus, flower water potentials may decline more quickly than leaf water potentials and possibly cause air embolisms to spread more quickly through the xylem in flowers than in leaves (Zhang and Brodribb, 2017; Bourbia et al., 2020), depending on the morphology of intervessel pit traits in flowers and leaves (Zhang et al., 2021). However, hydraulic capacitance can buffer declines in water potentials that lead to embolism spread, and flowers have significantly higher hydraulic capacitance than leaves (Roddy et al., 2019). Since flowers are short-lived but have high water demands (Roddy and Dawson, 2012; Lambrecht, 2013; Roddy et al., 2018; Bourbia et al., 2020), flowers might employ different hydraulic strategies than leaves and stems and exhibit different coordination between hydraulic traits than leaves. High water demands and greater reliance on stored water may physiologically buffer flowers from diurnal variability in the water status of other plant structures. Prior evidence based on 132 species has suggested that vegetative and reproductive structures may be developmentally modular, with independent evolution of vein density in flowers and leaves (Roddy et al., 2013). Similarly, based on data from about 20 species, flowers tend to have higher water contents and hydraulic capacitance than leaves. These differences in venation and pressure-volume traits may be linked to other differences in hydraulic anatomy and physiology. Yet remarkably little is known about the hydraulic strategies of flowers and their mechanisms of maintaining water balance.
In the present study, we characterized a diverse set of anatomical and physiological traits in both leaves and flowers of ten angiosperm species ( Tables 1 , 2 ). These traits included vein and stomatal traits, minimum diffusive conductance (g min), parameters derived from pressure-volume curves (Scholander et al., 1965; Tyree and Hammel, 1972), and pit traits measured using scanning electron microscopy (SEM). We used this diverse set of traits to address the following questions (1) Do species with higher g min have higher hydraulic capacitance, which could buffer water potential declines due to excessively high g min? (2) Do flowers and leaves exhibit differences in intervessel pit structure reflecting their different hydraulic strategies? (3) Are anatomical and physiological traits in leaves and flowers coordinated, which would indicate similar hydraulic strategies? We hypothesized that flowers would exhibit higher g min and higher hydraulic capacitance than leaves. Since flowers may rely on high water content and hydraulic capacitance to support high g min, we also hypothesized that intervessel pit traits and the coordination of anatomical and physiological traits would differ in flowers and leaves and indicate different hydraulic strategies.
Table 1.
List of species in this study.
| Species | Family | Genus | Code |
|---|---|---|---|
| Bauhinia × blakeana Dunn | Fabaceae | Bauhinia | Bb |
| Bidens pilosa L. | Asteraceae | Bidens | Bp |
| Bougainvillea spectabilis Willd. | Nyctaginaceae | Bougainvillea | Bs |
| Catharanthus roseus (L.) G. Don | Apocynaceae | Catharanthus | Cr |
| Ceiba speciosa (A.St.-Hil.) Ravenna | Malvaceae | Ceiba | Cs |
| Hibiscus rosa-sinensis L.var. rubro-plenus Sweet | Malvaceae | Hibiscus | Hr |
| Michelia × alba DC. | Magnoliaceae | Michelia | Ma |
| Rhododendron sp. | Ericaceae | Rhododendron | Rh |
| Rosa sp. | Rosaceae | Rosa | Ro |
| Ruellia simplex C.Wright | Acanthaceae | Ruellia | Rs |
Table 2.
List of major characters with definition and units.
| Symbol | Definition | Units | |
|---|---|---|---|
| Minimum diffusive conductance and theoretical hydraulic conductivity | |||
| g min,area | Minimum diffusive conductance (normalized by the projected area of each organ) | mmol m-2 s-1 | |
| g min,mass | Minimum diffusive conductance (normalized by the dry mass of each organ) | mmol g-1 s-1 | |
| K th | Theoretical hydraulic conductivity | kg m-1 MPa-1 s-1 | |
| Pressure–volume parameters | |||
| C T | Absolute capacitance, normalized by dry mass | mol kgs-1 MPa-1 | |
| SWC | Saturated water content | g g-1 | |
| Ψsft | Osmotic potential at full turgor | MPa | |
| Ψtlp | Water potential at turgor loss point | MPa | |
| SEM anatomical traits | |||
| D pml | Diameter of the outer pit membrane along the longest axis | µm | |
| D pms | Diameter of the outer pit membrane along the shortest axis | µm | |
| D pal | Diameter of the outer pit aperture along the longest axis | µm | |
| D pas | Diameter of the outer pit aperture along the shortest axis | µm | |
| A pit | Intervessel pit membrane surface area | µm2 | |
| A pa | Intervessel pit aperture surface area | µm2 | |
| R pa | Pit aperture shape = ratio of the longest axis of outer pit aperture to the shortest axis | ||
| R pit | Pit membrane shape = ratio of longest axis of outer pit membrane to the shortest axis | ||
| D p | Pit density = number of intervessel pits per vessel wall area | no.µm-2 | |
| LM anatomical traits | |||
| S s | Stomatal size | µm2 | |
| D s | Stomatal density | no.mm-2 | |
| D v | Vein density | mm mm-2 | |
| LT | Leaf thickness | µm | |
| FT | Flower thickness | µm | |
| D h | Hydraulically-weighted vessel diameter | µm | |
| T w | Double vessel wall thickness | µm | |
| VF | Vessel frequency | no.mm-2 | |
2. Materials and methods
2.1. Plant species and study site
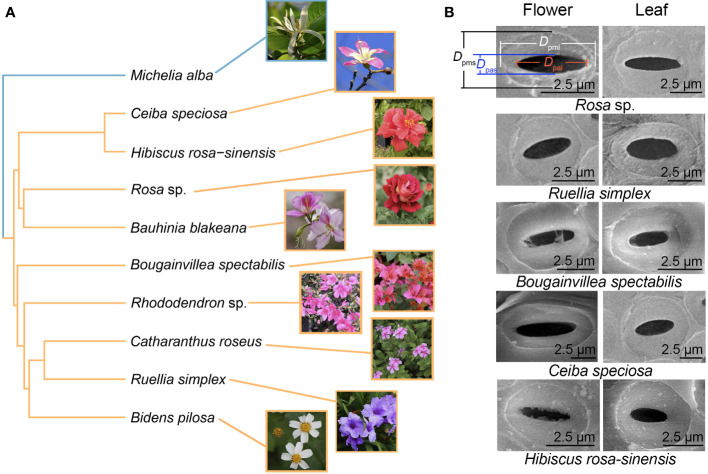
Flower and leaf samples of the 10 species in this study ( Figure 1 and Table 1 ) were collected on the campus of Guangxi University, Nanning (Guangxi, China, 22°50′N 108°17′E), which has a subtropical monsoon climate with a mean annual temperature of 21.8°C and a mean annual precipitation of 1,290 mm. Three to five randomly selected individuals per species were selected for sampling. On each plant, a sun-exposed branch with leaves and flowers was cut and immediately placed in a bucket with water in the evening or early morning and transported back to the laboratory on campus.
Figure 1.
Phylogenetic relationships of the 10 species (A) and scanning electron micrographs of intervessel pits (B). The bule and yellow branches represent magnoliids and eudicots, respectively.
2.2. Light microscopy of anatomical traits
All measurements were made on a fully expanded, healthy, sun-exposed branch with flowers and leaves for each of the 3-5 individuals sampled per species. From each leaf or flower, approximately 1-cm2 sections of lamina were excised, avoiding the margin and midrib. These sections were cleared in a 1:1 solution of H2O2 (30%) and CH3COOH (100%), then incubated at 70°C until all pigments had been removed. Sections were then removed from this solution and rinsed in water for 3 minutes, then the epidermises separated with forceps from the mesophyll and veins, allowing the upper and lower epidermises to be stained and mounted separately. To increase contrast, all samples were stained with Safranin O (0.5% w/v in water) for 5 min and Alcian Blue (1% w/v in 3% acetic acid) for 20 secs - 1 min, then washed in water and mounted on microscope slides.
Cross-sections of petals and leaves were made with a sliding microtome (RM225, Leica Inc., Germany) with a tissue thickness of 35 µm. Cross-sections with the same thickness were also made of peduncles and petioles. Sections were bleached for 10 min, rinsed in water, and then stained with Safranin O (0.5% w/v in water) for 5 min and with Alcian Blue (1% w/v in 3% acetic acid) for 20 secs - 1 min, rinsed, and then mounted on glass slides.
Images were taken at 5x, 10x, or 20x magnification, which had fields of view of approximately 3.99 mm2, 0.89 mm2, and 0.22 mm2, respectively, using a compound microscope outfitted with a digital camera (DM3000, Leica Inc., Germany). Both abaxial (lower) and adaxial (upper) leaf and petal surfaces were imaged for all species to determine whether they were amphistomatous. In subsequent analyses, we used sum of abaxial and adaxial stomatal densities for comparisons. We found no stomata on petals of Catharanthus roseus and Rosa sp.
All anatomical measurements from images were made using ImageJ (Rueden et al., 2017). From images of paradermal sections, vein density (D v) was measured as the total length of leaf or petal vascular tissue per mm2 of leaf area or petal area, stomatal density (D s) was measured by counting the number of stomata in the image and dividing by the area of the field of view, stomatal size (S s) (comprising a pair of guard cells) was directly measured on at least five stomata per image. Partial stomata and epidermal cells were included in the density counts if visible along the top and left borders of the photomicrographs and discarded if visible along the bottom and right borders (Carins Murphy et al., 2017). Leaf and petal thicknesses were directly measured from the cross-section images.
Vessel double wall thickness (T w) was measured on at least 10 pairs of connected vessels per image from cross-sections of peduncles and petioles. Mean hydraulically weighted vessel diameter (D h) for each species was calculated as (Tyree and Zimmermann, 2013):
where D is the equivalent circular diameter of a vessel whose area was calculated from its long and short diameters and N is the number of vessels measured. The D h is biased towards wider vessels that conduct the majority of water according to the Hagen–Poiseuille law. Average vessel frequency (VF) was calculated per image by dividing the total number of vessels by the image area. For each species, we calculated the theoretical hydraulic conductivity of as (Rakthai et al., 2020):
where ρ is fluid density (assumed to be 998.2 kg m-3 at 20°C) and η is viscosity of water (1.002 x 10–9 MPa s-1 at 20°C).
2.3. Scanning electron microscopy of anatomical traits
Upon returning samples to the lab, peduncles and petioles were immediately cut into small segments and placed in 100 ml of 5% FAA fixative (90:5:5 ratio of 70% ethanol, acetic acid, formaldehyde) at room temperature (25°C) to prevent expansion or shrinkage. Longitudinal sections of the segments were made with a sliding microtome (RM225, Leica Inc., Germany) at a thickness of 2-3 mm. The sections were fixed to aluminum sample holders with an electron-conductive carbon adhesive tape (Nisshin EM Co. Ltd., Tokyo), air-dried for 12 h at room temperature, and coated with gold using a sputter coater (Cressington 108Auto) for 40 secs at 0.08 mA to get a 20-nm-thick gold layer, under an argon atmosphere. A conventional scanning electron microscope (FEI Quattro S, US) with a voltage of 2 kV was used to visualize intervessel pit parameters according to standard protocols (Jansen et al., 2009; Lens et al., 2011; Zhang et al., 2021).
ImageJ (Rueden et al., 2017) was used to determine the following intervessel pit characteristics ( Table 2 ): intervessel pit aperture surface area (A pa), intervessel pit surface area or intervessel pit membrane surface area (A pit), pit aperture longest diameter (D pal), pit aperture shortest diameter (D pas), pit aperture shape (R pa = D pal/D pas), pit membrane longest diameter (D pml), pit membrane shortest diameter (D pms), pit shape or pit membrane shape (R pit = D pml/D pms) and pit density (D p). Mean values of these intervessel pit traits were calculated from at least 50 measurements from SEM images of various intervessel walls per individual.
2.4. Measurement of pressure–volume parameters
Shoots with leaves and flowers were collected from at least three individuals per species at night or at predawn and transported back to the laboratory. In the lab, all shoots were recut underwater to rehydrate for at least 2 h and covered with a black plastic bag during equilibration. Initial water potentials were checked and always close to -0.1 MPa. Pressure–volume curves were constructed for each sample by repeatedly measuring the bulk water potential using a pressure chamber (0.01 MPa resolution; PMS Instruments, Albany, OR, USA) and the mass to determine the relationship between water potential and water content following standard methods (Scholander et al., 1965; Tyree and Hammel, 1972; Sack and Pasquet-Kok, 2011; Roddy et al., 2019; Jiang et al., 2022). Prior to each water potential measurement, samples were enclosed in humidified plastic bags for about 20 min to allow equilibration. The pressure chamber was kept humidified with wet paper towels to prevent evaporation during the water potential measurement. After water potential measurement, the sample was weighed on a balance (± 0.0001g, model ML204T; Mettler Toledo). At the end of measurements, samples were oven-dried at 70°C for at least 72 h before determining dry mass. Because measuring flower surface area is difficult after turgor loss, pressure–volume parameters were expressed on a dry mass basis, according to previous analyses (Roddy et al., 2019). From these pressure-violume curves, we calculated saturated water content (SWC), absolute capacitance (CT), water potential at turgor loss point (Ψtlp), and osmotic potential at full turgor (Ψsft) ( Table 2 ).
2.5. Leaf and flower minimum diffusive conductance
Shoots with leaves and flowers were collected at night from at least five individuals per species, recut underwater, and rehydrated over night while covered with a black plastic bag. Leaf and flower samples were excised in the morning, including the petiole or peduncle. Immediately following excision, their cut ends were sealed with glue and the entire organ was weighed every 10 min using an electronic balance ( ± 0.0001g, model ML204T; Mettler Toledo) in a dark room. The room was equipped with an air-conditioning to control the temperature and humidity, and samples were hung in front of a large fan as they desiccated. The velocity of air flow was high enough to physically move the samples. A small temperature and humidity sensor was kept near the samples, and temperature (T) and relative humidity (RH) were recorded manually each time a sample was weighed. After ten measurements, samples were scanned to determine projected area and then oven-dried at 70°C for 72 hours before determining dry mass.
Minimum diffusive conductance (g min) was calculated as (Bourbia et al., 2020):
where WL is the water loss rate (mmol m-2 s-1) calculated as the slope of mass (g) over time (s) and normalized by the projected area (m2) or dry mass (g) of each organ; Patm is the atmospheric pressure (101.3 kPa); VPD is the vapor pressure deficit determined using the Arden Buck equation (Buck, 1981).
2.6. Data analysis
All statistical analyses were conducted in R (v. 4.0.3) (Core Team, 2022). Paired t-tests were used to determine differences between flowers and leaves. Differences of pit area and density among different plant lineages were tested through one-way ANOVA. We used linear regression and standard major axis (SMA) regression (R package ‘smatr’) to determine the relationships between traits (Warton et al., 2012). Principal component analysis (PCA) was carried out on centered and scaled trait data using the ‘vegan’ package. A phylogenetic tree was built using the R package ‘V.PhyloMaker’ and phylogenetic independent contrasts (PICs) were calculated using the ‘pic’ function in the R package ‘ape’ and PIC correlations tested using linear regression. All statistical tests were considered significant at P< 0.05. In order to contextualize our measurements of inter-conduit pit traits on leaves and flowers, we compiled published data reporting pit membrane surface area (A pit) and pit density (D p) for a diverse set of vascular plants ( Supplementary Table 1 ). We used this broad dataset to examine how pit membrane area and pit density vary among lineages and organs.
3. Results
3.1. Trait variation and physiological trait coordination
Minimum diffusive conductance (g min) was significantly higher in flowers than in leaves, whether it was normalized by dry mass (t = 5.48, P<0.001) or by projected area (t = 4.88, P<0.001) ( Figures 2A, B and Supplementary Table 2 ). In addition, traits from pressure-volume curves were also significantly higher in flowers than in leaves (P< 0.01) ( Figures 2C–F and Supplementary Table 2 ).
Figure 2.
Traits differences of 10 selected traits for g min (A, B), pressure-volume curves (C–F), and pit characteristics (G–J) in flowers and leaves. Mean values of listed traits from 10 species (n=10) were significantly different (P< 0.05) in flowers and leaves. See Table 2 for definitions of abbreviations.
SEM images were used to examine intervessel pits in peduncles and petioles ( Figure 1B ). Compared to petioles, peduncles had significantly smaller pit membrane diameters D pms (t = -2.36, P< 0.05) and D pml (t = -2.86, P< 0.01) and smaller pit area A pit (t = -3.09, P< 0.05), as well as differences in pit aperture shape R pa (t = -2.16, P< 0.05) ( Figures 2G–J and Supplementary Table 2 ). However, pit aperture diameters D pas (t = -0.43, P > 0.05) and D pal (t = -1.49, P > 0.05) and pit aperture area A pa (t = -1.15, P > 0.05) were not significantly different between petioles and peduncles ( Supplementary Table 2 ). Pit membrane shape R pit (t = 0.41, P > 0.05) and pit density D p (t = 2.02, P > 0.05) were similar in peduncles and petioles ( Supplementary Table 2 ). Peduncles and petioles differed significantly in the size of the pit membranes despite having similar pit aperture sizes ( Figure 2 and Supplementary Table 2 ).
In leaves, g min,mass was positively correlated with Ψtlp (R 2 = 0.56, P = 0.013) and Ψsft (R 2 = 0.50, P = 0.022), which remained significant after accounting for shared evolutionary history ( Figures 3A, B and Table 3 ). No similar relationships between g min,area or g min,mass and C T in leaves ( Figures 3C, D ) or g min,mass and Ψtlp or Ψsft in flowers ( Figures 3E, F ) were found. g min,area was positively correlated with C T in flowers (R 2 = 0.50, P = 0.034, Figure 3H ). The relationships between C T and both g min,mass and g min,area were significant after accounting for shared evolutionary history ( Table 3 ).
Figure 3.
Relationships among minimum diffusive conductance (gmin) and traits from pressure-volume curves (A–H, see Table 2 for definitions of abbreviations.). Each point represents the mean value in peduncles and petiole, respectively. The green circles represent leaf, the orange circles represent flower, and error bars represent standard error (n = 5 individual plants).
Table 3.
Phylogenetic independent contrast (PIC) results for paired traits of the 10 species studied, showing the PICs calculations between traits in flower and leaf.
| R2 | |||
|---|---|---|---|
| Flower | Leaf | ||
| D p | D pml | 0.93** | 0.90** |
| D pms | 0.89** | 0.89** | |
| A pit | 0.95** | 0.86** | |
| D pal | 0.84** | 0.84** | |
| D pas | 0.93** | 0.85** | |
| A pa | 0.84** | 0.81** | |
| A pit | A pa | 0.92** | 0.92** |
| D h | 0.26 | 0.56* | |
| K th | D pas | 0.30 | 0.71** |
| D pal | 0.19 | 0.79** | |
| A pit | 0.19 | 0.87** | |
| T w | A pit | 0.001 | 0.43 |
| D pal | T w | 0.03 | 0.49* |
| D h | 0.34 | 0.63* | |
| D s | 0.002 | 0.39 | |
| R pa | D h | 0.17 | 0.04 |
| K th | 0.26 | 0.25 | |
| SWC | 0.37 | 0.22 | |
| Ψsft | 0.23 | 0.22 | |
| Ψtlp | 0.20 | 0.38 | |
| C T | 0.26 | 0.02 | |
| FT/LT | 0.31 | 0.72** | |
| R pit | D v | 0.02 | 0.66** |
| g min,mass | Ψsft | 0.001 | 0.50* |
| Ψtlp | 0.01 | 0.45* | |
| C T | 0.61* | 0.72 | |
| g min,area | C T | 0.54* | 0.17 |
| VF | A pit | 0.10 | 0.09 |
| A pa | 0.19 | 0.09 | |
The numbers represent the correlation coefficients, *: significant correlation (P < 0.05), **: significant correlation (P < 0.01), ***: significant correlation (P < 0.001).
3.2. Trade off among intervessel pit traits
Pit density D p was negatively correlated with pit membrane diameters D pms (R 2 = 0.68, P< 0.001) and D pml (R 2 = 0.71, P< 0.001) and with pit area A pit (R 2 = 0.61, P< 0.001), as well as with pit aperture diameters D pas (R 2 = 0.58, P< 0.001) and D pal (R 2 = 0.5, P< 0.001) and pit aperture area A pa (R 2 = 0.47, P< 0.001) ( Figure 4 ). These correlations remained significant after accounting for shared evolutionary history (all P< 0.05, Table 3 ). Comparing these data to previously published data from stems and leaves of a broad sampling of angiosperms, gymnosperms, and ferns ( Supplementary Table 1 ) showed that the negative correlation between D p and A pit was common ( Supplementary Figure 1 ), with gymnosperms exhibiting larger pits that occur at lower density ( Table 4 and Supplementary Figure 1 ). A pit and D p in flowers and leaves measured here were within the range reported previously for angiosperms and differed significantly from only gymnosperms ( Table 4 ).
Figure 4.
Relationships between pit density (D p) and pit membrane (A–C) and pit aperture traits (D–F), and relationships between pit membrane area (A pa) with pit aperture area (A pit) (G). Each point represents the mean value in peduncles and petiole, respectively. The green circles represent leaf, the orange circles represent flower, and error bars represent standard error (n = 3-5 individual plants).
Table 4.
Variation in D p and A pit among Fern, Gymnosperm and original data.
| A pit (µm2) | D p (no.µm-2) | |
|---|---|---|
| Fern | 11.23 ± 3.65a | 0.073 ± 0.0179a |
| Gymnosperm | 174.74 ± 25.09b | 0.004 ± 0.0008b |
| Leaf | 13.29 ± 1.84a | 0.045 ± 0.0043a |
| Flower | 9.38 ± 0.90a | 0.052 ± 0.0035a |
Different lower-case letters following the values indicate significant differences between groups (P < 0.05, LSD’s post hoc test, one-way ANOVA, values are means ± SE). Data for angiosperms, gymnosperms, and ferns were collected from published references ( Table S1 ).
3.3. Relationships among pit traits and hydraulic traits
In petioles, like in other species (Lens et al., 2011; Mrad et al., 2018), K th was positively correlated with D pas (R 2 = 0.45, P = 0.004), D pal (R 2 = 0.58, P = 0.011), and A pit (R 2 = 0.74, P = 0.001), but in peduncles these relationships were not significant, mainly because of the relatively constant D pas and D pal ( Figures 5A-C ). These correlations were statistically similar after accounting for shared evolutionary history (P< 0.05, Table 3 ). D pal was positively correlated with D h (R 2 = 0.39, P = 0.003) and with T w (R 2 = 0.45, P = 0.001) in both petioles and peduncles ( Figures 5D, E ). These correlations remained significant only in leaves after accounting for shared evolutionary history (P< 0.05, Table 3 ). The positive relationships between D pal and D s (R 2 = 0.25, P = 0.005) ( Figure 5G ), A pit and T w (R 2 = 0.49, P = 0.025) ( Figure 5F ) became non-significant in leaves after accounting for shared evolutionary history ( Table 3 ).
Figure 5.
Relationships among pit apertures traits and theoretical hydraulic conductance (K th) (A, B), pit membrane area and K th (C) and double vessel wall thickness (T w) (F), pit apertures traits and vessel diameter (D), and double vessel wall thickness (E), and stomatal density (G). Each point represents the mean value in peduncles and petiole, respectively. The green circles represent leaf, the orange circles represent flower, and error bars represent standard error (n = 3-5 individual plants).
In peduncles, R pa was positively correlated with D h (R 2 = 0.43, P = 0.04), K th (R 2 = 0.43, P = 0.038), SWC (R 2 = 0.53, P = 0.018), Ψsft (R 2 = 0.54, P = 0.016), Ψtlp (R 2 = 0.50, P = 0.022), C T (R 2 = 0.43, P = 0.041), FT (R 2 = 0.54, P = 0.015) ( Figures 6A-G ). However, none of these correlations remained significant after accounting for shared evolutionary history ( Table 3 ). R pa was unrelated to any of these hydraulic traits in petioles, but R pa was positively correlated with leaf thickness after accounting for shared evolutionary history (R 2 = 0.72, P< 0.01) ( Table 3 ). Only R pit was negatively correlated with D v (R 2 = 0.42, P = 0.041) ( Figure 6H ), even after accounting for shared evolutionary history (R 2 = 0.72, P< 0.01) ( Table 3 ).
Figure 6.
Relationships among pit membrane shape (R pit), pit apertures shape (R pa) and hydraulic traits. (A–H, see Table 2 for definitions of abbreviations). Each point represents the mean value in peduncles and petiole, respectively. The green circles represent leaf, the orange circles represent flower, and error bars represent standard error (n = 3-5 individual plants).
3.4. Phylogenetic independent contrast correlations of all paired traits between flowers and leaves
Phylogenetic independent contrast correlations (PIC) were made between all 24 traits measured in both flowers and leaves. Positive correlations of D pms (R 2 = 0.62, P = 0.012), D pml (R 2 = 0.84, P = 0.001), A pit (R 2 = 0.86, P< 0.001), D pas (R 2 = 0.91, P< 0.001), D pal (R 2 = 0.79, P = 0.001), A pa (R 2 = 0.93, P< 0.001), D p (R 2 = 0.78, P = 0.002) between flowers and leaves were found ( Figures 7A–G ), and species with larger pit membranes and pit apertures in petioles also had larger pit membranes and pit apertures in peduncles ( Supplementary Figure 2 ). Interestingly, pit membrane (R 2 = 0.09, P = 0.444) and pit aperture shape (R 2 = 0.14, P = 0.33) showed non-significant relationships after accounting for shared evolutionary history ( Figures 7H, I ).
Figure 7.
Phylogenetic independent contrast (PIC) correlations for pit traits. (A–I, see Table 2 for definitions of abbreviations) of the 10 species studied, showing the PICs calculated for pit traits between flowers and leaves. Correlation coefficients and P values are shown for statistically significant correlations based on Pearson's product-moment correlation.
There were positive correlations in traits between organs for S s (R 2 = 0.50, P = 0.048), D v (R 2 = 0.41, P = 0.047), T w (R 2 = 0.49, P = 0.024), and g min,mass (R 2 = 0.46, P = 0.031) ( Supplementary Figure 3 ), which became non-significant after accounting for shared evolutionary history ( Table 5 ). Meanwhile some traits were not correlated among organs and remained uncorrelated even after accounting for shared evolutionary history: g min,area (R 2 = 0.13, P = 0.336), SWC (R 2 = 0.07, P = 477), K th (R 2 = 0.06, P = 0. 523), Ψsft (R 2 = 0.44, P = 0. 051), CT (R 2 = 0.02, P = 0.721), D s (R 2 = 0.13, P = 0.336), and LT/FT (R 2 = 0.04, P = 0.626) ( Table 5 ). Among physiological traits, only Ψtlp exhibited correlated evolution among flowers and leaves (R 2 = 0.54, P = 0.024) ( Table 5 ).
Table 5.
Phylogenetic independent contrast (PIC) correlations of all traits between flowers and leaves for the 10 species studied.
| Trait | p-value | R2 |
|---|---|---|
| D pms | <0.001 | 0.62** |
| D pml | 0.001 | 0.84** |
| A pit | <0.001 | 0.86** |
| D pas | <0.001 | 0.91** |
| D pal | 0.001 | 0.79** |
| A pa | <0.001 | 0.93** |
| R pit | 0.444 | 0.09 |
| R pa | 0.330 | 0.14 |
| D p | 0.002 | 0.78** |
| g min,area | 0.336 | 0.13 |
| g min,mass | 0.203 | 0.22 |
| SWC | 0.477 | 0.07 |
| K th | 0.523 | 0.06 |
| Ψsft | 0.051 | 0.44 |
| Ψtlp | 0.024 | 0.54** |
| C T | 0.721 | 0.02 |
| S s | 0.201 | 0.22 |
| D s | 0.336 | 0.13 |
| D v | 0.100 | 0.34 |
| LT/FT | 0.626 | 0.04 |
| D h | 0.058 | 0.42 |
| T w | 0.096 | 0.35 |
| VF | 0.101 | 0.34 |
The numbers represent the correlation coefficients, *, significant correlation (P < 0.05); **, significant correlation (P < 0.01); ***, significant correlation (P < 0.001).
3.5. Principal component analysis
The principal components analysis using all 24 traits revealed that the first two principal components explained 37.20% and 24.06% of the total variation, respectively. The first PC was driven by D h, K th, and some pit characters, including D p, D pms, and A pit. The second PC was largely driven by pressure-volume parameters, including SWC, CT, Ψtlp, Ψsft, as well as anatomical traits, including D v, D s, and LT. Flowers and leaves largely differed in the regions of trait space they occupied ( Figure 8A ).
Figure 8.

Principal component analysis (PCA) of all 24 traits on the first two principal component axes in flowers and leaves. The shaded regions indicate the total volume of trait space occupied by leaves (green) and flowers (orange). The red lines represent scanning electron microscopy anatomical traits, the black lines represent Pressure–volume parameters, the pink lines represent minimum diffusive conductance, and the blue lines represent light microscopy anatomical traits (A). Principal component analysis of 9 pit traits on the first two principal component axes in flowers and leaves. The green circles represent leaf, the orange circles represent flower, and the shaded regions indicate the total volume of trait space occupied by leaves (green) and flowers (orange) (B). See Table 2 for definitions of abbreviations.
Using only the pit traits in a principal components analysis revealed that the first two principal components explained 86.55% of the total variation among species and organs. The first PC (66.85%) was driven primarily by D p versus all of the other pit traits except Rpit and Rpa . The second PC (19.70%) was driven primarily by R pit and R pa. There was a high level of overlap among flowers and leaves in the regions of pit trait space they occupied ( Figure 8B ).
4. Discussion
Our results revealed that despite large differences in g min and pressure-volume traits between leaves and flowers, there were relatively small differences in pit traits between leaf petioles and flower peduncles ( Figure 8 ). While flowers have higher g min that is associated with higher hydraulic capacitance and higher turgor loss points than leaves ( Figures 2 , 3 and Supplementary Table 2 ), these differences in tissue water relations seem to be independent of differences in intervessel pit traits between organs. Thus, flowers rely on a cheap hydrostatic skeleton maintained by turgor pressure rather than a rigid, carbon-based skeleton (Roddy et al., 2019). High hydraulic capacitance of flowers prevents water potential declines that lead to xylem embolism and may have shielded selection from driving large divergences in intervessel pit traits between leaves and flowers.
4.1. Traits regulating water balance in flowers
In order for terminal organs to avoid desiccation, water loss must equal water supply, at least over diel timescales. In leaves, which maintain relatively high transpiration rates, the need to maintain water balance has resulted in coordinated evolution of key anatomical traits that influence both liquid water supply and water vapor loss, particularly leaf vein density (D v) and stomatal density and size (Sack et al., 2003; Brodribb et al., 2013; Simonin and Roddy, 2018; Zhang et al., 2018). While similar coordination between veins and stomata has been observed in flowers (Zhang et al., 2018), flowers often have few or no stomata, meaning that other traits, such as g min, may be more important to regulating water balance (Roddy et al., 2016; Roddy, 2019). Furthermore, in both flowers and leaves, higher water contents and hydraulic capacitance can buffer water potential declines and lengthen the time required to reach steady state transpiration when water supply equals water loss (Simonin et al., 2013; Roddy et al., 2018; Roddy et al., 2019). Because flowers can have higher g min than leaves ( Figure 2 ), we predicted that there may be coordination between g min and hydraulic capacitance, which would indicate that higher hydraulic capacitance can compensate for higher g min in flowers. Across organs, g min and hydraulic capacitance were correlated even after accounting for shared evolutionary history ( Figure 3 and Table 3 ), with flowers having both higher g min and higher capacitance than leaves ( Figure 2 ). These patterns suggest that multiple traits and hydraulic strategies may be employed to maintain water balance among leaves and flowers.
In the absence of high hydraulic capacitance to buffer water potential declines, high g min may cause water potentials to decline enough to initiate xylem embolism in flowers before leaves (Bourbia et al., 2020). If this were the case, we would predict that to prevent embolism in flowers, intervessel pit traits may have experienced selection to reduce embolism vulnerability. However, there were overall relatively small differences in intervessel pit traits between petioles and peduncles ( Figure 2 ). One possible explanation is that intervessel pit traits may be shielded from selection by high hydraulic capacitance in flowers that allows g min to be high without causing water potential declines and embolism spread. Because intervessel pit traits also influence hydraulic conductance (Choat et al., 2005; Ellmore et al., 2006; Hacke et al., 2006; Lens et al., 2011; Jacobsen et al., 2016), differences in intervessel pit traits between leaves and flowers may be due to divergent selection on hydraulic conductance among leaves and flowers. However, while flowers generally have relatively low hydraulic conductance, they are not necessarily outside the range of hydraulic conductance of leaves (Roddy et al., 2016), further suggesting that the strength of selection due to hydraulic efficiency acting on pit traits may be relatively weak.
4.2. Similar coordination of intervessel pit traits in leaves and flowers
In some cases, leaves and flowers exhibited similar coordination between intervessel pit traits despite the large morphological, anatomical, and physiological differences between these organs. We found similar coordination between A pit and A pa in both leaves and flowers ( Figure 4 ), with larger A pit being associated with higher theoretical hydraulic conductivity K th in leaves ( Figure 5 ). It is worth nothing that K th incorporates only vessel traits and not intervessel pit traits, so coordination between K th and A pit and A pa suggests that variation in vessel size and density are linked to intervessel pit variation. A broad sampling of vascular plants ( Supplementary Figure 1 ) revealed strong coordination between A pit and pit density (D p), due largely to packing constraints similar to those elucidated for stomata on the leaf surface and mesophyll cells inside the leaf (Franks and Beerling, 2009; Théroux-Rancourt et al., 2021; Borsuk et al., 2022; Jiang et al., 2023). Compared to other vascular plants, angiosperm A pit and D p were closer to the theoretical packing limit, which may be important to increasing hydraulic efficiency of angiosperm xylem regardless of other xylem traits. Coordination between A pit and D p was also found among flower peduncles and leaf petioles, similar to other angiosperms, regardless of the fact that previously published data were taken from both stems and leaves ( Supplementary Figure 1 and Supplementary Table 1 ). Previous studies have shown that larger A pit is associated with larger A pa, leading to higher hydraulic conductivity (Orians et al., 2004; Wheeler et al., 2005; Lens et al., 2011; Johnson et al., 2016). Similarly, we found that A pit was positively linked to K th in leaves but not in flowers (noted that A pit was positively correlated with D h but not VF in leaves, Table 3 ), suggesting that coordination in pit traits and vessel dimensions and packing were decoupled in flowers ( Figure 5 ). Thus, despite obeying similar biophysical packing principles as vegetative organs–i.e. stomatal and vein densities (Zhang et al., 2018)–flowers can deviate in other traits that also influence hydraulic performance.
4.3. Special pit traits and correlations in flowers
From the hydraulic efficiency perspective, plants that have larger diameter vessels will have higher hydraulic efficiency (Hargrave et al., 1994; Christenhusz et al., 2011; Liu et al., 2019), while larger conduit diameter, larger pit membrane area, and larger pit aperture area, will be expected to decrease hydraulic safety (Pittermann et al., 2010; Lens et al., 2011; Brodersen et al., 2014; Jacobsen et al., 2016). Conduit wall thickness is thought increase hydraulic safety (Hacke et al., 2001; Brodribb and Holbrook, 2005). In our results, both pit membrane and pit aperture traits were correlated with K th and D h in leaves, no such correlations were found in flowers ( Table 3 ). These results may indicate that leaves increase hydraulic efficiency with larger vessels and A pit, but they may increase hydraulic safety through thicker vessel walls and more elliptical pits ( Table 3 ). On the other hand, high hydraulic capacitance of flowers prevents water potential declines may relax the selective pressure of intervessel pit traits for hydraulic efficiency and safety. In general, elliptically shaped pit apertures are associated with greater embolism resistance, with more cavitation-resistant species exhibiting narrower and more elliptical pit apertures (Lens et al., 2011; Scholz et al., 2013; Jacobsen et al., 2016). Consequently, angiosperms adapted to dry environments might have smaller conduit diameters and thicker, denser, smaller, and more elliptical pit apertures (Wheeler et al., 2005; Hacke et al., 2007; Jansen et al., 2009; Lens et al., 2011; Scholz et al., 2013). While intervessel pit traits might influence both hydraulic safety and efficiency, none of the pit traits were correlated with hydraulic traits in flowers ( Table 3 ). It is highly likely, therefore, that traits exhibiting greater differences between leaves and flowers (e.g. pressure-volume traits) may be more important to flower water balance than intervessel pit traits.
Flowers have been shown to exhibit high diversity in hydraulic traits with higher water content and higher hydraulic capacitance than leaves (Roddy et al., 2019). We found similar patterns in our data, with flowers exhibiting greater variation in g min and pressure-volume traits than leaves ( Figure 2 ). However, flowers exhibited less variation in pit traits than leaves ( Figure 2 ), although there were some clear differences in intervessel pit traits between leaves and flowers. This was further validated by the PCA results ( Figure 8 ), in which R pit and R pa loaded on the same axis as the pressure-volume traits, in contrast to all other intervessel pit traits, which were orthogonal to other hydraulic traits except D h and K th. Further corroborating the role of R pit and R pa in causing the divergence in hydraulic strategies between leaves and flowers, R pit and R pa exhibited no correlated evolution between leaves and flowers, in contrast to all other intervessel pit traits ( Figure 7 ). Taken together, these results suggest that while most of the hydraulic differences between leaves and flowers is due to stomatal and vein anatomy and pressure-volume traits, differences in pit and pit aperture shape may also signify important differences.
5. Conclusions
The water dynamics of flowers are critical to successful reproduction and population viability, yet remarkably little is known about the hydraulic strategies of flowers and their mechanisms of maintaining water balance. Limiting water loss, storing large amounts of water, and building xylem safe from embolism are all ways of avoiding the detrimental effects of water limitation. Here we show that, compared to leaves, flowers are leakier and exhibit relatively few differences in intervessel pit traits that influence embolism vulnerability. Instead, flowers primarily use high water contents to prevent water potential declines. This drought-avoidant strategy employed by flowers may have protected their xylem from selection for greater differences from leaves. Furthermore, by quantifying a broad suite of anatomical and physiological traits among leaves and flowers, we show that with the exception of pit and pit aperture shape, intervessel pit traits are largely orthogonal to stomatal and vein traits and pressure-volume traits. These results highlight the many dimensions in which flowers have diverged from leaves under different functional demands and suggest that high water content and hydraulic capacitance are the primary traits that protect flowers from experiencing low water potentials that can cause failure in the hydraulic system.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
G-FJ conceived the ideas and designed the study. Y-DA, AR and G-FJ collected the data. Y-DA, T-HZ and G-FJ analyzed the data. G-FJ and Y-DA wrote the first manuscript, AR helped to improve final manuscript; and all authors reviewed each draft before giving approval for submission of the final version. All authors contributed to the article and approved the submitted version.
Acknowledgments
We gratefully thank the core facility center of State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources for providing technical supports.
Funding Statement
This work was supported by grants from the Natural Science Foundation of Guangxi Province (Key Program 2022GXNSFDA035059), the National Natural Science Foundation of China (grant number 31860195), and Bama county program for talents in science and technology, Guangxi, China (20210020, 20220011) to G-FJ. ABR was supported by grant CMMI-2029756 from the US National Science Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1130724/full#supplementary-material
References
- Adams H. D., Zeppel M. J. B., Anderegg W. R. L., Hartmann H., Landhäusser S. M., Tissue D. T., et al. (2017). A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 1 (9), 1285–1291. doi: 10.1038/s41559-017-0248-x [DOI] [PubMed] [Google Scholar]
- Ashman T. L., Schoen D. J. (1994). How long should flowers live? Nature 371 (6500), 788–791. doi: 10.1038/371788a0 [DOI] [Google Scholar]
- Bazzaz F. A., Chiariello N. R., Coley P. D., Pitelka L. F. (1987). Allocating resources to reproduction and defense: new assessments of the costs and benefits of allocation patterns in plants are relating ecological roles to resource use. BioScience 37 (1), 58–67. doi: 10.2307/1310178 [DOI] [Google Scholar]
- Blackman C. J., Brodribb T. J., Jordan G. J. (2010). Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol. 188 (4), 1113–1123. doi: 10.1111/j.1469-8137.2010.03439.x [DOI] [PubMed] [Google Scholar]
- Blanke M. M., Lovatt C. J. (1993). Anatomy and transpiration of the avocado inflorescence. Ann. Bot. 71 (6), 543–547. doi: 10.1006/anbo.1993.1070 [DOI] [Google Scholar]
- Borsuk A. M., Roddy A. B., Theroux-Rancourt G., Brodersen C. R. (2022). Structural organization of the spongy mesophyll. New Phytol. 234 (3), 946–960. doi: 10.1111/nph.17971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbia I., Carins-Murphy M. R., Gracie A., Brodribb T. J. (2020). Xylem cavitation isolates leaky flowers during water stress in pyrethrum. New Phytol. 227 (1), 146–155. doi: 10.1111/nph.16516 [DOI] [PubMed] [Google Scholar]
- Boyce C. K., Brodribb T. J., Feild T. S., Zwieniecki M. A. (2009). Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc. R. Soc. B: Biol. Sci. 276 (1663), 1771–1776. doi: 10.1098/rspb.2008.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen C., Jansen S., Choat B., Rico C., Pittermann J. (2014). Cavitation resistance in seedless vascular plants: the structure and function of interconduit pit membranes. Plant Physiol. 165 (2), 895–904. doi: 10.1104/pp.113.226522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T. J., Cochard H. (2009). Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 149 (1), 575–584. doi: 10.1104/pp.108.129783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T. J., Feild T. S., Jordan G. J. (2007). Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 144 (4), 1890–1898. doi: 10.1104/pp.107.101352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T. J., Holbrook N. M. (2005). Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiol. 137 (3), 1139–1146. doi: 10.1104/pp.104.058156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T. J., Jordan G. J., Carpenter R. J. (2013). Unified changes in cell size permit coordinated leaf evolution. New Phytol. 199 (2), 559–570. doi: 10.1111/nph.12300 [DOI] [PubMed] [Google Scholar]
- Brodribb T. J., Powers J., Cochard H., Choat B. (2020). Hanging by a thread? forests and drought. Science 368 (6488), 261–266. doi: 10.1126/science.aat7631 [DOI] [PubMed] [Google Scholar]
- Buck A. L. (1981). New equations for computing vapor pressure and enhancement factor. J. Appl. Meteorology 20 (12), 1527–1532. doi: [DOI] [Google Scholar]
- Burkle L. A., Runyon J. B. (2016). Drought and leaf herbivory influence floral volatiles and pollinator attraction. Global Change Biol. 22 (4), 1644–1654. doi: 10.1111/gcb.13149 [DOI] [PubMed] [Google Scholar]
- Carins Murphy M. R., Jordan G. J., Brodribb T. J. (2017). Ferns are less dependent on passive dilution by cell expansion to coordinate leaf vein and stomatal spacing than angiosperms. PloS One 12 (9), e0185648. doi: 10.1371/journal.pone.0185648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso C. M. (2006). Plasticity of inflorescence traits in lobelia siphilitica (Lobeliaceae) in response to soil water availability. Am. J. Bot. 93 (4), 531–538. doi: 10.3732/ajb.93.4.531 [DOI] [PubMed] [Google Scholar]
- Caruso C. M., Eisen K. E., Martin R. A., Sletvold N. (2019). A meta-analysis of the agents of selection on floral traits. Evolution 73 (1), 4–14. doi: 10.1111/evo.13639 [DOI] [PubMed] [Google Scholar]
- Choat B., Brodie T. W., Cobb A. R., Zwieniecki M. A., Holbrook N. M. (2006). Direct measurements of intervessel pit membrane hydraulic resistance in two angiosperm tree species. Am. J. Bot. 93 (7), 993–1000. doi: 10.3732/ajb.93.7.993 [DOI] [PubMed] [Google Scholar]
- Choat B., Brodribb T. J., Brodersen C. R., Duursma R. A., López R., Medlyn B. E. (2018). Triggers of tree mortality under drought. Nature 558 (7711), 531–539. doi: 10.1038/s41586-018-0240-x [DOI] [PubMed] [Google Scholar]
- Choat B., Lahr E. C., Melcher P. J., Zwieniecki M. A., Holbrook N. M. (2005). The spatial pattern of air seeding thresholds in mature sugar maple trees. Plant Cell Environ. 28 (9), 1082–1089. doi: 10.1111/j.1365-3040.2005.01336.x [DOI] [Google Scholar]
- Christenhusz M. J. M., Reveal J. L., Farjon A., Gardner M. F., Mill R. R., Chase M. W. (2011). A new classification and linear sequence of extant gymnosperms. Phytotaxa 19, 55–70. doi: 10.11646/phytotaxa.19.1.3 [DOI] [Google Scholar]
- Core Team R. (2022). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing; ). Available at: https://www.R-project.org/. [Google Scholar]
- Crane P. R., Friis E. M., Pedersen K. R. (1995). The origin and early diversification of angiosperms. Nature 374 (6517), 27–33. doi: 10.1038/374027a0 [DOI] [Google Scholar]
- de Boer H. J., Eppinga M. B., Wassen M. J., Dekker S. C. (2012). A critical transition in leaf evolution facilitated the Cretaceous angiosperm revolution. Nat. Commun. 3 (1), 1221. doi: 10.1038/ncomms2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H. H., Joly J. (1895). XII. on the ascent of sap. Philos. Trans. R. Soc. London (B.) 186, 563–576. doi: 10.1098/rstb.1895.0012 [DOI] [Google Scholar]
- Ellmore G. S., Zanne A. E., Orians C. M. (2006). Comparative sectoriality in temperate hardwoods: hydraulics and xylem anatomy. Botanical J. Linn. Soc. 150 (1), 61–71. doi: 10.1111/j.1095-8339.2006.00510.x [DOI] [Google Scholar]
- Fontes C. G., Pinto-Ledezma J., Jacobsen A. L., Pratt R. B., Cavender-Bares J. (2022). Adaptive variation among oaks in wood anatomical properties is shaped by climate of origin and shows limited plasticity across environments. Funct. Ecol. 36 (2), 326–340. doi: 10.1111/1365-2435.13964 [DOI] [Google Scholar]
- Franks P. J., Beerling D. J. (2009). Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. 106 (25), 10343–10347. doi: 10.1073/pnas.0904209106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C., Sherry R. A., Carroll A. B. (1999). Are flowers physiological sinks or faucets? costs and correlates of water use by flowers of polemonium viscosum. Oecologia 118 (4), 461–470. doi: 10.1007/s004420050749 [DOI] [PubMed] [Google Scholar]
- Gleason S. M. (2018). A blooming interest in the hydraulic traits of flowers. Plant Cell Environ. 41 (10), 2247–2249. doi: 10.1111/pce.13345 [DOI] [PubMed] [Google Scholar]
- Hacke U. G., Jacobsen A. L., Pratt R. B. (2009). Xylem function of arid-land shrubs from California, USA: an ecological and evolutionary analysis. Plant Cell Environ. 32 (10), 1324–1333. doi: 10.1111/j.1365-3040.2009.02000.x [DOI] [PubMed] [Google Scholar]
- Hacke U. G., Sperry J. S., Feild T. S., Sano Y., Sikkema E. H., Pittermann J. (2007). Water transport in vesselless angiosperms: conducting efficiency and cavitation safety. Int. J. Plant Sci. 168 (8), 1113–1126. doi: 10.1086/520724 [DOI] [Google Scholar]
- Hacke U. G., Sperry J. S., Pockman W. T., Davis S. D., McCulloh K. A. (2001). Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126 (4), 457–461. doi: 10.1007/s004420100628 [DOI] [PubMed] [Google Scholar]
- Hacke U. G., Sperry J. S., Wheeler J. K., Castro L. (2006). Scaling of angiosperm xylem structure with safety and efficiency. New Phytol. 26 (6), 689–701. doi: 10.1093/treephys/26.6.689 [DOI] [PubMed] [Google Scholar]
- Hargrave K. R., Kolb K. J., Ewers F. W., Davis S. D. (1994). Conduit diameter and drought-induced embolism in salvia mellifera Greene (Labiatae). New Phytol. 126 (4), 695–705. doi: 10.1111/j.1469-8137.1994.tb02964.x [DOI] [Google Scholar]
- Harrison Day B. L., Carins-Murphy M. R., Brodribb T. J. (2022). Reproductive water supply is prioritized during drought in tomato. Plant Cell Environ. 45 (1), 69–79. doi: 10.1111/pce.14206 [DOI] [PubMed] [Google Scholar]
- Jacobsen A. L., Tobin M. F., Toschi H. S., Percolla M. I., Pratt R. B. (2016). Structural determinants of increased susceptibility to dehydration-induced cavitation in post-fire resprouting chaparral shrubs. Plant Cell Environ. 39 (11), 2473–2485. doi: 10.1111/pce.12802 [DOI] [PubMed] [Google Scholar]
- Jansen S., Choat B., Pletsers A. (2009). Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am. J. Bot. 96 (2), 409–419. doi: 10.3732/ajb.0800248 [DOI] [PubMed] [Google Scholar]
- Jiang G. F., Li S. Y., Dinnage R., Cao K. F., Simonin K. A., Roddy A. B. (2023). Diverse mangroves deviate from other angiosperms in their genome size, leaf cell size, and cell packing density relationships. Ann. Bot 131, 347–360. doi: 10.1093/aob/mcac151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G. F., Li S. Y., Li Y. C., Roddy A. B. (2022). Coordination of hydraulic thresholds across roots, stems, and leaves of two co-occurring mangrove species. Plant Physiol 189, 2759–2174. doi: 10.1093/plphys/kiac240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. M., Wortemann R., McCulloh K. A., Jordan-Meille L., Ward E., Warren J. M., et al. (2016). A test of the hydraulic vulnerability segmentation hypothesis in angiosperm and conifer tree species. Tree Physiol. 36 (8), 983–993. doi: 10.1093/treephys/tpw031 [DOI] [PubMed] [Google Scholar]
- Jupa R., Plichta R., Paschová Z., Nadezhdina N., Gebauer R. (2017). Mechanisms underlying the long-term survival of the monocot dracaena marginata under drought conditions. Tree Physiol. 37 (9), 1182–1197. doi: 10.1093/treephys/tpx072 [DOI] [PubMed] [Google Scholar]
- Kaack L., Altaner C., Carmesin C., Diaz A., Holler M., Kranz C., et al. (2019). Function and three-dimensional structure of intervessel pit membranes in angiosperms: a review. IAWA J. 40, 673–702. doi: 10.1163/22941932-40190259 [DOI] [Google Scholar]
- Kerstiens G. (1996). Cuticular water permeability and its physiological significance. J. Exp. Bot. 47 (12), 1813–1832. doi: 10.1093/jxb/47.12.1813 [DOI] [Google Scholar]
- Kuppler J., Kotowska M. M. (2021. a). A meta-analysis of responses in floral traits and flower–visitor interactions to water deficit. Global Change Biol. 27, 3095–3108. doi: 10.1111/gcb.15621 [DOI] [PubMed] [Google Scholar]
- Kuppler J., Wieland J., Junker R. R., Ayasse M. (2021. b). Drought-induced reduction in flower size and abundance correlates with reduced flower visits by bumble bees. AoB Plants 13, 1–7. doi: 10.1093/aobpla/plab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht S. C. (2013). Floral water costs and size variation in the highly SelfingLeptosiphon bicolor(Polemoniaceae). Int. J. Plant Sci. 174 (1), 74–84. doi: 10.1086/668230 [DOI] [Google Scholar]
- Lambrecht S. C., Dawson T. E. (2007). Correlated variation of floral and leaf traits along a moisture availability gradient. Oecologia 151 (4), 574–583. doi: 10.1007/s00442-006-0617-7 [DOI] [PubMed] [Google Scholar]
- Lambrecht S. C., Morrow A., Hussey R. (2017). Variation in and adaptive plasticity of flower size and drought-coping traits. Plant Ecol. 218 (6), 647–660. doi: 10.1007/s11258-017-0718-x [DOI] [Google Scholar]
- Lens F., Sperry J. S., Christman M. A., Choat B., Rabaey D., Jansen S. (2011). Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus acer. New Phytol. 190 (3), 709–723. doi: 10.1111/j.1469-8137.2010.03518.x [DOI] [PubMed] [Google Scholar]
- Li S., Hao G. Y., Niinemets U., Harley P. C., Wanke S., Lens F., et al. (2019). The effects of intervessel pit characteristics on xylem hydraulic efficiency and photosynthesis in hemiepiphytic and non-hemiepiphytic ficus species. Physiol. Plant 167 (4), 661–675. doi: 10.1111/ppl.12923 [DOI] [PubMed] [Google Scholar]
- Li S., Lens F., Espino S., Karimi Z., Klepsch M., Schenk H., et al. (2016). Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA J. 37, 152–171. doi: 10.1163/22941932-20160128 [DOI] [Google Scholar]
- Lipayeva L. J. B. Z. (1989). On the anatomy of petals in angiosperms. Botanicheskii Zhurnal 74, 333, 9–18. [Google Scholar]
- Liu X., Liu H., Gleason S. M., Goldstein G., Zhu S., He P., et al. (2019). Water transport from stem to stomata: the coordination of hydraulic and gas exchange traits across 33 subtropical woody species. Tree Physiol. 39 (10), 1665–1674. doi: 10.1093/treephys/tpz076 [DOI] [PubMed] [Google Scholar]
- Meinzer F. C. (2002). Co-Ordination of vapour and liquid phase water transport properties in plants. Plant Cell Environ. 25 (2), 265–274. doi: 10.1046/j.1365-3040.2002.00781.x [DOI] [PubMed] [Google Scholar]
- Mrad A., Domec J. C., Huang C. W., Lens F., Katul G. (2018). A network model links wood anatomy to xylem tissue hydraulic behaviour and vulnerability to cavitation. Plant Cell Environ. 41 (12), 2718–2730. doi: 10.1111/pce.13415 [DOI] [PubMed] [Google Scholar]
- Noblin X., Mahadevan L., Coomaraswamy I. A., Weitz D. A., Holbrook N. M., Zwieniecki M. A. (2008). Optimal vein density in artificial and real leaves. Proc. Natl. Acad. Sci. 105 (27), 9140–9144. doi: 10.1073/pnas.0709194105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orians C., Vuuren M., Harris N., Babst B., Ellmore G. (2004). Differential sectoriality in long-distance transport in temperate tree species: evidence from dye flow, 15N transport, and vessel element pitting. Trees 18, 501–509. doi: 10.1007/s00468-004-0326-y [DOI] [Google Scholar]
- Pittermann J., Choat B., Jansen S., Stuart S. A., Lynn L., Dawson T. E. (2010). The relationships between xylem safety and hydraulic efficiency in the cupressaceae: the evolution of pit membrane form and function. Plant Physiol. 153 (4), 1919–1931. doi: 10.1104/pp.110.158824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittermann J., Sperry J., Hacke U., Wheeler J., Sikkema E. (2006). Torus-Margo pits help conifers compete with angiosperms. Sci. (New York N.Y.) 310, 1924. doi: 10.1126/science.1120479 [DOI] [PubMed] [Google Scholar]
- Rakthai S., Fu P. L., Fan Z. X., Gaire N. P., Pumijumnong N., Eiadthong W., et al. (2020). Increased drought sensitivity results in a declining tree growth of pinus latteri in northeastern Thailand. Forests 11 (3), 361. doi: 10.3390/f11030361 [DOI] [Google Scholar]
- Reekie E. G., Bazzaz F. A. (1987. a). Reproductive effort in plants. 1. carbon allocation to reproduction. Am. Nat. 129 (6), 876–896. doi: 10.1086/284681 [DOI] [Google Scholar]
- Reekie E. G., Bazzaz F. A. (1987. b). Reproductive effort in plants. 2. does carbon reflect the allocation of other resources? Am. Nat. 129 (6), 897–906. doi: 10.1086/284682 [DOI] [Google Scholar]
- Reekie E. G., Bazzaz F. A. (1987. c). Reproductive effort in plants. 3. effect of reproduction on vegetative activity. Am. Nat. 129 (6), 907–919. doi: 10.1086/284683 [DOI] [Google Scholar]
- Roddy A. B. (2019). Energy balance implications of floral traits involved in pollinator attraction and water balance. Int. J. Plant Sci. 180 (9), 944–953. doi: 10.1086/705586 [DOI] [Google Scholar]
- Roddy A., Brodersen C., Dawson T. (2016). Hydraulic conductance and the maintenance of water balance in flowers. Plant Cell Environ. 39, 2123–2132. doi: 10.1111/pce.12761 [DOI] [PubMed] [Google Scholar]
- Roddy A. B., Dawson T. E. (2012). Determining the water dynamics of flowering using miniature sap flow sensors. Acta Hortic. 951), 47–53. doi: 10.17660/ActaHortic.2012.951.4 [DOI] [Google Scholar]
- Roddy A. B., Guilliams C. M., Lilittham T., Farmer J., Wormser V., Pham T., et al. (2013). Uncorrelated evolution of leaf and petal venation patterns across the angiosperm phylogeny. J. Exp. Bot. 64 (13), 4081–4088. doi: 10.1093/jxb/ert247 [DOI] [PubMed] [Google Scholar]
- Roddy A. B., Jiang G. F., Cao K., Simonin K. A., Brodersen C. R. (2019). Hydraulic traits are more diverse in flowers than in leaves. New Phytol. 223 (1), 193–203. doi: 10.1111/nph.15749 [DOI] [PubMed] [Google Scholar]
- Roddy A. B., Simonin K. A., McCulloh K. A., Brodersen C. R., Dawson T. E. (2018). Water relations of calycanthus flowers: hydraulic conductance, capacitance, and embolism resistance. Plant Cell Environ. 41 (10), 2250–2262. doi: 10.1111/pce.13205 [DOI] [PubMed] [Google Scholar]
- Rueden C. T., Schindelin J., Hiner M. C., DeZonia B. E., Walter A. E., Arena E. T., et al. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 18 (1), 529. doi: 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L., Cowan P. D., Jaikumar N., Holbrook N. M. (2003). The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant Cell Environ. 26 (8), 1343–1356. doi: 10.1046/j.0016-8025.2003.01058.x [DOI] [Google Scholar]
- Sack L., Frole K. (2006). LEAF STRUCTURAL DIVERSITY IS RELATED TO HYDRAULIC CAPACITY IN TROPICAL RAIN FOREST TREES. Ecology 87 (2), 483–491. doi: 10.1890/05-0710 [DOI] [PubMed] [Google Scholar]
- Sack L., Pasquet-Kok J. (2011) Leaf pressure-volume curve parameters. Available at: http://www.publish.csiro.au/prometheuswiki (Accessed 1 May 2014).
- Sargent R. D., Ackerly D. D. (2008). Plant-pollinator interactions and the assembly of plant communities. Trends Ecol. Evol. 23 (3), 123–130. doi: 10.1016/j.tree.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Schmitz N., Jansen S., Verheyden A., Kairo J. G., Beeckman H., Koedam N. (2007). Comparative anatomy of intervessel pits in two mangrove species growing along a natural salinity gradient in gazi bay, Kenya. Ann. Bot. 100 (2), 271–281. doi: 10.1093/aob/mcm103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander P. F., Bradstreet E. D., Hemmingsen E. A., Hammel H. T. (1965). Sap pressure in vascular plants. Science 148 (3668), 339–346. doi: 10.1126/science.148.3668.339 [DOI] [PubMed] [Google Scholar]
- Scholz A., Rabaey D., Stein A., Cochard H., Smets E., Jansen S. (2013). The evolution and function of vessel and pit characters with respect to cavitation resistance across 10 prunus species. Tree Physiol. 33 (7), 684–694. doi: 10.1093/treephys/tpt050 [DOI] [PubMed] [Google Scholar]
- Simonin K. A., Roddy A. B. (2018). Genome downsizing, physiological novelty, and the global dominance of flowering plants. PloS Biol. 16 (1), e2003706. doi: 10.1371/journal.pbio.2003706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin K. A., Roddy A. B., Link P., Apodaca R., Tu K. P., Hu J. I. A., et al. (2013). Isotopic composition of transpiration and rates of change in leaf water isotopologue storage in response to environmental variables. Plant Cell Environ. 36 (12), 2190–2206. doi: 10.1111/pce.12129 [DOI] [PubMed] [Google Scholar]
- Song Y., Poorter L., Horsting A., Delzon S., Sterck F. (2021). Pit and tracheid anatomy explain hydraulic safety but not hydraulic efficiency of 28 conifer species. J. Exp. Botany 73(3), 1033–1048. doi: 10.1093/jxb/erab449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry J. S., Tyree M. T. (1988). Mechanism of water stress-induced xylem embolism 1. Plant Physiol. 88 (3), 581–587. doi: 10.1104/pp.88.3.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel C. K. (1996). “Discovery of the secret of nature in the structure and fertilization of flowers,” in Floral biology: studies on floral evolution in animal-pollinated plants. Eds. Lloyd D. G., Barrett S. C. H. (Boston, MA: Springer US; ), 3–43. [Google Scholar]
- Teixido A. L., Valladares F. (2014). Disproportionate carbon and water maintenance costs of large corollas in hot Mediterranean ecosystems. Perspect. Plant Ecology Evol. Systematics 16 (2), 83–92. doi: 10.1016/j.ppees.2014.02.002 [DOI] [Google Scholar]
- Théroux-Rancourt G., Roddy A. B., Earles J. M., Gilbert M. E., Zwieniecki M. A., Boyce C. K., et al. (2021). Maximum CO2 diffusion inside leaves is limited by the scaling of cell size and genome size. Proc. R. Soc. B: Biol. Sci. 288 (1945), 20203145. doi: 10.1098/rspb.2020.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree M. T., Hammel H. T. (1972). The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J. Exp. Bot. 23 (1), 267–282. doi: 10.1093/jxb/23.1.267 [DOI] [Google Scholar]
- Tyree M. T., Zimmermann M. H. (2013). Xylem structure and the ascent of sap (New York: Springer Science & Business Media; ). [Google Scholar]
- Warton D. I., Wright S. T., Wang Y. (2012). Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol. Evol. 3 (1), 89–101. doi: 10.1111/j.2041-210X.2011.00127.x [DOI] [Google Scholar]
- Waser N. M., Price M. V. (2016). Drought, pollen and nectar availability, and pollination success. Ecology 97 (6), 1400–1409. doi: 10.1890/15-1423.1 [DOI] [PubMed] [Google Scholar]
- Wheeler J. K., Sprerry J. S., Hacke U. G., Hoang N. (2005). Inter-vessel pitting and cavitation in woody rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ. 28 (6), 800–812. doi: 10.1111/j.1365-3040.2005.01330.x [DOI] [Google Scholar]
- Zhang F. P., Brodribb T. J. (2017). Are flowers vulnerable to xylem cavitation during drought? Proc. R. Soc. B-Biological Sci. 284 (1854), , 20162642–20162650. doi: 10.1098/rspb.2016.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. P., Carins Murphy M. R., Cardoso A. A., Jordan G. J., Brodribb T. J. (2018). Similar geometric rules govern the distribution of veins and stomata in petals, sepals and leaves. New Phytol. 219 (4), 1224–1234. doi: 10.1111/nph.15210 [DOI] [PubMed] [Google Scholar]
- Zhang F. P., Zhang J. L., Brodribb T. J., Hu H. (2021). Cavitation resistance of peduncle, petiole and stem is correlated with bordered pit dimensions in magnolia grandiflora. Plant Divers. 43 (4), 324–330. doi: 10.1016/j.pld.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.