Abstract
Introduction
Psoriatic alopecia is considered a type of hair loss occurring in patients with psoriasis. Adalimumab is a fully humanized recombinant anti-TNF-alpha monoclonal antibody approved for treatment of psoriasis and psoriatic arthritis (PsA), rarely related to the occurrence of dermatological disorders.
Case Presentation
We report the case of a 56-year-old female with PsA developing psoriatic alopecia and paradoxical psoriasis induced by adalimumab and successfully treated switching to certolizumab, evaluating response at both thrichoscopy and in vivo reflectance confocal microscopy.
Discussion
Among anti-TNF-α agents, certolizumab is the least involved in the development of paradoxical reactions such as psoriatic alopecia and showed to be an effective and safe alternative therapeutic options to manage psoriasis and PsA minimizing the risk of paradoxical reactions.
Keywords: Certolizumab pegol, Adalimumab, Psoriatic alopecia, Paradoxical psoriasis, In vivo reflectance confocal microscopy
Established Facts
Psoriatic alopecia is a form of hair loss or any other hair abnormality occurring in patients with psoriasis. Psoriatic alopecia is an emerging type of paradoxical reaction possibly being secondary to biological drug use.
Female sex, adalimumab or infliximab treatment, and inflammatory bowel disease represent the most common risk factors.
Novel Insights
Paradoxical reaction to certolizumab pegol, a Fab fragment of a recombinant humanized antibody directed against TNF-alpha and conjugated with polyethylene glycol, seems to be lower than other anti-TNF-alpha.
Different immunological properties of various TNF-alpha monoclonal antibodies and the lack of the Fc fraction characterizing certolizumab may explain the lower incidence of paradoxical reactions.
Certolizumab pegol may be efficacious in psoriatic alopecia due to TNF-alpha inhibitor.
Introduction
Tumor necrosis factor (TNF)-alpha inhibitors play an important role in chronic inflammatory diseases therapy such as Crohn's disease and ulcerative colitis, ankylosing spondylitis, psoriatic arthritis (PsA), psoriasis, and hidradenitis suppurativa [1]. TNF-alpha is a pro-inflammatory cytokine involved in host defense against infectious agents and in regulating inflammation and cell differentiation in many chronic immunologically-based inflammatory diseases [2].
Adalimumab is a fully humanized recombinant anti-TNF-alpha monoclonal antibody safe and effective in the treatment of moderate-to-severe plaque psoriasis and PsA. Dermatological disorders (lupus-like syndrome, paradoxical psoriasis, alopecia areata, and psoriatic alopecia) may rarely occur during treatment with adalimumab [3].
Psoriatic alopecia is not yet a clinically and histopathologically well-defined entity. It is considered a form of hair loss or any other hair abnormality occurring in patients with psoriasis and affecting the scalp. Three forms of scalp alopecia have been described in the literature: (i) lesional, the most frequent form confined to lesional psoriatic skin, (ii) generalized telogen effluvium, and (iii) scarring alopecia, the rarest one. The etiopathogenesis is still unclear, and the hair loss may be related to psoriasis itself, autoimmune conditions, and topic or systemic treatments used for psoriasis [4]. We herein report the case of a 56-year-old female patient affected by PsA who developed paradoxical psoriasis and psoriatic alopecia under adalimumab, successfully treated with certolizumab pegol.
Case Report
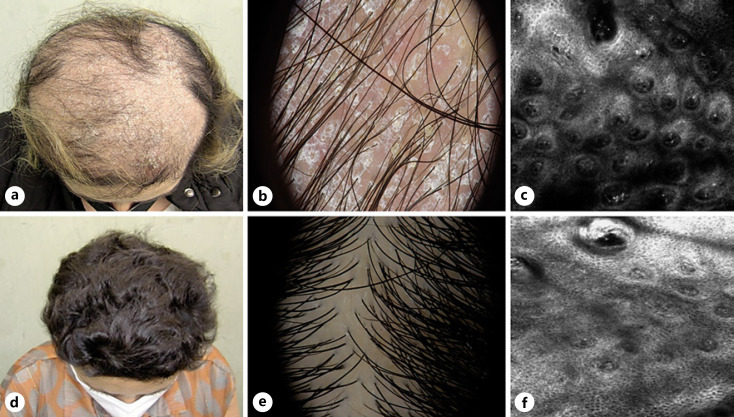
A 56-year-old female with PsA referred to our outpatient clinic for the abrupt development of erythemato-desquamative plaques over the body including the scalp and concomitant severe alopecia. The patient related and referred that these lesions developed after 12 weeks of adalimumab which was prescribed by her rheumatologist for PsA. Her medical history was unremarkable except for hypertension and PsA (diagnosed 7 years before) previously treated with systemic corticosteroids and methotrexate without consistent results. Dermatological examination showed erythematous scaly plaques on the scalp with nonscarring inflammatory alopecia characterized by a severe decrease in hair density and finer hairs at the vertex and the parietal regions (shown in Fig. 1a). Trichoscopy revealed a thick inter and perifollicular white-yellowish scales, arborizing and glomerular vessels, follicular units of single-hairs, and multiple upgrowing hairs (shown in Fig. 1b). The pull test was positive. She also presented psoriasis lesions on the trunk and limbs (psoriasis area and severity index = 22 and body surface area 38%). Routine laboratory investigations including thyroid function, celiac disease, and autoantibodies were within normal ranges. Since the patient was reluctant to undergo an incisional scalp biopsy for histological/pathological examination, we decided to perform in vivo reflectance confocal microscopy (RCM) which showed parakeratosis and inflammatory cells in the superficial layer, with papillomatosis and a high blood flow at the dermoepidermal junction (shown in Fig. 1c).
Fig. 1.
Patient at baseline: clinical (a), dermoscopic (b), and RCM (c) pictures; patient after 6 months of treatment: clinical (d), dermoscopic (e), and RCM (f) pictures.
A diagnosis of paradoxical psoriasis and psoriatic alopecia induced by adalimumab was performed. Since also her PsA symptoms were not under control (the patient complained of joint pain in elbows, wrists, hands, and feet), treatment was promptly switched to certolizumab (400 mg subcutaneously every 2 weeks for 3 times followed by 200 mg every 2 weeks). Despite the absence of alopecia treatment, a significant improvement was observed for both psoriatic skin lesions and alopecia with arrest of hair loss and progressive regrowth at 3 months. PsA symptoms were under control without swollen or tender joints at clinical examination. After 6 months, a complete skin clearance (psoriasis area and severity index and body surface area = 0) and complete hair regrowth were achieved (shown in Fig. 1d, e). RCM showed a decrease in parakeratosis and papillomatosis, as well as a return to normal dermal papillae configuration (shown in Fig. 1f). The patient maintained such controlled condition up to 12 months of treatment.
Discussion
Paradoxical reactions are defined as adverse events consisting in new onset or aggravation of a disease, caused by a biologic drug which is usually used to improve symptoms of that disease [5]. Anti-TNF-alpha is described as the most commonly biologic involved in the development of paradoxical psoriasis. The most common clinical variant is palmoplantar pustulosis (42.9%), followed by plaque psoriasis (14.7%), and less frequently scalp psoriasis (7%) [6].
Psoriatic alopecia is an emerging type of paradoxical reaction secondary to anti-TNF-alpha therapy, first reported by Doyle et al. in 2011 [7]. Female sex, adalimumab or infliximab treatment, and inflammatory bowel disease represent the most common risk factors [8]. Paradoxical reaction to certolizumab pegol, a Fab fragment of a recombinant humanized antibody directed against TNF-alpha and conjugated with polyethylene glycol, seems to be lower than other anti-TNF-alpha. Particularly, a study based on a combined analysis of clinical trial data in patients with Crohn's disease demonstrated the long-term safety of certolizumab and the low incidence of paradoxical psoriasis [9]. The different immunological properties of various TNF-alpha monoclonal antibodies and the lack of the Fc fraction characterizing certolizumab may explain the lower incidence of paradoxical reactions [9]. Only 5 cases of psoriatic alopecia secondary to certolizumab pegol are reported in the current literature [10, 11]. Among 52 cases of TNF-α antagonists-related alopecia collected by the French Pharmacovigilance Database, only 2 cases (3.8%) occurred during exposure to certolizumab [10]. Raquel Aragòn-Miguel et al. [11] described the development of psoriatic alopecia-like paradoxical reaction in female patient with sacroiliitis 6 months after starting certolizumab pegol with complete resolution achieved by switching to ustekinumab. In addition, Ferraresso et al. [12] described a case of scalp alopecia in a patient with Chron's disease who experienced hair regrowth after 4 weeks of treatment with topical steroids and discontinuation of certolizumab. Lauro W, et al. [13] as well reported a case of psoriatic alopecia occurred in a patient suffering from psoriasis after 10 months of treatment with certolizumab pegol and resolved with the discontinuation of certolizumab pegol and pulsed therapy with oral dexamethasone combined with topical clobetasol lotion for 8 weeks. Hence, psoriatic alopecia linked to certolizumab seems to be likely uncommon compared to other anti-TNF drugs. Moreover, paradoxical adverse events are not restricted to anti-TNF-α agents and drug-induced psoriatic alopecia has been also described in association with anti-interleukin-12/23 and anti-interleukin-17A agents [14, 15]. Considering this evidence, the presence of both active PsA and psoriasis apart from psoriatic alopecia, as well as the possibility of development of psoriatic alopecia with all existing biologic drug classes, we decided to switch our patient treatment from adalimumab to certolizumab in order to obtain an adequate control of PsA and psoriasis without worsening her psoriatic alopecia. To our knowledge, this is the first case reported in the literature of psoriatic alopecia due to TNF-alpha inhibitor with complete resolution after switching to another TNF-alpha blocking agent such as certolizumab. The treatment algorithm of paradoxical psoriatic alopecia is still lacking and not universally shared. Even if mild forms may improve with topicals, scarring alopecia may rarely occur so that switching biologic drug should often be considered [16]. Further studies are required to improve our knowledge about the occurrence of new or as yet undescribed emerging adverse events due to newly available biological drugs and to identify effective and safe alternative therapeutic options to manage the underlying disease minimizing the risk of paradoxical reactions.
Statement of Ethics
This article was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Ethics approval is not required in accordance with local/national guidelines. The patient's written informed consent to publish the case (including publication of images) was obtained. Information revealing the subject's identity was avoided.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding received.
Author Contributions
All authors have read and approved the final manuscript. Matteo Megna, Mario De Lucia, Lucia Gallo, Wanda Lauro, Vincenzo Picone: conceptualization, validation, visualization, writing-original draft preparation, and writing − review and editing. Gabriella Fabbrocini: conceptualization, validation, visualization, writing − original draft preparation, and writing − review and editing, supervision. Sonia Sofia Ocampo-Garza: data curation, investigation methodology, visualization, writing-original draft preparation, and writing − review and editing.
Data Availability Statement
Data are reported in the current study. Further inquiries can be directed to the corresponding author.
Funding Statement
No funding received.
References
- 1.Gerriets V, Bansal P, Goyal A, Khaddour K. StatPearls [internet] Treasure Island (FL): StatPearls Publishing; 2022. Jan, Tumor necrosis factor inhibitors. 2021 jul 18. [PubMed] [Google Scholar]
- 2.Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38) transforming growth factor β and TNF-α receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016 Oct;138((4)):984–1010. doi: 10.1016/j.jaci.2016.06.033. Epub 2016 Aug 28. [DOI] [PubMed] [Google Scholar]
- 3.Scheinfeld N. Adalimumab a review of side effects. Expert Opin Drug Saf. 2005 Jul;4((4)):637–641. doi: 10.1517/14740338.4.4.637. [DOI] [PubMed] [Google Scholar]
- 4.George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015 Oct;40((7)):717–721. doi: 10.1111/ced.12715. Epub 2015 Jul 23. [DOI] [PubMed] [Google Scholar]
- 5.Munera-Campos M, Ballesca F, Carrascosa JM. Paradoxical reactions to biologic therapy in psoriasis a review of the literature. Actas Dermosifiliogr(Engl Ed) 2018;109((9)):791–800. doi: 10.1016/j.ad.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Joyau C, Veyrac G, Dixneuf V, Jolliet P. Anti-tumour factor alpha therapy and increased risk of de novo psoriasis is it really a paradoxical side effect? Clin Exp Rheumatol. 2012;30((5)):700–706. [PubMed] [Google Scholar]
- 7.Doyle LA, Sperling LC, Baksh S, Lackey J, Thomas B, Vleugels RA, et al. Psoriatic alopecia/alopecia areata-like reactions secondary to anti-tumor necrosis factor-α therapy a novel cause of noncicatricial alopecia. Am J Dermatopathol. 2011;33((2)):161–166. doi: 10.1097/DAD.0b013e3181ef7403. [DOI] [PubMed] [Google Scholar]
- 8.Toda-Brito H, Lopes L, Soares-Almeida L, Filipe P. Adalimumab- induced psoriatic alopecia/alopecia areata-like reaction in a patient with Crohn's disease. Dermatol Online J. 2015;21((11)) [PubMed] [Google Scholar]
- 9.Loftus EV, Jr, Colombel JF, Schreiber S, Randall CW, Regueiro M, Ali T, et al. Safety of long- term treatment with certolizumab pegol in patients with Crohn's disease based on a pooled analysis of data from clinical trials. Clin Gastroenterol Hepatol. 2016;14((12)):1753–1762. doi: 10.1016/j.cgh.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Béné J, Moulis G, Auffret M, Lefevre G, Coquerelle P, Coupe P, et al. Alopecia induced by tumour necrosis factor-alpha antagonists description of 52 cases and disproportionality analysis in a nationwide pharmacovigilance database. Rheumatology (Oxford) 2014 Aug;53((8)):1465–1469. doi: 10.1093/rheumatology/keu145. [DOI] [PubMed] [Google Scholar]
- 11.Aragón-Miguel R, Calleja-Algarra A, Vico-Alonso C, Sánchez-Velázquez A, Garrido MC, Ortiz-Romero PL, et al. Psoriatic alopecia-like paradoxical reaction to certolizumab pegol. Int J Dermatol. 2019 Jun;58((6)):e118–e120. doi: 10.1111/ijd.14417. Epub 2019 Mar 1. [DOI] [PubMed] [Google Scholar]
- 12.Ferraresso MG, Garlatti MLB, Perez-Chada LM, Martin MS, Mazzuoccolo LD. Certolizumab-induced paradoxical psoriatic alopecia. J Psoriasis Psoriatic Arthritis. 2020;5((3)):86–92. [Google Scholar]
- 13.Lauro W, Picone V, Abategiovanni L, Vastarella M, Gallo L, Fabbrocini G, et al. A case of psoriatic alopecia secondary to certolizumab pegol clinical and trichoscopic evaluation. Int J Dermatol. 2022 May 17; doi: 10.1111/ijd.16275. [DOI] [PubMed] [Google Scholar]
- 14.Mihailescu M, Cibull T, Joyce J. Development of drug-induced psoriasiform alopecia in a pediatric patient on ustekinumab. J Cutan Pathol. 2021 Dec;48((12)):1523–1525. doi: 10.1111/cup.14122. Epub 2021 Sep 1. [DOI] [PubMed] [Google Scholar]
- 15.Tan TL, Taglia L, Yazdan P. Drug-induced psoriasiform alopecia associated with interleukin-17 inhibitor therapy. J Cutan Pathol. 2021 Jun;48((6)):771–774. doi: 10.1111/cup.13952. Epub 2021 Jan 12. [DOI] [PubMed] [Google Scholar]
- 16.Jeong KM, Seo JY, Kim A, Baek YS, Song HJ, Jeon J. Tumor necrosis factor-alpha inhibitor-associated psoriatic alopecia in a patient with ulcerative colitis a case report and review of the literature. Ann Dermatol. 2021 Feb;33((1)):82–85. doi: 10.5021/ad.2021.33.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are reported in the current study. Further inquiries can be directed to the corresponding author.