Abstract
Scalp seborrheic dermatitis (SSD) is a prevalent chronic, relapsing inflammatory skin disease. The etiology is related to sebum production, bacterial proliferation − Staphylococcus sp., Streptococcus, and M. restricta − and host immunity factors − NK1+, CD16+ cells, IL-1, and IL-8. Trichoscopy features include mostly arborizing vessels and yellowish scales. New trichoscopic findings were described to guide the diagnosis as dandelion vascular conglomerate, “cherry blossom” vascular pattern, and intrafollicular oily material. Antifungals and corticosteroids constitute the essential therapy, but new treatments have been described. This article aims to review and discuss the etiology, pathophysiology, trichoscopy, histopathologic findings, main differential diagnoses, and therapeutic options of SSD.
Keywords: Seborrheic dermatitis, Scalp, Dandruff, Microbiome
Introduction
Scalp seborrheic dermatitis (SSD) is a prevalent chronic, relapsing inflammatory skin disease [1−7]. It is the most common cause of scalp itching, characterized by scaling in an erythematous background. This condition can also lead to telogen effluvium and the progression of androgenetic alopecia [6]. Scalp SD has two peaks of incidence: one among newborns to infants up to 3 months of age and another among adults 30–60 years of age [3, 8]. Men are affected more often than women, and there is no preference for ethnicity [2−4, 6, 9]. The complex interplay of Malassezia, keratinocytes, and the immune response against an altered lipid composition in the skin has an essential role in the pathogenesis [2, 3]. This article aims to review seborrheic dermatitis (SD) with a specific focus on scalp involvement, highlighting the main points of practical interest for physicians.
Methods and Results
We performed a literature search in the scientific database PubMed between 2004 and 2022, using the terms “seborrheic dermatitis” AND “scalp”, “seborrheic dermatitis” AND “treatment”, “seborrheic dermatitis” AND “microbiome”. We limited the search to the more recent articles in English literature and excluded duplicate titles. We considered the largest studies focusing on SSD triggers, their relation to the skin microbiota, trichoscopic findings, and new treatments. We had 19 relevant articles, which included nine review articles, four clinical trials, three observational studies, two case reports, and one comparative study.
Discussion
Etiology
Several factors are involved in the development of this disorder: hormone levels, individual lipid compositions, overactivity of the sebaceous glands, fungal infections, nutritional deficits, neuropsychiatric factors, and environmental conditions, in addition to host susceptibilities such as sebum production [8−10]. SSD starts at puberty suggesting a hormonal influence of androgens on the pilosebaceous unit, which affects sebaceous gland activity and lipid composition, and promotes Malassezia growth [2, 3, 11]. Malassezia spp. is a lipophilic fungus that is part of the human skin's normal microbiota [2]. It produces lipases, which hydrolyze sebaceous lipids in arachidonic acid and fatty acids [2, 12]. These fatty acids become integrated into the fungal cell wall and are necessary for its growth [2, 7−9]. Furthermore, arachidonic acid activates immunity and stimulates keratinocytes to produce pro-inflammatory mediators [7, 8, 10].
Diet, in particular zinc deficiency, is also implicated in SSD [1−3]. A cross-sectional study has shown that the antioxidants found in fruit and vegetables may help prevent the expression of SD inflammatory genes [13].
Environmental factors such as high humidity − the strongest association − [1], as well as cloudy days and low temperatures, are linked to the emergence of SD [6]. Furthermore, UV light inhibits the growth of Malassezia yeasts [5].
SD is more common in immunosuppressed patients, suggesting that immune dysfunction plays a role in the development of the disease [1−3]. Rincón et al. reported a predominance of Malassezia globosa in HIV-positive patients with SD, whereas Malassezia furfur and Malassezia restricta were more prevalent in HIV-negative individuals. Passi et al. [9] reported a significant reduction in the skin content of squalene and an increase in the cholesterol esters in HIV-positive patients.
Patients with Parkinson's disease are usually affected by both SD and SSD due to inadequate dopamine levels leading to the lack of melanocyte-stimulating hormone inhibiting factors [12−14]. When treated with L-Dopa, these medications restore the production of melanocyte-stimulating hormone-inhibiting factors, and sebum production decreases [3].
During the COVID-19 pandemic, the scalp was affected in 19.9% of SD patients [15]. The increased production of interleukin-1, interleukin-6, and TNF-alfa may link COVID-19 to SD [14].
Immunogenetics
There is an increase in NK1+, CD16+ cells and interleukins − IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, beta-defensins, IFN-γ, nitric oxide, and histamine. Also, the complement is activated [2−4, 7, 9, 14]. A significant increase in the ratio of IL-1RA: IL-1a and IL8 and overproduction of histamine were detected in SSD [2]. Hereditary factors also predispose to SSD, such as HLA-AW30 and/or AW31 and HLA-B12 [9].
Microbiome
The healthy scalp microbiota has low bacterial diversity and is dominated by Cutibacterium acnes, Staphylococcus epidermidis, Corynebacterium, and yeasts, especially M. globosa [3]. The C. acnes to S. epidermidis ratio is higher in a healthy scalp [11]. Children's scalp microbiome is more diverse and has a lower content of Malassezia spp [11]. In SSD, M. restricta and M. globosa are the two prevalent species of Malassezia, and it is suggested that they have an increased expression of lipase genes [3, 8].
Moreover, changes in the skin pH can create a favorable environment for the growth of Staphylococcus aureus and influence lipid metabolism in the skin [12]. While Staphylococcus is strongly correlated with the disease severity in SSD, dandruff was associated with a higher incidence of S. epidermidis[1, 10].
Clinical Trial
SSD presents yellow scales surrounded by erythema, dandruff, and pruritus when there is more inflammation [3, 9, 12, 15]. Especially in infancy, the most common manifestation is the yellowish adherent scales, that can provoke alopecia, such as pseudo tinea amiantacea [9, 12, 15].
Trichoscopy
The most important trichoscopic findings of SSD are diffuse yellowish scales, single and clusters of scales on an erythematous background distributed among the follicular units, peripilar casts, multicomponent vascular pattern − multiple dotted, comma, linear, simple red loops, and thin arborizing vessels, which is the most frequent finding [6, 16]. A recent study described three new trichoscopy findings: (1) “Dandelion” vascular conglomerate: a yellow dot surrounded by glomerular and comma vessels − the only trichoscopic sign correlated to a high quantity of Malassezia sp. in this study; (2) “cherry blossom” vascular pattern: a conglomerate of arborizing vessels, with multiple glomerular and comma vessels surrounding a vascular pattern; (3) intrafollicular oily material − the most frequent trichoscopic feature − described as a cumulus of sebum and keratin in the hair infundibulum [16].
Differential Diagnoses
The differential diagnoses of SSD include pytiriasis sicca, psoriasis, sebopsoriasis, and tinea capitis [12]. Pytiriasis sicca, also called dandruff, is the main differential diagnosis. It is restricted to the scalp, involving itchy and flaking skin without visible inflammation. Psoriasis presents demarcated thick plaques with a silvery scale [12]. Trichoscopy findings are usually glomerular vessels and red globules. The biopsy can help in this diagnosis. Sebopsoriasis is another differential and overlaps between SD and psoriasis [12].
Tinea capitis is characterized by the presence of scaly, red, slightly raised patches with a central clearing [12]. There may be vesicles at the active border [12]. In trichoscopy, the presence of broken hairs, common hairs, corkscrews, and “zig-zag” hairs can help the diagnosis. Furthermore, in some cases, a mycological exam should be requested [12].
Histopathology
SSD reveals a pattern of spongiotic dermatitis, with the classical evolution of the other eczemas (acute, subacute, and chronic phases). Infundibular ostia parakeratosis is frequent. There are lymphocytes and rarely neutrophils exocytosis, vascular dilatation at the superficial dermis, and mild edema. In the chronic phase, psoriasiform hyperplasia is more common. The most important differential diagnosis is scalp psoriasis, which reveals hypogranulosis, confluent parakeratosis, and neutrophils at the corneal layer [3, 9, 12].
Treatment
Topical Treatment
Antifungals and corticosteroids usually are the first-line treatment for SSD. 2% fluconazole shampoo, 1% bifonazole ointment, 2% miconazole, and 2% ketoconazole reduce Malassezia proliferation and the inflammatory response [1, 2, 17]. Ciclopirox olamine 1% also has antibacterial effects [2−4, 10]. Ketoconazole foaming gel seems to be more effective than 0.05% betamethasone dipropionate. In addition, 2% ketoconazole cream is as effective as 1% hydrocortisone cream [3]. Topical corticosteroids should be reserved for the control of acute flares and to reduce inflammation [8, 12]. However, relapses are frequent [8, 15].
Keratolytics − coaltar, salicylic acid (2–6%), urea, propylene glycol, lactic acid, and zinc pyrithione − removed the adherent flakes [3, 4, 15]. Zinc pyrithione 1-2% has keratolytic activity and inhibits fungal growth [2, 3, 9, 10, 12, 15]. Selenium disulfide 1–2.5% shampoo is fungicidal to Pityrosporum ovale and M. furfur, keratolytic effects [2−4, 9, 11, 12].
Topical calcineurin inhibitors − tacrolimus and pimecrolimus − have immunomodulatory and anti-inflammatory properties [2, 4, 9, 17]. Topical 0.1% tacrolimus also inhibited the growth of M. furfur and P. ovale[2, 4, 17]. In a study, tacrolimus was as effective as betamethasone lotion or zinc pyrithione shampoo; however, tacrolimus offered more prolonged remission [2, 9, 17]. Statins have demonstrated anti-inflammatory and immunomodulatory effects. Inhibiting HMG-CoA reductase class I and cholesterol synthesis, statins reduce the production of ergosterol which have a role in the survival of fungi. 5% atorvastatin lotion had efficacy comparable to 0.1% betamethasone lotion in the SSD [7]. Cannabidiol shampoo demonstrated efficacy in mild to moderate SSD. It normalizes lipogenesis, suppresses cell proliferation, and prevents the elevation of pro-inflammatory cytokine [6]. Glycyrrhetinic acid has anti-inflammatory and antimicrobial effects. Shampoo may be suitable as initial therapy or maintenance for scalp SD [4, 10, 16].
Oral Treatment
Oral antifungals have been used in extensive and resistant conditions [2, 13]. Azoles (itraconazole, fluconazole, and ketoconazole) inhibit the conversion of lanosterol to ergosterol by suppressing the cytochrome P450 [4]. As a result, the cell membrane is disrupted, compromising function and cell growth [4]. Oral ketoconazole 200 mg daily for 4 weeks is an effective treatment [4]. However, side effects are common when used for more than 4 weeks [4]. Thus, itraconazole 200 mg daily for 7 days is also efficient with less hepatotoxicity when compared to ketoconazole [4].
Gupta et al. [3, 4] described that itraconazole achieves much higher serum levels than ketoconazole and griseofulvin [3, 4]. It can last up to 2 weeks in the patient's plasma after completion of therapy [3, 4]
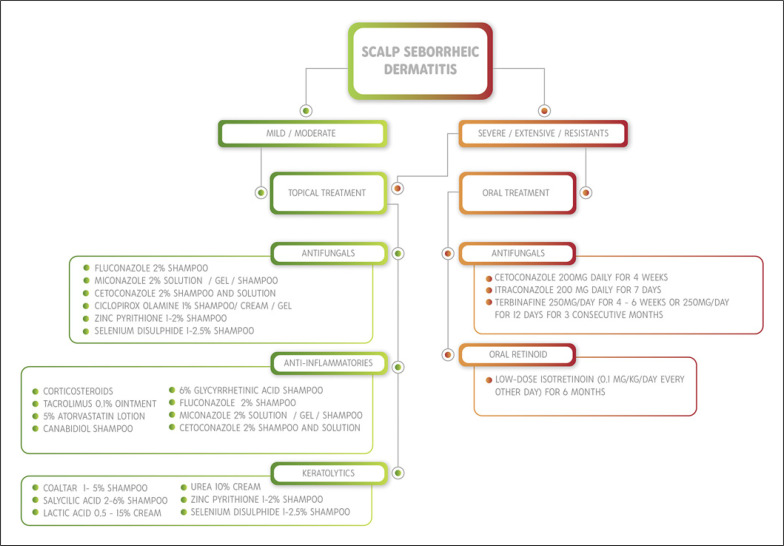
Another option is terbinafine at a dosage of 250 mg/day for 4–6 weeks or 250 mg/day for 12 days for 3 consecutive months (pulse regimen) [18]. For resistant, moderate, or severe cases, low-dose oral isotretinoin (0.1 mg/kg/day every other day) over the course of 6 months should be considered [19]. A practical algorithm for SSD therapy is provided in Figure 1.
Fig. 1.
Practical algorithm for SSD therapy according to the disease severity.
Conclusion
SSD is a prevalent relapsing inflammatory condition. Full pathogenesis is still unclear, although correlated with alterations in sebaceous gland activity, lipid composition, androgens, humidity, and immunity. Dysbiosis of the skin microbiome also plays an important role.
Trichoscopy remains an auxiliary tool for this condition. SSD should be considered especially when showing scales associated with thin arborizing vessels. Antifungals and corticosteroids constitute the essential therapy for SSD, but new treatments have been proposed recently, such as cannabidiol shampoo and statins lotions.
In conclusion, SSD is a chronic and distressful condition that significantly impacts patients' quality of life. This article provides a comprehensive overview of SD with a specific focus on scalp involvement to guide dermatologists in clinical practice.
Statement of Ethics
The study complies with the internationally accepted standards for research practice and reporting. Ethical approval was not required for this study as per national guidelines.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
The authors did not receive any funding.
Author Contributions
Anne Kelly Leroy Pinto prepared the draft, wrote the article, and approved the final version to be published. Rita Fernanda Cortez de Almeida conceived the study, wrote the article, and approved the final version to be published. Sidney Frattini and Daniel Obadia wrote and reviewed the article and approved the final version to be published. Daniel Fernandes Melo conceived the study, critically reviewed the article, and approved the final version to be published.
Funding Statement
The authors did not receive any funding.
References
- 1.Tao R, Li R, Wang R. Skin microbiome alterations in seborrheic dermatitis and dandruff a systematic review. Exp Dermatol. 2021 Oct;30(10):1546–1553. doi: 10.1111/exd.14450. [DOI] [PubMed] [Google Scholar]
- 2.Dessinioti C, Katsambas A. Seborrheic dermatitis etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013 Jul-Aug;31(4):343–351. doi: 10.1016/j.clindermatol.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004 Jan;18(1):13–26. doi: 10.1111/j.1468-3083.2004.00693.x. quiz 19-20. [DOI] [PubMed] [Google Scholar]
- 4.Gupta AK, Nicol K, Batra R. Role of antifungal agents in the treatment of seborrheic dermatitis. Am J Clin Dermatol. 2004;5(6):417–422. doi: 10.2165/00128071-200405060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kutlu Ö. Evaluation of the correlation between frequency of seborrheic dermatitis and quantitative climate data. Int J Dermatol. 2022 Feb;61(2):e65–7. doi: 10.1111/ijd.15783. [DOI] [PubMed] [Google Scholar]
- 6.Vincenzi C, Tosti A. Efficacy and tolerability of a shampoo containing broad-spectrum Cannabidiol in the treatment of scalp inflammation in patients with mild to moderate scalp psoriasis or seborrheic dermatitis. Skin Appendage Disord. 2020 Nov;6(6):355–361. doi: 10.1159/000510896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobhan M, Gholampoor G, Firozian F, Mohammadi Y, Mehrpooya M. Comparison of efficacy and safety of atorvastatin 5% lotion and betamethasone 0.1% lotion in the treatment of scalp seborrheic dermatitis. Clin Cosmet Investig Dermatol. 2019 Apr 29;12:267–275. doi: 10.2147/CCID.S196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Q, Panchamukhi A, Li P, Shan W, Zhou H, Hou L, et al. Malassezia and Staphylococcus dominate scalp microbiome for seborrheic dermatitis. Bioprocess Biosyst Eng. 2021 May;44(5):965–975. doi: 10.1007/s00449-020-02333-5. [DOI] [PubMed] [Google Scholar]
- 9.Sampaio ALSB, Mameri ACA, Vargas TJd S, Ramos-e-Silva M, Nunes AP, Carneiro SCd S. Seborrheic dermatitis. An Bras Dermatol. 2011 Nov-Dec;86(6):1061–1071. doi: 10.1590/s0365-05962011000600002. quiz 1072–4. [DOI] [PubMed] [Google Scholar]
- 10.Wang HC, Wang CS, Hsieh SC, Hung YT, Chen HH. Evaluation of a new-formula shampoo containing 6% glycyrrhetinic acid complex for scalp seborrheic dermatitis a pilot study. J Cosmet Dermatol. 2022 Aug;21(8):3423–3430. doi: 10.1111/jocd.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polak-Witka K, Rudnicka L, Blume-Peytavi U, Vogt A. The role of the microbiome in scalp hair follicle biology and disease. Exp Dermatol. 2020 Mar;29(3):286–294. doi: 10.1111/exd.13935. [DOI] [PubMed] [Google Scholar]
- 12.Ijaz N, Fitzgerald D. Seborrhoeic dermatitis. Br J Hosp Med. 2017 Jun 2;78(6):C88–C91. doi: 10.12968/hmed.2017.78.6.C88. [DOI] [PubMed] [Google Scholar]
- 13.Sanders MGH, Pardo LM, Ginger RS, Kiefte-de Jong JC, Nijsten T. Association between diet and seborrheic dermatitis a cross-sectional study. J Invest Dermatol. 2019 Jan;139(1):108–114. doi: 10.1016/j.jid.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Alpalhão M, Gaibino N, Filipe P. Seborrheic dermatitis in COVID-19 a case report. Int J Dermatol. 2020 Dec;59(12):1543–1544. doi: 10.1111/ijd.15256. [DOI] [PubMed] [Google Scholar]
- 15.Turkmen D, Altunisik N, Sener S, Colak C. Evaluation of the effects of COVID-19 pandemic on hair diseases through a web-based questionnaire. Dermatol Ther. 2020 Nov;33(6):e13923. doi: 10.1111/dth.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Arriaga LF, Arenas R, Vega-Sánchez DC, Asz-Sigall D, Martínez-Velazco MA. Seborrheic dermatitis three novel trichoscopic signs and its correlation to Malassezia sp. colonization. Skin Appendage Disord. 2019 Aug;5(5):288–292. doi: 10.1159/000497782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin H, Kwon OS, Won CH, Kim BJ, Lee YW, Choe YB, et al. Clinical efficacies of topical agents for the treatment of seborrheic dermatitis of the scalp a comparative study. J Dermatol. 2009 Mar;36(3):131–137. doi: 10.1111/j.1346-8138.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 18.Dall'Oglio F, Nasca MR, Gerbino C, Micali G. An overview of the diagnosis and management of seborrheic dermatitis. Clin Cosmet Investig Dermatol. 2022 Aug 6;15:1537–1548. doi: 10.2147/CCID.S284671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis a comprehensive review. J Dermatolog Treat. 2019 Mar;30(2):158–169. doi: 10.1080/09546634.2018.1473554. [DOI] [PubMed] [Google Scholar]