Abstract
Diverse representation in clinical trials is crucial to understand the efficacy and safety of drugs in minority groups. This review aims to (1) describe research participants' sex, racial, and ethnic diversity in clinical drug trials and (2) describe the sex distribution of researchers conducting the research. We reviewed all clinical drug trials published in the journals “Clinical Pharmacology and Therapeutics” and “Clinical and Translational Science” in 2000–2001 and 2020–2021 and analyzed the research participants' and researchers' demographics. We compared the race of the research participants with the concurrent race diversity of the reference population in the countries where the research was conducted. We identified 281 articles with 17,639 research participants. Approximately one‐third of the research participants were women in both 2000–2001 and 2020–2021. The representation from racial minorities of Black and Asian people increased from 2000–2001 to 2020–2021, but Asian and Native American people are still under‐represented in clinical drug trials today. The proportion of female authors increased, but female authors still made up less than 40% of the total number of authors in 2020–2021. In conclusion, men are still over‐represented in clinical pharmacology research, and some races are still vastly under‐represented. Furthermore, although the proportion of female authors increased with time, they are still under‐represented as first and last authors.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Representation from minorities in clinical trials is essential because sex‐dependent and inter‐racial/ethnic differences in pharmacokinetics/pharmacodynamics can affect drug response in individuals from different population groups, leading to increased risk of drug failure or toxicity in unrepresented groups.
WHAT QUESTION DID THIS STUDY ADDRESS?
How do the sex, racial, and ethnic diversity of research participants and sex distribution of researchers look in clinical drug trials published in 2000–2001 and 2020–2021?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Women are still under‐represented as participants in clinical drug trials. The representation of minority groups of Black and Asian people increased over 20 years. However, Asian people and Native Americans are still under‐represented. More women author clinical drug trials but are still vastly under‐represented, especially as the last authors.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Awareness regarding the under‐representation of women and minorities is essential in combatting the lack of diversity in clinical research, thereby increasing drug treatment safety for minority populations.
INTRODUCTION
Clinical trials are essential when investigating the efficacy and safety of drugs in humans. Pharmacokinetics and pharmacodynamics differ between population groups, and the diversity of research participants is important for the generalizability of the results obtained in a clinical trial. Historically, primarily White men have been included in clinical trials. 1 Thus, the under‐representation of minority groups in clinical drug trials is a cause for concern and might lead to minorities being at higher risk of adverse reactions or reduced drug efficacy.
Women and men are genetically and physiologically different. Sex‐dependent differences in renal clearance, body weight, and body fat percentage 2 result in differences in drug disposition. 3 The genes encoding the cytochrome P450 (CYP) enzymes are located on autosomal chromosomes leading to sex differences in regulation of expression and activity. 4 For example, CYP3A4 activity is 20–50% higher in women, resulting in faster clearance of drugs metabolized through this enzyme. 5 In addition, CYP enzyme activity may be influenced by oral contraceptives. 6 These internal and external factors emphasize the need for a diverse sex representation in clinical trials.
The terms race and ethnicity are often used synonymously. The race is biologically focused and relates to physical traits in people with a common ancestry. 7 Ethnicity is a broader term taking cultural expression, religion, and shared beliefs into account. 8 Pharmacogenetics is one of the most critical factors for inter‐racial differences in drug efficacy and safety. Genetic polymorphisms are identified for drug transporters and drug‐metabolizing enzymes (e.g., CYP or phase II enzymes). 9 Some polymorphisms are found with increased frequency in specific populations, making these racial groups more susceptible to altered activity. 5 Pathophysiological differences between races have also been reported. For example, differences are observed in the renin‐angiotensin‐aldosterone system (RAAS), leading to a lower effect of angiotensin‐converting enzyme (ACE) inhibitors and beta‐blockers in Black people. 10 Race‐specific dosing recommendations are already seen for a range of drugs approved by the US Food and Drug Administration (FDA), such as rosuvastatin and warfarin, for which a lower dose is recommended for Asian people. 11 However, current guidelines are limited by the available data, and representative clinical trials are needed to facilitate the development of guidelines directed toward minority groups. In ethnic groups, simultaneous use of other medications, medical practices, and diet may vary, contributing to the differences in the outcome of a given medical treatment. 12
“Diversity, Equity, and Inclusion” (DEI) has become a crucial element of most societies and has gained increasing attention in the scientific communities. 13 , 14 The movement seeks to promote the fair treatment of individuals who historically have been discriminated against in the context of, for example, race, sex, or identity. 15 When conducting clinical trials, there has been an increased focus on diversity among research participants, and the idea of diversity in clinical trials is not new. The National Institutes of Health (NIH) Revitalization Act of 1993 stated that more women and minorities should be included as subjects in clinical research, 1 to ensure that research findings are generalizable to the entire population.
This review aims to describe the development of sex, racial, and ethnic diversity of research participants in clinical drug trials published in the American Society for Clinical Pharmacology and Therapeutics (ASCPT) family journals. Furthermore, we aim to describe the sex distribution of the researchers conducting the research.
METHODS
We reviewed clinical drug trials published in the journals Clinical Pharmacology and Therapeutics (CPT) or Clinical and Translational Science (CTS) hosted by the ASCPT.
We searched PubMed on March 21, 2022, with the following search terms: (“Clinical pharmacology and therapeutics”[Journal]) OR (“Clinical and translational science”[Journal]) AND (pharmacogenetics OR interaction OR phase OR clinical trial). To explore the development in diversity over 20 years, we carried out two separate searches applying filters. The first search included articles published between 2000 and 2001, and the second search was limited to publications from 2020 and 2021. CTS was not yet established in 2000–2001, so only articles published in CPT are included in the first search.
Inclusion and exclusion criteria
We included articles with participants above 18 years of age and a year of publication matching the predefined timepoints (2000, 2001, 2020, or 2021). We excluded retrospective trials and trials investigating diseases or drugs specific to one sex, such as prostate cancer or oral contraceptives. Trials examining interventions (e.g., vitamin supplements) other than drugs were also excluded.
Selection and data extraction
The articles were selected by two authors (A.D. and J.B.). First, the titles and abstracts were screened, and articles not complying with inclusion and exclusion criteria were deemed irrelevant. Any disagreement was handled by discussion and mutual agreement. Author J.B. carried out a single‐person full‐text screen, and predefined data points were extracted. The data points included the year of publication, type of study, number of participants, population demographics (sex [female or male], race [White, Black, Asian, Hispanic, or Native American], ethnicity [Hispanic or non‐Hispanic]), region of study, recruiting information, intervention information, and notes on inclusion or exclusion criteria. We extracted data on the sex of the authors by reviewing the first names of the authors. For unisex names, we searched for the specific author to determine the sex. If it was impossible to distinguish the sex of authors, the sex was labeled as “Unknown.”
Racial and ethnic representation
To compare the diversity of the research participants with the diversity of the reference population, we calculated the representation ratio (RR) and the representation quotient (RQ). 16 The RR was assessed by country; for each country, we calculated the race in the study populations as the ratio between the number of research participants in the specific race group and the total number of research participants. We then compared the study population to the reference population by calculating the RR for each race:
An RR = 1 demonstrates that the racial group is equally represented in the clinical trials and reference population. An RR greater than 1 indicates an overrepresentation, whereas an RR less than 1 indicates an under‐representation of the racial group relative to the reference population. 17
The RQ was calculated for the individual studies. To calculate the RQ, the diversity index D is calculated for the research participants, D research, and the reference population, D reference (Equation 1). D research is then divided by D reference to obtain the RQ (Equation 2).
| (1) |
| (2) |
where s is the individual racial subgroups, n is the total number of participants in each subgroup, and N is the total number of participants in all subgroups. 16 An RQ greater than or equal to 1 indicates diversity in the clinical trial that matches or is higher than that of the reference population. An RQ less than 1 indicates a lack of diversity in the clinical trial compared with the reference population.
For the reference populations, the race distributions of the individual countries were used. For the 2000–2001 reference population, we obtained demographic data from the year 2000 using the Central Intelligence Agency's (CIA's) “The World Factbook Archives.” 18 Race distributions for France and Sweden were obtained from other resources. 19 , 20 The 2020–2021 reference populations were obtained using the CIA's online database “The World Factbook.” 21 Data from France, Canada, and Colombia were obtained from other resources. 19 , 22 , 23 Ethnic representation was measured by extracting information about the Hispanic ethnicity of the research participants.
Statistical analysis and subgroup analysis
Data are described using medians and interquartile ranges (IQRs). A subgroup analysis examining the sex distribution was performed for articles not having female sex as an exclusion criterion. The analyses were conducted in Excel (version 16.47.1) and RStudio (version 2022.02.1).
RESULTS
We included 281 articles in this review. The selection process is described in Figure 1. A total of 151 articles from 25 countries were published from 2000–2001. In 2020–2021, 130 articles descending from 22 countries were published. Most trials were conducted in North America and Europe, whereas the fewest articles were from Africa and South America (Figure 2).
FIGURE 1.
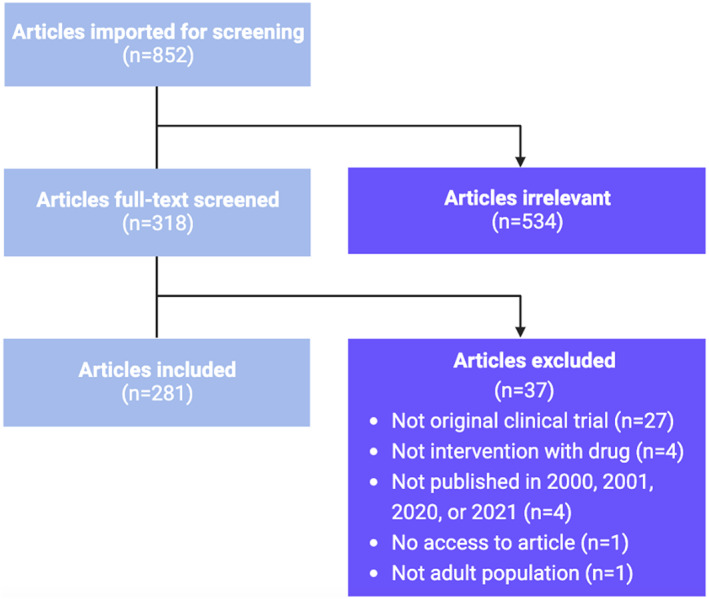
Flow diagram of the screening process for articles published by Clinical Pharmacology and Therapeutics and Clinical and Translational Science in 2000–2001 and 2020–2021. n = number of articles.
FIGURE 2.
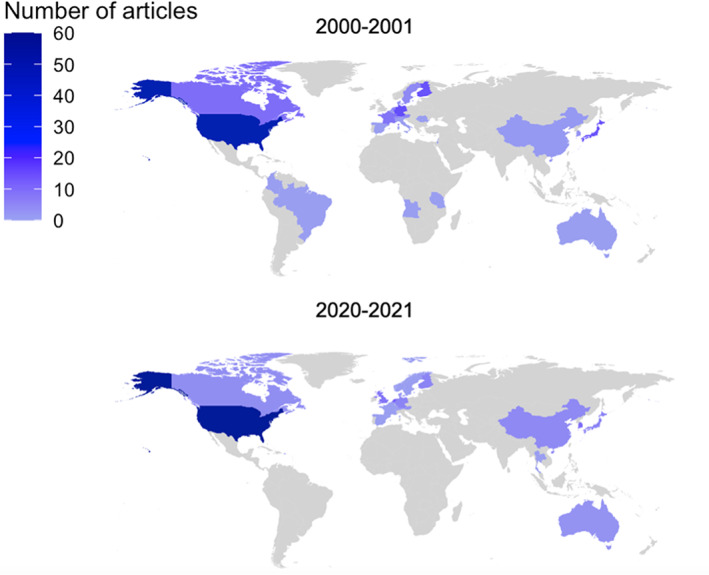
Choropleth map showing the geographical distribution of the 281 clinical drug trials published by Clinical Pharmacology and Therapeutics and Clinical and Translational Science in 2000–2001 and 2020–2021.
A total of 6779 and 10,860 research participants were included in 2000–2001 and 2020–2021, respectively. Generally, the trials from 2020–2021 had a greater number of participants, and the median number of participants increased from 14 participants in 2000–2001 to 32 participants in 2020–2021. Most participants were healthy volunteers (74% and 69%, respectively). The complete characteristics of the included articles are found in Table S1.
Diversity of research participants
Approximately one‐third of the research participants were women in both 2000–2001 and 2020–2021 (Table 1). In 2000–2001, 51 trials (34%) only included men, of which 39 articles had female sex as an exclusion criterion. In 2020–2021, 26 trials (20%) only included men, and 20 of these had female sex as an exclusion criterion. The proportion of female research participants did not change in the subgroup analysis, only assessing the 222 studies not having female sex as an exclusion criterion (Table 1).
TABLE 1.
Sex characteristics of the 281 clinical drug trials published in Clinical Pharmacology and Therapeutics or Clinical and Translational Science in 2000–2001 or 2020–2021.
| 2000–2001 | 2020–2021 | |||
|---|---|---|---|---|
| n | % | n | % | |
| Research participants | ||||
| All included articles | 6779 | 100.0 | 10,860 | 100.0 |
| Female | 2374 | 35.0 | 3936 | 36.2 |
| Male | 4405 | 65.0 | 6924 | 63.8 |
| Subgroup analysis a | 6204 | 100.0 | 10,390 | 100 |
| Female | 2374 | 38.3 | 3936 | 37.8 |
| Male | 3830 | 61.7 | 6454 | 62.2 |
| Authors b | ||||
| All authors | 943 | 100.0 | 1267 | 100.0 |
| Female | 243 | 25.8 | 489 | 38.6 |
| Male | 685 | 72.6 | 699 | 55.2 |
| First author | 151 | 100.0 | 130 | 100.0 |
| Female | 47 | 31.1 | 49 | 37.7 |
| Male | 100 | 66.2 | 77 | 59.2 |
| Last author | 151 | 100.0 | 130 | 100.0 |
| Female | 19 | 12.6 | 37 | 28.5 |
| Male | 131 | 86.8 | 91 | 70.0 |
Abbreviation: n, number of individuals.
Subgroup analysis: the sex distribution for 222 articles not having female sex as an exclusion criterion.
The remaining proportion not accounted for is due to unknown sex of the author.
Among racial groups, White research participants were the most abundantly represented race in total numbers in both 2000–2001 and 2020–2021 (Table 2). However, in 2020–2021, Black and Hispanic people were over‐represented relative to the reference population. This was primarily caused by a significant representation of Black and Hispanic people in trials conducted in the United States (Table 2). This contrasts with the RQ, which shows the United States to have a well‐balanced representation (Table 2). The representation for Black and Asian people increased by 125% and 67% from 2000–2001 to 2020–2021, respectively, whereas the representation for White, Hispanic, and Native American people decreased from 2000–2001 to 2020–2021 (Table 2).
TABLE 2.
Racial representation of research participants included in clinical drug trials published by Clinical Pharmacology and Therapeutics or Clinical and Translational Science in 2000–2001 and 2020–2021.
| Race | 2000–2001 | 2020–2021 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Study population, % | Reference population, % | RR | RQ | n | Study population, % | Reference population, % | RR | RQ | |
| All countries | ||||||||||
| White | 3651 | 71.5 | 75.1 | 0.95 | 0.36 | 9730 | 61.2 | 61.3 | 1.00 | 0.27 |
| Black | 8.3 | 9.7 | 0.86 | 18.7 | 9.9 | 1.89 | ||||
| Asian | 5.8 | 8.2 | 0.71 | 9.7 | 11.6 | 0.84 | ||||
| Hispanic | 12.0 | 1.0 | 12.0 | 5.2 | 0.1 | 52.0 | ||||
| Native American | 0.6 | 1.1 | 0.55 | 0.2 | 1.0 | 0.20 | ||||
| Canada | ||||||||||
| White | 36 | 100.0 | 66.0 | 1.52 | 0.00 | 80 | 71.3 | 52.9 | 1.35 | 0.83 |
| Black | 0.0 | ‐ | 11.2 | ‐ | ||||||
| Asian | 0.0 | ‐ | 17.5 | 12.5 | 1.40 | |||||
| Hispanic | 0.0 | ‐ | 0.0 | ‐ | ||||||
| Native American | 0.0 | 2.0 | 0.00 | 0.0 | 2.6 | 0.00 | ||||
| China | ||||||||||
| White | 26 | 0.0 | 0.0 | ‐ | 0.00 | 128 | 0.0 | 0.0 | ‐ | 0.00 |
| Black | 0.0 | 0.0 | ‐ | 0.0 | 0.0 | ‐ | ||||
| Asian | 100.0 | 100.0 | 1.00 | 100.0 | 100.0 | 1.00 | ||||
| Hispanic | 0.0 | 0.0 | ‐ | 0.0 | 0.0 | ‐ | ||||
| Native American | 0.0 | 0.0 | ‐ | 0.0 | 0.0 | ‐ | ||||
| Germany | ||||||||||
| White | 32 | 100.0 | 91.5 | 1.09 | 0.00 | 156 | 96.8 | 89.1 | 1.09 | 0.32 |
| Black | 0.0 | ‐ | 0.6 | ‐ | ||||||
| Asian | 0.0 | ‐ | 2.6 | ‐ | ||||||
| Hispanic | 0.0 | ‐ | 0.0 | ‐ | ||||||
| Native American | 0.0 | ‐ | 0.0 | ‐ | ||||||
| Japan | ||||||||||
| White | 157 | 0.0 | 0.0 | ‐ | 0.00 | 84 | 0.0 | ‐ | 0.00 | |
| Black | 0.0 | 0.0 | ‐ | 0.0 | ‐ | |||||
| Asian | 100.0 | 100.0 | 1.00 | 100.0 | 98.9 | 1.01 | ||||
| Hispanic | 0.0 | 0.0 | ‐ | 0.0 | ‐ | |||||
| Native American | 0.0 | 0.0 | ‐ | 0.0 | ‐ | |||||
| South Korea | ||||||||||
| White | 20 | 50.0 | 0.0 | ‐ | 0.00 | 342 | 0.0 | 0.0 | ‐ | 0.00 |
| Black | 0.0 | 0.0 | ‐ | 0.0 | 0.0 | ‐ | ||||
| Asian | 50.0 | 100.0 | 0.50 | 100.0 | 100.0 | 1.00 | ||||
| Hispanic | 0.0 | 0.0 | ‐ | 0.0 | 0.0 | ‐ | ||||
| Native American | 0.0 | 0.0 | ‐ | 0.0 | 0.0 | ‐ | ||||
| United States | ||||||||||
| White | 2212 | 64.9 | 77.1 | 0.84 | 0.85 | 7451 | 62.1 | 61.6 | 1.01 | 0.96 |
| Black | 12.6 | 12.9 | 0.98 | 23.3 | 12.4 | 1.88 | ||||
| Asian | 0.9 | 4.2 | 0.21 | 2.7 | 6.0 | 0.45 | ||||
| Hispanic | 18.8 | ‐ | 6.7 | ‐ | ||||||
| Native American | 0.0 | 1.8 | 0.00 | 0.2 | 1.3 | 0.15 | ||||
Abbreviations: n, number; RR, representation ratio; RQ, representation quotient.
A total of 138 articles (49%) failed to report the ethnicity or race of the research participants. In 2000–2001, 48 articles (32%) reported the participants' race. However, none of the articles reported ethnicity. In 2020–2021, 95 articles (73%) reported the ethnicity or race of the participants, but only 26 articles (20% of the total) reported ethnicity and race separately, thus contributing to estimates on ethnicity. Through all continents, most research participants identified as ethnic non‐Hispanic (79.2%), with North America having the highest percentage of Hispanic research participants (31.2%; Table S2).
Diversity of the researchers
The proportion of female authors increased from 25.8% to 38.6% between 2000–2001 and 2020–2021. Despite an increase in female authors, most authors are still men. The ratio between men and women as the last authors was more skewed than for all the authors in both 2000–2001 and 2020–2021 (Table 1).
DISCUSSION
This review summarizes the sex, racial, and ethnic diversity of clinical trials published in CPT and CTS in 2000–2001 and 2020–2021. Approximately one‐third of the research participants were women. White research participants were the most abundantly represented race in total numbers. The racial representation of Black and Asian research participants increased from 2000–2001 to 2020–2021, but Asian and Native American people are still under‐represented as research participants in clinical trials. Most of the research participants were ethnic non‐Hispanic, with most Hispanic research participants in the North American continent. The proportion of female authors increased from 2000–2001 to 2020–2021, but female authors still constitute less than 40% of all authors. Despite both the NIH calling for more women and minorities in clinical trials 1 and the DEI movement increasing awareness to include minority groups in clinical trials, there is still a lack of diversity in clinical drug trials published in CPT and CTS.
There is a continuous lack of female research participants in clinical trials. Following the thalidomide scandal in the late 1950s and early 1960s, causing severe limb abnormalities in newborns, 24 researchers became cautious including women of childbearing potential in clinical drug trials. Of the 281 articles included, 59 trials directly exclude women, whereas others exclude participants with a “usage of any daily medication,” which would exclude women using, for example, oral contraceptives. One article even required female participants to be sterile to participate in the trial. 25 Maintenance of such restrictions might explain why the distribution of female participants did not change in the subgroup analysis of only trials where women could be included. However, we emphasize the potential to recruit women of childbearing potential and have requirements for adequate contraceptive methods in the trial protocol (e.g., oral contraceptives).
Different methods have previously been used to describe racial diversity in clinical trials. 16 , 17 Here, we have used the RR and RQ as diversity measures, although they both have limitations. The premise of the RQ is that two individuals from a population are equally likely to belong to different racial or ethnic groups. This is not in alignment with reality. For instance, the research population in China was very uniform, resulting in low RQs (Table 2). However, this does reflect the reference population, which in China is also very uniform. The RR also gives rise to bias. For large racial groups, it is mathematically impossible to obtain large RRs—even if the research participants were 100% White, the high proportion of White people in the reference population would restrict the RR of White people. Thus, large racial groups may become underestimated. For minority racial groups, a slight increase in representation of the research participants can result in large RRs, and the representation becomes overestimated. Our review highlights that Black people in clinical trials are over‐represented compared to the reference population, which conflicts with previous studies. 26 , 27 However, most Black people are included in trials conducted in the United States; the racial distribution varies significantly between the states, with more Black people in urban areas. 28 Inaccuracy of the reference population potentially skews the calculations of representation. It is outside the scope of this review to investigate if the individual trials reflect the reference population in the state or city in which they are conducted.
Although the sex distribution within clinical trials did not change, the proportion of female authors increased significantly between 2000–2001 and 2020–2021. However, men are still over‐represented as authors. The gap in equality is mainly seen among the last authors, where the under‐representation of women is more pronounced. The last author is often recognized as the intellectual and financial source of the research. 29 Women in research receive less funding than men at the same scientific level, 30 which may contribute to the under‐representation of female authors.
In both 2000–2001 and 2020–2021, most articles came from the United States. The United States is the third largest country by population 31 and the journals CPT and CTS are hosted in the United States. In 2000–2001, articles from more countries were included, compared to 2020–2021 (25 and 22 countries, respectively). For example, Africa, South America, and the Middle East were not represented in 2020–2021. African people are the most genetically diverse population group, 32 emphasizing the need to conduct clinical research including African participants.
Strengths and limitations
The main strength of this review is the inclusion of 281 articles with more than 13,000 research participants contributing demographic information about sex and race. The articles represent different types of clinical drug trials, making this review's results generalizable to a broader range of clinical drug trials. We collected data and showed the diversity of clinical trials from 2000–2001 and 2020–2021. This allows us to assess the development in the diversity of clinical trials. Results from 2 years of each timepoint increase the number of articles, thus amplifying the results. Another strength is that we only included articles investigating diseases or drugs not specific to one sex. Such articles would not contribute to knowledge on whether male and female subjects are recruited equally in clinical drug trials, as the premise of recruitment would be altered. However, we did not consider the specific diseases in the analysis. Some diseases are unequally distributed among sexes (e.g., rheumatoid arthritis is more prevalent among women). In line, some diseases are more prevalent among racial minorities. In both cases, the reference population might not reflect the affected population, thus potentially causing deviations in the reported representation. Another limitation is the limited number of racial and ethnic categories applied in the included papers. The complexity of ethnic and racial minorities is insufficiently represented in the published literature and the categories only partly reflect the racial and ethnic diversity in the population. Furthermore, the data for the reference population are associated with some uncertainty. Information on some racial groups was absent, especially for countries with limited access to demographic details. 19 , 20 This prevents the comparison of research participants to the reference population in that given country. Data on the authors' sex was sometimes unobtainable, leaving some authors categorized as “Unknown.” We do not expect the “Unknown” category to affect the total results as the number of unknown authors is confined. Last, a fundamental limitation of this review is that it only includes articles published in the journals CPT and CTS.
CONCLUSION
In conclusion, far more male than female subjects are still included in clinical drug trials as research participants. Racial representation from minority groups of Black and Asian people in clinical drug trials has increased over the last 20 years, but Asian and Native Americans are still under‐represented today. The increase in representation from minority racial groups is essential, as more robust representation can lead to increased efficacy and safety in minorities. Even though the proportion of female authors has increased significantly over the last 20 years, the largest proportion of authors is still men. Women are even less represented as the last authors of articles, which may be linked to women in research receiving less funding than men. Representation from minorities is of utmost importance so that sex‐dependent and inter‐racial/ethnic differences in drug response can be discovered. Different dosing regimens between population groups may be suggested to provide safe and efficient health care to all, not just the majority group.
AUTHOR CONTRIBUTIONS
J.B. wrote the manuscript. J.B., T.B., and A.D. designed the research. J.B. and A.D. performed the research. J.B. analyzed the data.
FUNDING INFORMATION
This work was supported by the Lundbeck Foundation Fellowship (Grant R307‐2018‐2980).
CONFLICT OF INTEREST STATEMENT
A.D. has given paid lectures for Astellas Pharma. T.S has given paid lectures for Pfizer and Eisai and consulted for Pfizer. All other authors declared no competing interests for this work.
Supporting information
Data S1
ACKNOWLEDGMENTS
The authors are deeply grateful for APRN, Stephani L. Stancil, PhD, insightful comments and advice in the early stages of this research.
Bøttern J, Stage TB, Dunvald A‐CD. Sex, racial, and ethnic diversity in clinical trials. Clin Transl Sci. 2023;16:937‐945. doi: 10.1111/cts.13513
REFERENCES
- 1. NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research | grants.nih.gov [Internet]. Available at: https://grants.nih.gov/policy/inclusion/women‐and‐minorities/guidelines.htm. Accessed March 15, 2022.
- 2. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spoletini I, Vitale C, Malorni W, Rosano GMC. Sex differences in drug effects: interaction with sex hormones in adult life. Handb Exp Pharmacol. 2012;214:91‐105. [DOI] [PubMed] [Google Scholar]
- 4. Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55(2):81‐95. [DOI] [PubMed] [Google Scholar]
- 5. Anderson GD. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health. 2005;14(1):19‐29. [DOI] [PubMed] [Google Scholar]
- 6. Hägg S, Spigset O, Dahlqvist R. Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br J Clin Pharmacol. 2001;51(2):169‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Definition of RACE [Internet] . Available at: https://www.merriam‐webster.com/dictionary/race. Accessed March 18, 2022.
- 8. Definition of ETHNIC [Internet] . Available at: https://www.merriam‐webster.com/dictionary/ethnic. Accessed March 18, 2022.
- 9. Yasuda S, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84(3):417‐423. [DOI] [PubMed] [Google Scholar]
- 10. Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr. 2007;18(4):241‐247. [PMC free article] [PubMed] [Google Scholar]
- 11. Huang SM, Temple R. Is this the drug or dose for you?: impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clin Pharmacol Ther. 2008;84(3):287‐294. [DOI] [PubMed] [Google Scholar]
- 12. Ramamoorthy A, Pacanowski M, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther. 2015;97(3):263‐273. [DOI] [PubMed] [Google Scholar]
- 13. Boulware LE, Corbie G, Aguilar‐Gaxiola S, et al. Combating structural inequities — diversity, equity, and inclusion in clinical and translational research. N Engl J Med. 2022;386(3):201‐203. [DOI] [PubMed] [Google Scholar]
- 14. Swartz TH, Palermo AGS, Masur SK, Aberg JA. The science and value of diversity: closing the gaps in our understanding of inclusion and diversity. J Infect Dis. 2019;220(Suppl 2):S33‐S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Definition of DEI | Dictionary.com [Internet]. Available at: https://www.dictionary.com/browse/dei. Accessed April 6, 2022.
- 16. Abdel‐Rahman SM, Wimes MP, Curran T. A call to action: issuing a diversity and inclusion challenge to research organizations. Clin Transl Sci. 2021;14(6):2095‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raine S, Liu A, Mintz J, Wahood W, Huntley K, Haffizulla F. Racial and ethnic disparities in COVID‐19 outcomes: social determination of health. Int J Environ Res Public Health. 2020;17(21):E8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The World Factbook Archives ‐ The World Factbook [Internet]. Available at: https://www.cia.gov/the‐world‐factbook/about/archives/. Accessed August 3, 2022.
- 19. France Population 2022 (Demographics, Maps, Graphs) [Internet]. Available at: https://worldpopulationreview.com/countries/france‐population. Accessed March 14, 2022.
- 20. Andersson R. Understanding ethnic Minorities' settlement and geographical mobility patterns in Sweden using longitudinal data. 2012. p. 263–91.
- 21. The World Factbook ‐ The World Factbook [Internet]. Available at: https://www.cia.gov/the‐world‐factbook/. Accessed March 14, 2022.
- 22. Government of Canada SC . Immigration and Ethnocultural Diversity Highlight Tables ‐ Ethnic Origin, both sexes, age (total), Canada, 2016 Census – 25% Sample data [Internet]. 2017. Available at: https://www12.statcan.gc.ca/census‐recensement/2016/dp‐pd/hlt‐fst/imm/Table.cfm?Lang=E&T=31&Geo=01&SO=4D. Accessed April 6, 2022.
- 23. Ethnic groups of Colombia [internet] . WorldAtlas. 2019. Available at: https://www.worldatlas.com/articles/ethnic‐groups‐of‐colombia.html. Accessed April 6, 2022.
- 24. Ridings JE. The thalidomide disaster, lessons from the past. In: Barrow PC, ed. Teratogenicity Testing: Methods and Protocols. Humana Press; 2013:575‐586 (Methods in Molecular Biology; vol. 947). [DOI] [PubMed] [Google Scholar]
- 25. Mazzu AL, Lasseter KC, Shamblen EC, Agarwal V, Lettieri J, Sundaresen P. Itraconazole alters the pharmacokinetics of atorvastatin to a greater extent than either cerivastatin or pravastatin. Clin Pharmacol Ther. 2000;68(4):391‐400. [DOI] [PubMed] [Google Scholar]
- 26. Knepper TC, McLeod HL. When will clinical trials finally reflect diversity? Nature. 2018;557(7704):157‐159. [DOI] [PubMed] [Google Scholar]
- 27. Yekedüz E, Trapani D, Xu W, et al. Assessing population diversity in phase III trials of cancer drugs supporting Food and Drug Administration approval in solid tumors. Int J Cancer. 2021;149(7):1455‐1462. [DOI] [PubMed] [Google Scholar]
- 28. U.S. Census Bureau QuickFacts: United States [Internet] . Available at: https://www.census.gov/quickfacts/fact/map/US/RHI225220. Accessed April 27, 2022.
- 29. Tscharntke T, Hochberg ME, Rand TA, Resh VH, Krauss J. Author sequence and credit for Contributions in multiauthored publications. PLoS Biol. 2007;5(1):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oliveira DFM, Ma Y, Woodruff TK, Uzzi B. Comparison of National Institutes of Health Grant amounts to first‐time male and female principal investigators. Jama. 2019;321(9):898‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Total Population by Country 2022. Available at: https://worldpopulationreview.com/countries. Accessed May 6, 2022.
- 32. Fatumo S, Chikowore T, Choudhury A, Ayub M, Martin AR, Kuchenbaecker K. A roadmap to increase diversity in genomic studies. Nat Med. 2022;10:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


