Abstract
Dopamine transporter (DAT) imaging is an in vivo tool to assess presynaptic dopaminergic function in the clinical practices of Parkinson's disease (PD). Current clinical practices focused on qualitatively visual interpretation of DAT imaging, whereas quantitative analyses are potentially more helpful when monitoring the progression of PD. Previous cross‐sectional studies indicated certain motor and non‐motor features were associated with striatal DAT binding, whereas limited data were reported in terms of the longitudinal correlation between clinical features of PD with striatal DAT binding. The purpose of our study is to clarify current and longitudinal correlations between striatal DAT binding and clinical measures. A total of 352 untreated PD individuals and 167 healthy controls with complete baseline clinical measures and neuroimaging data were identified from the Parkinson's Progression and Markers Initiative (PPMI) database. Patients with PD underwent DAT imaging at the screening visit and following months 12, 24, and 48. Multiple linear regression models and linear mixed‐effect models were respectively conducted to investigate the cross‐sectional and longitudinal correlation between clinical characteristics and DAT binding. Associations between changes in clinical characteristics and changes in DAT binding were further evaluated and the Spearman rank correlation coefficients were reported. In the cross‐sectional analysis, baseline striatal DAT binding was significantly associated with the Hoehn and Yahr scale, the Movement Disorder Society‐Sponsored Revision of the Unified Parkinson Disease Rating Scale (MDS‐UPDRS) scores, the rigidity scores, and the axial scores in PD individuals (false discovery rate [FDR]‐adjusted p = 0.0017 for all above). Patients who developed freezing of gait had lower striatal DAT binding (FDR‐adjusted p = 0.0161). Healthy controls who had higher tremor scores and suffered more severe olfactory dysfunction had lower striatal DAT binding (FDR‐adjusted p = 0.0257 for all above). Longitudinal analysis indicated that baseline severity of rapid‐eye‐movement sleep behavior disorder was significantly associated with longitudinal striatal DAT binding in patients with PD (FDR‐adjusted p = 0.0120). Furthermore, changes in MDS‐UPDRS scores and the State–Trait Anxiety Inventory (STAI) scores demonstrated significant correlations with changes in striatal DAT binding over 4 years (p = 0.005 and p = 0.032, respectively). Our findings clarified quantitative associations between certain motor and non‐motor features with current and future striatal dopamine binding, suggesting the feasibility of using DAT images as a progression predictive marker for PD. Further studies are needed to investigate correlations between different regional dopamine binding with specific clinical features.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Dopamine transporter (DAT) imaging is a tool to evaluate presynaptic dopaminergic function in Parkinson's disease (PD) and has been linked with certain motor and non‐motor symptoms by previous cross‐sectional analyses, whereas limited data on longitudinal correlations between PD clinical features and DAT uptake were reported. Pathological studies have confirmed a population of dopaminergic neurons was potentially recoverable at the early stage.
WHAT QUESTION DID THIS STUDY ADDRESS?
Our study aimed to define the cross‐sectional and longitudinal correlation between PD clinical characteristics and DAT binding at PD at the early stage.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Our study demonstrated that patients with severer rigidity, axial, and gait symptoms had a greater dopaminergic dysfunction at the current stage. Patients with rapid‐eye‐movement sleep behavior disorder (RBD) and healthy controls with olfactory dysfunction also demonstrated severer DAT deficits. Additionally, severer RBD at diagnosis correlated with more rapid deterioration of dopaminergic dysfunction over 4 years. Greater changes in Movement Disorder Society‐Sponsored Revision of the Unified Parkinson Disease Rating Scale (MDS‐UPDRS) and State–Trait Anxiety Inventory (STAI) scores were linked with greater deterioration of DAT deficits.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Our study reported quantitative correlations between certain clinical features of PD and DAT deficits. Patients who had higher risks of severer dopaminergic dysfunction could be easily identified at the early stage via assessments of certain motor and non‐motor symptoms. These patients can benefit more from early identification and more aggressive treatment as the potential recoverability of certain dopaminergic neurons at the early stage.
INTRODUCTION
Parkinson's disease (PD) is a complex neurological disorder characterized by cardinal parkinsonian motor signs. Loss of dopaminergic neurons within the substantia nigra pars compacta (SNpc) is one of the crucial pathological hallmarks of PD. 1 The absence of accurate and objective markers of PD development and progression has long been considered an obstacle to the early detection and surveillance of PD progression. 1 Previous studies have confirmed that striatal dopaminergic denervation correlated with disease progression. The moderate loss of dopaminergic neurons was reported to be present about 1–3 years before the onset of cardinal motor symptoms, whereas the same study also provided evidence indicating a population of these neurons was potentially recoverable. 2 Pathological and cross‐sectionally clinical studies also suggested a positive linear correlation between the loss of striatal dopaminergic neurons with the severity of motor symptoms. 3 , 4 Thus, assessment of the striatal dopaminergic function is vital to clinical practices of PD for accurate diagnosis and objective evaluation of disease progression. Dopamine transporter (DAT) imaging, such as [123I]FP‐CIT single‐photon emission computed tomography (SPECT), has been used as an in vivo tool to visualize and monitor the severity of dopaminergic denervation. However, previous clinical practices mostly focused on qualitatively visual interpretation of DAT imaging, whereas quantitative analysis may be more helpful in the early detection and progression tracking of PD. 5
Several motor and non‐motor symptoms (NMSs) have been reported to be associated with striatal DAT binding levels. NMSs, such as cognitive impairment, sleep disorders, autonomic dysfunction, psychiatric symptoms, and olfactory dysfunction, were frequently present years or even decades before the diagnosis of PD, some of which were reported to be associated with dopaminergic deficits. 1 , 6 Freezing of gait (FOG) is one of the common and disabling postural and gait impairments of PD. Previous studies reported a lower level of DAT uptake related to the onset of FOG in drug‐naïve PD, indicating that striatal dopaminergic depletion may be exclusively associated with the development of postural and gait impairment of PD. 7 Liu and colleagues reported correlations among baseline NMSs, including rapid eye movement (REM) sleep behavior disorder (RBD), cognitive impairments, anxiety, olfactory disability, and autonomic dysfunction, with baseline and longitudinal DAT bindings. However, associations between changes in these NMSs and changes in DAT bindings over time were not investigated. 8 Longitudinal analysis considering motor symptoms and DAT bindings suggested a small but significant correlation between changes in striatal DAT binding and the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) total score, whereas the longitudinal association between specific motor symptoms and DAT bindings was under‐defined. 9
In the present study, we investigated correlations between motor and NMSs with striatal DAT bindings both at baseline and during follow‐up visits, using data extracted from the Parkinson's Progression Markers Initiative (PPMI) database. We also studied the potential associations between changes in clinical variables with changes in DAT uptakes over time. We believe a comprehensive and accurate clarification of cross‐sectional and longitudinal correlation between clinical characteristics with DAT bindings is essential for DAT imaging to be used as a biomarker in clinical trials.
MATERIALS AND METHODS
Participants from PPMI
We requested and obtained approval to access clinical and neuroimaging data from PPMI, an ongoing, longitudinal, observational, and multicenter investigation aiming to identify biological markers of PD progression. Detailed pieces of information about study designs, study cohorts, operation manuals, and other protocols have been published 10 and are available online (http://www.ppmi‐info.org/study‐design). A total of 352 untreated PD individuals and 167 healthy controls (HCs) with complete baseline motor and nonmotor assessment data, and DAT imaging data at screening visits were identified for the cross‐sectional analysis. All patients with PD in this cohort have a clinical diagnosis of PD and a positive DAT SPECT. Longitudinal analysis was conducted among PD individuals, with the sample consisting of 266 patients with PD at 12‐ and 24‐month visits, and 232 patients at 48‐month visits (Figure 1). The PPMI study has been registered with ClinicalTrials.gov (NCT01141023) and written informed permission has been collected from all participants or guardians before enrollment.
FIGURE 1.
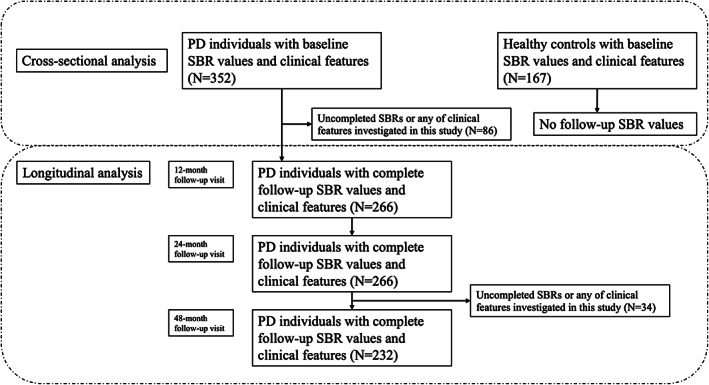
Participants selected in cross‐sectional analysis and longitudinal analysis. PD, Parkinson's disease; SBR, striatal binding ratio.
Clinical characteristics from PPMI
For all participants, we recorded baseline demographic data, including age, sex, years of education, and disease duration. The global motor impairment severity was assessed using section III of the MDS‐UPDRS scale and the Hoehn and Yahr scale. We also computed various subsets to analyze different phenotypes of motor impairment including tremor score, rigidity score, axial score, and postural instability and gait difficulty (PIGD) score. Tremor score was calculated by the sum of the following 11 items, including 2.10 (tremor), 3.15a (postural tremor right upper extremity, RUE), 3.15b (postural tremor left upper extremity, LUE), 3.16a (kinetic tremor RUE), 3.16b (kinetic tremor LUE), 3.17a (rest tremor RUE), 3.17b (rest tremor LUE), 3.17c (rest tremor right lower extremity, RLE), 3.17d (rest tremor left lower extremity, LLE), 3.17e (rest tremor lip/jaw), and 3.18 (rest consistency). The PIGD score was computed by the sum of five items including 2.12 (walking and balance), 2.13 (freezing), 3.10 (gait), 3.11 (FOG), and 3.12 (postural stability). 11 The axial score was the sum of speech, neck rigidity, posture stability, and gait items in MDS‐UPDRS part III. 12 Initial symptoms, including resting tremors, rigidity, bradykinesia, and postural instability, were also considered as potential baseline predictors in this study. We also used MDS‐UPDRS item 2.13 (freezing) and item 3.11 (FOG) to evaluate the onset of FOG. Individuals with the sum score of the two items greater than or equal to one were considered to be developing FOG. 7 Five major areas of NMSs, including sleep, autonomic function, neurobehavioral, olfaction, and cognition, were also included in this study. The RBD Screening Questionnaire (RBDSQ) and the Epworth Sleepiness Scale (ESS) were used to evaluate the status of sleep disturbance. The Scales for Outcomes in Parkinson's disease – Autonomic (SCOPA‐AUT) was used to assess autonomic dysfunction. The State–Trait Anxiety Inventory (STAI) and the Geriatric Depression Scale (GDS) were assessments of neurobehavioral dysfunction. The University of Pennsylvania Smell Identification Test (UPSIT) and the Montreal Cognitive Assessment (MoCA) were used to assess olfaction and global cognitive function. Individuals with scores of greater than or equal to 6 on the RBDSQ scale, greater than or equal to 10 on the ESS scale, and greater than or equal to 5 on the GDS scale were respectively considered to develop probable RBD, excessive daytime sleepiness (EDS), and depression. 13 , 14 , 15
Neuroimaging acquisition and processing in PPMI
For each participant, the DaTscan SPECT imaging was acquired at the screening visit and following 12‐, 24‐, and 48‐month visits at specific PPMI imaging sites according to modality‐specific protocols provided by PPMI (https://www.ppmi‐info.org/study‐design/research‐documents‐and‐sops). Iterative reconstruction was performed centrally at the Institute for Neurodegenerative Disorders, New Haven, Connecticut, using the raw projection data imported to a HERMES (Hermes Medical Solutions) system without any filtering applied. The reconstructed files were then transferred to the PMOD (PMOD Technologies) software, where attenuation correction ellipses were drawn. A Chang zero attenuation correction was performed utilizing a site‐specific attenuation coefficient derived from phantom data acquired during site initiation for the trial. After attenuation correction, files were normalized to standard Montreal Neurologic Institute (MNI) space to ensure the same anatomic alignment. A single slice image was generated by averaging the eight striatal slices surrounding the transaxial slice with the highest striatal uptake. Count densities for each region of interest (including the left and right caudate, and the left and right putamen), and the occipital cortex (set as the reference tissue) were extracted for calculating striatal binding ratios (SBRs). SBR is calculated as (target region/reference region)‐1. In the present study, we treated the mean striatal SBR (the average of SBRs from the four interest regions mentioned above) as the main outcome measure.
Statistical analysis
The demographic and clinical characteristics were summarized using descriptive statistical methods. For continuous variables, we provided means and standard deviations, and for categorical variables, we provided proportions. SBR measurements with a skewed distribution (Shapiro–Wilk test < 0.05) were stabilized to resemble the normal distribution using the Box‐Cox transformation via the “powerTransform” function of the “car” package in R software. We compared baseline demographic and clinical characteristics between groups using t‐tests or chi‐square tests for normally distributed data and Wilcoxon rank‐sum test for nonparametric data. Three or more groups were compared using Kruskal–Wallis tests.
In the cross‐sectional analysis, multivariate linear regression models were conducted individually to investigate the relationship between each normalized SBR value and each clinical characteristic, with SBR values as dependent variables and clinical characteristics as independent variables, whereas adjusting for potential confounding variables, such as sex, age, years of education, and disease duration. We also conducted multivariate linear regression models respectively on caudate and putamen SBR values given that the denervation of the two subregions could have a different association with clinical features. For the longitudinal analyses, we use the linear mixed‐effect model to investigate the effect of each baseline clinical variable on the striatal SBR value over time separately for patients with PD. Association between baseline striatal SBR value and longitudinal normalized non‐motor features were also investigated using the linear mixed‐effect model. We further established linear mixed‐effect models respectively on the caudate and the putamen SBR values to see whether the two subregions have different effects on motor and nonmotor features. The HCs were excluded from the longitudinal analysis due to the lack of follow‐up SBR measures. Accounting for correlations existing in repeated measurements of SBR for each patient, time and with‐in‐subject variation were treated as random factors, using a random slope for time and a random intercept for individuals. Time and baseline clinical characteristics, together with their interactions were treated as predictors and entered as fixed effects in this model. Age, sex, years of education, and disease duration were entered as covariates in this model to adjust their possible influence.
Additionally, associations between longitudinal changes in SBR levels and follow‐up changes in clinical variables were investigated after adjusting for confounding factors. Then, we reported the Spearman rank‐correlation coefficients and p values. For all the above analyses, we conducted further subgroup analyses stratified by sex.
The statistical significance threshold of each test was set as a p value < 0.05. For all models, the p values were adjusted for multiple comparisons by controlling the false discovery rate (FDR) via the Benjamini and Hochberg method. All the statistical analyses and visualization were performed using R version 4.2.0 (R Project for Statistical Computing; http://www.r‐project.org).
Ethics statement
The studies involving human participants were reviewed and approved by the local institutional review boards of the participating centers. The patients/participants provided their written informed consent to participate in this study.
RESULTS
Baseline demographic and clinical characteristics of participants
As shown in Table 1, a total of 519 participants, including 352 patients with PD (mean age: 62.03 ± 9.48 years; 65.6% men) and 167 HCs (mean age: 61.59 ± 11.03 years; 63.5% men) were enrolled in this study. For PD individuals, the global MDS‐UPDRS score together with each subsection of the MDS‐UPDRS score was significantly higher than those of HCs (p < 0.001). Among NMS assessments, PD individuals presented significantly higher scores in GDS, RBDSQ, SCOPA‐AUT, and MoCA tests in comparison to HCs (p < 0.001), whereas lower scores in UPSIT and MoCA (p < 0.001), for which lower scores indicated deterioration of respective functions. No significant difference in ESS scores was found between the two groups. The SBRs of caudate, putamen, and global striatum in patients with PD were significantly lower than those in the HCs (p < 0.001).
TABLE 1.
Demographic, DAT SPECT, and clinical characteristics of included participants at baseline.
| Variables | PD (N = 352) | HCs (N = 167) | p Values |
|---|---|---|---|
| Age | |||
| Mean ± SD | 62.03 ± 9.48 | 61.59 ± 11.03 | 0.963 |
| (Minimum, maximum) | (34, 85) | (31, 84) | |
| Sex (n, %) | |||
| Male | 231 (65.6%) | 106 (63.5%) | 0.703 |
| Female | 121 (34.4%) | 61 (36.5%) | |
| Education years | |||
| Mean ± SD | 15.55 ± 2.93 | 16.12 ± 2.86 | 0.047 |
| (Minimum, maximum) | (5, 26) | (8, 24) | |
| MDS‐UPDRS total score | |||
| Mean ± SD | 31.95 ± 12.82 | 4.65 ± 4.30 | <0.001 |
| (Minimum, maximum) | (7, 72) | (0, 20) | |
| MDS‐UPDRS I score | |||
| Mean ± SD | 5.54 ± 4.04 | 2.86 ± 2.62 | <0.001 |
| (Minimum, maximum) | (0, 24) | (0, 12) | |
| MDS‐UPDRS II score | |||
| Mean ± SD | 5.86 ± 4.15 | 0.46 ± 1.05 | <0.001 |
| (Minimum, maximum) | (0, 20) | (0, 6) | |
| MDS‐UPDRS III score | |||
| Mean ± SD | 20.56 ± 8.61 | 1.33 ± 2.32 | <0.001 |
| (Minimum, maximum) | (4, 51) | (0, 13) | |
| MDS‐UPDRS tremor score | |||
| Mean ± SD | 4.31 ± 3.13 | 0.32 ± 0.89 | <0.001 |
| (Minimum, maximum) | (0, 18) | (0, 7) | |
| MDS‐UPDRS rigidity score | |||
| Mean ± SD | 3.74 ± 2.56 | 0.25 ± 0.69 | <0.001 |
| (Minimum, maximum) | (0, 13) | (0, 4) | |
| MDS‐UPDRS PIGD score | |||
| Mean ± SD | 1.15 ± 1.11 | 0.10 ± 0.46 | <0.001 |
| (Minimum, maximum) | (0, 7) | (0, 4) | |
| MDS‐UPDRS axial score | |||
| Mean ± SD | 2.52 ± 1.71 | 0.22 ± 0.55 | <0.001 |
| (Minimum, maximum) | (0, 8) | (0, 3) | |
| UPSIT score | |||
| Mean ± SD | 22.15 ± 8.16 | 33.87 ± 5.07 | <0.001 |
| (Minimum, maximum) | (1, 40) | (11, 40) | |
| UPSIT categories | |||
| Normosmia (n, %) | 30 (8.5%) | 105 (62.9%) | <0.001 |
| Hyposmia (n, %) | 199 (56.5%) | 57 (34.1%) | |
| Anosmia (n, %) | 123 (34.9%) | 5 (3.0%) | |
| ESS score | |||
| Mean ± SD | 5.76 ± 3.45 | 5.59 ± 3.24 | 0.704 |
| (Minimum, maximum) | (0, 20) | (0, 15) | |
| ESS categories | |||
| Not EDS, ESS < 10 (n, %) | 300 (85.2%) | 148 (88.6%) | 0.360 |
| EDS, ESS ≥10 (n, %) | 52 (14.8%) | 19 (11.4%) | |
| GDS score | |||
| Mean ± SD | 2.30 ± 2.45 | 1.20 ± 1.85 | <0.001 |
| (Minimum, maximum) | (0, 14) | (0, 14) | |
| GDS categories | |||
| Not depressed, GDS < 5 (n, %) | 305 (86.6%) | 158 (94.6%) | 0.010 |
| Depressed, GDS ≥ 5 (n, %) | 47 (13.4%) | 9 (5.4%) | |
| RBDSQ score | |||
| Mean ± SD | 4.09 ± 2.68 | 2.90 ± 2.31 | <0.001 |
| (Minimum, maximum) | (0, 12) | (0, 11) | |
| RBD categories | |||
| No RBD, RBDSQ <6 (n, %) | 218 (61.9%) | 130 (77.8%) | <0.001 |
| RBD, RBDSQ ≥6 (n, %) | 134 (38.1%) | 37 (22.2%) | |
| SCOPA‐AUT score | |||
| Mean ± SD | 9.56 ± 6.18 | 5.75 ± 3.57 | <0.001 |
| (Minimum, maximum) | (0, 39) | (0, 19) | |
| STAI score | |||
| Mean ± SD | 65.03 ± 18.56 | 56.04 ± 12.61 | <0.001 |
| (Minimum, maximum) | (40, 137) | (40, 96) | |
| MoCA score | |||
| Mean ± SD | 27.15 ± 2.27 | 28.22 ± 1.11 | <0.001 |
| (Minimum, maximum) | (17, 30) | (26, 30) | |
| Mean caudate (SBR) | |||
| Mean ± SD | 2.03 ± 0.55 | 2.99 ± 0.64 | <0.001 |
| (Minimum, maximum) | (0.5, 4) | (1.3, 5) | |
| Mean putamen (SBR) | |||
| Mean ± SD | 0.84 ± 0.30 | 2.15 ± 0.56 | <0.001 |
| (Minimum, maximum) | (0.3, 2) | (0.6, 4) | |
| Mean striatum (SBR) | |||
| Mean ± SD | 1.43 ± 0.40 | 2.57 ± 0.58 | <0.001 |
| (Minimum, maximum) | (0.4, 3) | (1.0, 4) | |
Note: Significance at p‐value <0.05 were reported in bold. Except for UPSIT, SCOPA‐AUT, and MoCA, for which lower baseline scores indicate deterioration of respective functions, for all remaining non‐motor symptoms, higher baseline scores indicate deterioration of respective functions. The t‐test or chi‐square test assessed differences between normally distributed group values. Nonparametric data were compared using the Wilcoxon rank‐sum test. Three or more groups were compared using Kruskal–Wallis tests.
Abbreviations: DAT, dopamine transporter; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale Score; GDS, Geriatric Depression Scale Score; HCs, health controls; MDS‐UPDRS, Movement Disorder Society‐Sponsored Revision of the Unified Parkinson Disease Rating Scale; MoCA, Montreal Cognitive Assessment (adjusted for education); PD, Parkinson's disease; PIGD, postural instability and gait difficulty; RBD, rapid‐eye‐movement sleep behavior disorder; RBDSQ, rapid eye movement Sleep Behavior Disorder Screening Questionnaire; SCOPA‐AUT, Scales for Outcomes in Parkinson's disease ‐ Autonomic; SD, standard deviation; SBR, striatal binding ratio; SPECT, single‐photon emission computed tomography; STAI, State–Trait Anxiety Inventory; UPSIT, University of Pennsylvania Smell Identification Test.
Among all the 352 PD individuals who provided clinical and neuroimaging data at baseline, 232 cases completed all the follow‐up visits at 12, 24, and 48 months (Table 2). Individuals who dropped out during follow‐up visits presented higher scores in total MDS‐UPDRS, MDS‐UPDRS part II, and MDS‐UPDRS part III tests than those who had completed all four visits (p = 0.003, p = 0.025, and p = 0.015, respectively). Baseline demographic, non‐motor characteristics, or neuroimaging data did not differ significantly between non‐completers and completers.
TABLE 2.
Demographic, DAT SPECT, and clinical characteristics of included participants at baseline.
| Variables | Completers (N = 232) | Non‐completers (N = 120) | p Values |
|---|---|---|---|
| Age | |||
| Mean ± SD | 61.55 ± 9.83 | 63.17 ± 8.68 | 0.077 |
| (Minimum, maximum) | (34, 85) | (39, 80) | |
| Sex (n, %) | |||
| Male | 153 (65.9%) | 78 (65.0%) | 0.953 |
| Female | 79 (34.1%) | 42 (35.0%) | |
| Education years | |||
| Mean ± SD | 15.53 ± 2.82 | 15.57 ± 3.13 | 0.894 |
| (Minimum, maximum) | (5, 26) | (5, 24) | |
| MDS‐UPDRS total score | |||
| Mean ± SD | 30.30 ± 12.03 | 35.13 ± 13.73 | 0.003 |
| (Minimum, maximum) | (7, 68) | (11, 72) | |
| MDS‐UPDRS I score | |||
| Mean ± SD | 5.22 ± 3.62 | 6.14 ± 4.71 | 0.257 |
| (Minimum, maximum) | (0, 18) | (0, 24) | |
| MDS‐UPDRS II score | |||
| Mean ± SD | 5.50 ± 4.01 | 6.53 ± 4.33 | 0.025 |
| (Minimum, maximum) | (0, 20) | (1, 18) | |
| MDS‐UPDRS III score | |||
| Mean ± SD | 19.57 ± 7.94 | 22.46 ± 9.51 | 0.015 |
| (Minimum, maximum) | (4, 41) | (6, 51) | |
| MDS‐UPDRS tremor score | |||
| Mean ± SD | 4.17 ± 2.94 | 4.58 ± 3.45 | 0.432 |
| (Minimum, maximum) | (0, 15) | (0, 18) | |
| MDS‐UPDRS rigidity score | |||
| Mean ± SD | 3.74 ± 2.57 | 3.72 ± 2.80 | 0.667 |
| (Minimum, maximum) | (0, 12) | (0, 13) | |
| MDS‐UPDRS PIGD score | |||
| Mean ± SD | 1.09 ± 1.02 | 1.27 ± 1.28 | 0.358 |
| (Minimum, maximum) | (0, 6) | (0, 7) | |
| MDS‐UPDRS axial score | |||
| Mean ± SD | 2.48 ± 1.66 | 2.59 ± 1.79 | 0.729 |
| (Minimum, maximum) | (0, 7) | (0, 8) | |
| UPSIT score | |||
| Mean ± SD | 21.86 ± 8.02 | 22.71 ± 8.44 | 0.429 |
| (Minimum, maximum) | (1, 38) | (5, 40) | |
| UPSIT categories | |||
| Normosmia (n, %) | 17 (7.3%) | 13 (10.8%) | 0.484 |
| Hyposmia (n, %) | 131 (56.5%) | 68 (56.7%) | |
| Anosmia (n, %) | 84 (36.2%) | 39 (32.5%) | |
| ESS score | |||
| Mean ± SD | 5.66 ± 3.36 | 5.96 ± 3.62 | 0.438 |
| (Minimum, maximum) | (0, 15) | (0, 20) | |
| ESS categories | |||
| Not EDS, ESS < 10 (n, %) | 218 (85.3%) | 102 (85.0%) | 1 |
| EDS, ESS ≥10 (n, %) | 34 (14.7%) | 18 (15.0%) | |
| GDS score | |||
| Mean ± SD | 2.34 ± 2.41 | 2.21 ± 2.54 | 0.284 |
| (Minimum, maximum) | (0, 14) | (0, 12) | |
| GDS categories | |||
| Not depressed, GDS <5 (n, %) | 201 (86.6%) | 104 (86.7%) | 1 |
| Depressed, GDS ≥5 (n, %) | 31 (13.4%) | 16 (13.3%) | |
| RBDSQ score | |||
| Mean ± SD | 4.07 ± 2.57 | 4.13 ± 2.88 | 0.751 |
| (Minimum, maximum) | (0, 12) | (0, 12) | |
| RBD categories | |||
| No RBD, RBDSQ <6 (n, %) | 143 (61.6%) | 75 (62.5%) | 0.966 |
| RBD, RBDSQ ≥6 (n, %) | 89 (38.4%) | 45 (37.5%) | |
| SCOPA‐AUT score | |||
| Mean ± SD | 9.34 ± 5.95 | 10.00 ± 6.61 | 0.327 |
| (Minimum, maximum) | (0, 32) | (0, 39) | |
| STAI score | |||
| Mean ± SD | 64.97 ± 17.92 | 65.15 ± 19.80 | 0.753 |
| (Minimum, maximum) | (40, 121) | (40, 137) | |
| MoCA score | |||
| Mean ± SD | 27.11 ± 2.28 | 27.23 ± 2.27 | 0.606 |
| (Minimum, maximum) | (17, 30) | (19, 30) | |
| Mean caudate (SBR) | |||
| Mean ± SD | 2.02 ± 0.53 | 2.04 ± 0.59 | 0.815 |
| (Minimum, maximum) | (0.5, 4) | (0.6, 4) | |
| Mean putamen (SBR) | |||
| Mean ± SD | 0.83 ± 0.27 | 0.86 ± 0.36 | 0.848 |
| (Minimum, maximum) | (0.3, 2) | (0.3, 2) | |
| Mean striatum (SBR) | |||
| Mean ± SD | 1.42 ± 0.38 | 1.45 ± 0.44 | 0.789 |
| (Minimum, maximum) | (0.4, 3) | (0.5, 3) | |
Note: Significance at p value < 0.05 were reported in bold. Except for UPSIT and MoCA, for which lower baseline scores indicate deterioration of respective functions, for all remaining non‐motor symptoms, higher baseline scores indicate deterioration of respective functions. The t‐test or chi‐square test assessed differences between normally distributed group values. Nonparametric data were compared using the Wilcoxon rank‐sum test. Three or more groups were compared using Kruskal–Wallis tests.
Abbreviations: DAT, dopamine transporter; ESS, Epworth Sleepiness Scale Score; GDS, Geriatric Depression Scale Score; MDS‐UPDRS, Movement Disorder Society‐Sponsored Revision of the Unified Parkinson Disease Rating Scale; MoCA, Montreal Cognitive Assessment (adjusted for education); PIGD, postural instability and gait difficulty; RBD, rapid‐eye‐movement sleep behavior disorder; RBDSQ, REM Sleep Behavior Disorder Screening Questionnaire; SBR, striatal binding ratio; SCOPA‐AUT, Scales for Outcomes in Parkinson's disease – Autonomic; SD, Standard deviation; SPECT, single‐photon emission computed tomography; STAI, State–Trait Anxiety Inventory; UPSIT, University of Pennsylvania Smell Identification Test.
Associations between baseline clinical characteristics and SBR values in the cross‐sectional analysis
In the cross‐sectional analysis, mean striatal SBR values were transformed to resemble the normal distribution by Box‐Cox transformation (λ = 0.6319). The associations between baseline clinical characteristics and mean striatal SBR levels for PD individuals were concluded in Figure 2a and Table S1. Generally, PD individuals who were in a higher Hoehn and Yahr scale stage (β = −0.1295, FDR‐adjusted p = 0.0017; Figure 2b) and had higher MDS‐UPDRS global scores (β = −0.0054, FDR‐adjusted p = 0.0017) had lower mean striatal SBR levels. In terms of sub‐sectional scores of MDS‐UPDRS, PD individuals with higher scores for MDS‐UPDRS part II, MDS‐UPDRS part III, rigidity, and axial MDS‐UPDRS presented lower SBR levels (β = −0.0152, β = −7.385e‐03, β = −0.0249, and β = −0.0405, respectively, FDR‐adjusted p = 0.0017). Patients who developed FOG at the screening visit had lower SBR values (β = −0.2368, FDR‐adjusted p = 0.0161; Figure 2c). Initial rigidity symptom was also associated with a lower SBR level, whereas this association failed to remain significant after FDR adjustment (β = −0.0430, nominal p = 0.0432, FDR‐adjusted p = 0.0017; Figure 2d). When stratified by sex, associations between motor symptoms and SBR levels were the same as those in the whole PD group (Hoehn and Yahr scale: β = −0.1592, FDR‐adjusted p = 0.0015, Figure 2e; MDS‐UPDRS total score: β = −0.0073, FDR‐adjusted p = 0.0013; MDS‐UPDRS part II score: β = −0.0197, FDR‐adjusted p = 0.0013; MDS‐UPDRS part III score: β = −9.037e‐03, FDR‐adjusted p = 0.0015; rigidity MDS‐UPDRS score: β = −2.830e‐02, FDR‐adjusted p = 0.0017; axial MDS‐UPDRS score: β = −0.0487, FDR‐adjusted p = 0.0015). Men with PD who developed FOG at baseline were more likely to have reduced SBR levels (β = −0.3553, FDR‐adjusted p = 0.0018; Figure 2f). Additionally, higher PIGD scores and higher RBDSQ scores had negative relationships with mean striatal SBR levels, although the significance did not remain after multiple test corrections (PIGD score: β = −0.0430, nominal p = 0.0432, FDR‐adjusted p = 0.0015; RBDSQ score: β = −0.0170, nominal p = 0.0307, FDR‐adjusted p = 0.0959). There was no significant association between any clinical characteristic and mean striatal SBR levels in female PD individuals.
FIGURE 2.
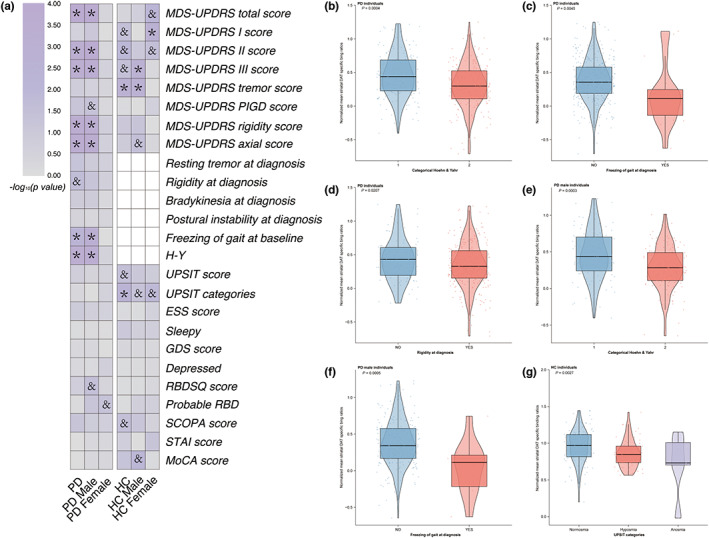
Associations of mean striatal DAT binding with clinical measures in cross‐sectional analysis. Cross‐sectional associations of mean striatal DAT binding with clinical measures were shown (a). PD individuals with higher Hoehn and Yahr scales (b), freezing of gait (c), and initial rigidity symptoms (d) contribute to lower striatal DAT binding levels. PD men who had higher Hoehn and Yahr scales (e) and freezing of gait (f) showed lower striatal DAT binding. Healthy controls who had severe olfactory dysfunction presented lower striatal DAT binding (g). *FDR‐adjusted p < 0.05; and nominal p < 0.05 whereas FDR‐adjusted p ≥ 0.05. Nominal p values were demonstrated in (b–f). DAT, dopamine transporter; ESS, Epworth Sleepiness Scale; FDR, false discovery rate; GDS, Geriatric Depression Scale; HC, healthy control; MDS‐UPDRS, Movement Disorder Society‐Sponsored Revision of the Unified Parkinson Disease Rating Scale; MoCA, Montreal Cognitive Assessment; PD, Parkinson's disease; RBD, rapid‐eye‐movement sleep behavior disorder; RBDSQ, rapid‐eye‐movement sleep behavior disorder screening questionnaire; SCOPA, Scales for Outcomes in Parkinson's disease – Autonomic.
The cross‐sectional analysis conducted respectively on the caudate and the putamen SBR values showed similar results to the analysis conducted on the striatal SBR levels (Tables S3 and S5). Among all the motor scales, the MDS‐UPDRS total scores, MDS‐UPDRS part II scores, MDS‐UPDRS part III scores, rigidity scores, and axial MDS‐UPDRS scores showed negative correlation with SBR levels (the caudate SBRs: FDR‐adjusted p = 0.037, the putamen SBRs: FDR‐adjusted p < 0.0001, respectively, see Tables S3 and S5). PD individuals who had FOG showed lower caudate and putamen SBR levels (FDR‐adjusted p = 0.0379, FDR‐adjusted p = 0.0118, respectively). Notably, PD individuals whose initial symptom was rigidity had lower putamen SBRs (β = −0.1050, FDR‐adjusted p = 0.0150), whereas this association failed to be detected on caudate SBR levels. When stratified by sex, we found similar results on the PD male group to those on whole PD individuals, although we failed to detect any association between SBRs and clinical features in female groups.
Healthy individuals who had higher MDS‐UPDRS tremor scores presented lower mean striatal SBR levels at baseline (β = −0.0578, FDR‐adjusted p = 0.0257; Table S2). Other sub‐sectional scores of the MDS‐UPDRS scale including the MDS‐UPDRS part I score, the MDS‐UPDRS part II score, and the UPDRS part III score, also demonstrated relationships with SBR levels of HCs (MDS‐UPDRS part I score: β = 0.0140, nominal p = 0.0322, FDR‐adjusted p = 0.0888; MDS‐UPDRS part II score: β = 0.0404, nominal p = 0.0113, FDR‐adjusted p = 0.0716; MDS‐UPDRS part III score: β = −0.0161, nominal p = 0.0303, FDR‐adjusted p = 0.0888), although those associations did not reach significance after FDR adjustment. Among NMS assessments, HCs with more severe olfactory impairment were more likely to have lower SBR levels (β = −0.0916, FDR‐adjusted p = 0.0257; Figure 2g). Among HC men, scores of MDS‐UPDRS part III and tremor MDS‐UPDRS demonstrated negative associations with mean striatal SBR values (β = −0.0298, FDR‐adjusted p = 0.0171; β = −0.6390, FDR‐adjusted p = 0.0171, respectively). No significant associations were found between clinical characteristics of HCs women and their SBR levels except for MDS‐UPDRS part I scores (β = 0.0275, FDR‐adjusted p = 0.0342).
HCs with heavier olfactory impairment showed lower caudate SBR levels at the screening visit (β = −0.0899, FDR‐adjusted p = 0.0342; Table S4). Higher baseline tremor scores were associated with lower putamen SBR values (β = −0.0942, FDR‐adjusted p = 0.0494; Table S6). Female HCs with higher MDS‐UPDRS total scores, higher MDS‐UPDRS part I scores, and more severe olfactory impairment had lower caudate SBR levels (MDS‐UPDRS total score: β = 0.0163, FDR‐adjusted p = 0.0298; MDS‐UPDRS part I score: β = 0.0262, FDR‐adjusted p = 0.0298; UPSIT categories: β = −0.1442, FDR‐adjusted p = 0.0298, respectively; Table S4). Male HCs with higher MDS‐UPDRS part III scores, higher tremor scores, and lower MoCA scores showed lower putamen SBR values at the screening visit (MDS‐UPDRS part III score: β = −0.0467, FDR‐adjusted p = 0.0295; tremor score: β = 0.1058, FDR‐adjusted p = 0.0228; MoCA score: β = 0.0841, FDR‐adjusted p = 0.0424; Table S6).
Associations between clinical characteristics and SBR values in longitudinal analysis
Changes in normalized mean striatal SBRs in PD individuals, PD men, and PD women over time are shown in Figure 3. Associations between baseline clinical variables and normalized mean striatal SBR levels in the longitudinal analysis were revealed in Table S7 and Figure 4a. For total PD individuals, higher baseline RBDSQ scores were associated with greater negative growth rates of normalized mean striatal SBR values. For each increased score in the baseline RBDSQ scale, the annual decline rate of SBR values increased by 0.0126 (β = −0.0126, FDR‐adjusted p = 0.0120). Higher scores in the MDS‐UPDRS part II scale also presented a potential association with a higher annual decrease rate of mean striatal SBRs, whereas this association failed to pass the significance threshold after multiple testing corrections (β = −0.0056, nominal p = 0.0183, FDR‐adjusted p = 0.1464).
FIGURE 3.
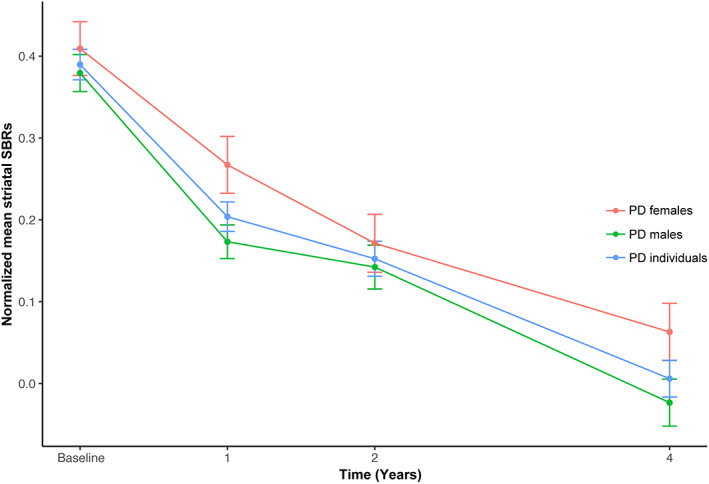
Changes in normalized mean striatal SBRs in PD individuals, PD men, and PD women over time. PD, Parkinson's disease; SBR, striatal binding ratio.
FIGURE 4.
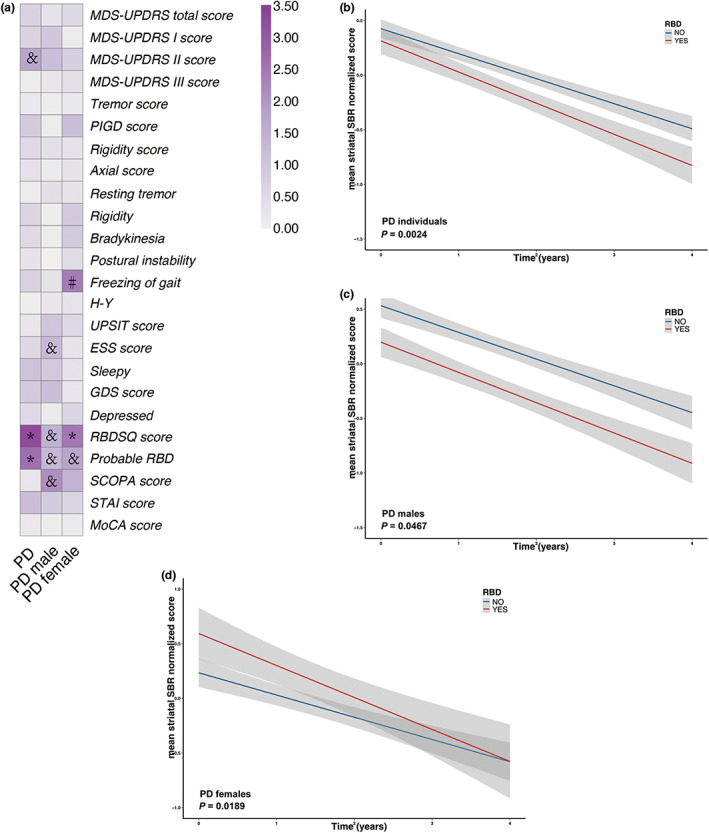
Associations of mean striatal DAT binding with clinical measures in longitudinal analysis. Longitudinal associations of mean striatal DAT binding with clinical measures were shown (a). RBD contributed to a greater decrease in mean striatal DAT binding in PD individuals (b), PD men (c), and PD women (d) respectively. *FDR‐adjusted p < 0.05; and nominal p < 0.05 whereas FDR‐adjusted p ≥ 0.05. #: Unreliable significant results with insufficient sample size in some subgroups. Nominal p values were demonstrated in (b–d). DAT, dopamine transporter; ESS, Epworth Sleepiness Scale; MDS‐UPDRS, Movement Disorder Society‐Sponsored Revision of the Unified Parkinson Disease Rating Scale; PD, Parkinson's disease; PIGD, postural instability and gait difficulty; RBD, rapid‐eye‐movement sleep behavior disorder; SBR, striatal binding ratio; STAI, State–Trait Anxiety Inventory; UPSIT, University of Pennsylvania Smell Identification Test.
Subgroup analysis stratified by sex found no significant association between baseline clinical characteristics and annual changes of normalized mean striatal SBR values among PD men. Among PD women, a higher score on the RBDSQ scale at baseline presented a greater downward trend of longitudinal SBRs (β = −0.0257, FDR‐adjusted p = 0.0444). PD men and women who had developed probable RBD presented a potentially higher annual drop rate of SBR levels, although this relationship did not remain significant after FDR adjustment (PD men: β = −0.0421, nominal p = 0.0467, FDR‐adjusted p = 0.2848; PD women: β = −0.0966, nominal p = 0.0189, FDR‐adjusted p = 0.1512, respectively; Figure 4c,d).
Similar results were found in longitudinal analysis conducted on the association between baseline clinical features and longitudinal caudate striatal SBR values (Table S8). Patients with PD with higher baseline RBDSQ scores showed larger reduction rate of caudate SBR values (β = −0.0126, FDR‐adjusted p = 0.007). Patients who had developed probable RBD at screening visit presented a potentially higher annual drop rate of caudate SBR levels (β = −0.0606, FDR‐adjusted p = 0.0276). No significant correlation between baseline clinical features and longitudinal putamen SBR values was detected in this analysis (Table S9).
Linear mixed‐effect models were also established to evaluate the relationship between baseline SBR values and longitudinal normalized non‐motor characteristics. For each decreased value in the normalized baseline caudate SBR, the annual decline rate of normalized MoCA scores increased by 0.0589 (β = 0.0589, FDR‐adjusted p = 0.0372; Table S11). We failed to detect other significant associations between baseline striatal and putamen SBR values and longitudinal non‐motor clinical features (Tables S10 and S12).
Associations between changes in clinical characteristics and changes in SBRs over time
Associations between longitudinal changes in normalized mean striatal SBR values and follow‐up changes in clinical variables are concluded in Table S13. Among PD individuals, a greater increase in scores on the MDS‐UPDRS scale and its sub‐sectional scales related to a greater decline of mean striatal SBRs over the 48‐month follow‐up (MDS‐UPDRS total score: ρ = −0.187, p = 0.005; MDS‐UPDRS part I score: ρ = −0.168, p = 0.011; MDS‐UPDRS part II score: ρ = −0.160, p = 0.016, respectively; Figure 5a–c). Additionally, changes in STAI scores showed a negative correlation with changes in SBRs both over the 24‐month and over the 48‐month follow‐ups (ρ = −0.091, p = 0.041; ρ = −0.097, p = 0.032, respectively; Figure 5d).
FIGURE 5.
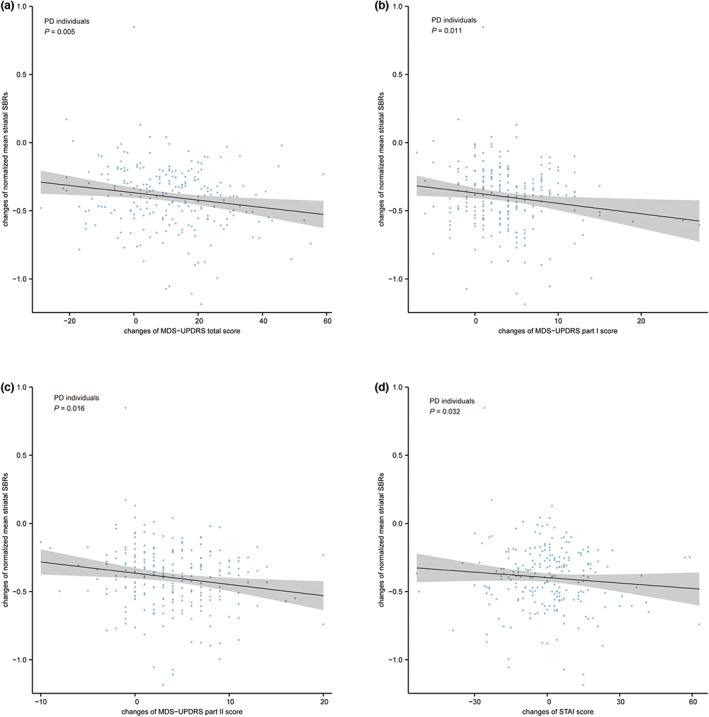
Associations between changes in striatal DAT binding and changes in clinical measures in PD individuals. A greater increase in the MDS‐UPDRS total scores (a), the MDS‐UPDRS part I scores (b), the MDS‐UPDRS part II scores (c), and the STAI scores (d) related to a greater decline in mean striatal SBRs over the 48‐month follow‐up. Nominal p values were demonstrated in (a–d). DAT, dopamine transporter; MDS‐UPDRS, Movement Disorder Society‐Sponsored Revision of the Unified Parkinson Disease Rating Scale; PD, Parkinson's disease; SBR, striatal binding ratio; STAI, State–Trait Anxiety Inventory.
PD men who had higher annual increase rates in MDS‐UPDRS scores and MDS‐UPDRS sub‐sectional scores also demonstrated a greater decrease in mean striatal SBRs values over the 48‐month follow‐up visit (MDS‐UPDRS total score: ρ = −0.026, p = 0.001; MDS‐UPDRS part I score: ρ = −0.201, p = 0.015; MDS‐UPDRS part II score: ρ = −0.215, p = 0.009; MDS‐UPDRS part III score: ρ = −0.172, p = 0.037; Figure 6a–d). Notably, PD men who experienced a greater increase in tremor scores had more severe declines in mean striatal SBR levels (ρ = −0.183, p = 0.027; Figure 6e). There was no significant association between changes in clinical characteristics and changes in mean striatal SBRs over 48‐month follow‐up visits among PD women. However, changes in MDS‐UPDRS part II scores, PIGD scores, and axial scores presented negative correlations with changes in SBRs over the 24‐month follow‐up visits (MDS‐UPDRS part II score: ρ = −0.246, p = 0.022; PIGD score: ρ = −0.211, p = 0.049; axial score: ρ = −0.217, p = 0.043; STAI score: ρ = −0.130, p = 0.011).
FIGURE 6.
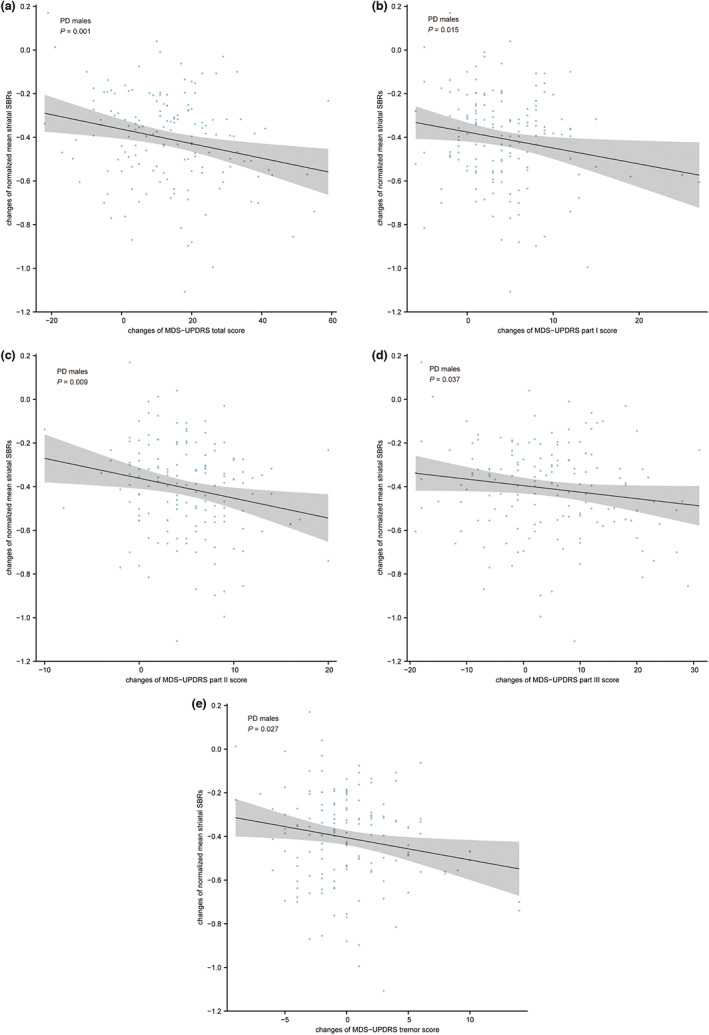
Associations between changes in striatal DAT binding and changes in clinical measures in PD men. A greater increase in the MDS‐UPDRS total scores (a), the MDS‐UPDRS part I scores (b), the MDS‐UPDRS part II scores (c), the MDS‐UPDRS part III scores (d), and the tremor scores (e) related to a greater decline in mean striatal SBRs over the 48‐month follow‐up. Nominal p values were demonstrated in (a–e). DAT, dopamine transporter; MDS‐UPDRS, Movement Disorder Society‐Sponsored Revision of the Unified Parkinson Disease Rating Scale; PD, Parkinson's disease; SBR, striatal binding ratio.
Similar results were found when taken the caudate subregion into account. Among PD individuals, a greater increase in scores on the MDS‐UPDRS scale and its sub‐sectional scales related to a greater decline of mean caudate SBRs over the 48‐month follow‐up (MDS‐UPDRS total score: ρ = −0.203, p = 0.002; MDS‐UPDRS part I score: ρ = −0.171, p = 0.009; MDS‐UPDRS part II score: ρ = −0.156, p = 0.018; MDS‐UPDRS part III score: ρ = −0.133, p = 0.044; rigidity score: ρ = −0.137, p = 0.039, respectively; Table S16). When stratified by sex, changes in MDS‐UPDRS part I scores showed a negative correlation with changes in caudate SBRs over the 48‐month follow‐ups in PD men, whereas changes in rigidity scores showed a negative correlation with changes in caudate SBRs over the 48‐month follow‐ups in PD women (MDS‐UPDRS part I score: ρ = −0.151, p = 0.048; rigidity score: ρ = −0.258, p = 0.025; Tables S17 and S18, respectively). Regarding non‐motor characteristics, changes in STAI and MoCA scores were significantly associated with changes in caudate SBRs over 24‐month follow‐ups (STAI: ρ = −0.129, p = 0.037; MoCA: ρ = 0.136, p = 0.027; Table S16). Both changes in ESS scores and changes in SCOPA scores were related to changes in caudate SBR values over 24‐month follow‐ups (ESS: ρ = −0.215, p = 0.041; SCOPA: ρ = −0.212, p = 0.045; Table S18). The same associations were also found in the putamen SBR values over 24‐month follow‐up visits (ESS: ρ = −0.208, p = 0.049; SCOPA: ρ = −0.222, p = 0.035; Table S21).
DISCUSSION
Our study systematically investigated both cross‐sectional and longitudinal correlations between motor and NMSs with striatal DAT binding levels. To the best of our knowledge, this study is the first to study associations between changes in NMSs and changes in DAT uptakes over time. It is also the first investigation to link baseline and longitudinal striatal DAT binding to the severity of specific motor symptoms.
The cross‐sectional analysis indicated negative correlations between the current severity of motor symptoms and the current striatal DAT binding for early PD individuals and PD men. Among all types of motor symptoms, the mean striatal DAT binding exclusively demonstrated negative associations with the severity of rigidity and axial dysfunction in PD individuals and PD men. Patients who have developed FOG had significantly lower striatal DAT binding levels. Additionally, the severity of tremors and olfactory dysfunction showed a significant association with DAT binding in total HCs and HC men.
In the longitudinal analysis, we found a significant and comprehensive reduction of mean striatal DAT binding over 4 years after PD diagnosis. This data were consistent with a previous pathological study suggesting a comprehensive loss of dopamine markers by 4–5 years after the diagnosis. 2 Additionally, we reported that the yearly change in DAT uptake was largest in year 1 compared with years 2 and 4. Moreover, we reported a higher current RBDSQ score potentially predicted a greater decline in DAT uptake over 4 years. PD individuals with RBD had a more severe drop in DAT binding during the following 4 years. Furthermore, changes in the severity of motor symptoms demonstrated negative relationships with changes in DAT uptake over time, with a higher increase of MDS‐UPDRS scores associated with a greater decline of striatal DAT binding over 4 years for both PD individuals and PD men. Changes in tremor severity were correlated with changes in DAT uptake for PD men. Among the NMSs, only changes in anxiety demonstrated a significant negative association with changes in DAT binding. Additionally, when respectively taking caudate and putamen subregions into account, we found that caudate SBR values showed more significant associations with both motor and non‐motor clinical features.
In terms of motor symptoms, our observations were consistent with a previous longitudinal study suggesting a modest but significant correlation between change in MDS‐UPDRS total score and change in mean striatum binding. 9 Previous clinical studies indicated significant associations between striatal DAT activity with specific motor symptoms, including bradykinesia, rigidity, gait, and axial symptoms, whereas tremors did not associate with DAT binding. 16 , 17 Our results lend further support to the correlation between DAT binding with rigidity, gait, and axial symptoms, whereas contradictions exist in bradykinesia and tremor symptoms. Notably, all the previous investigations were cross‐sectional and used DAT imaging at an only one timepoint in a small sample size. Our study was conducted on a much larger sample size and investigated DAT binding levels at four timepoints. Additionally, longitudinal studies using baseline DAT binding as a predictive factor suggested a lower baseline DAT binding level well correlated with increased severity of motor symptoms, including motor‐related disability, falling, and postural instability. 18 , 19 Our study reported associations between longitudinal severity of motor symptoms with longitudinal striatal DAT binding, providing novel evidence for the clinical usage of DAT binding as an imaging marker of PD progression.
Liu and colleagues reported several NMSs associated with current and future mean striatal DAT binding levels, including RBD, memory and visuospatial cognitive domains, and total anxiety. 8 Our study confirmed the same correlation as the previous study in terms of RBD, whereas significant associations were found between neither cognitive dysfunction nor total anxiety with DAT binding. Different assessment methods of cognitive function may partly contribute to this contradiction, with Liu using the Hopkins Verbal Learning Test‐Revised (HVLT‐R) and the Benton Judgment of Line Orientation (JOLO) for memory and visuospatial cognitive domains, respectively. When considering the global cognitive dysfunction using MoCA, our result is identical to that of Liu's. For the potential explanation of the inconsistency regarding total anxiety, the previous study established mixed‐effect models separately at different follow‐up visits, whereas we established the whole model based on 4‐year data. Another previous study reported a negative correlation between EDS and striatal DAT binding, which was inconsistent with our results. 20 Different identity methods of EDS and sample sizes may cause this contradiction. With the presence of EDS defined as the ESS score greater than or equal to 10 and relatively bigger sample size, we found no significant association between EDS and striatal DAT uptake levels. Longitudinal studies conducted on patients with idiopathic RBD (iRBD) revealed reduced striatal DAT binding over time compared to controls. 21 , 22 , 23 Additionally, previous studies suggested that reduced striatal DAT uptake was associated with phenotypical progression of iRBD to several neurodegenerative disorders. 21 , 22 , 24 Moreover, a recent study confirmed the validity of the baseline and longitudinal DAT binding as a useful biomarker in detecting iRBD individuals with a high risk of phenotypical conversion. 23 Our findings highlighted the significant linear correlation between the severity of the RBD symptom with current and future striatal DAT depletion in de novo PD, lending further support to these clinical observations.
There are also several limitations in our study needed to be acknowledged. First, participants with early PD enrolled in the PPMI database were relatively younger and less disabled at baseline than the general PD population. 9 Therefore, our results may not be readily applicable to the general PD population. Second, dopamine replacement therapy (DRT) potentially has an impact on DAT availability over years, whereas patients recruited in the PPMI were all drug naïve at enrollment. However, part of the PD cohort took DRT during follow‐up visiting. It is important to further deal with the effect that DRT had on DAT uptakes in longitudinal analysis. Notably, an early PPMI publication revealed that over the first year, when half of the patients began DRT, the change in DAT availability was not significantly different between those who initiated DRT and those who did not. 25 Third, as a more generalized DAT imaging marker, the mean striatal SBR always demonstrated significant association with motor and nonmotor symptoms in previous studies, whereas such association sometimes failed to be detected in terms of the mean caudate or putamen SBRs. 8 , 9 Therefore, we only investigated the association between the mean striatal DAT binding and different clinical characteristics in the present study. However, due to the hemispheric asymmetry in motor symptoms and the heterogenous of NMSs, further studies are needed to clarify whether different subregional striatal or even extrastriatal DAT binding are associated with different motor or NMSs respectively. 8 , 26
CONCLUSION
Our study revealed the longitudinal change of nigrostriatal dopamine binding and pointed out that certain motor and non‐motor features were correlated with current and future nigrostriatal dopamine binding in patients newly diagnosed with PD, lending further support to the usage of DAT imaging as a predictor of disease progression. Further studies are warranted to clarify the associations between different regional dopamine binding with specific clinical features.
AUTHOR CONTRIBUTIONS
Z.Y. wrote the manuscript. Z.Y., Y.X., and L.Y performed the research. K.D. and A.X. designed the research. Z.Y., Y.X., and L.Y analyzed the data.
FUNDING INFORMATION
This work was funded by the National Natural Science Foundation of China (grant no. 81971192).
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Data S1
ACKNOWLEDGMENTS
The authors would like to thank all subjects for their participation in this study. We thank the associate editor and the reviewers for their useful feedback that improved this paper.
Yang Z, Xie Y, Dou K, Yang L, Xie A. Associations of striatal dopamine transporter binding with motor and non‐motor symptoms in early Parkinson's disease. Clin Transl Sci. 2023;16:1021‐1038. doi: 10.1111/cts.13508
DATA AVAILABILITY STATEMENT
We confirm that the data supporting the findings of this study are available within the article and supporting information.
REFERENCES
- 1. Kalia LV, Lang AE. Parkinson's disease. Lancet Lond Engl. 2015;386:896‐912. doi: 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 2. Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease, brain. J Neurol. 2013;136:2419‐2431. doi: 10.1093/brain/awt192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150‐1157. doi: 10.1016/S1474-4422(09)70238-8 [DOI] [PubMed] [Google Scholar]
- 4. Kaasinen V, Vahlberg T. Striatal dopamine in Parkinson disease: a meta‐analysis of imaging studies. Ann Neurol. 2017;82:873‐882. doi: 10.1002/ana.25103 [DOI] [PubMed] [Google Scholar]
- 5. Mitchell T, Lehéricy S, Chiu SY, Strafella AP, Stoessl AJ, Vaillancourt DE. Emerging neuroimaging biomarkers across disease stage in Parkinson disease: a review. JAMA Neurol. 2021;78:1262‐1272. doi: 10.1001/jamaneurol.2021.1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Postuma RB, Aarsland D, Barone P, et al. Identifying prodromal Parkinson's disease: pre‐motor disorders in Parkinson's disease. Mov Disord. 2012;27:617‐626. doi: 10.1002/mds.24996 [DOI] [PubMed] [Google Scholar]
- 7. Kim R, Lee J, Kim Y, et al. Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson's disease: an analysis of the PPMI cohort. Parkinsonism Relat Disord. 2018;51:49‐54. doi: 10.1016/j.parkreldis.2018.02.047 [DOI] [PubMed] [Google Scholar]
- 8. Liu R, Umbach DM, Tröster AI, Huang X, Chen H. Non‐motor symptoms and striatal dopamine transporter binding in early Parkinson's disease. Parkinsonism Relat Disord. 2020;72:23‐30. doi: 10.1016/j.parkreldis.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simuni T, Siderowf A, Lasch S, et al. Parkinson's progression marker initiative*, longitudinal change of clinical and biological measures in early Parkinson's disease: Parkinson's Progression Markers Initiative cohort. Mov Disord. 2018;33:771‐782. doi: 10.1002/mds.27361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parkinson Progression Marker Initiative . The Parkinson progression marker initiative (PPMI). Prog Neurobiol. 2011;95:629‐635. doi: 10.1016/j.pneurobio.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord. 2013;28:668‐670. doi: 10.1002/mds.25383 [DOI] [PubMed] [Google Scholar]
- 12. Gallea C, Wicki B, Ewenczyk C, et al. Antisaccade, a predictive marker for freezing of gait in Parkinson's disease and gait/gaze network connectivity, brain. J Neurol. 2021;144:504‐514. doi: 10.1093/brain/awaa407 [DOI] [PubMed] [Google Scholar]
- 13. Nomura T, Inoue Y, Kagimura T, Kusumi M, Nakashima K. Validity of the Japanese version of the REM sleep behavior disorder (RBD) screening questionnaire for detecting probable RBD in the general population. Psychiatry Clin Neurosci. 2015;69:477‐482. doi: 10.1111/pcn.12286 [DOI] [PubMed] [Google Scholar]
- 14. Simuni T, Caspell‐Garcia C, Coffey C, et al. PPMI sleep working group on behalf of the PPMI investigators, correlates of excessive daytime sleepiness in de novo Parkinson's disease: a case control study. Mov Disord. 2015;30:1371‐1381. doi: 10.1002/mds.26248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerin JM, Copersino ML, Schretlen DJ. Clinical utility of the 15‐item geriatric depression scale (GDS‐15) for use with young and middle‐aged adults. J Affect Disord. 2018;241:59‐62. doi: 10.1016/j.jad.2018.07.038 [DOI] [PubMed] [Google Scholar]
- 16. Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson's disease severity and duration with 123I‐FP‐CIT SPECT striatal uptake. Mov Disord. 2000;15:692‐698. doi: [DOI] [PubMed] [Google Scholar]
- 17. Pirker W. Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord. 2003;18(Suppl 7):S43‐S51. doi: 10.1002/mds.10579 [DOI] [PubMed] [Google Scholar]
- 18. Ravina B, Marek K, Eberly S, et al. Dopamine transporter imaging is associated with long‐term outcomes in Parkinson's disease. Mov Disord. 2012;27:1392‐1397. doi: 10.1002/mds.25157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vogt T, Kramer K, Gartenschlaeger M, Schreckenberger M. Estimation of further disease progression of Parkinson's disease by dopamin transporter scan vs clinical rating. Parkinsonism Relat Disord. 2011;17:459‐463. doi: 10.1016/j.parkreldis.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 20. Yousaf T, Pagano G, Niccolini F, Politis M. Excessive daytime sleepiness may be associated with caudate denervation in Parkinson disease. J Neurol Sci. 2018;387:220‐227. doi: 10.1016/j.jns.2018.02.032 [DOI] [PubMed] [Google Scholar]
- 21. Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid‐eye‐movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10:797‐805. doi: 10.1016/S1474-4422(11)70152-1 [DOI] [PubMed] [Google Scholar]
- 22. Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post‐mortem pathology in idiopathic rapid‐eye‐movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443‐453. doi: 10.1016/S1474-4422(13)70056-5 [DOI] [PubMed] [Google Scholar]
- 23. Shin JH, Lee J‐Y, Kim Y‐K, et al. Longitudinal change in dopamine transporter availability in idiopathic REM sleep behavior disorder. Neurology. 2020;95:e3081‐e3092. doi: 10.1212/WNL.0000000000010942 [DOI] [PubMed] [Google Scholar]
- 24. Iranzo A, Lomeña F, Stockner H, et al. Sleep Innsbruck Barcelona (SINBAR) group, decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid‐eye‐movement sleep behaviour disorder: a prospective study [corrected]. Lancet Neurol. 2010;9:1070‐1077. doi: 10.1016/S1474-4422(10)70216-7 [DOI] [PubMed] [Google Scholar]
- 25. Smith KM, Xie SX, Weintraub D. Incident impulse control disorder symptoms and dopamine transporter imaging in Parkinson disease. J Neurol Neurosurg Psychiatry. 2016;87:864‐870. doi: 10.1136/jnnp-2015-311827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fiorenzato E, Antonini A, Bisiacchi P, Weis L, Biundo R. Asymmetric dopamine transporter loss affects cognitive and motor progression in Parkinson's disease. Mov Disord. 2021;36:2303‐2313. doi: 10.1002/mds.28682 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
We confirm that the data supporting the findings of this study are available within the article and supporting information.


