Abstract
Brain metastases (BMs) are associated with poor prognosis in epidermal growth factor receptor mutation‐positive (EGFRm) non‐small cell lung cancer (NSCLC). Osimertinib is a third‐generation, irreversible, EGFR‐tyrosine kinase inhibitor that potently and selectively inhibits EGFR‐sensitizing and T790M resistance mutations with efficacy in EGFRm NSCLC including central nervous system (CNS) metastases. The open‐label phase I positron emission tomography (PET) and magnetic resonance imaging (MRI) study (ODIN‐BM) assessed [11C]osimertinib brain exposure and distribution in patients with EGFRm NSCLC and BMs. Three dynamic 90‐min [11C]osimertinib PET examinations were acquired together with metabolite‐corrected arterial plasma input functions at: baseline, after first oral osimertinib 80 mg dose, and after greater than or equal to 21 days of osimertinib 80 mg q.d. treatment. Contrast‐enhanced MRI was performed at screening and after 25–35 days of osimertinib 80 mg q.d.; treatment effect was assessed per CNS Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 and per volumetric changes in total BM using a novel analysis approach. Four patients (aged 51–77 years) completed the study. At baseline, ~1.5% injected radioactivity reached the brain (IDmax[brain]) 22 min (median, T max[brain]) after injection. Total volume of distribution (V T) in whole brain was numerically higher compared with the BM regions. After a single oral osimertinib 80 mg dose, there was no consistent decrease in V T in whole brain or BMs. After greater than or equal to 21 days' daily treatment, V T in whole brain and BMs were numerically higher versus baseline. MRI revealed 56%–95% reduction in total BMs volume after 25–35 days of osimertinib 80 mg q.d. treatment. The [11C]osimertinib crossed the blood–brain and brain‐tumor barriers and had a high, homogeneous brain distribution in patients with EGFRm NSCLC and BMs.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Brain metastases (BMs) are associated with poor prognosis in epidermal growth factor receptor mutation‐positive (EGFRm) non‐small cell lung cancer (NSCLC). Osimertinib has demonstrated efficacy in EGFRm NSCLC, including central nervous system metastases. However, the exposure and exact distribution of osimertinib in whole brain following administration in patients with BMs is unknown.
WHAT QUESTION DID THIS STUDY ADDRESS?
ODIN‐BM was a phase I multimodal imaging study combining positron emission tomography (PET) and magnetic resonance imaging to examine osimertinib brain exposure in patients with EGFRm NSCLC and BMs. The results demonstrated homogenous distribution of radiolabeled osimertinib in the whole brain and BMs.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
These findings corroborate preclinical data and observations in healthy volunteers with an intact blood brain barrier to patients with BMs treated with osimertinib. Notably, our study demonstrated that presence of [11C]osimertinib exposure in BMs shell and core regions was similar to that in the surrounding brain tissue, suggesting that [11C]osimertinib passes the blood–brain and blood‐tumor barriers.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Findings of this study offer more information regarding osimertinib brain exposure and its radiologically assessed efficacy in BMs as reported in large clinical trials and recent case study reports. Data suggesting that [11C]osimertinib passes the blood–brain and brain‐tumor barriers has not been reported with any other anticancer treatment to the best of our knowledge. Additionally, this study also illustrates the potential of microdosing PET studies in the development of drugs targeting brain malignancies.
INTRODUCTION
Brain metastases (BMs) are common in patients with non‐small cell lung cancer (NSCLC) and can negatively impact prognosis and quality of life. 1 In patients with advanced epidermal growth factor receptor mutation‐positive (EGFRm) NSCLC, risk of BMs is even higher (incidence, ~60%–70%) compared with EGFR wild‐type disease. 2 , 3 Furthermore, 40%–50% of patients develop BMs within 3 years of diagnosis despite treatment with first‐ or second‐generation EGFR tyrosine kinase inhibitors (EGFR‐TKIs). 4
Osimertinib is a third‐generation, irreversible, oral EGFR‐TKI that potently and selectively inhibits EGFR TKI‐sensitizing and T790M EGFR mutations. 5 , 6 , 7 , 8 , 9 Initial case observations of effects of osimertinib in BMs 10 were confirmed in clinical trials in patients with advanced EGFRm NSCLC and central nervous system (CNS) metastases. 6 , 7 , 8 , 11 Furthermore, in the phase III ADAURA trial, clinical benefit of adjuvant osimertinib in the CNS (82% reduction in risk of CNS disease recurrence or death) versus placebo was observed in patients with completely resected EGFRm stage IB–IIIA NSCLC. 12
Molecular brain imaging using positron emission tomography (PET) can be applied to trace an administered microdose of radiolabeled drug and measure its tissue concentration with high spatial resolution. This approach is commonly used in CNS drug development, 13 such as in neuro‐oncology drug‐candidate and dose selection, 14 , 15 and to help understand blood–brain barrier (BBB) penetration of drugs used to treat CNS tumors. 16 , 17 Recently, brain exposure of 11C‐labeled osimertinib ([11C]osimertinib) was examined following intravenous (i.v.) administration of a microdose in non‐human primates (NHPs) 14 , 18 and healthy volunteers. 19 In healthy volunteers, radioactivity in the brain at time to maximum brain radioactivity concentration (T max[brain]) was 1.7%–2.4% (n = 8) of injected radioactivity, 19 comparable with that observed for established CNS drugs. 20 In our recent study, osimertinib had the highest brain exposure compared with 15 other EGFR‐TKIs in several species, including NHPs, 14 and resulted in significant brain exposure compared with other tested EGFR‐TKIs. This indicates that the prominent CNS efficacy of osimertinib is likely attributed to its high brain exposure. Similar studies have not been conducted in patients with EGFRm NSCLC and BMs.
The primary aim of the present study was to assess i.v. administered [11C]osimertinib exposure in whole brain and BMs under drug‐naïve conditions in patients with EGFRm NSCLC and BMs after a single dose of oral osimertinib 80 mg and after at least 21 days' once daily (q.d.) treatment with oral osimertinib. The study also examined whether specific binding of [11C]osimertinib to mutated EGFR could be demonstrated by PET. Data were interpreted by simple descriptive PET image analysis as well as compartmental analysis using a metabolite‐corrected arterial input function. Effects of osimertinib treatment on BM size after 25–35 days' oral osimertinib 80 mg q.d. treatment on magnetic resonance imaging (MRI) scans were also explored using CNS Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 and volumetric analyses of total BMs.
MATERIALS AND METHODS
The study was approved by the Medical Products Agency in Sweden, the Ethical Committee of the Stockholm region, and the Radiation Safety Committee of the Karolinska University Hospital, Stockholm, Sweden (ODIN‐BM; NCT03463525). Patients were recruited and the trial was conducted at Uppsala University Hospital and Karolinska University Hospital in Stockholm, Sweden, from October 2018 to March 2020, in accordance with current amendments of the Declaration of Helsinki and the International Conference on Harmonization/Good Clinical Practice guidelines. Written informed consent was obtained from all study participants.
Patients
Eligible patients were greater than or equal to 18 years old with a World Health Organization performance status of 0–2, histological or cytological confirmation of diagnosis of EGFRm NSCLC, and MRI‐confirmed, treatment‐naïve BMs. Patients did not have prior brain surgery or brain radiotherapy. Full inclusion and exclusion criteria are provided in Table S1. In total, ~12 patients were planned to participate in the study, and it was expected that at least eight patients would complete all planned study assessment procedures.
Summary of study design
ODIN‐BM was an open‐label, single‐center phase I study, consisting of two phases: an imaging and a continued‐access phase (Figure 1a). During the imaging phase, three brain [11C]osimertinib PET examinations were performed, PET1 at baseline (i.e., before osimertinib [unlabeled] 80 mg q.d. treatment initiation [day 1]); PET2 after first osimertinib 80 mg q.d. administration (days 2–8); and PET3 after greater than or equal to 21 days' osimertinib 80 mg q.d. treatment (days 22–29). Osimertinib 80 mg q.d. was administered by the investigator. For PET2 and PET3, [11C]osimertinib was injected 6 h after oral osimertinib 80 mg dose administration (i.e., at the predicted median time of maximum drug concentration [T max]). 21 Osimertinib was radiolabeled with 11C for PET imaging as described previously. 19 At each PET examination, saline solution containing [11C]osimertinib with mean radioactivity of 289 MBq (range, 221–363 MBq) was injected in the cubital vein as a bolus for 10 s. Mean molar radioactivity at injection time was 262 GBq/μmol (range 120–589 GBq/μmol), corresponding to mean injected mass of 0.67 μg (range 0.26–1.27 μg). After imaging study completion, patients continued to receive oral osimertinib 80 mg q.d. until they no longer derived clinical benefit.
FIGURE 1.
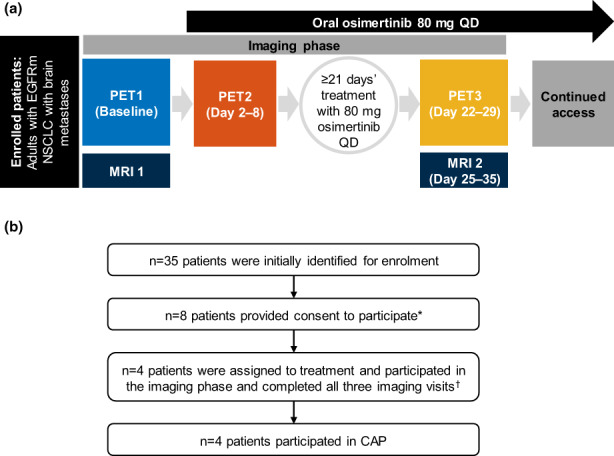
(a) ODIN‐BM study design. At PET1, 2, and 3, patients were administered with an i.v. microdose of [11C]osimertinib. Patients continued receiving osimertinib 80 mg tablets q.d. until they no longer benefitted, or chose to stop treatment. (b) Patient disposition. *Four patients failed screening; †PET1–3. CAP, continued‐access phase; EGFRm, epidermal growth factor receptor mutation‐positive; MRI, magnetic resonance imaging; NSCLC, non‐small cell lung cancer; PET, positron‐emission tomography; q.d., once‐daily.
End points and assessments
To determine the brain exposure of [11C]osimertinib in tumor after a single [11C]osimertinib i.v. dose and after single and multiple therapeutic doses of osimertinib, the primary outcomes measured were: the percent of injected dose in the whole brain and brain standard uptake value to describe the maximal radioactivity concentration in the brain (C max[brain]); and the brain to plasma partition coefficient as area under the concentration curve (AUC0–90min brain/plasma). The secondary outcome was to determine pharmacokinetics of osimertinib and its metabolite (AZ5104) after multiple doses of osimertinib. Exploratory outcomes included the total volume of distribution (V T) of [11C]osimertinib, the change in percent of [11C]osimertinib exposure by brain region in the course of treatment, and assessment of BM volume changes according to CNS RECIST version 1.1 and exploratory volumetric analysis, using brain MRI.
Following [11C]osimertinib i.v. injection, emission data were acquired in list mode over 90 min. During this time, manual and automatic radioactivity measurements were obtained for arterial blood and plasma, and samples were drawn for radio‐metabolite analysis as described previously. 19
MRI examinations were completed at baseline and after treatment period. Radiological response on MRI was assessed using CNS RECIST version 1.1 criteria and a novel volumetric analysis (all detectable BMs considered); the Response Assessment in Neuro‐Oncology Brain Metastases (RANO‐BM) criteria were considered retrospectively in a descriptive manner.
Follow‐up examinations were completed according to routine clinical practice.
Full details of study procedures, data acquisition, and image analyses, including the novel volumetric MRI analyses, are provided in the supplementary information and Figures S1 and S2.
Statistical methods
Descriptive statistics were used to depict imaging and pharmacokinetic parameters using the SAS® version 9.4. program.
RESULTS
Patient disposition
Thirty‐five patients were identified for enrollment; eight provided consent to participate, of which four failed screening (Figure 1b). The remaining four patients (2 men and 2 women; age, 51–77 years) received all osimertinib doses during the imaging phase and completed all imaging assessments. Patient demographics and baseline disease characteristics are in Table S2.
Recruitment was discontinued at the onset of the coronavirus disease 2019 (COVID‐19) pandemic. Due to low interpatient variability in the imaging results, data collected were considered sufficient to provide evidence for [11C]osimertinib brain exposure.
Brain exposure of [11 C]osimertinib
Visual inspection of PET images (Figure 2a) showed that [11C]osimertinib entered the brain, crossing the BBB and blood‐tumor barrier (BTB) and resulting in uniform radioactivity concentration across brain regions. The concentration was generally higher in gray versus white matter (Figure 2b). Brain exposure parameters were quantified from time activity curves (TACs). At baseline, median T max[brain] was 22 min (range, 11–42 min); at this time, 1.5% (range, 1.4%–1.6%, n = 4) of injected [11C]osimertinib radioactivity was measured in the brain (Table 1). Radioactivity concentration in BMs was similar to that in surrounding gray matter. Signal was generally higher in BM shell versus core regions (Figure 2b). The ratio of brain‐to‐plasma concentration leveled out at ~50–80 min after injection (Figure S3).
FIGURE 2.
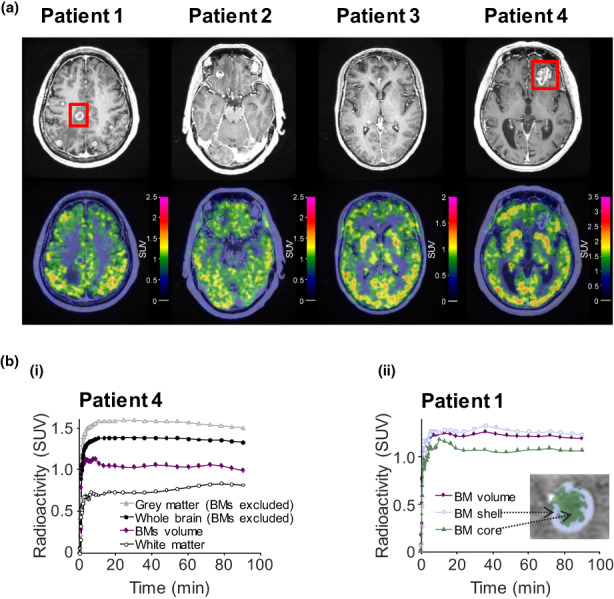
(a) Contrast‐enhanced T1‐weighted MR and PET images illustrating brain distribution [11C]osimertinib radioactivity. Color‐coded PET images overlaid with co‐registered MR images are provided; transaxial sections presented were selected according to the largest BM location. (b) Regional TACs of [11C]osimertinib in individual patients: curves for (i) anatomic brain regions and BMs in patient 4; and (ii) a BM and its subdivisions in patient 1. Red squares on MR images denote BMs illustrated in TACs. BM, brain metastasis; MR, magnetic resonance; NSCLC, non‐small cell lung cancer; PET, positron emission tomography; SUV, standard uptake value; TAC, time‐activity curve.
TABLE 1.
Whole brain exposure parameters for [11C]osimertinib in individual patients.
| Patient | %IDmax, brain | C max, brain (SUV) | T max, brain (min) | AUC0–90min brain/plasma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PET1 | PET2 | PET3 | PET1 | PET2 | PET3 | PET1 | PET2 | PET3 | PET1 | PET2 | PET3 | |
| 1 | 1.4 | 1.7 | 1.4 | 0.9 | 1.2 | 1.0 | 25 | 36 | 17 | 3.2 | 3.8 | 3.8 |
| 2 | 1.6 | 1.7 | 1.9 | 1.1 | 1.2 | 1.4 | 11 | 9 | 20 | 4.6 | 5.2 | 7.0 |
| 3 | 1.4 | 1.5 | 1.3 | 0.7 | 0.8 | 0.7 | 42 | 78 | 60 | 2.9 | 2.9 | 4.0 |
| 4 | 1.6 | 1.6 | 1.4 | 1.4 | 1.3 | 1.2 | 20 | 36 | 54 | 4.3 | 4.0 | 3.9 |
| Median | 1.5 | 1.6 | 1.4 | 1.0 | 1.2 | 1.1 | 22 | 36 | 37 | 3.8 | 3.9 | 3.9 |
| Mean (SD) | 1.5 (0.1) | 1.6 (0.1) | 1.5 (0.2) | 1.0 (0.3) | 1.1 (0.2) | 1.1 (0.3) | 24 (13) | 40 (29) | 38 (23) | 3.8 (0.8) | 4.0 (0.9) | 4.7 (1.5) |
Abbreviations: AUC, area under the curve; C max, maximum concentration after dosing; ID, injected dose; PET, positron‐emission tomography; SD, standard deviation; SUV, standard uptake volume; T max, time of maximum drug concentration after dosing.
Exposure of [11C]osimertinib in the brain was similar following single and multiple dose osimertinib treatment across patients, as evidenced by the whole brain TACs. There was no evident reduction in TACs after first osimertinib 80 mg q.d. dose (PET2) versus baseline (PET1). Likewise, there was no clear difference in TACs after multiple oral osimertinib 80 mg q.d. doses at PET3 versus baseline (Figure 3a). There were small intra‐individual differences in TACs among the three PET examinations but no consistent differences among patients. Similarly, there were no consistent differences across BM‐region TACs (Figure 3b).
FIGURE 3.
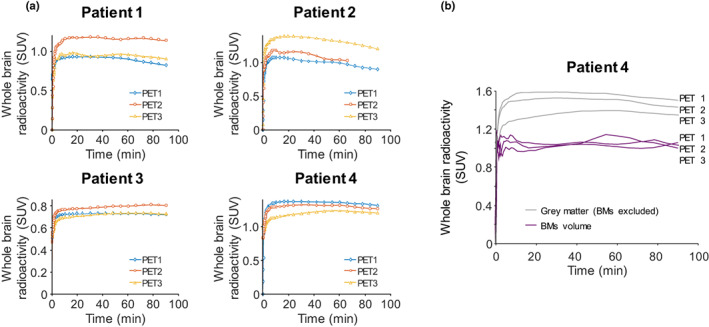
(a) Regional whole brain TACs of [11C]osimertinib in all patients at PET1–3. (b) Regional TACs of [11C]osimertinib in gray matter and BMs in patient 4 at PET1–3. BM, brain metastasis; PET, positron‐emission tomography; SUV, standard uptake value; TAC, time‐activity curve.
Kinetic compartment model‐based interpretation of TACs showed that the two‐tissue compartment model (2TCM) was preferred statistically over the one‐tissue compartment model, and that the 2TCM for reversible binding was preferred statistically over a 2TCM with irreversible binding to the second compartment (i.e., k 4 set to zero). Regional influx rate constant K 1, rate constants k 2, k 3, and k 4, and V T values, were each of a similar range across PET examinations (Table S3).
At baseline, V T of [11C]osimertinib in whole brain was numerically higher versus V T in BM regions (Figure 4). However, within larger BMs, standard uptake value and V T of [11C]osimertinib were higher in shell versus core regions and on average similar to that of the whole brain (Figures 2b and 4). After a single oral osimertinib 80 mg dose versus baseline, V T was similar in the whole brain and BMs (Figure 4a). After greater than or equal to 21 days' daily osimertinib treatment versus baseline, V T for whole brain and BMs were numerically higher in all patients (Figure 4b).
FIGURE 4.
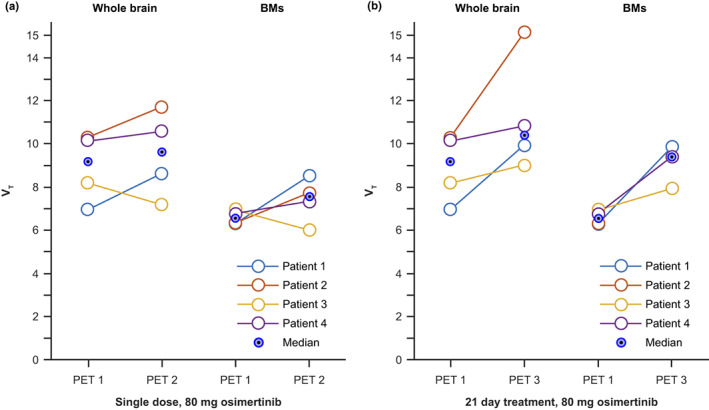
[11C]osimertinib V T in whole brain and BMs (a) after a single oral osimertinib 80 mg dose (PET2) and (b) after greater than or equal to 21 days' oral treatment (PET3) versus baseline (PET1). Individual data points are shown using circles and connecting lines. Eye markers (blue circles with black dots) represent median values. BMs, brain metastases; PET, positron emission tomography; V T, total value of distribution.
Osimertinib effect on tumor size volumes (MRI analyses)
CNS RECIST 1.1‐based assessment
The sum of the longest diameters (SoD) of brain target lesions at baseline in patients 1, 2, and 4 were 32, 13, and 45 mm, respectively. At follow‐up MRI, SoD decreased by 31%, 62%, and 29%, respectively, versus baseline. In patient 3, the two largest metastases were 6 and 5 mm at baseline, respectively, both below the 10 mm minimum limit required by CNS RECIST version 1.1 to be considered target lesions. However, they were located again at follow‐up MRI and remeasured to assess radiological response to therapy. The SoD of these two largest lesions in patient 3 decreased by 36% (non‐complete response/non‐progressive disease). Overall and according to CNS RECIST version 1.1, two patients (patients 1 and 2) achieved a partial response (≥30% decrease), one (patient 4) had stable disease (29% decrease), and one (patient 3) did not have target lesions and was assessed as non‐complete response/non‐progressive disease (i.e., stable disease).
Volumetric MRI analysis of total BMs
Volumetric tumor analysis of contrast‐enhanced MRI scans in patient 1 confirmed BMs shrinkage (core and shell regions) and brain tissue recovery (Figure 5a; Figure S4; Video S1) after 35 days' oral osimertinib 80 mg q.d. treatment versus baseline. Across patients, there was 56%–95% reduction in total BM volume after 25–35 days' oral osimertinib 80 mg q.d. treatment versus baseline (Figure 5b).
FIGURE 5.
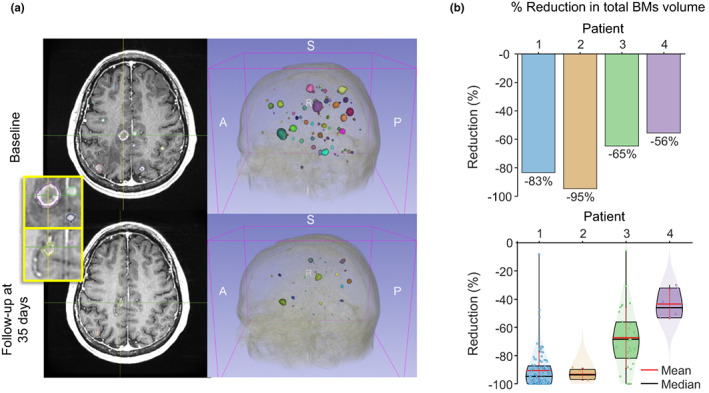
(a) Contrast‐enhanced MRI in patient 1 with 3D BM visualization at baseline and after 35 days' osimertinib 80 mg q.d. treatment (BM colors have been assigned arbitrarily to aid visualization). Inset presents magnifications of BM regions with delineation of total tumor area (contrast enhanced shell and core area). (b) Volumetric reduction in BMs (in %) versus baseline in all patients: reductions in total BM volume are shown in the upper panel and reductions in separate BMs are shown in the lower panel. BMs, brain metastases; MRI, magnetic resonance imaging; q.d., once‐daily.
Peritumoral edema regions
T2 FLAIR brain images and contrast‐enhanced MRI have been provided for each patient. Axial slices were selected to highlight the largest tumors and peritumoral edema regions at baseline. The corresponding slices after 25–35 days' oral osimertinib 80 mg q.d. treatment are provided for comparison (Figure S5).
Other results
For all patients, neurological symptoms improved or remained stable clinically and corticosteroid usage could be decreased or stopped (Table S4). Thoracic/abdominal computed tomography was conducted to provide a broader description of radiological response; the results are in the supplementary information. All adverse events (AEs) were of Common Terminology Criteria for Adverse Events (CTCAE) grade 1 or 2; there were no AEs of CTCAE grade 3 or higher. Pharmacokinetics, safety, and tolerability data are also in the supplementary information.
DISCUSSION
The ODIN‐BM study showed that [11C]osimertinib crossed the BBB in all four patients with EGFRm NSCLC and BMs. At baseline, a mean 1.5% injected dose (ID; n = 4) was present in whole brain at T max. This brain exposure is similar to the mean 2.2% ID (n = 8) reported previously in healthy volunteers 19 and 1.3% ID (n = 3) in NHPs, 18 and comparable with that of established CNS drugs (range, 1%–6% ID). 20
Once in the brain, drug access to BM regions is critical for clinical effectiveness. Several conditions have been suggested to influence drug distribution to a brain tumor. These include a compromised BBB, tumor growth properties (compressive, edema‐inducing vs. infiltrating type), and presence of a BTB with additional efflux transporters. 22 , 23 Importantly, our study demonstrated that presence of [11C]osimertinib exposure in BM shell and core regions was similar to that in the surrounding brain tissue, suggesting that [11C]osimertinib not only passes the intact BBB, as previously demonstrated in healthy volunteers, 19 but also the BTB. To our knowledge, this has not been reported with any other anticancer treatment.
Regarding kinetics, [11C]osimertinib distributed rapidly and uniformly throughout the brain. Two PET studies in patients with radiolabeled early generation EGFR/human epidermal growth factor receptor (HER)‐TKIs have been reported previously. In patients with HER2‐positive breast cancer and BMs, the distribution of [11C]lapatinib was limited to BMs and was related to the local BBB impairment, with no detectable radioactivity signal in intact brain regions. 16 In patients with advanced solid tumors and no BMs, [11C]erlotinib showed that whole brain V T was low regardless of ABCB1/ABCG2 efflux transporter inhibition by elacridar. 17 As such and compared with these earlier‐generation EGFR‐TKIs, high and spatially unrestricted brain exposure in humans may distinguish osimertinib from other EGFR‐TKIs. 14 , 18
With respect to changes in the brain PET signal, V T of [11C]osimertinib increased in whole brain and BMs in three of four patients when steady‐state for oral osimertinib was reached. Several factors may have contributed to this observation. For instance, osimertinib is a weak substrate for the efflux P‐glycoprotein and the BCRP transporter. 14 Thus, it cannot be excluded that the clinical osimertinib dose at least partially saturates some efflux transporters at the BBB, leading to slightly elevated V T. Another consideration is that an increased V T may be related to concomitant oral osimertinib clinical treatment leading to diminished oedema and necrotic tissue clearance following tumor regression, thereby restoring blood circulation in the region of interest. This interpretation is supported by compartmental analysis. The kinetic rate constant (K 1) describing [11C]osimertinib passage across BBB was higher at PET2 and PET3 versus baseline.
PET microdosing demonstrated previously that some drug molecules have inadequate affinity or selectivity to provide sufficient signal for detecting specific binding. 24 However, the high affinity of [11C]osimertinib for EGFR Ex19Del and L858R mutations (half‐maximal inhibitory concentration: 13–54 nM as confirmed in cell lines harboring these EGFR‐TKI sensitizing mutations, which is superior to its affinity for wild‐type EGFR) 5 raised the hypothesis that part of the imaging signal could represent specific binding to mutated EGFR. Specific binding of [11C]osimertinib could not be confirmed in whole brain or BMs as the signal was not inhibited after oral administration of single or repeated osimertinib 80 mg q.d. doses. It is possible that osimertinib affinity was not sufficiently high, or that the mutated‐EGFR density was too low, for PET quantification of specific binding over the background (i.e., non‐displaceable) signal.
In a previous PET study in which brain exposure of i.v. administered osimertinib metabolite [11C]AZ5104 was examined in NHPs, brain radioactivity concentrations plateaued at ~10% of that measured separately for the parent [11C]osimertinib molecule. 18 In the present study, plasma concentration of unlabeled AZ5104 at steady‐state was ~13% that of unlabeled osimertinib. Overall, these data suggest that contribution of radiolabeled AZ5104 to total radioactivity concentration in the brain was likely negligible.
Following 25–35 days' treatment with orally administered osimertinib (unlabeled) 80 mg q.d., we explored early radiological response in total detectable BMs using a volumetric MRI analysis approach. Clear volumetric decreases (56%–95%) in total BMs volume were observed in all patients; these findings are in line with the early case report in a patient with EGFRm NSCLC with a large, symptomatic BM, in whom osimertinib treatment over 15 days resulted in a 94% decrease in BM volume. 25 Notably, volumetric MRI analysis included all detectable BMs, below 10 mm. Routine radiological evaluation of BMs using CNS RECIST version 1.1. criteria focus on larger target lesions (greater than 10 mm), therefore, patients with smaller disseminated BMs, typical of NSCLC, can pose challenges in treatment effect analyses. The volumetric MRI analysis enabled delineation of BMs at the size of a few voxels and suggests potential of this approach as more sensitive for evaluation of treatment effect.
All patients improved or remained stable clinically regarding neurological symptoms, and corticosteroid usage could be decreased or stopped. Together with the imaging data, these observations were consistent with RANO‐BM‐defined partial responses in two patients and stable disease in two patients. Our data align with CNS efficacy results in AURA 9 , 11 and FLAURA trials. 7 , 8 In these large studies, however, patients with stable and asymptomatic BMs were eligible for enrollment, irrespective of whether the patient received prior brain radiotherapy. This is different to our study, in which all patients had no prior CNS‐directed treatments, such as surgery, stereotactic radiosurgery, or whole brain radiation therapy, which otherwise could have affected BM volumes. Collectively, our data and these previous reports support the hypothesis that BMs respond early to osimertinib treatment.
Throughout the study, no safety or tolerability concerns were apparent, nor were any unexpected safety signals reported compared with the known profile of osimertinib. 6
Limitations
The studied population was small but representative of patients with EGFRm NSCLC and BMs in terms of untreated BM characteristics (i.e., included both patients with large BMs and smaller, disseminated, non‐infiltrating BMs). Notably, PET results were consistent across patients.
CONCLUSIONS
This study demonstrated that [11C]osimertinib crosses the BBB and BTB in patients with EGFRm NSCLC and BMs. At baseline, ~1.5% (range, 1.4%–1.6%) of i.v. bolus injected radioactivity reached the brain after a median of 22 min, with homogeneous exposure across whole brain and BMs; this is comparable with that of well‐established CNS drugs. This study also illustrates the potential of microdosing in the development of drugs targeting brain malignancies.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript; all authors designed the research; and all authors performed the research. S.E., Z.C., H.M., K.V., and L.F. analyzed the data. S.E., A.V., H.M., M.S., P.J., and R.L. contributed new reagents/analytical tools.
FUNDING INFORMATION
This study was supported by AstraZeneca.
CONFLICT OF INTEREST STATEMENT
The authors have completed the ICMJE uniform disclosure form. Z.C., A.J., M.S., P.J., and K.V. are AstraZeneca employees. Z.C., M.S., P.J., J.vdA., and K.V. own AstraZeneca stocks. J.vdA. also declares membership of AstraZeneca advisory councils or committees. G.L., L.F., J.vdA., and A.P.B. are past AstraZeneca employees and own AstraZeneca stocks. A.P.B. also declares membership of Radiomics advisory councils or committees and receipt of consulting fees from GlaxoSmithKline, Johnson and Johnson, Adaptimmune, Brainomix, and Targovax. G.L. and J.vdA. were employed by AstraZeneca at the time of study conduct, G.L is a now an employee of Merus N.V. and J.vdA. of GSK. All other authors declared no competing interests for this work.
ETHICAL APPROVAL
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with current amendments of the Declaration of Helsinki and the International Conference on Harmonization/Good Clinical Practice guidelines. The study was approved by the Medical Products Agency in Sweden, the Ethical Committee of the Stockholm region, and the Radiation Safety Committee of the Karolinska University Hospital, Stockholm, Sweden (ODIN‐BM; NCT03463525). Written informed consent was obtained from all study participants.
Supporting information
Video S1
Video S2
Appendix S1
ACKNOWLEDGMENTS
The authors send thanks to all patients and their families. The authors acknowledge Clare Venter in helping to finalize the study and the staff of the PET‐Centre at Karolinska Institutet for technical support. The authors acknowledge Leon Newman, PhD, of Ashfield Health, Macclesfield, UK, for medical writing support, funded by AstraZeneca, Cambridge, UK.
Ekman S, Cselényi Z, Varrone A, et al. Brain exposure of osimertinib in patients with epidermal growth factor receptor mutation non‐small cell lung cancer and brain metastases: A positron emission tomography and magnetic resonance imaging study. Clin Transl Sci. 2023;16:955‐965. doi: 10.1111/cts.13500
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
REFERENCES
- 1. Peters S, Bexelius C, Munk V, Leighl N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non‐small‐cell lung cancer. Cancer Treat Rev. 2016;45:139‐162. [DOI] [PubMed] [Google Scholar]
- 2. Ge M, Zhuang Y, Zhou X, Huang R, Liang X, Zhan Q. High probability and frequency of EGFR mutations in non‐small cell lung cancer with brain metastases. J Neurooncol. 2017;135:413‐418. [DOI] [PubMed] [Google Scholar]
- 3. Chooback N, Lefresne S, Lau SC, Ho C. CNS metastases in epidermal growth factor receptor mutation–positive non–small‐cell lung cancer: impact on health resource utilization. J Onc Pract. 2018;14:e612‐e620. [DOI] [PubMed] [Google Scholar]
- 4. Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR‐mutated or ALK‐rearranged non‐small‐cell lung cancers. Lung Cancer. 2015;88:108‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M‐mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378:113‐125. [DOI] [PubMed] [Google Scholar]
- 7. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR‐mutated advanced non–small‐cell lung cancer. J Clin Oncol. 2018;36:3290‐3297. [DOI] [PubMed] [Google Scholar]
- 8. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382:41‐50. [DOI] [PubMed] [Google Scholar]
- 9. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M‐positive advanced non‐small‐cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36:2702‐2709. [DOI] [PubMed] [Google Scholar]
- 10. Reichegger H, Jochum W, Forbs D, Hader C, Fruh M. Rapid intracranial response to osimertinib in a patient with epidermal growth factor receptor T790M‐positive adenocarcinoma of the lung. Oncol Res Treat. 2016;39:461‐463. [DOI] [PubMed] [Google Scholar]
- 11. Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M‐positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol. 2018;29:687‐693. [DOI] [PubMed] [Google Scholar]
- 12. Wu Y‐L, Tsuboi M, He J, et al. Osimertinib in resected EGFR‐mutated non–small‐cell lung cancer. N Engl J Med. 2020;383:1711‐1723. [DOI] [PubMed] [Google Scholar]
- 13. Lee C‐M, Farde L. Using positron emission tomography to facilitate CNS drug development. Trends Pharmacol Sci. 2006;27:310‐316. [DOI] [PubMed] [Google Scholar]
- 14. Colclough N, Chen K, Johnström P, et al. Preclinical comparison of the blood‐brain barrier permeability of osimertinib with other EGFR TKIs. Clin Cancer Res. 2021;27:189‐201. [DOI] [PubMed] [Google Scholar]
- 15. Jucaite A, Stenkrona P, Cselényi Z, et al. Brain exposure of the ATM inhibitor AZD1390 in humans—a positron emission tomography study. Neuro Oncol. 2020;23:687‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saleem A, Searle GE, Kenny LM, et al. Lapatinib access into normal brain and brain metastases in patients with her‐2 overexpressing breast cancer. EJNMMI Res. 2015;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verheijen RB, Yaqub M, Sawicki E, et al. Molecular imaging of ABCB1 and ABCG2 inhibition at the human blood‐brain barrier using elacridar and (11)C‐erlotinib PET. J Nucl Med. 2018;59:973‐979. [DOI] [PubMed] [Google Scholar]
- 18. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR‐TKIs in EGFR‐mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130‐5140. [DOI] [PubMed] [Google Scholar]
- 19. Varrone A, Varnäs K, Jucaite A, et al. A PET study in healthy subjects of brain exposure of (11)C‐labelled osimertinib ‐ a drug intended for treatment of brain metastases in non‐small cell lung cancer. J Cereb Blood Flow Metab. 2020;40:799‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schou M, Varnas K, Lundquist S, et al. Large variation in brain exposure of reference CNS drugs: a pet study in nonhuman primates. Int J Neuropsychopharmacol. 2015;18:pyv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Planchard D, Brown KH, Kim DW, et al. Osimertinib Western and Asian clinical pharmacokinetics in patients and healthy volunteers: implications for formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother Pharmacol. 2016;77:767‐776. [DOI] [PubMed] [Google Scholar]
- 22. Levin VA, Ellingson BM. Understanding brain penetrance of anticancer drugs. Neuro Oncol. 2018;20:589‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arvanitis CD, Ferraro GB, Jain RK. The blood‐brain barrier and blood‐tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farde L, von Bahr C. Distribution of remoxipride to the human brain and central D2‐dopamine receptor binding examined in vivo by PET. Acta Psychiatr Scand Suppl. 1990;358:67‐71. [DOI] [PubMed] [Google Scholar]
- 25. Ozair MZ, Larsen AMG, Eng J, Moss NS. Exceptional response of a large and symptomatic EGFR‐mutant brain metastasis to osimertinib: case report and review of the literature. JCO Precis Oncologia. 2021;5:585‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Video S2
Appendix S1
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.


