Abstract
Kratom is a widely used Asian botanical that has gained popularity in the United States due to a perception that it can treat pain, anxiety, and opioid withdrawal symptoms. The American Kratom Association estimates 10–16 million people use kratom. Kratom‐associated adverse drug reactions (ADRs) continue to be reported and raise concerns about the safety profile of kratom. However, studies are lacking that describe the overall pattern of kratom‐associated adverse events and quantify the association between kratom and adverse events. ADRs reported to the US Food and Drug Administration Adverse Event Reporting System from January 2004 through September 2021 were used to address these knowledge gaps. Descriptive analysis was conducted to analyze kratom‐related adverse reactions. Conservative pharmacovigilance signals based on observed‐to‐expected ratios with shrinkage were estimated by comparing kratom to all other natural products and drugs. Based on 489 deduplicated kratom‐related ADR reports, users were young (mean age 35.5 years), and more often male (67.5%) than female patients (23.5%). Cases were predominantly reported since 2018 (94.2%). Fifty‐two disproportionate reporting signals in 17 system‐organ‐class categories were generated. The observed/reported number of kratom‐related accidental death reports was 63‐fold greater than expected. There were eight strong signals related to addiction or drug withdrawal. An excess proportion of ADR reports were about kratom‐related drug complaints, toxicity to various agents, and seizures. Although further research is needed to assess the safety of kratom, clinicians and consumers should be aware that real‐world evidence points to potential safety threats.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Kratom, an Asian botanical that has gained popularity in the United States due to a perception that it can treat pain, anxiety, and opioid withdrawal symptoms, was reported to induce adverse reactions and event death in some case reports and case series. However, the overall pattern of kratom‐related adverse reactions in the United States is unknown, and the association between kratom and adverse reactions is not quantified yet.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study used US Food and Drug Administration Adverse Event Reporting System (FAERS) data to address this knowledge gap by summarizing the characteristics of kratom‐related adverse reactions and detecting disproportionate reporting signals of kratom.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study found that in the United States, patients who reported with kratom‐related adverse reactions were relatively young (35.5 ± 11.5 years). Kratom‐related adverse reactions reported in male patients were about three times those in female patients. Most kratom‐related cases (94.2%) were reported since 2018 and death was reported to be the outcome of 49.9% of the cases. The observed number of kratom‐related accidental death was estimated to be 63.1‐fold greater than expected. Eight signals indicate there were excess number of reports about addiction or drug withdrawal. The system‐organ‐class “psychiatric disorders” accounted for the largest proportion of the 10 most reported adverse reactions (4/10), of the 10 strongest signals (6/10), and of all detected signals (17/52). Future studies should be conducted to provide more valid evidence to assess kratom‐associated death and psychiatric disorders.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Although further research is needed to assess the safety of kratom, clinicians and consumers should be aware that real‐world evidence points to potential safety threats.
INTRODUCTION
Kratom is a botanical product derived from the tropical tree Mitragyna speciosa that is native to Southeast Asia, particularly Thailand, Malaysia, Indonesia, Myanmar, and Papua New Guinea. It is available as several forms, including dried or crushed leaves, powder, capsules, tablets, liquids, and gum or resin. Kratom also is added to cocktails, caffeinated beverages, cough syrup, and cannabinoid preparations. A member of the coffee family, kratom contains several alkaloids that have shown multiple physiological, psychoactive, and behavioral effects. 1
The two most extensively studied kratom alkaloids are the abundant alkaloid mitragynine (typically more than 60% of the alkaloid content in kratom) and the minor alkaloid 7‐hydroxymitragynine, which is also formed in vivo from mitragynine. 2 , 3 Mitragynine has been shown to elicit mixed activity at various receptors, including several opioid receptors, as well as adrenergic, serotonergic, and adenosine receptors. Its action as an α2‐adrenergic agonist may mimic adjunctive therapies for opioid withdrawal. 4 , 5 The 7‐hydroxymitragynine, typically accounting for no more than 2% of total alkaloid content, was reported to have similar effect but be more potent than mitragynine, leading to the uncertainty that whether mitragynine or 7‐hydroxymitragynine is the main functioning phytoconstituent. 2 , 3 , 6 , 7 Other kratom alkaloids, including paynantheine, speciogynine, and speciociliatine, were also reported to have effects at opioid receptors, but it is uncertain the roles they have due to the limited concentration they have been found in kratom. 1 Dozens of other alkaloids in kratom are even more negligible and thus were not well studied. 6 Kratom has a rapid onset (5–10 min) and relatively long duration (2–5 h) of effect. 2 Although there is no standardized dosage, low to moderate doses (1–5 g) typically produce stimulant effects, such as increased sociability, alertness, and energy, whereas higher doses (5–15 g) have been associated with opioid‐like effects.
Kratom has become readily available in the United States, where the American Kratom Association estimates 10–16 million people use kratom regularly as an opioid alternative and for recreational purposes. 8 , 9 , 10 It is frequently publicized as a psychoactive alternative to other stimulant‐type and sedative drugs, the latter including opioids. However, there are no US Food and Drug Administration (FDA)‐approved medical uses for kratom, 11 and only two clinical trials involving kratom have been registered in clinicaltrials.gov to date. 3 The US Drug Enforcement Administration (DEA) listed kratom among its “Drugs of Concern” because of its sedative effects and addiction potential, leading to efforts to ban the sale of kratom in some US cities and states (11). In 2016, the DEA published a notice of intent to temporarily place mitragynine and 7‐hydroxymitragynine into Schedule I of the Controlled Substances Act. 12 There was substantial push back from the American Kratom Association, who highlighted potential therapeutic uses of kratom and opposed the DEA's intent. 13 The DEA subsequently withdrew its intent and then sought input from the FDA, the National Institute on Drug Abuse (NIDA), and the public to develop an assessment of abuse potential and provide regulatory recommendations. 14 At the time of preparation of this report, kratom is banned or criminalized in six US states: Alabama, Arkansas, Indiana, Rhode Island, Vermont, and Wisconsin. 6
Kratom‐associated adverse events continue to be reported, raising concerns about the safety profile of kratom. Poison center reports involving kratom exposures identified possible adverse reactions, including altered mental status, tachycardia, agitation or irritability, central nervous system (CNS) depression, drowsiness, nausea, seizure, and hypertension. 15 , 16 A recent review identified 33 published case reports of liver toxicity. 16 The Centers for Disease Control and Prevention (CDC) reported that, of 27,338 overdose deaths occurring from July 2016 to December 2017, 152 (0.56%) of these cases tested positive for mitragynine on postmortem toxicology of residual blood samples. Kratom was evaluated to be the cause of death of 91 of the 152 (59.9%) kratom‐positive death cases by coroners or medical examiners, including seven for which mitragynine was the only substance detected. 17
These descriptive reports suggest potential repercussions from using kratom. But they are insufficient to provide a comprehensive picture of the adverse reaction and evaluate severity. There is a need for further research to better characterize the safety profile of kratom. Using adverse drug reaction (ADR) reports obtained from the FAERS, we conducted this study to describe patterns of kratom‐associated ADRs and assessed whether there is an excessive number of kratom‐related adverse reaction reports compared to other drugs and natural products through disproportionality analysis.
METHODS
Data and preprocessing
The FAERS reports consist of de‐identified demographic information about the person experiencing ADRs, information about drugs and other xenobiotics taken by the person at the time of the reaction, reported ADRs coded using preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA) terminology, clinical outcomes for the event (e.g., death, life‐threatening injury, and hospitalization), and information about the submitter of the report. 18 The open‐source database contains de‐identified data, thus ethics approval was not required for this study.
The FAERS data from January 2004 through September 2021 were loaded into a relational database using a previously published workflow, which includes the mapping of drug and natural product strings present in reports to the RxNorm terminology. 19 To obtain a more complete capture of reports mentioning natural product, we compiled a comprehensive list of variations on natural product names as reported in FAERS by querying all reports that contained a list of the 73 natural products, including 40 top‐marketed natural products 20 and 33 others of interest to the project funder (National Center for Complementary and Integrative Health). The queries for natural products included plant Latin binomials and common names. We observed that the mapping process missed some spelling variations (including misspellings) for natural products and drugs, so we allowed character variation using the Levenshtein distance measure. 21 The Levenshtein distance computes the difference between two words and is calculated as the minimum number of character changes required to change one word into another. The candidate natural product mentions were manually reviewed to arrive at a reference set mapping the 73 natural products to 3460 variations (see Table S1). 22
Duplicated ADR reports were identified with the following algorithm: (1) cases with the same identifier but different versions; (2) cases referring to the same literature reference (many FAERS reports provide a literature citation), reported drugs, reactions, age, and sex; and (3) FAERS cases identified as duplicates using the likelihood‐based World Health Organization vigiMatch algorithm. 23 An automated deduplication process retained a single report in each cluster of duplicates.
Descriptive analysis
All cases with kratom as suspected or concomitant drugs were included in the descriptive analysis. Cases reported from October 2012 to September 2021 were included. This date range was chosen because the FDA used an older system before October 2012, thus cases reported earlier to this date were less complete and did not contain a literature reference field. In addition, there was a change in the FAERS data beginning in Q4 2021, when nearly all reports involving kratom herbal products or any products containing its constituents were referred to as “HERBALS\MITRAGYNINE.” Because this change made it impossible to distinguish those involving kratom herbal products from the reports involving a selected phytoconstituent present in kratom (e.g., mitragynine or 7‐hydroxymitragynine), we focused only on data preceding this change.
The output of the automated deduplication process was visually inspected to identify any remaining potential duplicate reports. Regarding reports from the same literature, the original full‐text articles were checked. Other cases believed to be duplicates satisfied three criteria: (1) same age and sex; (2) highly similar lists of drugs and adverse events; and (3) same event date or same body weight. The more informative report in each cluster of duplicates was retained in the analysis.
Descriptive statistics were used to analyze patient demographics and clinical characteristics, occupation, and county of reporters, as well as the reporting years. For reports listing multiple adverse reactions and outcomes, all reactions and outcomes were included in the analysis. For reports using the MedDRA term “drug interaction” as the adverse reaction, possible mechanism for interactions between kratom and drugs were proposed if there was a common metabolic pathway or effect.
Disproportionality analysis
The disproportionality analysis compared kratom to all other drugs and natural products using cases reported from January 2004 through September 2021. Reports were deduplicated only via the automated deduplication process, as it was not feasible to visually inspect all FAERS reports. There is no gold standard for signal detection using spontaneous reports and each method has advantages, disadvantages, and limitations. 24 , 25 Two different disproportionality signal detection methods were used in sequence. First, reporting odds ratios were used to identify preliminary signals because the method tends to have a relatively high sensitivity. Reporting odds ratio (ROR) produces a greater number of false positive results compared to other disproportionality measures and this problem is more significant when there are small case counts. 26 Norén et al. have proposed a method that is more conservative (and produces fewer false positive signals) by regularizing for unstable variance, especially when there are small case counts. 27 Therefore, the data for preliminary signals were then subjected to further analysis, using the more conservative observed‐to‐expected (OE) ratio with shrinkage to define the final signal. The details of each method are described below.
The ROR measures of reporting disproportionality and 95% confidence intervals (CIs) were calculated to detect preliminary pharmacovigilance signals. 28 The ROR for the pair (kratom, adverse reaction R) was calculated as (A/C)/(B/D) where:
A = case count for the pair: kratom, adverse reaction R.
B = sum (case count) for all pairs: kratom, all events except adverse reaction R.
C = sum (case count) for all pairs: all other drug and natural products in FAERS except kratom, adverse reaction R.
D = sum (case count) for all pairs: all drugs and natural products in FAERS except kratom, all events except adverse reaction R.
If C was zero for any pair, then the resulting ROR value was set to null. CIs were obtained using the following two formulas:
An ROR no less than 1.0 for a given pair would indicate that kratom has a disproportionally greater odds of being reported for the ADR relative to other natural products. The higher the ROR, the stronger the disproportionality in reporting. 29
For this analysis, we defined a preliminary signal if the lower 95% CI of the ROR for a given pair was greater than 1.0 and there were at least five spontaneous reports involving the pair. Pairs with significant preliminary signals were further evaluated with the logarithm (log) OE ratio with shrinkage, which was calculated as , to define final signal. O is the observed count for the given pair, which equals to A in the ROR estimation. E means the expected count for the given pair, which is calculated as (A + B) × (A + C)/(A + B + C + D). Credibility interval (CrI) were estimated by the following two formulas:
The log OE ratio with shrinkage and its 95% CrI were estimated with the R pharmsignal package, using the default alpha as 0.05 (https://github.com/tystan/pharmsignal). A signal was defined if the lower 95% CI of log OE ratio exceeded zero. A log OE ratio of 5 indicates the OE ratio is 25, suggesting the observed number of reports mentioning the pair of interest is 25 = 32 times greater than the expected number in this conservative estimation.
For strong signals with a lower limit of 95% CrI greater than 1.0 (OE ratio significantly >2.0), a treemap was plotted to show the relative number and strength of signals within the system‐organ‐class (SOC) categories provided by the MedDRA terminology.
RESULTS
Descriptive review of kratom spontaneous reports
A total of 570 reports with unique case identifiers were obtained from FAERS from October 2012 through September 2021. After 33 duplicates were removed by the machine‐based algorithm and another 50 duplicates removed by visual inspection, 489 reports were included in the descriptive analysis.
A total of 330 reports (67.5%) involved male patients, whereas 115 (23.5%) involved female patients, and 44 (9.0%) reported unknown biological sex. The average age was 35.5 ± 11.5 years among 415 patients with valid age information. There were two reports with the elderly (no less than 70 years old) and six cases with the minor (less than 18 years old). The majority of the cases (474 or 96.9%) originated in the United States, with 10 cases (2.0%) that were reported in Canada. Most cases (195 or 39.9%) were reported by consumers, with 128 (26.2%) reported by healthcare professionals, and the remaining 166 (33.9%) reported by people with missing occupation information. The number of reports by year are shown in Table S2. Most kratom‐associated cases were reported since 2018 (460 or 94.2%). Of the 489 reports, 220 (45.0%) listed kratom as the only drug, 356 (72.8%) listed kratom as the primary suspected cause of the indicated adverse reaction, and 32 (6.5%) identified kratom as a concomitant drug rather than the cause of the adverse reaction. In 29 (5.9%) cases, patients used 10 or more drugs.
Adverse drug reactions
Among the 489 reports, there were 1649 adverse reactions, with 494 distinct reactions (i.e., distinct preferred terms). Table 1 shows the 10 most commonly mentioned adverse reactions in kratom‐related reports, with the full list provided in Table S3. The most commonly reported reaction was death, accounting for 102 (20.9%) cases. Toxicity to various agents ranked second (78 or 16.0%), followed by drug dependence (53 or 10.8%). Five of the 10 top‐reported adverse reactions belonged in the SOC of psychiatric disorders (drug dependence, anxiety, withdrawal syndrome, and drug abuse) or nerve system disorders (seizure), and two belong in the SOC of gastrointestinal disorders (vomiting and nausea).
TABLE 1.
Ten most mentioned reactions in 489 kratom‐related adverse reaction reports.
| MedDRA preferred term for the adverse reaction | Frequency of mention n (%) |
|---|---|
| Death | 102 (20.9) |
| Toxicity to various agents | 78 (16.0) |
| Drug dependence | 53 (10.8) |
| Accidental death | 34 (7.0) |
| Vomiting | 32 (6.5) |
| Anxiety | 27 (5.5) |
| Withdrawal syndrome | 26 (5.3) |
| Drug abuse | 26 (5.3) |
| Seizure | 26 (5.3) |
| Nausea | 22 (4.5) |
Death also ranked first among the top reported adverse reactions in cases with kratom as the primary suspect drug (Table S4). Diarrhea rather than drug abuse entered the top 10 reactions in these cases.
Outcomes
Table 2 shows the frequency of outcomes in kratom‐associated reports. The most reported outcome of the 489 cases was death (244 or 49.9%), followed by other serious medical event (137 or 28.0%) and hospitalization (99 or 20.2%; Table 3). A small subset (10.4%) of the reports had missing outcome values. Among 356 reports with kratom as the primary suspect drug, the proportion of death, other serious medical event, and hospitalization were 52.0%, 19.9%, and 19.1%, respectively (Table S5).
TABLE 2.
Outcomes of 489 kratom‐related reports.
| Outcome | Frequency of mention n (%) |
|---|---|
| Death | 244 (49.9) |
| Other serious event | 137 (28.0) |
| Hospitalization | 99 (20.2) |
| Disability | 48 (9.8) |
| Life threatening | 44 (9.0) |
| Required intervention to prevent permanent impairment/damage | 11 (2.2) |
| Congenital anomaly | 0 (0.0) |
| Unknown/missing | 51 (10.4) |
TABLE 3.
Ten disproportionate reporting signals with the highest shrinkage observed‐to‐expected ratios.
| MedDRA preferred term for the adverse reaction | Kratom‐related report counts | Log OE ratios (95% CrI) | OE ratios (95% CrI) | ROR (95% CI) |
|---|---|---|---|---|
| Accidental death | 35 | 5.98 (5.41, 6.38) | 63.1 (42.5, 83.3) | 563.5 (402.7, 788.3) |
| Drug screen positive | 15 | 4.43 (3.56, 5.03) | 21.6 (11.8, 32.7) | 68.8 (41.4, 114.3) |
| Product complaint | 12 | 4.37 (3.39, 5.03) | 20.7 (10.5, 32.7) | 113.6 (64.4, 200.5) |
| Withdrawal syndrome | 26 | 4.2 (3.54, 4.66) | 18.4 (11.6, 25.3) | 28.0 (19.0, 41.2) |
| Toxicity to various agents | 87 | 4.02 (3.66, 4.27) | 16.2 (12.6, 19.3) | 18.6 (15.0, 23.1) |
| Drug dependence | 53 | 3.86 (3.41, 4.19) | 14.5 (10.6, 18.3) | 17.2 (13.1, 22.6) |
| Drug abuse | 33 | 3.78 (3.2, 4.19) | 13.7 (9.2, 18.3) | 17.4 (12.3, 24.5) |
| Drug withdrawal syndrome | 23 | 3.38 (2.68, 3.87) | 10.4 (6.4, 14.6) | 13.3 (8.8, 20.0) |
| Dependence | 7 | 3.35 (2.04, 4.2) | 10.2 (4.1, 18.4) | 29.5 (14.1, 62.0) |
| Drug withdrawal syndrome neonatal | 9 | 3.32 (2.18, 4.08) | 10.0 (4.5, 16.9) | 19.9 (10.3, 38.3) |
Abbreviations: CI, confidence intervals; CrI, credibility intervals; Log, logarithm; OE, observed‐to‐expected; ROR, reporting odds ratio.
Drug interactions
Ten (2.3%) reports specifically identified the adverse reaction as “drug interaction.” Most of these reports were reported by healthcare professionals (7 or 70%). In four (40%) cases, kratom was explicitly suspected to be the interacting drug. Two reports mentioning drug interactions reported death as an outcome. A total of 30 medications/natural products suggesting an interaction were reported. Table S6 shows probable mechanisms of these interactions based on the reported effect of kratom and metabolic pathway of these drugs.
Pharmacovigilance signals
A total of 59 preliminary signals were identified in the FAERS database using the lower bounds of the 95% CI of ROR results. Fifty‐two (88.1%) of these adverse reactions involving 18 SOC categories remained significant in the final results of log OE ratio with shrinkage, with 34 having a lower 95% CrI greater than or equal to 1.0, suggestive of a strong signal (Table S7). Adverse reactions with the greatest log OE ratios are shown in Table 3. The highest log OE ratio was 5.98 (95% CrI: 5.41–6.38) of accidental death, suggesting the observed count of FAERS reports mentioning both accidental death and kratom was ~63.1 times greater than the expected count for this pair in the conservative estimation. Drug screen positive (log OE ratio 4.43, 95% CrI: 3.56–5.03) ranked second, suggesting the reporting of this event was 21.6 times greater than expected. Product complaint ranked the third (log OE ratio 4.37, 95% CrI: 3.39–5.03), indicating an OE ratio of 20.7.
Based on log OE ratio results, there were eight strong signals about addiction or withdrawal: withdrawal syndrome (4.20, 95% CrI: 3.54–4.66), drug dependence (3.86, 95% CrI: 3.41–4.19), drug abuse (3.78, 95% CrI: 3.20–4.19), drug withdrawal syndrome (3.38, 95% CrI: 2.68–3.87), dependence (3.35, 95% CrI: 2.04–4.20), drug withdrawal syndrome neonatal (3.32, 95% CrI: 2.18–4.08), drug use disorder (3.19, 95% CrI: 1.62–4.17), and intentional overdose (2.32, 95% CrI: 1.11–3.12). These signals indicated that the observed number of kratom‐related reports about addiction or withdrawal was more than five times greater than the expected number. Six of them were among the 10 strongest signals. The SOC category with the most signals was psychiatric disorders (n = 17), followed by general disorders, and administration site conditions (n = 10), and injury and poisoning and procedural complications (n = 9). A treemap diagram (Figure 1) shows the relative number and strength of strong signals based on log OE ratios (lower 95% CrI >1.0) within the SOC categories provided by the MedDRA terminology.
FIGURE 1.
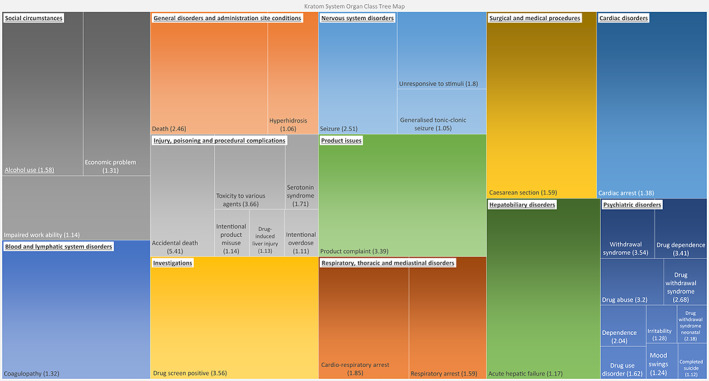
A treemap of strong signals of the disproportionate reporting adverse drug reactions mentioning kratom within the US Food and Drug Administration (FDA) Adverse Event Reporting System from January 2004 through September 2021. *The number in each parenthesis is the lower bounds of the 95% credibility interval of the logarithm of observed‐to‐expected (OE) ratio with shrinkage for the signal. The signals are organized by the system‐organ‐class (SOC) according to the MedDRA terminology. The area of each box represents the relative strength of the signal within the SOC represented by the color group that the box resides in (the relative contribution of each SOC to all signals is not shown).
Accidental death, drug screen positive, product complaint, withdrawal syndrome, toxicity to various agents, dependence, and neonatal drug withdrawal syndrome were among the top 10 disproportionately reported adverse reactions using both ROR and OE with shrinkage. Nine of the 10 most mentioned adverse reactions were associated with an excess number of reports based on log OE ratios significantly greater than zero, with nausea as the only exception.
DISCUSSION
Based on increasing concerns about the safety of kratom, we conducted a descriptive analysis of kratom‐related adverse reactions and disproportionality analyses to detect pharmacovigilance disproportional reporting signals. The analyses were conducted after capture of kratom spelling variations in the FAERS database and careful report deduplication. The average age of patients with kratom‐related adverse reactions was relatively young (35.5 ± 11.5 years). Kratom‐related adverse reactions reported in male patients were about three times those in female patients. Most kratom‐related cases (94.2%) were reported since 2018 and death was reported to be the outcome of 49.9% cases. The OE shrinkage ratio for kratom‐related accidental death was estimated to be 63, suggesting observed numbers of this pairs in FAERS are much greater than expected numbers. There were eight strong signals related to addiction or drug withdrawal. The SOC of psychiatric disorders accounted for the largest proportion of the 10 most reported adverse reactions (4/10), of the 10 strongest signals (6/10), and of all detected signals (17/52).
Young age of patients in this study is consistent with a previous study reporting a mean age of 32.3 years for in kratom‐associated death in eight western countries. 30 This mean age was much younger than the average age determined in analyses of reports related to other medications: 65.8 years in reports related to cardiovascular medications 31 and greater than 65 years in reports related to respiratory agents. 32 This younger population in kratom‐related cases is concordant with a cross‐sectional survey indicated kratom users were significantly younger than non‐users. 33 Sex distribution in this study is also aligned with the cross‐sectional study reporting that male patients account for a larger proportion in kratom users (61.0 vs. 48.6%) 33 and the case series showing male patients with kratom‐associated death (80.0%). 30
Although kratom is commonly used to mitigate symptoms of opioid withdrawal, 34 kratom alone may cause another addiction or withdrawal problem. The six strong signals of kratom‐related addiction or withdrawal in this study align with the retrospective review from Thailand reported that 23.1% of 52 kratom‐related adverse reactions were withdrawal symptoms, with specific complaints including myalgia, insomnia, fatigue, and chest discomfort. 35 Additional research is needed to determine the absolute risk and severity of kratom‐related addiction or withdrawal problems.
The current study identified significant signals of seizure (log OE ratio 3.15, 95% credibility interval: 2.50–3.60) and generalized tonic–clonic seizure (log OE ratio 2.6, 95% credibility interval: 1.05–3.59). There have been several reports of kratom‐associated seizures. 36 , 37 A 2018 case report suggested that kratom abuse might be able to induce structural brain lesions and recurrent seizures. 38 The mechanism of how these seizures occurred is not well‐understood, but adrenergic and stimulant effects are possible explanations.
In a previous study, authors found that kratom was only detected in less than 1% of all overdose deaths based on data from State Unintentional Drug Overdose Reporting System, but suspected the kratom‐positive deaths was underestimated because the detection of kratom is method‐dependent. 17 The significant signals of accidental death and death in our study contributes to this concern. Although the retrospective review from Thailand reported palpitations accounted for 22.5% of all adverse events except for withdrawal symptoms, 35 we found no disproportionate reporting signal of palpitation.
Research on the pharmacology of kratom and its constituent alkaloids extends beyond mitragynine and 7‐hydroxymitragynine. Kratom has shown dose‐dependent effects, acting as a stimulant at low doses and exerting opioid‐like effects at higher doses. 39 , 40 Constituent indole alkaloids may bind differently to opioid, serotonin, and adrenergic receptors. This variable receptor binding could possibly offer synergy and/or pharmacologically different effects that complicate the effects of kratom. 41
The presence of cases of polysubstance use concurrent with kratom raises concerns for adverse kratom‐drug interactions. For example, kratom extracts and mitragynine have been shown to inhibit multiple cytochrome P450 (CYP) enzymes in vitro, including CYP1A2, CYP2D6, CYP2C9, CYP2C19, and CYP3A. 2 , 42 Such inhibition could result in clinically significant pharmacokinetic interactions with drugs metabolized by these enzymes. Indeed, a recent case report reported that the antidepressant venlafaxine (dual CYP2D6/3A substrate) and anti‐psychotic quetiapine (CYP3A substrate) in combination with kratom may have led to the electrocardiogram abnormalities and serotonin syndrome experienced by the patient. 43 Mitragynine has also been shown to inhibit UDP‐glucuronosyltransferases (UGTs) in vitro, including UGT2B7 and UGT1A1. 44 , 45 As such, kratom may precipitate interactions with drugs that undergo glucuronidation, including lorazepam, which is extensively conjugated to the inactive 3‐O‐phenolic glucuronide in the liver. 46 The UGT inhibitor valproate has been shown to decrease the oral clearance of lorazepam and increase systemic concentrations of lorazepam. 47
The incidence of kratom use in the US population was estimated at 0.8% annually and the life‐time prevalence was reported to be 1.3% in recent years. 33 Such wide and unregulated use of a natural product with pharmacological effect but weak evidence with respect to efficacy and safety might lead to unforeseen consequences. The FDA has not placed the main constituent of kratom on Schedule I of the Controlled Substances Act. Similarly, the World Health Organization Expert Committee on Drug Dependence determined that there is not adequate evidence to recommend a critical review of kratom for its safety concern. 48 In spite of this, we think that spontaneous reporting evidence suggests a need for further studies that generate the robust evidence needed to weigh the benefits and risks of kratom use.
To the best of our knowledge, this study is the first to summarize the characteristics of kratom‐related adverse reactions in FAERS data while also providing a disproportionality analysis to quantify the association between kratom and adverse reactions. Because kratom is not FDA‐approved, and there is no routine surveillance of it, evidence generated in this study using spontaneous reporting data represents the most comprehensive profile of the real‐world safety of kratom. Signals detected in this study can serve as valuable hypotheses for future studies.
There are several limitations in this study, mostly inherent with FAERS data. First, given that FAERS is a spontaneous reporting system, it may be affected by patient's or reporters' perception of the drug/natural product. Second, FAERS reports do not necessarily involve rigorous evaluation. No causal relationship or precise temporal relationships can be proven between the outcome and the suspected drug using such data. Third, given the spontaneous nature of the reports analyzed in this study, it is possible that some duplicate reports were included in the disproportionality analysis. Although we took great care to remove duplicates from the descriptive analysis, some duplicate reports may have remained. Misclassification can bias the result as there were many variations on drug and natural product names. Finally, this analysis did not include data past September of 2021 because a change in how FAERS reports captured herbal reports led to a potential ambiguity between kratom herbal products (complex mixtures of chemical constituents) and products containing selected chemical constituents, such as mitragynine or 7‐OH‐mitragynine. We think that the latter should be the focus of a separate study.
This study comprehensively summarizes the characteristics of kratom‐related adverse reactions in FAERS data and detected disproportionate reporting signals of kratom‐related death, addiction/withdrawal problem, and some other syndromes of psychiatric disorders. Further research addressing this public health issue, including potential interactions between kratom and pharmaceutical drugs, will provide critical evidence for the rational use of this readily available botanical natural product.
AUTHOR CONTRIBUTIONS
X.L., P.N., S.L.K.G., and M.F.P. wrote the manuscript. R.D.B., S.L.K.G., P.N., S.B.T., M.C., K.A., S.E., and M.F.P. designed the research. X.L., P.N., R.D.B., S.B.T., K.A., and S.L.K.G. performed the research. X.L., R.D.B., P.N., S.L.K.G., and S.B.T. analyzed the data. R.D.B., X.L., and S.B.T. contributed new reagents/analytical tools.
FUNDING INFORMATION
This work was supported by the National Institutes of Health National Center for Complementary and Integrative Health and the Office of Dietary Supplements via the Center of Excellence for Natural Product Drug Interaction Research (U54 AT008909).
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Table S1
Li X, Ndungu P, Taneja SB, et al. An evaluation of adverse drug reactions and outcomes attributed to kratom in the US Food and Drug Administration Adverse Event Reporting System from January 2004 through September 2021. Clin Transl Sci. 2023;16:1002‐1011. doi: 10.1111/cts.13505
REFERENCES
- 1. Cinosi E, Martinotti G, Simonato P, et al. Following “the roots” of kratom (Mitragyna speciosa): the evolution of an enhancer from a traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in Western countries. Biomed Res Int. 2015;2015:1‐11. doi: 10.1155/2015/968786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Todd DA, Kellogg JJ, Wallace ED, et al. Chemical composition and biological effects of kratom (Mitragyna speciosa): In vitro studies with implications for efficacy and drug interactions. Sci Rep. 2020;10(1):19158. doi: 10.1038/s41598-020-76119-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanna RS, Nguyen JT, Hadi DL, et al. Clinical pharmacokinetic assessment of kratom (Mitragyna speciosa), a botanical product with opioid‐like effects, in healthy adult participants. Pharmaceutics. 2022;14(3):620. doi: 10.3390/pharmaceutics14030620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eastlack SC, Cornett EM, Kaye AD. Kratom‐pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9(1):55‐69. doi: 10.1007/s40122-020-00151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grundmann O. Patterns of kratom use and health impact in the US‐results from an online survey. Drug Alcohol Depend. 2017;176:63‐70. doi: 10.1016/j.drugalcdep.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 6. Swogger MT, Smith KE, Garcia‐Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855. doi: 10.3389/fphar.2022.801855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adkins JE, Boyer EW, McCurdy CR. Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. Curr Top Med Chem. 2011;11(9):1165‐1175. doi: 10.2174/156802611795371305 [DOI] [PubMed] [Google Scholar]
- 8. Demick DS, Lee TT, Summers AT, El‐Mallakh RS. Kratom: a growing substance of abuse in the United States. Ann Clin Psychiatry. 2020;32(4):275‐280. doi: 10.12788/acp.0012 [DOI] [PubMed] [Google Scholar]
- 9. Henningfield JE, Grundmann O, Babin JK, Fant RV, Wang DW, Cone EJ. Risk of death associated with kratom use compared to opioids. Prev Med. 2019;128:105851. doi: 10.1016/j.ypmed.2019.105851 [DOI] [PubMed] [Google Scholar]
- 10. Singh D, Yeou Chear NJ, Narayanan S, et al. Patterns and reasons for kratom (Mitragyna speciosa) use among current and former opioid poly‐drug users. J Ethnopharmacol. 2020;249:112462. doi: 10.1016/j.jep.2019.112462 [DOI] [PubMed] [Google Scholar]
- 11. Commissioner O of the. FDA and Kratom. FDA . 2022. Accessed January 18, 2023. https://www.fda.gov/news‐events/public‐health‐focus/fda‐and‐kratom
- 12. Drug Enforcement Administration, Drug Enforcement Administration, U.S. Department of Justice . DEA Announces Intent To Schedule Kratom. 2016. Accessed September 26, 2022. https://www.dea.gov/press‐releases/2016/08/30/dea‐announces‐intent‐schedule‐kratom
- 13. American Kratom Association . American Kratom Association Fact Sheet. 2016. Accessed January 18, 2023. https://d3n8a8pro7vhmx.cloudfront.net/americankratomassociation/pages/86/attachments/original/1475527128/AKA_Factsheet_10_03_2016.pdf?1475527128
- 14. Withdrawal of Notice of Intent to Temporarily Place Mitragynine and 7‐Hydroxymitragynine Into Schedule I. Federal Register . Accessed January 18, 2023. https://www.federalregister.gov/documents/2016/10/13/2016‐24659/withdrawal‐of‐notice‐of‐intent‐to‐temporarily‐place‐mitragynine‐and‐7‐hydroxymitragynine‐into
- 15. Anwar M, Law R, Schier J. Notes from the field: kratom (Mitragyna speciosa) exposures reported to poison centers – United States, 2010‐2015. MMWR Morb Mortal Wkly Rep. 2016;65(29):748‐749. doi: 10.15585/mmwr.mm6529a4 [DOI] [PubMed] [Google Scholar]
- 16. Ballotin VR, Bigarella LG, de Brandão AB M, Balbinot RA, Balbinot SS, Soldera J. Herb‐induced liver injury: systematic review and meta‐analysis. World J Clin Cases. 2021;9(20):5490‐5513. doi: 10.12998/wjcc.v9.i20.5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olsen EO, O'Donnell J, Mattson CL, Schier JG, Wilson N. Notes from the field: unintentional drug overdose deaths with kratom detected – 27 states, July 2016‐December 2017. MMWR Morb Mortal Wkly Rep. 2019;68(14):326‐327. doi: 10.15585/mmwr.mm6814a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use . MedDRA Terminology Home Page. Accessed September 26, 2022. https://www.meddra.org/
- 19. Banda JM, Evans L, Vanguri RS, Tatonetti NP, Ryan PB, Shah NH. A curated and standardized adverse drug event resource to accelerate drug safety research. Sci Data. 2016;3:160026. doi: 10.1038/sdata.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith T, Majid F, Eckl V, Reynolds CM. Herbal supplement sales in US increase by record‐breaking 17.3% in 2020. HerbalGram. 2021;131:52‐65. [Google Scholar]
- 21. Nam E. Understanding the Levenshtein distance equation for beginners. 2019. Accessed September 26, 2022. https://medium.com/@ethannam/understanding‐the‐levenshtein‐distance‐equation‐for‐beginners‐c4285a5604f0
- 22. Taneja SB, Paine MF, Kane‐Gill SL, Boyce RD. Extending the OMOP standard vocabulary to include botanical natural products. 2022.
- 23. Tregunno PM, Fink DB, Fernandez‐Fernandez C, Lázaro‐Bengoa E, Norén GN. Performance of probabilistic method to detect duplicate individual case safety reports. Drug Saf. 2014;37(4):249‐258. doi: 10.1007/s40264-014-0146-y [DOI] [PubMed] [Google Scholar]
- 24. Ibrahim H, Abdo A, El Kerdawy AM, Eldin AS. Signal detection in pharmacovigilance: a review of informatics‐driven approaches for the discovery of drug‐drug interaction signals in different data sources. Artificial Intelligence in the Life Sciences. 2021;1:100005. doi: 10.1016/j.ailsci.2021.100005 [DOI] [Google Scholar]
- 25. Kim HR, Sung M, Park JA, et al. Analyzing adverse drug reaction using statistical and machine learning methods: a systematic review. Medicine. 2022;101(25):e29387. doi: 10.1097/MD.0000000000029387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ang PS, Chen Z, Chan CL, Tai BC. Data mining spontaneous adverse drug event reports for safety signals in Singapore – a comparison of three different disproportionality measures. Expert Opin Drug Saf. 2016;15(5):583‐590. doi: 10.1517/14740338.2016.1167184 [DOI] [PubMed] [Google Scholar]
- 27. Norén GN, Hopstadius J, Bate A. Shrinkage observed‐to‐expected ratios for robust and transparent large‐scale pattern discovery. Stat Methods Med Res. 2013;22(1):57‐69. doi: 10.1177/0962280211403604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519‐523. doi: 10.1002/pds.1001 [DOI] [PubMed] [Google Scholar]
- 29. DailyMed – DEPO‐TESTOSTERONE‐testosterone cypionate injection, solution. 2020. Accessed September 26, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=735b5bf3‐4464‐412c‐a072‐1bd02f2e5380
- 30. Corkery JM, Streete P, Claridge H, et al. Characteristics of deaths associated with kratom use. J Psychopharmacol. 2019;33(9):1102‐1123. doi: 10.1177/0269881119862530 [DOI] [PubMed] [Google Scholar]
- 31. Patel NM, Stottlemyer BA, Gray MP, Boyce RD, Kane‐Gill SL. A pharmacovigilance study of adverse drug reactions reported for cardiovascular disease medications approved between 2012 and 2017 in the United States Food and Drug Administration adverse event reporting system (FAERS) database. Cardiovasc Drugs Ther. 2022;36(2):309‐322. doi: 10.1007/s10557-021-07157-3 [DOI] [PubMed] [Google Scholar]
- 32. Kim H, Pfeiffer CM, Gray MP, Stottlemyer BA, Boyce RD, Kane‐Gill SL. Assessing adverse drug reactions reported for new respiratory medications in the FDA adverse event reporting system database. Respir Care. 2021;66(11):1739‐1745. doi: 10.4187/respcare.08809 [DOI] [PubMed] [Google Scholar]
- 33. Schimmel J, Amioka E, Rockhill K, et al. Prevalence and description of kratom (Mitragyna speciosa) use in the United States: a cross‐sectional study. Addiction. 2021;116(1):176‐181. doi: 10.1111/add.15082 [DOI] [PubMed] [Google Scholar]
- 34. Palamar JJ. Past‐year kratom use in the U.S.: estimates from a nationally representative sample. Am J Prev Med. 2021;61(2):240‐245. doi: 10.1016/j.amepre.2021.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trakulsrichai S, Tongpo A, Sriapha C, et al. Kratom abuse in Ramathibodi poison center, Thailand: a five‐year experience. J Psychoactive Drugs. 2013;45(5):404‐408. doi: 10.1080/02791072.2013.844532 [DOI] [PubMed] [Google Scholar]
- 36. Afzal H, Esang M, Rahman S. A case of kratom‐induced seizures. Cureus. 2020;12(1):e6588. doi: 10.7759/cureus.6588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burke D, Shearer A, Cott AV. Two cases of provoked seizure associated with kratom ingestion (P4). Neurology. 2019;92(15 Supplement) Accessed September 26, 2022: https://n.neurology.org/content/92/15_Supplement/P4.5‐030 [Google Scholar]
- 38. Tatum WO, Hasan TF, Coonan EE, Smelick CP. Recurrent seizures from chronic kratom use, an atypical herbal opioid. Epilepsy Behav Case Rep. 2018;10:18‐20. doi: 10.1016/j.ebcr.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trakulsrichai S, Sathirakul K, Auparakkitanon S, et al. Pharmacokinetics of mitragynine in man. Drug Des Devel Ther. 2015;9:2421‐2429. doi: 10.2147/DDDT.S79658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vicknasingam B, Chooi WT, Rahim AA, et al. Kratom and pain tolerance: a randomized, placebo‐controlled, double‐blind study. Yale J Biol Med. 2020;93(2):229‐238. [PMC free article] [PubMed] [Google Scholar]
- 41. Prevete E, Kuypers KPC, Theunissen EL, Corazza O, Bersani G, Ramaekers JG. A systematic review of (pre)clinical studies on the therapeutic potential and safety profile of kratom in humans. Hum Psychopharmacol. 2022;37(1):e2805. doi: 10.1002/hup.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanna RS, Tian DD, Cech NB, et al. Refined prediction of pharmacokinetic kratom‐drug interactions: time‐dependent inhibition considerations. J Pharmacol Exp Ther. 2021;376(1):64‐73. doi: 10.1124/jpet.120.000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brogdon HD, McPhee MM, Paine MF, Cox EJ, Burns AG. A case of potential pharmacokinetic kratom‐drug interactions resulting in toxicity and subsequent treatment of kratom use disorder with buprenorphine/naloxone. J Addict Med. 2022;14:606‐609. doi: 10.1097/ADM.0000000000000968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim EL, Seah TC, Koe XF, et al. In vitro evaluation of cytochrome P450 induction and the inhibition potential of mitragynine, a stimulant alkaloid. Toxicol In Vitro. 2013;27(2):812‐824. doi: 10.1016/j.tiv.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 45. Chittrakarn S, Sawangjaroen K, Prasettho S, Janchawee B, Keawpradub N. Inhibitory effects of kratom leaf extract (Mitragyna speciosa Korth.) on the rat gastrointestinal tract. J Ethnopharmacol. 2008;116(1):173‐178. doi: 10.1016/j.jep.2007.11.032 [DOI] [PubMed] [Google Scholar]
- 46. Anderson GD, Gidal BE, Kantor ED, Wilensky AJ. Lorazepam‐valproate interaction: studies in normal subjects and isolated perfused rat liver. Epilepsia. 1994;35(1):221‐225. doi: 10.1111/j.1528-1157.1994.tb02937.x [DOI] [PubMed] [Google Scholar]
- 47. Tang JY, Kiang TKL, Ensom MHH. Pharmacokinetic interactions between valproic acid and lorazepam (PIVOtAL study): a review of site‐specific practices. Can J Hosp Pharm. 2017;70(3):171‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. WHO Expert Committee on Drug Dependence . 44th WHO ECDD Summary assessments, findings and recommendations. 2021. Accessed September 26, 2022. https://cdn.who.int/media/docs/default‐source/controlled‐substances/44ecdd_unsg_annex1.pdf?sfvrsn=9c380ac2_
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


