Summary
Extensive research across fields has repeatedly confirmed that early life adversity (ELA) is a major selective force for many taxa, in part via its ties to adult health and longevity1–3. Negative effects of ELA on adult outcomes have been documented in a wide range of species, from fish to birds to humans4. We used 55 years of long-term data collected on 253 wild mountain gorillas, to examine the effects of six putative sources of ELA on survival, both individually and cumulatively. Although cumulative ELA was associated with high mortality in early life, we found no evidence that it had detrimental consequences for survival later in life. Experiencing three or more forms of ELA was associated with greater longevity, with a 70% reduction in the risk of death across adulthood, driven specifically by greater longevity in males. Whilst this higher survival in later life is likely a consequence of sex-specific viability selection5 during early life due to the immediate mortality consequences of adverse experiences, patterns in our data also suggest that gorillas have significant resilience to ELA. Our findings demonstrate that detrimental consequences of ELA on later life survival are not universal, and indeed largely absent in one of humans’ closest living relatives. This raises important questions about the biological roots of sensitivity to early experiences, and the protective mechanisms that contribute to resiliency in gorillas, which could be critical for understanding how best to encourage similar resiliency to early-life shocks in humans.
eTOC blurb
The eTOC blurb is a short summary that describes the context and significance of the findings for the broader readership. Please see the “in brief” links in the table of contents for examples. The blurb should be 350 characters or less.
Adverse experiences in early life are linked with poor health and shortened adult lifespans across a range of taxa, including humans. Morrison et al. demonstrate that, contingent upon surviving early adversity, negative survival consequences in adulthood are not universal and indeed are largely absent in gorillas, one of humans’ closest living relatives.
Graphical Abstract
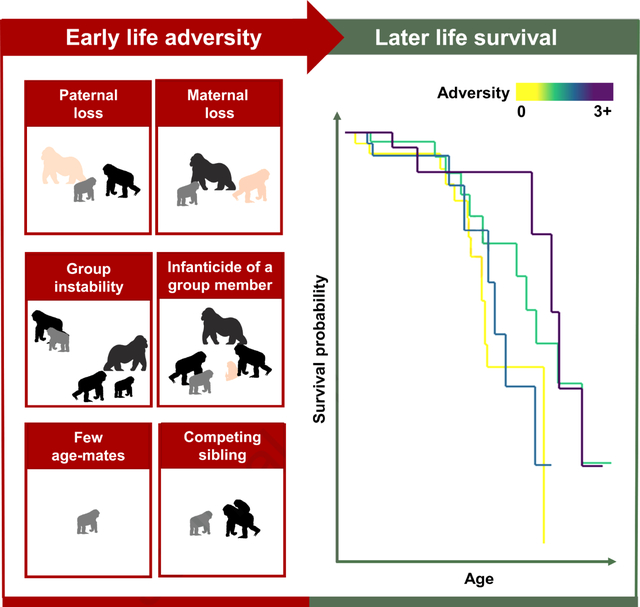
Results and discussion
To examine the consequences of ELA on longevity in one of our closest evolutionary relatives, we (1) identified six putative sources of ELA for mountain gorillas; (2) examined their effect on survival during early life both independently and cumulatively; and (3) examined their long-term effect on survival later in life both independently and cumulatively. We used 55 years of prospective, longitudinal data to generate a dataset mapping ELA to survival outcomes in 135 male and 118 female wild mountain gorillas, monitored since birth in Volcanoes National Park, Rwanda.
Combining an extensive cross-species literature review of ELA with expert knowledge of mountain gorilla socioecology, we identified: 1. paternal loss; 2. maternal loss; 3. infanticide of a group member; 4. group instability; 5. few age-mates; and 6. a competing sibling, as putative sources of ELA in mountain gorillas (Table 1). Most of these adversities were largely independent, showing no or only slight agreement with each other (Cohen’s Kappa <0.2). The two exceptions were paternal loss and maternal loss, which showed fair agreement (K=0.278), and paternal loss and group instability, which showed moderate agreement (K=0.585, Table S1).
Table 1.
Putative sources of early life adversity in gorillas.
| Sample size | ||||
|---|---|---|---|---|
|
| ||||
| Adverse event | Definition | Parallels in other species | Early life (n=253) | Later life (n=164) |
|
| ||||
| Paternal loss | The dominant male during the individual's first year of life dies or leaves the group when the individual is <6 years old. | Humans32–34 | 69 35F, 34M |
53 28F, 25M |
| Maternal loss | Mother dies or leaves the group when the individual is under <6 years old. | Humans33,34; chimpanzees7,35; multiple primates36,37; baboons6,38; elephants12; hyenas10 | 54 32F, 22M |
50 30F, 20M |
| Infanticide | A group member dies by infanticide when the individual is under six years of age. | Humans34,39,40; mice41 | 84 40F, 44M |
70 34F, 36M |
| Group instability | A substantial proportion of group members change when the individual is <6 years old (Jaccard similarity index of group members <0.64). | Humans33,34,42 but see43, elephants12. | 49 23F, 26M |
41 22F, 19M |
| Few age-mates | A mean of fewer than two age-mates present within the group when the individual is between two and six years old. Age-mates classified as group members within two years age difference. | Humans44; macaques45; badgers46. | 48 20F, 28M |
19 8F, 11M |
|
Competing
sibling |
Younger sibling born when the individual is still an infant (<3.5 years) | Humans47, baboons6,38; zebra finches48. | 36 26F, 10M |
35 25F, 10M |
|
| ||||
| Cumulative ELA | The total number of adverse events experienced in early life. | Humans1, baboons6, hyenas11 |
0: 85 33F, 52M 1: 67 32F, 35M 2: 51 28F, 23M 3+: 50 25F, 25M |
0: 37 23F, 24M 1: 47 23F, 24M 2: 41 21F, 20M 3+: 39 22F, 17M |
Sources of ELA examined in this study, their definitions, their parallels in other species, and their sample sizes. F indicates sample size for females, M indicates sample size for males. See also Table S1 for agreement between these sources of ELA.
We examined the effect of ELA on survival during early life (<6 years) via three time-dependent Cox proportional hazards models, in which the adversities each gorilla had experienced were updated at each time point they experienced one. The first model used each adversity as an individual predictor. The second examined cumulative ELA categorically (0, 1, 2, or 3+ sources of ELA). The third examined cumulative ELA numerically, as the total number of ELAs experienced (0–6).
The individual-adversity model demonstrated that paternal loss and having few age-mates were both associated with an increased risk of death during early life. There was also a borderline increased risk of death associated with group instability (Figure 1A, Table. S2A). Examining adverse events cumulatively revealed a strong association between the number of ELAs experienced and death during early life. Animals that experienced two adversities faced a 3.1-fold increase in the risk of death, and those that experienced three or more faced a 26.6-fold increase in the risk of death, relative to animals that faced none (Figure 2A; Table S2A). Overall, each additional ELA was associated with a 2.2-fold increase in the risk of death (Table S2A). Individual and cumulative effect models had similar levels of goodness-of-fit (model concordance: individual = 0.615, cumulative categorical = 0.610, cumulative numeric = 0.610). See Table S3 for additional analyses that examine the sensitivity of the results to removal of individual sources of adversity; results remain qualitatively similar.
Figure 1. The effect of individual ELAs on mortality.
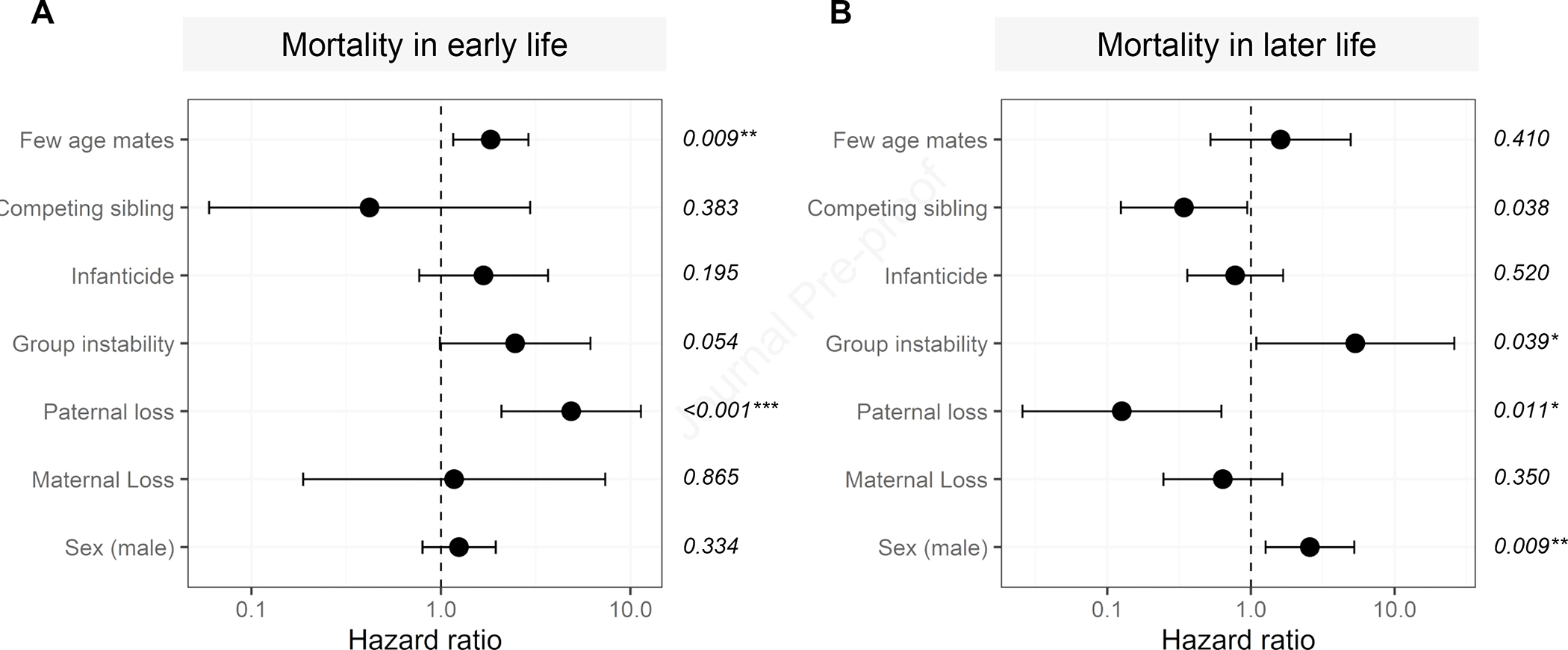
Hazard ratios with 95% confidence intervals for the individual effect of ELAs and sex on the risk of death A) in early life and B) in later life. Confidence intervals overlapping one indicate no significant relationship between the predictor and the risk of death. Values less than one indicate a reduced risk of dying and values greater than one indicate an increased risk of dying. P-values are reported on the right-hand side. Survival during early life was modelled from 253 individuals, 85 of which died before reaching the age of six years. Survival in later life was modelled from 164 individuals, of which 40 died. See also Table S2 for full model output, Table S3 for the robustness of the models to exclusion of certain ELAs, and Figure S1 for the sensitivity of these effects to the age by which adversities were experienced.
Figure 2. The effect of cumulative ELA on mortality.
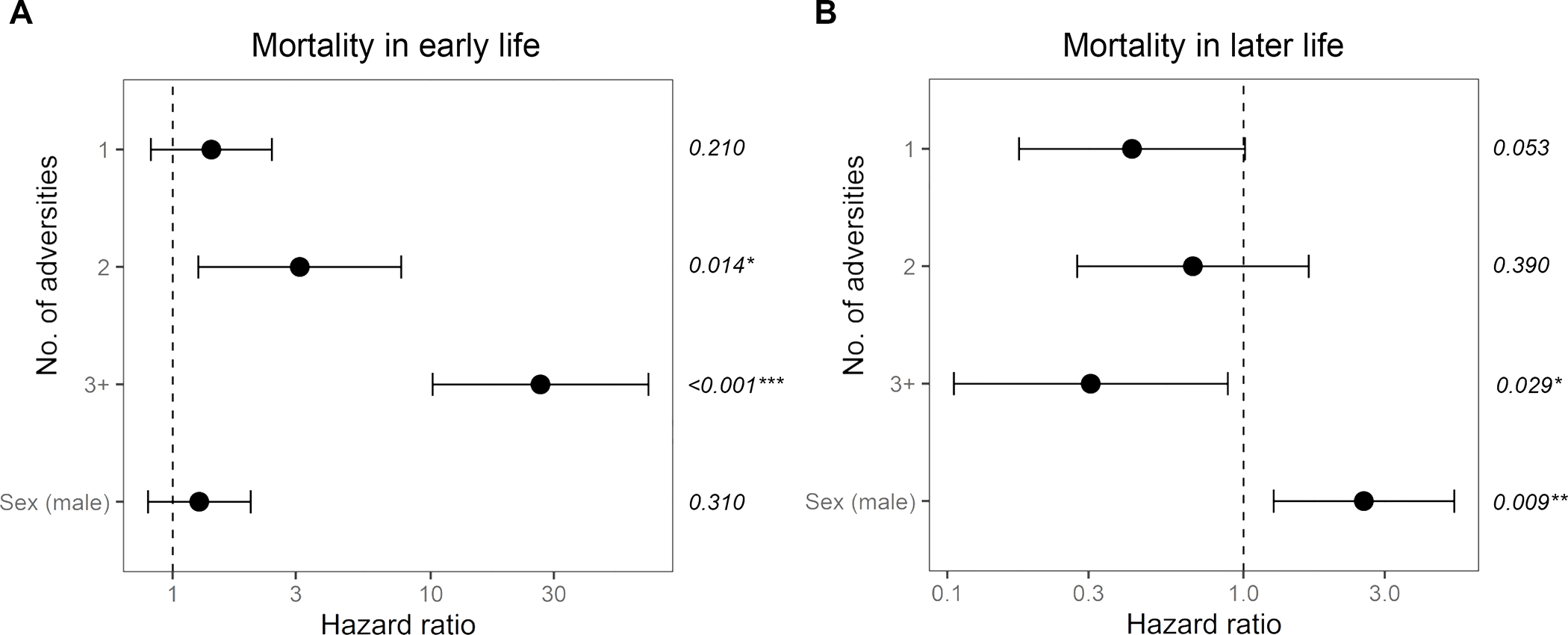
Hazard ratios with 95% confidence intervals for the effect of cumulative ELAs (0, 1, 2 or 3+) and sex on the risk of mortality A) during early life and B) later in life. Confidence intervals overlapping one indicate no significant relationship between experiencing the adverse event and the risk of death. Values less than one indicate a reduced risk of dying and values greater than one indicate an increased risk of dying. P-values are reported on the right-hand side. Sample sizes are the same as in Figure 1. See also Table S2 for full model output, Table S3 for the robustness of the models to exclusion of certain ELAs, Figure S1 for the sensitivity of these effects to the age by which adversities were experienced, and Figure S2 for the power of the numeric cumulative early life adversity model.
To investigate long-term longevity consequences, we examined the effect of ELA on survival from subadulthood onwards (age six years and over, n=164, 80 females, 84 males), via three Cox proportional hazards models structured as described above: individual predictors, cumulative ELA categories, and numeric cumulative ELA. These models were not time-dependent, as all ELAs had occurred when the animals were under the age of 6 and did not change during later life.
Examined as individual predictors, only group instability was associated with increased mortality in later life (hazard ratio=5.326; 95% CI=(1.089,26.049); p=0.039; Figure 1B; Table S2B). However, this relationship disappeared if paternal loss (with which group instability showed overlap; Table S1) was removed from the model (Table S3). This finding should therefore be interpreted with caution. Two individual sources of adversity predicted a lower risk of mortality later in life: suffering paternal loss (hazard ratio=0.126; 95% CI=(0.026,0.622); p=0.011) and having a competing sibling (hazard ratio=0.342; 95% CI=(0.124, 0.942); p=0.038). While having a competing sibling represents a likely source of competition for young animals6, it is also true that having a competing sibling may be indicative of a healthy, high-quality mother, faster development, and/or greater social support14–17, potentially helping to explain the observed relationship to longer lifespans.
Examined cumulatively, ELA was not associated with increased mortality in later life (Figure 2B; Figure 3; Table S2B). In fact, experiencing three or more ELAs was associated with a 70% reduction in the risk of death in later life relative to those experiencing none (Figure 2B). However, this effect was no longer significant if paternal loss, competing sibling or both were excluded as sources of adversity (Table S3). Furthermore, analysis of ELA numerically found no change in the risk of death with each additional adversity experienced (hazard ratio=0.798; 95% CI=(0.605,1.054); p=0.110; Table S2B). Individual and cumulative models again had similar levels of goodness-of-fit (model concordance: individual = 0.656, cumulative categorical = 0.599, cumulative numeric = 0.593).
Figure 3. Survival probability in later life by cumulative ELA.
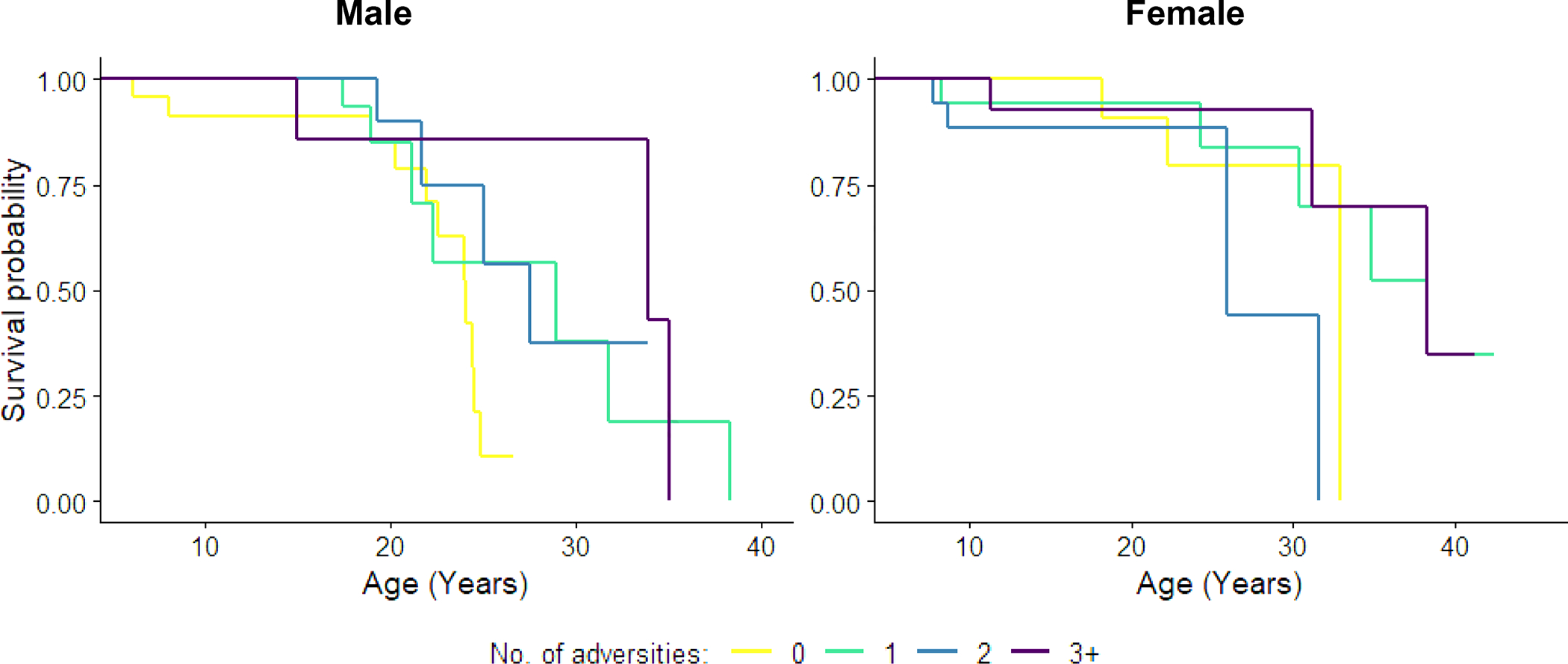
Kaplan-Meier survival curves by sex for the cumulative effect of early life adversity on mortality over the age of six years in 164 mountain gorillas (124 right-censored and 40 deaths). See also Figure S3, for Kaplan-Meier survival curves by sex alone.
Power analyses indicated that the cumulative numeric model had a >99.9% probability of detecting an effect size equivalent to that identified in previous similar analyses in baboons6 (Figure S2). The smallest effect detectable with 80% power through our analysis was a hazard ratio of 1.38 (i.e. a 38% increase in the risk of death with each additional ELA). Thus, it is unlikely we did not detect an ELA – later life longevity relationship due to insufficient power.
Survival curves plotted separately for each sex (Figure 3) suggested that the possible decreased risk of death effect that we observed was driven particularly by males. To examine this possibility quantitatively, we reran both cumulative models and added an interaction between sex and ELA. For later life survival, there was a significant interaction between experiencing two sources of ELA and sex in the categorical model (z=−2.25 p=0.025), but no significant interaction between other ELA categories and sex, and no interaction between number of ELAs and sex in the numeric model. For comparison, in early life survival models, neither categorical nor numeric models identified such an interaction.
In order to address whether our selected age cut-off for ELA influenced our results, we ran sensitivity analyses, varying the age before which an individual must have experienced an adverse event to classify as ELA between 3 and 6 years in steps of 0.1 years. We ran both individual and cumulative numeric models for survival in early and later life across the range of age cut-offs. All model findings remained highly consistent except for the effects of some individual ELAs on survival later in life (Figure S1). Maternal loss was associated with reduced survival in later life only when it occurred before the age of 3.3 years (very close to the age at which animals are typically weaned16), whilst group instability was associated with reduced survival only when it included individuals that experienced this ELA after the age of 5.2 years. The positive relationship between paternal loss and later life survival was also only statistically significant when including individuals that experienced paternal loss after the age of 5.2 years.
Our findings demonstrate that detrimental consequences of ELA on survival in later life, believed to be a significant selective force in large-bodied, long-lived mammals1,6–12, are perhaps less universal than is often assumed. Whilst we detect considerable consequences of ELA for survival in the short-term, we find no negative effect of cumulative ELA on later life survival in gorillas, one of the most evolutionarily-relevant models for human aging13. This is despite having more than adequate power to detect the sizes of ELA effects found in other wild mammals6,10, and examining ELAs that are both ecologically relevant to mountain gorillas and have clear parallels to those examined in other species. In gorillas, depending on the ELAs examined and models used, we find either no relationship or a positive relationship between ELA and adult survival. This contrasts dramatically with substantial evidence linking ELA to key sources of adult mortality in humans1, as well as consistent support for a relationship between ELA and adult mortality in a variety of wild mammals, including baboons6, chimpanzees7, bighorn ewes8,9, spotted hyenas10,11 and elephants12. The fact that ELA appears to have such different consequences in gorillas than in other mammals who share key life history characteristics begs interesting questions about the ecological and social factors that permit ‘escape’ from this evolutionarily consequential force.
Though there has been considerable speculation that male mammals may experience greater viability selection than female mammals18,19, concrete evidence for this has remained scarce. In stark contrast to previous studies, we detected a slight increase in adult survival for gorillas that experienced more sources of ELA, which appeared to be driven particularly by males. We do not think that it is plausible that such adversity truly provides survival benefits to those individuals. Though more research will be needed, sex-specific viability selection is a plausible explanation for this finding and the wider lack of negative adult survival consequences associated with ELA. Gorillas that reach adulthood despite experiencing multiple sources of ELA may be of higher quality than the population average5, and thus show higher survivorship through adulthood. Males show marginally higher mortality in early life (Figure S3) and face greater risk of infectious disease20 and injury during inter-group violence21 in later life than females do. Higher quality males that survived multiple sources of ELA may be better equipped to avoid these sources of mortality.
The potential presence of viability selection is supported by the finding that paternal loss, the strongest predictor of increased mortality during early life, is also the only form of ELA associated with increased adult survival when examined individually (Figure 1). Gorillas that are able to avoid the particularly strong mortality risks associated with paternal loss in early life, might be of especially high-quality, resulting in increased adult survival relative to those that did not experience paternal loss and its associated mortality risk in early life. However, whilst viability selection likely contributes to the lack of long-term survival consequences, it is unlikely to be the only contributor. Although further research is needed, there is no clear reason why viability selection would be occurring to a far greater extent in gorillas when compared to other mammals with slow life histories, especially given how relatively benign their environment is compared to that of many other mammals in which this has been studied23–25. There are also forms of ELA for which viability selection does not appear to be present. For example, previous research found that maternal loss under the age of 2.45 years resulted in 100% mortality, whilst maternal loss above this age had no detectable effect on survival22, effectively ruling out any strong viability selection and suggesting that the lack of influence of maternal loss on adult survival has its roots in the species’ resiliency to early experiences.
What could be responsible for this greater resiliency to early life effects in gorillas, relative to other long-lived mammals? Two aspects of mountain gorillas’ socioecology represent potential sources of resiliency: their high-resource habitat, with minimal feeding competition23–25, and their highly cohesive, affiliative “family” groups22. These potential drivers of resiliency are also likely to be linked, given the tendency for patterns of resource distribution to shape patterns of competition both within and between groups, and ultimately individuals’ social relationships26.
If the later life consequences of ELA in other species are rooted in the immediate energetic constraints imposed following an adverse event, e.g. stunting growth, restricting organ development, etc.27,28, mountain gorillas’ stable availability of food may buffer these detrimental consequences. The sources of ELA we identified for this species were predominantly social, as no environmental variables, such as drought or group size (as a proxy for feeding competition), represented plausible sources of adversity in this system within the 55-year timeframe of the study. Arguably, the only time a young gorilla may face a sudden change in food availability could be if they lose their mother prior to being weaned. We therefore find preliminary support for an energetic constraints hypothesis in our sensitivity analysis, which found that maternal loss was only associated with significantly lower survival in later life when it occurred under the age of 3.3 years. This is the mean age at being weaned in mountain gorillas16.
Gorillas’ resiliency to ELA may also stem from their capacity for social buffering. In humans, close family relationships are suggested to buffer developmental stress29, whilst in baboons, strong social relationships can buffer some of the negative consequences of maternal loss on survival30. In mountain gorillas, social relationships with fellow group members strengthen following maternal loss, resulting in motherless immature gorillas becoming more central in their groups’ social networks22. The current findings provide some support for this theory given that group instability (i.e. the breakdown of these stable affiliative relationships) was the only ELA associated with increased risk of mortality in later life (Figure 1). This effect only became significant when individuals that experienced group instability over the age of 5.2 years were included, suggesting these broader social relationships may become more important as young gorillas age and become less reliant on their closest relatives31. Further research is needed to examine whether the level of social buffering occurring in gorillas is considerably greater than that in other long-lived mammals, to determine whether this could be responsible for the very different consequences of ELA for later life survival.
Overall, our findings demonstrate that the link between ELA and increased mortality in later life is not universal, and indeed is largely absent in one of humans’ closest living relatives. Gorillas may provide valuable insight into the mechanisms through which early life conditions shape the life course. Further detailed studies examining how early life experiences shape health, reproduction, and sociality across the life course in a species in which cumulative ELA does not reduce adult survival will provide a new angle with which to understand the biological roots of sensitivity to early experiences. In particular, the potential resiliency found in such a close evolutionary relative suggests the presence of protective mechanisms that, if identified, could inform our understanding of how best to encourage resiliency to ELA in humans.
STAR Methods
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Robin E. Morrison (rmorrison@gorillafund.org).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Demographic data have been deposited at Zenodo and are publicly available as of the date of publication. DOIs are listed in the key resources table.
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental subjects | ||
| Gorilla beringei beringei | Population monitored by Dian Fossey Gorilla Fund in Volcanoes National Park, Rwanda |
https://gorillafund.org |
| Deposited data | ||
| Raw data | This paper | https://doi.org/10.5281/zenodo.7780540 |
| Software and algorithms | ||
| R | R core team68 | www.r-project.org |
| survival | Therneau, 202269 | https://cran.r-project.org/web/packages/survival/ |
| coxme | Therneau, 202270 | https://cran.r-project.org/web/packages/coxme/ |
| powerSurvEpi | Qiu et al, 202171 | https://cran.r-project.org/web/packages/powerSurvEpi/ |
| EloRating | Neumann and Lars, 201458 | https://cran.r-project.org/web/packages/EloRating/ |
Experimental model and subject details
We conducted this study on a population of Virunga mountain gorillas in Volcanoes National Park, Rwanda, which have been monitored by the Karisoke Research Center of the Dian Fossey Gorilla Fund (Fossey Fund) since 1967. Gorillas in this study population are individually known by physical characteristics and habituated to the presence of human observers. They have been visited on a near daily basis across the last 55 years, enabling detailed demographic and behavioural data to be collected. This data collection is approved by the Rwanda Development Board and conducted in accordance with the ethical standards of the Dian Fossey Gorilla Fund and the International Primatological Society’s Code of Best Practices for Field Primatology. Our study uses data collected between September 1967 and December 2021, on 135 male and 118 female gorillas across their lifespans.
Method details
Identifying sources of early life adversity (ELA)
We conducted an extensive literature review to identify commonly-studied sources of ELA in other species, including humans. Our goals were to 1) maximize comparability to existing literature; 2) identify sources of adversity that were relevant to mountain gorillas given the specifics of their socioecology, and 3) identify sources of adversity that could be quantified using the long-term data of the Fossey Fund (Table 1). We deliberately did not check any relationships between potential sources of adversity and survival in our data until after the sources of adversity were settled on (with the exception of maternal loss, which had already been studied22, in order to avoid basing our choices on our expected conclusions. We defined early life in mountain gorillas as less than six years of age. This is prior to female menarche, which typically occurs between the age of 6.0–7.5 years49. From six onwards, mountain gorillas are considered subadults or adults50. Commonly studied sources of ELA that could not be satisfactorily quantified or did not seem ecologically valid for mountain gorillas included psychological, physical or sexual abuse; mental illness of parents; domestic violence; natural or man-made disasters; maternal dominance rank; and extreme temperatures or drought. Feeding competition or meaningful caloric restriction, e.g. due to drought, did not represent likely sources of ELA for the mountain gorillas followed during the study. This is because Virunga mountain gorillas feed on resources that are highly abundant year round. Prior studies have found little evidence for within group feeding competition23–25 or any sustained periods of low food availability51,52.
ELA in the study population
We used information on all gorillas born into the study population by the end of 2021, for whom date of birth was known to within an accuracy of ± 6 months (85.3% known to the week ± 4 days, 11.1% known to the month ± 15 days, 2.4% known ± 3 months and 1.2% known ± 6 months). Dates of transfer out of the study population and death were extracted from the demographic database. Transfers between habituated groups within Rwanda were confirmed by discussion with the Rwanda Development Board, who monitor all other habituated gorilla groups within the country. Transfers across the border into the Democratic Republic of Congo were confirmed where possible using genetic samples during census work53,54. Deaths were confirmed by observation of recovered corpses after extensive searches carried out following the disappearance of any studied gorilla. Any disappearances in which transfer or death could not be determined were excluded from analyses (n=3). The only exceptions were when the individual was severely ill prior to their disappearance, in which case death was assumed (n=2).
For each individual, their exposure to each of the six putative sources of ELA (Table 1) was categorized as a binary variable (1=exposed, 0=not exposed) using the following classifications:
Paternal loss32–34: the male which held the highest dominance rank during the individual’s first year of life died or left the group when the individual was less than six years old. We used the dominant male of the group rather than the genetic father of the individual because dominance has been found to be a better predictor of relationships between males and immatures than genetic paternity in this mountain gorilla population55. In single-male groups, this was the only adult male present within their group. In multi-male groups, from 2003 onwards, dominant males were classified as the highest ranking male based on displacements and avoidances using the Elo-rating method and the R package ‘EloRating’56–58. Prior to 2003, dominant males in multi-male groups were identified based solely on observations by researchers as the data required to calculate Elo-ratings was not consistently recorded. However, there is strong agreement between current Elo-rating scores and researcher observation, suggesting that this limitation is unlikely to considerably alter classifications of the dominant male.
Maternal loss6,7,59,10,12,33–38: the mother of the individual either died or transferred out of the social group without them, when the individual was under six years of age. Mothers of all individuals born into the study population are known from observation due to near-daily monitoring of all individuals.
Infanticide34,39–41: one or more other group member(s) died by infanticide when the individual was under six years of age. This adversity is included as a potential parallel to exposure to violence and threat early in life in humans39,40, and also likely captures information about the individual’s own risk of death by infanticide. Whilst direct measures of violent inter-group encounters could provide a more precise estimate of exposure to violence and threat, comprehensive data on these events only dates back to 2003, and as such infanticide was instead used as a proxy. Infanticide of a group member was extracted from the infanticide database which includes all known cases of infanticide, the group it occurred in, and the identity of the infant that died, since observations began in 1967. This was cross-referenced with individual age and group memberships to verify whether these occurred within a group an individual was present in while they were under the age of six. If this occurred at least once, the individual was classified as having experienced the infanticide of a group member.
Group instability12,33,34,42: a substantial proportion of group members (> 1/3rd) changed from the previous year, when the individual was under six years of age. This was calculated for each individual in each year they were under the age of six, by extracting all gorillas that were present in the group for at least half of the previous year, and all gorillas present in the group for at least half of the data year in question. The similarity in group composition, based on all individuals over the age of one in the previous year and over the age of two in the data year, was then calculated using the Jaccard Similarity Index60. Excluding the youngest individuals meant that changes in group composition due to births or deaths of individuals under the age of one did not influence group stability estimates. The Jaccard Similarity Index (JSI) was calculated as the number of gorillas present in the group in both consecutive years divided by the total number of gorillas present in either year, resulting in an index between zero and one. Zero meant that all group members changed, and one meant that no group members changed. We extracted the lowest JSI that occurred in each individual’s group when they were under the age of 6 years. Individuals were classified as having experienced group instability early in life if this JSI value was in the bottom quartile for all individuals in the ELA dataset (JSI scores of <0.64). This meant that more than one third of group members had changed, e.g. 9 group members leaving a group of 24. These incidents of group instability were primarily the result of group disintegrations or group fissions.
Few age-mates22,44–46,59: the individual had a mean of fewer than two fellow group members within two years age difference during early life. This is a measure of social isolation for young gorillas, who typically spend extensive time in ‘play groups’ with similar-aged peers, which are often centered around adult males rather than mothers61,62. In other studies, maternal social isolation has been used as a form of ELA6, but we contend that our measure is more relevant for this species given the specifics of their social dynamics. This was calculated by extracting the number of other group members born within two years of the individual, from annual group composition data and taking the mean across all years they were present in the population and between the age of two and six. Values were not calculated when individuals were under the age of 2, as they would be biased by potential age-mates not yet born. For individuals that died before reaching the age of two, their expected mean number of age-mates was estimated based on that of the closest-in-age group member. The two-year age gap for classification of age-mates was consistent with previous studies on this population22.
Competing sibling6,38,47,48: the age difference between the individual and their younger maternal sibling was in the bottom quartile of individuals in the ELA dataset (<3.5 years difference). This was calculated by extracting the age of each individual when their mother gave birth to her next offspring.
Data on all six putative sources of ELA were available from September 1967 to December 2021 due to consistent daily long-term monitoring of the population. The only breaks to consistent monitoring occurred in April-July 1994 and June 1997-December 1998, due to political instability. Following these breaks, the dates of any deaths, births or changes in group composition were estimated as the midpoint of the period in which they occurred. This therefore should not have resulted in any missed cases of paternal loss, maternal loss or group instability, or considerably altered estimated numbers of age-mates. Incidences of infanticide could feasibly have been missed. However, infanticide has been shown to be strongly driven by social dynamics and group density, and therefore follows broader trends over time63,64. The gaps in monitoring occurred during a period of low group density and high group stability, when no cases of infanticide were recorded between March 1987 and August 2005. It is therefore highly unlikely that cases of infanticide were missed. It is also feasible that a competing younger sibling could have been missed during these gaps if a younger sibling was both born and died during a break in monitoring. This is again unlikely but cannot be entirely ruled out. If cases of either infanticide or a competing sibling were missed, we are underestimating the number of adversities an individual faced in early life. However, this would apply to both early life and later life models and therefore could not be driving different patterns across different life stages.
Quantification and statistical analysis
Agreement between sources of ELA
We examined the level of agreement between each pair of ELAs using Cohen’s Kappa65. All pairwise comparisons showed no or only slight agreement (Cohen’s Kappa <0.2), except for two: paternal loss and maternal loss (K=0.278), and paternal loss and group instability (K=0.585, Table S1). Despite this agreement, we elected to keep paternal loss as a source of ELA in our study as we believe it represents a fundamentally different source of adversity from maternal loss and group instability. Previous research has demonstrated the integral role that the dominant male plays in buffering the social consequences of maternal loss22, such that, experiencing both maternal and paternal loss is likely to be considerably worse than experiencing only one of these events. This research also demonstrated the smaller but substantial contribution to social buffering made by individuals in the wider social group. By examining both paternal loss and group instability, we are attempting to disentangle the effects specifically of losing the ‘social father’ (who is usually a key social relationship66) versus major disruption to the social environment more broadly. However, given the substantial overlap between paternal loss and other sources of adversity, all models were also run excluding paternal loss as a source of adversity (see below).
The effect of ELA on survival during early life
We examined the effect of all six sources of ELA on early life survival by modelling mortality under the age of six years using three models: individual predictors, cumulative ELA categories, and numeric cumulative ELA. These analyses included all individuals of known sex born within the population (n=253, 135 males, 118 females). This included gorillas born in 19 different social groups, with the majority of individuals born in groups containing multiple adult males (205 out of 253). We examined the effect of each ELA individually in a cox proportional hazards model that included sex, to account for well-established sex differences in mortality risk67 (Figure S3). Each event was included as a binary, time-dependent variable accounting for when the event occurred, except for few age-mates which was treated as constant from birth as it was primarily determined by the date and group of birth.
Each row in the dataset represented an individual during a period when there was no change to the early life adversities they had experienced, accurate to the day (or as close as possible e.g., the midpoint of the period during which the adversity could have occurred, if daily observation was not possible). If an individual experienced no adversities, they had only one row beginning at age 0 and ending at either 6 years or their age when they died. An individual that experienced (e.g.) maternal loss at age 3.6 and group instability at age 4.4 would have three rows: one with zero ELAs before age 3.6, one with 1 ELA from 3.6–4.4 years, and one row with 2 ELAs from 4.4–6 years or their age when they died.
Our models included gorilla ID, birth year, mother, and natal group as random effects. The infanticide ELA violated the proportional hazards assumption (i.e., risk of infanticide is not constant across ages), so the model was rerun including a time-dependent coefficient for infanticide to account for this. Of 253 individuals, 85 died before age six while 168 were right censored (i.e., they survived until at least age six or left the study population). Cumulative effects of ELA on survival under the age of six were then examined as a categorical time-dependent variable. Categories were based on whether individuals experienced zero, one, two, or three or more of the six putative sources of ELA, and were updated at each time point an individual experienced an additional adverse event. This was run in a cox proportional hazards model accounting for sex, with gorilla ID, birth year, mother, and natal group included as random effects. Cumulative effects of ELA were also examined numerically, based on the total number of adverse events (1–6) an individual experienced across early life. This was structured identically to the categorical model.
The effect of ELA on survival during later life
We examined the effects of ELA on mortality in later life with three models: individual predictors, cumulative ELA categories, and numeric cumulative ELA, using all individuals that were monitored until they were at least six years old (n=164 gorillas, 84 males, 80 female). Survival models for the effect of ELA in later life were not time-dependent, as all ELAs had occurred under the age of 6 years, and thus by definition did not change during later life. Therefore, each individual was only present once in the dataset, with a fixed set of ELAs that they had experienced when they were young. We examined the effect of each ELA individually, with each event as a binary predictor in a cox-proportional hazards model along with sex, plus random effects for birth year, mother and natal group, to predict risk of mortality from the age of six years. Of 164 gorillas, 40 had died whilst the remaining 124 were either still alive at the end of the study or had transferred out of the population and were therefore right-censored in the model. We examined the effect of these ELAs cumulatively by summing the total number of adversities experienced. They were then analysed as a categorical variable (zero, one, two, or three or more sources of adversity) along with sex in a cox proportional hazards model predicting mortality from the age of six years onwards. Kaplan-Meier survival curves were plotted for each sex based on the cumulative number of ELAs experienced. Cumulative ELA was also examined numerically (as the total number of adversities an individual had experienced in early life), structured identically to the categorical model.
Model specification and robustness
All cox proportional hazards models were run and Kaplan-Meier curves fitted in R version 4.1.368 using the ‘Survival ‘ package version 3.2–1369 and the ‘coxme’ package version 2.2–18.170. Whenever relevant the proportional hazards assumption was tested using the ‘cox.zph’ function in the ‘Survival’ package. All models were also run excluding either paternal loss, competing sibling, or both as sources of ELA (see Table S3). We did this because paternal loss overlapped with other sources of adversity, and because having a competing sibling, whilst a possible source of competition for food and maternal care6, may also be indicative of having a particularly healthy or high-quality mother, developing faster, and/or having social support from a close-in-age relative14–17. As Kaplan-Meier curves indicated potential sex differences in the effect of ELA on survival in later life, we also reran categorical and numeric cumulative models for both early and later life survival with the addition of an interaction term between sex and ELA.
Power analysis
We ran power analyses to identify the minimum effect size that could be detected with 80% power given our data, and our power to detect a range of effect sizes (hazard ratios of 1.3–1.9) across sample sizes of 20–200 individuals, using the ‘ssizeEpiCont.default’ and ‘powerEpiCont.default’ functions of the ‘powerSurvEpi’ R package version 0.1.1371. We assumed a type one error rate of 0.05. Power analyses were based on the model predicting mortality over the age of six from the cumulative numerical (i.e., not categorical) number of ELAs experienced (1–6). Analyses accounted for the proportion of individuals that had died (40 out of 164), the variance in cumulative number of ELAs experienced (1.804), and the correlation between sex and cumulative ELA (0.148) in our data.
Model sensitivity to age at adverse event
To examine the sensitivity of the effects of ELA on survival to the age at which an adverse event was experienced, we varied the age cut-off before which an adverse event needed to have occurred to be considered ELA, between 3 and 6 years in steps of 0.1 years. The categorisation of individuals as having experienced few age-mates and a competing sibling in early life were not altered, as the number of age-mates was largely determined at birth, and competing siblings were all born when the individual was between the age of 2.8 and 3.5 years. For all other adversities (paternal loss, maternal loss, infanticide of a group member and group instability), the date on which these events occurred was used to determine whether the event had occurred when the individual was under the age cut-off. The effect of each ELA on survival in early life and survival in later life across the range of age cut-offs were modelled using cox proportional hazards as described above. The effect size and 95% confidence intervals were extracted and plotted for paternal loss, maternal loss, infanticide of a group member and group instability. The cumulative effect of ELA in early life and later life was also modelled numerically across the range of age cut-offs using cox proportional hazards models as above. The effect size and 95% confidence intervals for cumulative ELA at each age cut-off were extracted and plotted.
Supplementary Material
Highlights.
Highlights are bullet points that convey the core findings of your paper. You may include up to four highlights. The length of each highlight cannot exceed 85 characters (including spaces).
Gorillas are exposed to a range of forms of adversity in early life
Those exposed to more forms of adversity suffer greater mortality in early life
But, unlike many other species, they do not suffer greater mortality as adults
Experiencing three or more adversities predicts lower adult mortality for males
Acknowledgements
We thank the many students, researchers and Dian Fossey Gorilla Fund (Fossey Fund) staff members who contributed to data collection and the Rwanda Development Board for their long-term support, as well as four anonymous reviewers for their helpful feedback on the manuscript. We are grateful to members of the Fossey Fund gorilla program: Didier Abavandimwe, Jonas Anthony, Jean Paul Hirwa, Honorine Ihimbazwe, Laban Kayitete, Godfrey Kwibuka, Samedi Jean Pierre Mucyo, Thadee Muhire, James Munyawera, Epiphanie Musabirema, Yvonne Mushimiyimana, Nadia Niyonizeye, Jean de Dieu Nsanzineza, Gudula Nyirandayambaje, Jean de Dieu Tuyizere, Cedric Ujeneza, Marie Rose Umuhoza, and Veronica Vecellio, along with Jenny Tung, Elizabeth Archie, and Susan Alberts, for feedback and discussion on the project. This work was supported by a pilot project grant from the Animal Models for the Social Dimensions of Health and Aging Research Network (NIH/NIH R24 AG065172) to S.R. and a Ben Barres Spotlight award from eLife to R.E.M..
Footnotes
Declaration of interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, and Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. Am. J. Prev. Med 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 2.Lynch J, and Smith GD (2005). A life course approach to chronic disease epidemiology. Annu. Rev. Public Health. 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- 3.Roseboom T, de Rooij S, and Painter R (2006). The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Eyck HJF, Buchanan KL, Crino OL, and Jessop TS (2019). Effects of developmental stress on animal phenotype and performance: a quantitative review. Biol. Rev. 10.1111/brv.12496. [DOI] [PubMed] [Google Scholar]
- 5.Douhard M, Plard F, Gaillard JM, Capron G, Delorme D, Klein F, Duncan P, Loe LE, and Bonenfant C (2014). Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate. Proc. R. Soc. B Biol. Sci. 10.1098/rspb.2014.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tung J, Archie EA, Altmann J, and Alberts SC (2016). Cumulative early life adversity predicts longevity in wild baboons. Nat. Commun. 10.1038/ncomms11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanton MA, Lonsdorf EV, Murray CM, and Pusey AE (2020). Consequences of maternal loss before and after weaning in male and female wild chimpanzees. Behav. Ecol. Sociobiol. 10.1007/s00265-020-2804-7. [DOI] [Google Scholar]
- 8.Pigeon G, Festa-Bianchet M, and Pelletier F (2017). Long-term fitness consequences of early environment in a long-lived ungulate. Proc. R. Soc. B Biol. Sci. 10.1098/rspb.2017.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douhard M, Festa-Bianchet M, Landes J, and Pelletier F (2019). Trophy hunting mediates sex-specific associations between early-life environmental conditions and adult mortality in bighorn sheep. J. Anim. Ecol. 10.1111/1365-2656.12970. [DOI] [PubMed] [Google Scholar]
- 10.Strauss ED, Shizuka D, and Holekamp KE (2020). Juvenile rank acquisition is associated with fitness independent of adult rank. Proc. R. Soc. B Biol. Sci. 10.1098/rspb.2019.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gicquel M, East ML, Hofer H, and Benhaiem S (2022). Early-life adversity predicts performance and fitness in a wild social carnivore. J. Anim. Ecol, 1–13. 10.1111/1365-2656.13785. [DOI] [PubMed] [Google Scholar]
- 12.Lee PC, Moss CJ, Njiraini N, Poole JH, Sayialel K, and Fishlock VL (2022). Cohort consequences of drought and family disruption for male and female African elephants. Behav. Ecol. 33, 408–418. 10.1093/beheco/arab148. [DOI] [Google Scholar]
- 13.Emery Thompson M, Rosati AG, and Snyder-Mackler N (2020). Insights from evolutionarily relevant models for human ageing. Philos. Trans. R. Soc. B Biol. Sci. 10.1098/rstb.2019.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grebe NM, Hirwa JP, Stoinski TS, Vigilant L, and Rosenbaum S (2022). Mountain gorillas maintain strong affiliative biases for maternal siblings despite high male reproductive skew and extensive exposure to paternal kin. Elife 11, 1–24. 10.7554/eLife.80820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campos FA, Altmann J, Cords M, Fedigan LM, Lawler R, Lonsdorf EV, Stoinski TS, Strier KB, Bronikowski AM, Pusey AE, et al. (2022). Female reproductive aging in seven primate species: Patterns and consequences. Proc. Natl. Acad. Sci. U. S. A. 119, 1–10. 10.1073/pnas.2117669119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckardt W, Fawcett K, and Fletcher AW (2016). Weaned age variation in the Virunga mountain gorillas (Gorilla beringei beringei): influential factors. Behav. Ecol. Sociobiol. 10.1007/s00265-016-2066-6. [DOI] [Google Scholar]
- 17.Patterson SK, Hinde K, Bond AB, Trumble BC, Strum SC, and Silk JB (2021). Effects of early life adversity on maternal effort and glucocorticoids in wild olive baboons. Behav. Ecol. Sociobiol. 10.1007/s00265-021-03056-7. [DOI] [Google Scholar]
- 18.Gamelon M, Focardi S, Gaillard JM, Gimenez O, Bonenfant C, Franzetti B, Choquet R, Ronchi F, Baubet E, and Lemaître JF (2014). Do age-specific survival patterns of wild boar fit current evolutionary theories of senescence? Evolution (N. Y). 10.1111/evo.12519. [DOI] [PubMed] [Google Scholar]
- 19.Lemaître JF, Ronget V, Tidière M, Allainé D, Berger V, Cohas A, Colchero F, Conde DA, Garratt M, Liker A, et al. (2020). Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc. Natl. Acad. Sci. U. S. A. 10.1073/pnas.1911999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison RE, Mushimiyimana Y, Stoinski TS, and Eckardt W (2021). Rapid transmission of respiratory infections within but not between mountain gorilla groups. Sci. Rep. 10.1038/s41598-021-98969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirville MO, Ridley AR, Samedi JPM, Vecellio V, Ndagijimana F, Stoinski TS, and Grueter CC (2018). Factors influencing individual participation during intergroup interactions in mountain gorillas. Anim. Behav. 144, 75–86. 10.1016/j.anbehav.2018.08.003. [DOI] [Google Scholar]
- 22.Morrison RE, Eckardt W, Colchero F, Vecellio V, and Stoinski TS (2021). Social groups buffer maternal loss in mountain gorillas. Elife 10, 1–22. 10.7554/eLife.62939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caillaud D, Ndagijimana F, Giarrusso AJ, Vecellio V, and Stoinski TS (2014). Mountain gorilla ranging patterns: Influence of group size and group dynamics. Am. J. Primatol. 76, 730–746. 10.1002/ajp.22265. [DOI] [PubMed] [Google Scholar]
- 24.Watts DP (1985). Relations between group size and composition and feeding competition in mountain gorilla groups. Anim. Behav. 10.1016/S0003-3472(85)80121-4. [DOI] [Google Scholar]
- 25.Robbins AM, Stoinski TS, Fawcett KA, and Robbins MM (2009). Socioecological influences on the dispersal of female mountain gorillas - Evidence of a second folivore paradox. Behav. Ecol. Sociobiol. 10.1007/s00265-008-0679-0. [DOI] [Google Scholar]
- 26.Snaith TV, and Chapman CA (2007). Primate group size and interpreting socioecological models: Do folivores really play by different rules? Evol. Anthropol 10.1002/evan.20132. [DOI] [Google Scholar]
- 27.Parker JM, and Wittemyer G (2022). Orphaning stunts growth in wild African elephants. Conserv. Physiol. 10, 1–10. 10.1093/conphys/coac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy EJ, Lee A, Siodi IL, Helmich EC, McLean EM, Malone EJ, Pickard MJ, Ranjithkumar R, Tung J, Archie EA, et al. (2023). Early life drought predicts components of adult body size in wild female baboons. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen E, Brody GH, and Miller GE (2017). Childhood close family relationships and health. Am. Psychol. 10.1037/amp0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lange EC, Zeng S, Campos FA, Li F, Tung J, Archie EA, and Alberts SC (2022). Early life adversity and adult social relationships have independent effects on survival in a wild animal model of aging. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison RE, Eckardt W, Stoinski TS, and Brent LJN (2020). Comparing measures of social complexity: larger mountain gorilla groups do not have a greater diversity of relationships. Proc. R. Soc. B Biol. Sci. 10.1098/rspb.2020.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webster GD, Graber JA, Gesselman AN, Crosier BS, and Schember TO (2014). Life history theory of father absence and menarche: A meta-analysis. Evol. Psychol. 10.1177/147470491401200202. [DOI] [PubMed] [Google Scholar]
- 33.Pilowsky DJ, Keyes KM, and Hasin DS (2009). Adverse childhood events and lifetime alcohol dependence. Am. J. Public Health. 10.2105/AJPH.2008.139006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessler RC, Davis CG, and Kendler KS (1997). Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol. Med. 10.1017/S0033291797005588. [DOI] [PubMed] [Google Scholar]
- 35.Girard-Buttoz C, Tkaczynski PJ, Samuni L, Fedurek P, Gomes C, Löhrich T, Manin V, Preis A, and Valé PF (2021). Early maternal loss leads to short- but not long- - term effects on diurnal cortisol slopes in wild chimpanzees. Elife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zipple MN, Altmann J, Campos FA, Cords M, Fedigan LM, Lawler RR, Lonsdorf EV, Perry S, Pusey AE, Stoinski TS, et al. (2020). Maternal death and offspring fitness in multiple wild primates. Proc. Natl. Acad. Sci. U. S. A. 10.1073/pnas.2015317118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.French JA, and Carp SB (2016). Early-life social adversity and developmental processes in nonhuman primates. Curr. Opin. Behav. Sci. 10.1016/j.cobeha.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson SK, Strum SC, and Silk JB (2022). Early life adversity has long-term effects on sociality and interaction style in female baboons. Proc. R. Soc. B Biol. Sci. 10.1098/rspb.2021.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumner JA, Colich NL, Uddin M, Armstrong D, and McLaughlin KA (2019). Early Experiences of Threat, but Not Deprivation, Are Associated With Accelerated Biological Aging in Children and Adolescents. Biol. Psychiatry. 10.1016/j.biopsych.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jovanovic T, Vance LA, Cross D, Knight AK, Kilaru V, Michopoulos V, Klengel T, and Smith AK (2017). Exposure to Violence Accelerates Epigenetic Aging in Children. Sci. Rep. 10.1038/s41598-017-09235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heiming RS, Jansen F, Lewejohann L, Kaiser S, Schmitt A, Lesch KP, and Sachser N (2009). Living in a dangerous world: The shaping of behavioral profile by early environment and 5-HTT genotype. Front. Behav. Neurosci. 10.3389/neuro.08.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavanagh SE, Stritzel H, Smith C, and Crosnoe R (2018). Family Instability and Exposure to Violence in the Early Life Course. J. Res. Adolesc. 10.1111/jora.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaydosh L, and Harris KM (2018). Childhood Family Instability and Young Adult Health. J. Health Soc. Behav. 10.1177/0022146518785174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Criss MM, Pettit GS, Bates JE, Dodge KA, and Lapp AL (2002). Family adversity, positive peer relationships, and children’s externalizing behavior: A longitudinal perspective on risk and resilience. Child Dev. 10.1111/1467-8624.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, and Capitanio JP (2009). What is an “Adverse” Environment? Interactions of Rearing Experiences and MAOA Genotype in Rhesus Monkeys. Biol. Psychiatry. 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lieshout SHJ, Badás EP, Bright Ross JG, Bretman A, Newman C, Buesching CD, Burke T, Macdonald DW, and Dugdale HL (2021). Early-life seasonal, weather and social effects on telomere length in a wild mammal. Mol. Ecol. 10.1111/mec.16014. [DOI] [PubMed] [Google Scholar]
- 47.Gillette MT, Lohman BJ, and Neppl TK (2017). Lower levels of maternal capital in early life predict offspring obesity in adulthood. Ann. Hum. Biol. 10.1080/03014460.2016.1213314. [DOI] [PubMed] [Google Scholar]
- 48.Brandl HB, Farine DR, Funghi C, Schuett W, and Griffith SC (2019). Early-life social environment predicts social network position in wild zebra finches. Proc. R. Soc. B Biol. Sci. 10.1098/rspb.2018.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watts DP (1991). Mountain gorilla reproduction and sexual behavior. Am. J. Primatol. 10.1002/ajp.1350240307. [DOI] [PubMed] [Google Scholar]
- 50.Breuer T, Hockemba MBN, Olejniczak C, Parnell RJ, and Stokes EJ (2009). Physical maturation, life-history classes and age estimates of free-ranging western gorillas - Insights from Mbeli Bai, Republic of Congo. Am. J. Primatol. 71, 106–119. 10.1002/ajp.20628. [DOI] [PubMed] [Google Scholar]
- 51.Grueter CC, Deschner T, Behringer V, Fawcett K, and Robbins MM (2014). Socioecological correlates of energy balance using urinary C-peptide measurements in wild female mountain gorillas. Physiol. Behav. 10.1016/j.physbeh.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Wright E, Grueter CC, Seiler N, Abavandimwe D, Stoinski TS, Ortmann S, and Robbins MM (2015). Energetic responses to variation in food availability in the two mountain gorilla populations (Gorilla beringei beringei). Am. J. Phys. Anthropol. 10.1002/ajpa.22808. [DOI] [PubMed] [Google Scholar]
- 53.Granjon AC, Robbins MM, Arinaitwe J, Cranfield MR, Eckardt W, Mburanumwe I, Musana A, Robbins AM, Roy J, Sollmann R, et al. (2020). Estimating abundance and growth rates in a wild mountain gorilla population. Anim. Conserv. 10.1111/acv.12559. [DOI] [Google Scholar]
- 54.Gray M, Roy J, Vigilant L, Fawcett K, Basabose A, Cranfield M, Uwingeli P, Mburanumwe I, Kagoda E, and Robbins MM (2013). Genetic census reveals increased but uneven growth of a critically endangered mountain gorilla population. Biol. Conserv. 10.1016/j.biocon.2012.09.018. [DOI] [Google Scholar]
- 55.Rosenbaum S, Hirwa JP, Silk JB, Vigilant L, and Stoinski TS (2015). Male rank, not paternity, predicts male-immature relationships inmountain gorillas, Gorilla beringei beringei. Anim. Behav. 104, 13–24. 10.1016/j.anbehav.2015.02.025. [DOI] [Google Scholar]
- 56.Albers PCH, and De Vries H (2001). Elo-rating as a tool in the sequential estimation of dominance strengths. Anim. Behav. 10.1006/anbe.2000.1571. [DOI] [Google Scholar]
- 57.Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, and Engelhardt A (2011). Assessing dominance hierarchies: Validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 10.1016/j.anbehav.2011.07.016. [DOI] [Google Scholar]
- 58.Neumann C, and Lars K (2014). EloRating: Animal Dominance Hierarchies by Elo Rating. R Packag. version 0.43. [Google Scholar]
- 59.Dettmer AM, Suomi SJ, and Hinde K (2014). Nonhuman primate models of mental health: Early life experiences affect developmental trajectories. In Ancestral landscapes in human evolution: Culture, childrearing and social wellbeing. [Google Scholar]
- 60.Real R, and Vargas JM (1996). The probabilistic basis of Jaccard’s index of similarity. Syst. Biol. 10.1093/sysbio/45.3.380. [DOI] [Google Scholar]
- 61.Rosenbaum S, and Silk JB (2022). Pathways to paternal care in primates. Evol. Anthropol. 10.1002/evan.21942. [DOI] [PubMed] [Google Scholar]
- 62.Stewart KJ (2001). Social relationships of immature gorillas and silverbacks. In Mountain Gorillas: Three Decades of Research at Karisoke (Cambridge University Press; ). 10.2307/3802725. [DOI] [Google Scholar]
- 63.Caillaud D, Eckardt W, Vecellio V, Ndagijimana F, Mucyo JP, Hirwa JP, and Stoinski T (2020). Violent encounters between social units hinder the growth of a high-density mountain gorilla population. Sci. Adv. 10.1126/SCIADV.ABA0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morrison RE, Hirwa JP, Ndagijimana F, Vecellio V, Eckardt W, and Stoinski TS (2022). Cascading effects of social dynamics on the reproduction, survival, and population growth of mountain gorillas. Anim. Conserv, 1–14. 10.1111/acv.12830. [DOI] [Google Scholar]
- 65.Cohen J (1960). A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 10.1177/001316446002000104. [DOI] [Google Scholar]
- 66.Rosenbaum S, Silk JB, and Stoinski TS (2011). Male-immature relationships in multi-male groups of mountain gorillas (Gorilla beringei beringei). Am. J. Primatol. 10.1002/ajp.20905. [DOI] [PubMed] [Google Scholar]
- 67.Robbins MM, and Robbins AM (2004). Simulation of the population dynamics and social structure of the Virunga Mountain gorillas. Am. J. Primatol. 10.1002/ajp.20052. [DOI] [PubMed] [Google Scholar]
- 68.R Core Team (2022). R: A language and environment for statistical computing. URL 10.1038/sj.hdy.6800737. [DOI]
- 69.Therneau TM (2022). A package for survival analysis in R; [“survival” version 3.2–13]. Compr. R Arch. Netw. [Google Scholar]
- 70.Therneau TM (2022). coxme: Mixed Effects Cox Models. R package version 2.2–18.1. Cran. [Google Scholar]
- 71.Qiu W, Chavarro J, Lazarus R, Rosner B, and Ma J (2021). powerSurvEpi: Power and Sample Size Calculation for Survival Analysis of Epidemiological Studies.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Demographic data have been deposited at Zenodo and are publicly available as of the date of publication. DOIs are listed in the key resources table.
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental subjects | ||
| Gorilla beringei beringei | Population monitored by Dian Fossey Gorilla Fund in Volcanoes National Park, Rwanda |
https://gorillafund.org |
| Deposited data | ||
| Raw data | This paper | https://doi.org/10.5281/zenodo.7780540 |
| Software and algorithms | ||
| R | R core team68 | www.r-project.org |
| survival | Therneau, 202269 | https://cran.r-project.org/web/packages/survival/ |
| coxme | Therneau, 202270 | https://cran.r-project.org/web/packages/coxme/ |
| powerSurvEpi | Qiu et al, 202171 | https://cran.r-project.org/web/packages/powerSurvEpi/ |
| EloRating | Neumann and Lars, 201458 | https://cran.r-project.org/web/packages/EloRating/ |


