Abstract
目的
:比较多种抗抑郁药治疗儿童青少年抑郁症的效果,为儿童青少年抑郁症的治疗提供疗效与耐受性方面的依据。
方法:
在PubMed、Cochrane Library、EMBASE、Web of Science、PsycINFO、中国生物医学文献数据库、中国知网和万方数据知识服务平台等数据库中检索关于抗抑郁药治疗儿童青少年抑郁症的随机对照试验,检索时间截至2021年12月。对纳入文献进行质量评价及数据提取,采用Stata 15.1软件对有效率、耐受性进行分析,采用累积排序概率曲线下面积(SUCAR)评估药物有效率和耐受性的累积概率。
结果:
最终纳入32篇文献的33项随机对照试验研究,共6949例患者,涉及阿米替林、维拉唑酮、氟西汀、司来吉兰、帕罗西汀、丙咪嗪、地昔帕明、舍曲林、去甲替林、艾司西酞普兰、西酞普兰、文拉法辛、度洛西汀等13种抗抑郁药。网状meta分析结果显示,度洛西汀( OR=1.95, 95% CI: 1.41~2.69)、氟西汀( OR=1.73,95% CI:1.40~2.14)、文拉法辛( OR=1.37, 95% CI: 1.04~1.80)、艾司西酞普兰( OR=1.48,95% CI:1.12~1.95)的有效率显著高于安慰剂(均 P<0.05),网状meta的累积概率排序为度洛西汀(87.0%)、阿米替林(83.3%)、氟西汀(79.0%)、艾司西酞普兰(62.7%)等。丙咪嗪( OR=0.15,95% CI:0.08~0.27)、舍曲林( OR=0.33, 95% CI: 0.16~0.71)、文拉法辛( OR=0.35,95% CI:0.17~0.72)、度洛西汀( OR=0.35, 95% CI: 0.17~0.73)、帕罗西汀( OR=0.52,95% CI:0.30~0.88)的不耐受发生率显著高于安慰剂(均 P<0.05),网状meta的累积概率排序为丙咪嗪(95.7%)、舍曲林(69.6%)、文拉法辛(68.6%)、度洛西汀(68.2%)等。
结论:
在13种抗抑郁药中,度洛西汀、氟西汀、艾司西酞普兰和文拉法辛治疗儿童青少年抑郁症的效果优于安慰剂,但度洛西汀和文拉法辛的耐受性较差。
Abstract
Objective
: To evaluate the efficacy and safety of antidepressants in treatment of depression disorder in children and adolescents by network meta-analysis.
Methods
: Databases of PubMed, Cochrane Library, EMBASE, Web of Science, PsycINFO, CBM, CNKI and Wanfang Data were searched for randomized controlled trials (RCT) related to antidepressants in treatment of children and adolescents with depression from inception to December 2021. Quality assessment and data extraction from the included RCTs were performed. Statistical analyses of efficacy and tolerability were conducted with Stata 15.1 software. Surface under the cumulative ranking (SUCAR) was used to rank the value of the antidepressants.
Results
: A total of 33 RCTs were included in 32 articles, involving 6949 patients. There are 13 antidepressants used in total, including amitriptyline, vilazodone, fluoxetine, selegiline, paroxetine, imipramine, desipramine, sertraline, nortriptyline, escitalopram, citalopram, venlafaxine and duloxetine. The results of network meta-analysis showed that the efficacy of duloxetine ( OR=1.95, 95% CI: 1.41–2.69), fluoxetine ( OR=1.73, 95% CI: 1.40–2.14), venlafaxine ( OR=1.37, 95% CI: 1.04–1.80) and escitalopram ( OR=1.48, 95% CI: 1.12–1.95) were significantly higher than that of placebos (all P<0.05); the probability cumulative ranks were duloxetine (87.0%), amitriptyline (83.3%), fluoxetine (79.0%), escitalopram (62.7%), etc. The results showed that the intolerability of patients receiving imipramine ( OR=0.15, 95% CI: 0.08–0.27), sertraline ( OR=0.33, 95% CI: 0.16–0.71), venlafaxine ( OR=0.35, 95% CI: 0.17–0.72), duloxetine ( OR=0.35, 95% CI: 0.17–0.73) and paroxetine ( OR=0.52, 95% CI: 0.30–0.88) were significantly higher than that of placebos (all P<0.05), and the probability cumulative ranks were imipramine (95.7%), sertraline (69.6%), venlafaxine (68.6%), duloxetine (68.2%), etc.
Conclusion
: Among 13 antidepressants, duloxetine, fluoxetine, escitalopram and venlafaxine are significantly better than placebo in terms of efficacy, but duloxetine and venlafaxine are less well tolerated.
Keywords: Children, Adolescents, Depression, Antidepressant, Efficacy, Tolerance, Meta-analysis
羟色胺(hydroxytryptamin, HT);比值比(odds ratio, OR);置信区间(confidence interval, CI);累积排序概率曲线下面积(surface under the cumulative ranking curve, SUCAR);
抑郁症又称抑郁障碍,可由各种原因引起,是一种表现为显著而持久的情绪低落的精神障碍。流行病学调查结果显示,全球抑郁症的患病率高达4.7%,约有3.4亿抑郁症患者 [1] ,抑郁症患病率逐年递增且患病年龄逐年递减,对儿童青少年健康的影响增加 [ 2- 3] 。抑郁症的病程长,需要长期规范的治疗,给家庭造成沉重的经济和精神负担,预测2030年将成为世界第二大疾病负担 [4] 。
对比成人抑郁症,儿童青少年抑郁症病程更长,首发年龄越小,复发率及自杀风险越高,将严重影响患者的学业、家庭关系、人际交往等 [5] 。药物是治疗儿童青少年抑郁症的主要方法之一,然而儿童青少年正处在生长发育期,神经系统中的5-HT和去甲肾上腺素递质系统尚未发育成熟,对于抗抑郁药的反应与成人有所不同 [ 6- 8] 。
目前抗抑郁药物主要有三环和四环类抗抑郁药、单胺氧化酶抑制剂、选择性5-HT再摄取抑制剂、5-HT和去甲肾上腺素再摄取抑制剂等 [9] 。但这些药物在儿童青少年抑郁症治疗中的疗效和不良反应还需要大样本分析证实。有研究结果显示,选择性5-HT再摄取抑制剂类药物氟西汀对儿童青少年抑郁症的疗效和耐受性较好 [ 10- 11] ,但也有研究显示氟西汀治疗儿童青少年抑郁症的疗效与安慰剂相比无明显差异 [12] ;5-HT和去甲肾上腺素再摄取抑制剂类药物、三环类、单胺氧化酶抑制剂抗抑郁药治疗儿童青少年抑郁症的疗效和耐受性目前也未能得出一致结论 [ 13- 17] 。本研究采用网状meta分析法分析了抗抑郁药在儿童青少年抑郁症中的疗效和耐受性,以期为临床用药提供循证依据。
资料与方法
文献纳入与排除标准
纳入标准:①研究对象为根据精神障碍诊断与统计手册 [ 18- 21] 、精神与行为障碍分类 [22] 和《中国精神障碍分类与诊断标准》 [23] 等诊断为抑郁症的儿童青少年,年龄在6~18岁。②研究类型为随机对照试验。③治疗措施为一种抗抑郁药与另一种抗抑郁药或安慰剂比较,患者的抗抑郁药物治疗剂量不限,治疗疗程为4~16周。④研究结果选取治疗第8周或距第8周最接近的结果,结局指标包括有效率和耐受性。有效率即抑郁症状量表得分降低50%及以上的患者数占总人数的比例;当研究中无有效率可选用缓解率代替,即抑郁症状量表得分降低至既定阈值以下的患者数占总人数的比例 [24] 。耐受性通过在治疗结束前因副作用退出并失访的患者数占总人数的比例来衡量。排除标准:①研究对象为难治性抑郁症患者;②排除总样本量小于10的试验;③报道的数据与结局指标不一致的文献;④重复发表的文献;⑤文献类型为个案报道、综述、meta分析等。
文献检索策略
检索PubMed、Cochrane Library、Web of Science、EMBASE、PsycINFO、中国生物医学文献数据库、中国知网和万方数据知识服务平台截至2021年12月的文献。中文检索词包括“抑郁症”“适应障碍”“抑郁障碍”“情感障碍”“情绪障碍”“恶劣心境障碍”“儿童”“青少年”“未成年”“氟西汀”“舍曲林”“帕罗西汀”“氟伏沙明”“西酞普兰”“艾司西酞普兰”“文拉法辛”“度洛西汀”“米那普仑”“安非他酮”“瑞波西汀”“米氮平”“米安舍林”“曲唑酮”“奈法唑酮”“丙咪嗪”“反苯环丙胺”“异卡波肼”“司来吉兰”“氯米帕明”“米帕明”“阿米替林”“去甲替林”“普罗替林”“马普替林”“阿莫沙平”“多塞平”“地昔帕明”“阿戈美拉汀”“维拉唑酮”“氢溴酸氟硫西汀”;英文检索词包括“affective disorders”“dysthymic disorder”“depressive disorder”“mood disorders”“adolescent”“child”“young adult”“minors”“pediatrics”“fluoxetine” “sertraline”“paroxetine”“citalopram”“escitalopram”“venlafaxine”“duloxetine”“milnacipran”“fluvoxamine”“bupropion”“reboxetine”“mirtazapine”“trazodone”“nefazodone”“tranylcypromine”“isocarboxazid”“selegiline”“clomipramine”“amitriptyline”“nortriptyline”“protriptyline”“maprotiline”“amoxapine”“doxepin”“desipramine”“agomelatine”“vilazodone”“vortioxetinehydrobromide”“imipramine”“mianserin”“phenelzine”。检索词根据具体数据库调整 , 采用MeSH(PubMed)、Emtree(EMBASE)等主题词与自由词相结合的方式。
文献筛选及数据提取
根据纳入和排除标准由两位研究者分别进行文献筛选、采用Excel提取数据并整理资料。两位研究者提取的数据有分歧时,通过全体讨论解决。提取以下内容:①基本情况:第一作者、发表期刊、发表年份、国家;②患者信息:诊断标准、年龄、性别、人数;③治疗措施:抗抑郁药、剂量、疗程、有效率、耐受性。
纳入文献质量评价
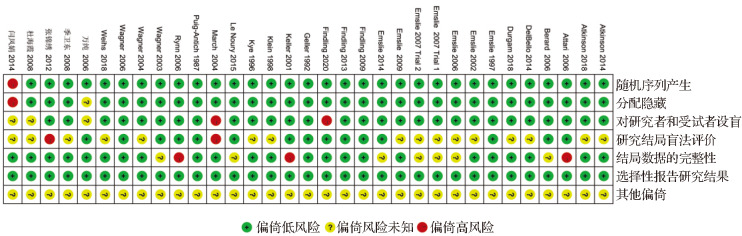
由两位研究者按照Cochrane系统评价员手册5.1.0推荐的风险偏倚评价工具独立评价研究质量。如评价存在分歧时,通过全体讨论解决。评价内容包括:随机方法、分配隐藏、对受试者和研究者施盲、结局评估的盲法、结果数据完整、选择性报道结果、其他偏倚来源。每个内容的评定分为“偏倚风险未知”、“偏倚低风险”和“偏倚高风险” [25] 。
统计学方法
采用Stata 15.1软件进行网状meta分析并绘制网状关系图,以呈现不同治疗措施间存在的直接比较和间接比较的关系。二分类变量采用 OR值及其95% CI表示。研究结果的整体异质性通过 I 2 检验评估, I 2 <50%提示各研究间无明显异质性。采用节点分裂法进行不一致性检验,若差异无统计学意义,提示直接比较和间接比较结果一致 [26] ;采用SUCAR评估每种治疗措施的累积概率,SUCAR值越大,说明该治疗措施的结局指标越靠前 [27] 。以 P<0.05为差异有统计学意义。
结果
文献筛选结果与纳入文献基本信息
数据库检索共2637篇摘要,排除重复文献1170篇,阅读文题和摘要后排除与主题不相符的文献1326篇,阅读全文后排除不符合纳入标准的文献109篇,最终纳入32篇文献,描述了共 33个随机对照试验研究 [ 10- 17, 28- 51] ,共计6949例患者 ( 图1)。 其中安慰剂组2726例、阿米替林组55例、司来吉兰组152例、氟西汀组833例、帕罗西汀组469例、维拉唑酮组542例、丙咪嗪组210例、舍曲林组327例、地昔帕明组23例、西酞普兰组185例、去甲替林组46例、艾司西酞普兰组441例、文拉法辛组599例、度洛西汀组341例。纳入文献的基本信息及治疗方案见 表1。
图 1.
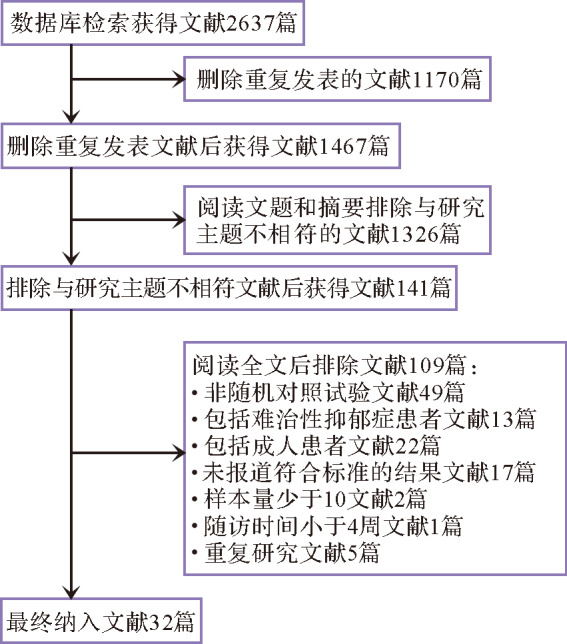
抗抑郁药物治疗儿童青少年抑郁症相关文献筛选流程图
|
编号 |
第一作者 [文献序号] |
发表年份 |
国家 |
诊断标准 |
分组及例数(用药剂量) |
治疗时间(周) |
年龄范围(岁) |
女性比例(%) |
结局指标 |
|
1 |
Findling [10] |
2009 |
美国 |
DSM-Ⅳ |
氟西汀 =18(10~20 mg/d)安慰剂=16 |
8 |
12~17 |
14.7 |
有效率 |
|
2 |
March [11] |
2004 |
美国 |
DSM-Ⅳ |
氟西汀 =109(10~40 mg/d)安慰剂=112 |
12 |
12~17 |
54.4 |
有效率、耐受性 |
|
3 |
Atkinson [12] |
2014 |
美国、俄罗斯、法国、芬兰、德国、乌克兰、爱沙尼亚、斯洛伐克 |
DSM-Ⅳ-TR |
度洛西汀 =117(60~120 mg/d)氟西汀 =117(20~40 mg/d)安慰剂=103 |
10 |
7~17 |
52.2 |
有效率、耐受性 |
|
4 |
Attari [13] |
2006 |
伊朗 |
DSM-Ⅳ |
去甲替林 =20(2 mg·kg –1·d –1) 氟西汀 =20(1 mg·kg –1·d –1) |
8 |
7~16 |
50.0 |
有效率 |
|
5 |
DelBello [14] |
2014 |
美国 |
DSM-Ⅳ-TR |
司来吉兰 =152(6~24 mg/d)安慰剂=156 |
12 |
12~17 |
64.0 |
有效率、耐受性 |
|
6 |
Kye [15] |
1996 |
美国 |
K-SADS和RDC |
阿米替林 =18(5 mg·kg –1·d –1) 安慰剂=13 |
8 |
13~18 |
29.0 |
有效率、耐受性 |
|
7 |
Weihs [16] |
2018 |
美国、墨西哥 |
DSM-Ⅳ-TR |
文拉法辛 =115(25~50 mg/d)氟西汀 =112(20 mg/d)安慰剂=112 |
8 |
7~18 |
54.3 |
有效率、耐受性 |
|
8 |
杜海霞 [17] |
2008 |
中国 |
CCMD-3 |
文拉法辛 =32(100~250 mg/d)氟西汀 =33(20~40 mg/d) |
6 |
9~16 |
60.0 |
有效率 |
|
9 |
Wagner [28] |
2006 |
美国 |
DSM-IV |
艾司西酞普兰=131(10~20 mg/d)安慰剂=133 |
8 |
6~17 |
51.9 |
有效率、耐受性 |
|
10 |
Puig-Antich [29] |
1987 |
美国 |
K-SADS和RDC |
丙咪嗪 =20(2~5 mg·kg –1·d –1) 安慰剂=22 |
5 |
6~18 |
36.8 |
有效率 |
|
11 |
Geller [30] |
1992 |
未提及 |
DSM-Ⅲ |
去甲替林 =26(140 mg/d)安慰剂=24 |
10 |
6~12 |
30.0 |
有效率 |
|
12 |
Findling [31] |
2013 |
美国 |
DSM-Ⅳ-TR |
艾司西酞普兰=155(10~20 mg/d)安慰剂=157 |
8 |
12~17 |
59.0 |
有效率、耐受性 |
|
13 |
Emslie [32] |
1997 |
美国 |
DSM-Ⅲ-R |
氟西汀 =48(20 mg/d)安慰剂=48 |
8 |
7~17 |
45.8 |
有效率、耐受性 |
|
14 |
Wagner [33] |
2003 |
美国、印度、加拿大、哥斯达黎加、墨西哥 |
DSM-Ⅳ |
舍曲林 =189(200 mg/d)安慰剂=187 |
10 |
6~17 |
51.1 |
有效率、耐受性 |
|
15 |
Rynn [34] |
2006 |
美国、加拿大、哥斯达黎加、墨西哥 |
DSM-Ⅳ |
舍曲林 =100(50~200 mg/d)安慰剂=126 |
10 |
6~17 |
48.0 |
有效率、耐受性 |
|
16 |
Le Noury [35] |
2015 |
美国、加拿大 |
DSM-Ⅳ |
帕罗西汀 =93(20~40 mg/d)丙咪嗪 =95(200~300 mg/d)安慰剂=87 |
8 |
12~18 |
62.2 |
有效率、耐受性 |
|
17 |
Keller [36] |
2001 |
美国、加拿大 |
DSM-Ⅳ |
帕罗西汀 =93(20~40 mg/d)丙咪嗪 =95(200~300 mg/d)安慰剂=87 |
8 |
12~18 |
62.2 |
有效率、耐受性 |
|
18 |
Emslie [37] |
2014 |
美国、加拿大、墨西哥 |
DSM-Ⅳ-TR |
度洛西汀 =224(30~60 mg/d)氟西汀 =117(20 mg/d)安慰剂=122 |
10 |
7~17 |
51.2 |
有效率、耐受性 |
|
19 |
Berard [38] |
2006 |
英国、加拿大、意大利、墨西哥、西班牙、荷兰、比利时、阿根廷、南非、阿联酋 |
DSM-Ⅳ |
帕罗西汀 =182(20~40 mg/d)安慰剂=93 |
8 |
13~18 |
66.5 |
有效率、耐受性 |
|
20 |
Durgam [39] |
2018 |
美国 |
DSM-Ⅳ-TR |
维拉唑酮 =355(15~30 mg/d)安慰剂=171 |
8 |
12~17 |
59.5 |
有效率、耐受性 |
|
21 |
Emslie [40] |
2002 |
美国 |
DSM-Ⅳ |
氟西汀 =109(20 mg/d)安慰剂=110 |
10 |
8~18 |
49.3 |
有效率、耐受性 |
|
22 |
Emslie [41] |
2009 |
美国 |
DSM-Ⅳ |
艾司西酞普兰=155(10~20 mg/d)安慰剂=157 |
8 |
12~17 |
59.0 |
有效率、耐受性 |
|
23 |
Wagner [42] |
2004 |
美国 |
DSM-Ⅳ |
西酞普兰=89 (20~40 mg/d)安慰剂=85 |
8 |
7~17 |
53.4 |
有效率、耐受性 |
|
24 |
张锦绣 [43] |
2012 |
中国 |
ICD-10 |
西酞普兰 =36(10~20 mg/d)舍曲林 =38(50~150 mg/d) |
6 |
14~18 |
55.4 |
有效率 |
|
25 |
季卫东 [44] |
2008 |
中国 |
CCMD-3 |
文拉法辛 =30(150 mg/d)氟西汀 =30(20 mg/d) |
8 |
12~18 |
46.7 |
有效率 |
|
26 |
Emslie [45]* |
2007 |
美国 |
DSM-Ⅳ |
文拉法辛 =179(225 mg/d)安慰剂=175 |
8 |
7~17 |
45.5 |
有效率、耐受性 |
|
27 |
Emslie [46] |
2006 |
美国、加拿大 |
DSM-Ⅳ |
帕罗西汀 =101(10~50 mg/d)安慰剂=102 |
8 |
7~17 |
46.8 |
有效率、耐受性 |
|
28 |
Findling [47] |
2020 |
美国、加拿大 |
DSM-Ⅳ-TR |
维拉唑酮 =187(15~30 mg/d)氟西汀 =97(20 mg/d)安慰剂=186 |
8 |
7~17 |
60.2 |
有效率、耐受性 |
|
29 |
万纯 [48] |
2006 |
中国 |
CCMD-3 |
西酞普兰 =38(10~30 mg/d)阿米替林 =37(50~150 mg/d) |
8 |
14~18 |
42.7 |
有效率 |
|
30 |
闫凤娟 [49] |
2014 |
中国 |
CCMD-3 |
西酞普兰 =22(10~20 mg/d)氟西汀 =23(20~40 mg/d) |
6 |
14~18 |
48.9 |
有效率 |
|
31 |
Klein [50] |
1998 |
美国 |
DSM-Ⅲ-R |
地昔帕明 =23(50~300 mg/d)安慰剂=22 |
6 |
6~17 |
67.0 |
有效率、耐受性 |
|
32 |
Atkinson [51] |
2018 |
美国、智利 |
DSM-Ⅲ-R |
文拉法辛 =243(25~50 mg/d)安慰剂=120 |
8 |
7~18 |
56.5 |
有效率、耐受性 |
纳入文献的质量评价结果
33项研究均为随机对照试验 [ 10- 17, 28- 51] 。 其中32项研究 描述了正确的随机序列产生方 法 [ 10- 17, 28- 48, 50- 51] ; 31项研究使 用了分配隐藏,受试 者和研究者均无法预测分配结果 [ 10- 17, 28- 47, 50- 51] ; 28项研究 对受试者、研究人员设 盲 [ 10, 12- 16, 28- 46, 50- 51] ; 15项研究对结局进行盲法评定 [ 10, 13, 28- 38, 47- 48] ;23项研究的结局数据完整 [ 10- 12, 14- 17, 28- 32, 39- 44, 47- 51] 。33项研究均未选择性报道研究结果,是否存在其他偏倚来源均不清楚。见 图2。
图2 .
纳入研究偏倚风险分析结果
网状meta分析结果
纳入研究的证据关系
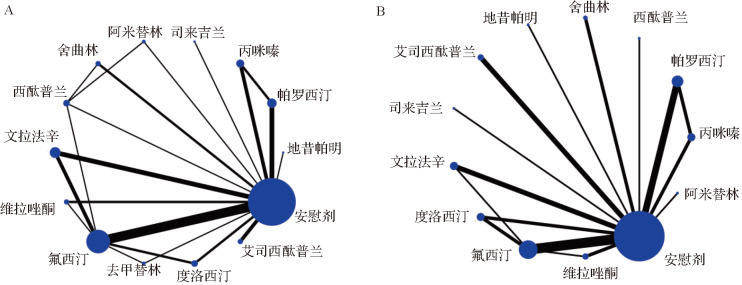
本研究共包含 13种药物:阿米替林、地昔帕明、氟西汀、帕罗西汀、丙咪嗪、舍曲林、艾司西酞普兰、文拉法辛、度洛西汀、司来吉兰、去甲替林、西酞普兰、维拉唑酮,所有结局指标的证据关系图见 图3。
图3 .
抗抑郁药物有效率和耐受性证据关系图
A:13种抗抑郁药的有效率证据关系图;B:12种抗抑郁药物的耐受性证据关系图.
纳入研究的异质性和不一致性检验结果
研究结果指标的整体异质性 I 2 值为有效率40%、耐受性19.5%,提示无明显异质性。整体不一致性检测显示,有效率的一致模型和不一致模型差异具有统计学意义( P=0.025),耐受性差异无统计学意义( P=0.575)。局部不一致性检测显示:有效率的8个闭合环(氟西汀-去甲替林-安慰剂、西酞普兰-阿米替林-安慰剂、度洛西汀-氟西汀-安慰剂、西酞普兰-氟西汀-安慰剂、西酞普兰-舍曲林-安慰剂、丙咪嗪-帕罗西汀-安慰剂、氟西汀-安慰剂-文拉法辛、氟西汀-安慰剂-维拉唑酮)中有2个差异具有统计学意义,分别为:氟西汀-去甲替林-安慰剂( P=0.029)、度洛西汀-氟西汀-安慰剂( P=0.014);耐受性的4个闭合环(维拉唑酮-氟西汀-安慰剂、文拉法辛-氟西汀-安慰剂、度洛西汀-氟西汀-安慰剂、丙咪嗪-帕罗西汀-安慰剂)差异均无统计学意义( P>0.05);点分裂模型不一致性检测结果显示,有效率氟西汀与度洛西汀( P=0.005)、安慰剂与度洛西汀( P=0.005)、去甲替林与氟西汀( P<0.027)、安慰剂与去甲替林( P<0.027)差异具有统计学意义;耐受性差异均无统计学意义(均 P>0.05)。
抗抑郁药有效率比较
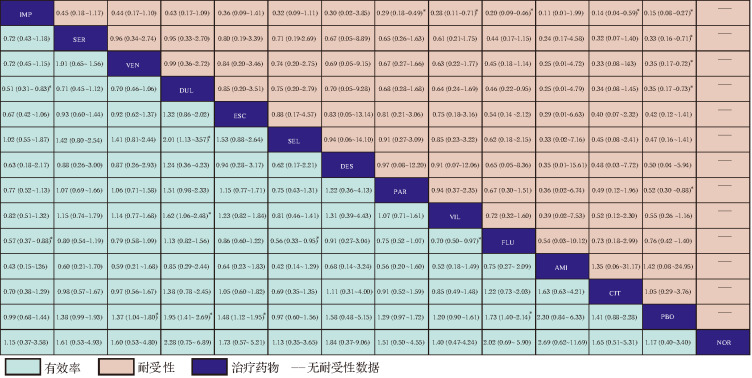
33项研究报道了临床有效率,共涉及13种抗抑郁药。网状meta分析结果显示,度洛西汀( OR=1.95, 95% CI: 1.41~2.69)、氟西汀( OR=1.73,95% CI:1.40~2.14)、文拉法辛( OR=1.37,95% CI:1.04~1.80)、艾司西酞普兰( OR=1.48,95% CI:1.12~1.95)的有效率显著高于安慰剂(均 P<0.05),见 图4。网状meta的累积概率排序为:度洛西汀(87.0%)、阿米替林(83.3%)、氟西汀(79.0%)、艾司西酞普兰(62.7%)、地昔帕明(60.1%)、西酞普兰(55.9%)、舍曲林(54.2%)、文拉法辛(52.8%)、帕罗西汀(46.9%)、维拉唑酮(38.7%)、去甲替林(22.6%)、司来吉兰(20.0%)、丙咪嗪(19.7%)、安慰剂(17.2%)。
图4 .
抗抑郁药物有效率和耐受性网状meta分析结果 [ OR(95% CI)]
AMI:阿米替林;DES:地昔帕明;FLU:氟西汀;PAR:帕罗西汀;IMP:丙咪嗪;SER:舍曲林;ESC:艾司西酞普兰;VEN:文拉法辛;DUL:度洛西汀;SEL:司来吉兰;NOR:去甲替林;CIT:西酞普兰;VIL:维拉唑酮;PBO:安慰剂; *差异有统计学意义.
抗抑郁药耐受性比较
23项研究报道了临床耐受性,共涉及除去甲替林外的12种抗抑郁药。网状meta分析结果( 图4)显示,丙咪嗪( OR=0.15,95% CI: 0.08~0.27)、舍曲林( OR=0.33,95% CI: 0.16~0.71)、文拉法辛( OR=0.35,95% CI: 0.17~0.72)、度洛西汀( OR=0.35,95% CI: 0.17~0.73)、帕罗西汀( OR=0.52,95% CI: 0.30~0.88)的不耐受发生率显著高于安慰剂(均 P<0.05)。网状meta的累积概率排序为:丙咪嗪(95.7%)、舍曲林(69.6%)、文拉法辛(68.6%)、度洛西汀(68.2%)、艾司西酞普兰(58.8%)、司来吉兰(52.8%)、地昔帕明(51.1%)、帕罗西汀(48.5%)、维拉唑酮(46.2%)、氟西汀(28.3%)、阿米替林(25.4%)、西酞普兰(21.4%)、安慰剂(15.2%)。
讨论
抑郁障碍以显著而持久的心境低落为主要临床特征,表现可从闷闷不乐到悲痛欲绝,常有躯体、精神病性症状,对学习、社交等功能造成一定的损害,也是导致青少年自杀、自伤的常见原因 [52] 。抑郁症的发病机制与脑内5-HT、去甲肾上腺素、多巴胺的浓度下降或功能低下有关。因此抗抑郁药以增加5-HT、去甲肾上腺素和多巴胺中的一种或几种单胺递质的突出功能,即阻断突出前膜转运体的再摄取,从而使患者恢复到正常状态。
选择性5-HT再摄取抑制剂通过抑制5-HT转运体,拮抗突触前膜对5-HT再摄取,从而增加突出间隙5-HT水平来产生效果,但同时会使所有通路和所有受体处的5-HT均增加,导致不良反应产生。如刺激脑干或下丘脑的5-HT 3受体可能会引起恶心、呕吐等不适,当刺激到胃肠道的5-HT 3受体及5-HT 4受体会引起肠蠕动增加、腹泻等不适。这些不良反应虽然没有很大的危险,但也可能会导致患者因不能耐受而停药 [53] 。本文资料显示,选择性5-HT再摄取抑制剂类抗抑郁药中的氟西汀对于儿童青少年抑郁症患者具有显著的有效率和良好的耐受性。Zhou等 [54] 系统评价同样表明氟西汀可有效减轻儿童青少年抑郁症患者的抑郁症状,且具有较好的耐受性。现有指南也已推荐氟西汀作为治疗儿童青少年抑郁症的一线药物。本研究结果还显示,艾司西酞普兰与氟西汀效果相当。目前其他儿童青少年抗抑郁药的系统评价中尚无艾司西酞普兰相关报道,可能的原因是艾司西酞普兰在2009年才被美国食品药品监督管理局批准用于青少年抑郁症治疗。从药理学机制上看,艾司西酞普兰是5-HT转运体抑制剂,对细胞色素酶P450的影响小,因此其耐受性较好,安全性较高。综上所述,氟西汀和艾司西酞普兰是儿童青少年抑郁症治疗的最佳选择。
本文资料显示,在疗效方面,度洛西汀和文拉法辛同样显著优于安慰剂,但其耐受性明显差于安慰剂,意味其不良反应较严重,导致部分患者不能耐受而停药。Boaden等 [55] 通过meta分析显示,文拉法辛和度洛西汀在儿童青少年抑郁症中耐受性较差。度洛西汀和文拉法辛属于5-HT和去甲肾上腺素再摄取抑制剂,在5-HT转运体抑制基础上加上去甲肾上腺素转运体抑制,提升了全脑各脑区三种胺类神经递质的稳定性,相应疗效增加。但同样也存在去甲肾上腺素转运体抑制后产生的不良反应,如刺激脑干心血管中心和下行到脊髓的去甲肾上腺素受体会改变血压,刺激腹侧前额叶皮质的去甲肾上腺素受体可引起激越 [53] 。因此,与选择性5-HT再摄取抑制剂比较,5-HT和去甲肾上腺素再摄取抑制剂类药物更容易引起高血压、失眠、呕吐等不良反应 [56] 。目前,度洛西汀和文拉法西治疗儿童青少年抑郁症均属超说明书用药,尤其对于药物耐受较差的儿童青少年抑郁症患者需谨慎使用。
此外,Hetrick等 [57] 研究显示,帕罗西汀及维拉唑酮的疗效优于文拉法辛,与本文结果有所不同,可能的原因是以上研究纳入了维拉唑酮未发表的临床试验,以及纳入帕罗西汀文献较少。因此,帕罗西汀及维拉唑酮治疗儿童青少年抑郁症患者的疗效和安全性仍有待更多的研究来证实。
综上所述,在13种抗抑郁药中,度洛西汀、氟西汀、艾司西酞普兰和文拉法辛治疗儿童青少年抑郁症的效果优于安慰剂,但度洛西汀和文拉法辛的耐受性较差。本研究的局限性为:①有效率指标中度洛西汀-氟西汀-安慰剂和去甲替林-氟西汀-安慰剂这两个闭合环的直接结果和间接结果不一致。去甲替林-氟西汀-安慰剂环路很可能是由时间效应引起的,本研究纳入去甲替林试验较为早期,其临床试验质量有所不足,且文献中纳入患者数有限,可能会造成偏倚,对结果产生误差,其他网络meta分析有相似的发现 [54] 。度洛西汀-氟西汀-安慰剂的环路考虑为度洛西汀相关研究的纳入量不足从而导致结果不一致。②本研究排除了仅纳入难治性抑郁症患者的研究,可能会夸大药物对儿童青少年抑郁症的治疗效果。③研究中13种抗抑郁药中缺乏去甲替林的耐受性数据,因此对其的综合评估存在一定的偏差,仍需更多高质量、大样本的随机对照试验予以验证。
COMPETING INTERESTS
所有作者均声明不存在利益冲突
Funding Statement
国家自然科学基金(81973060)
References
- 1.FERRARI A J, SOMERVILLE A J, BAXTER A J, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature[J] Psychol Med. . 2013;43(3):471–481. doi: 10.1017/S0033291712001511. [DOI] [PubMed] [Google Scholar]
- 2.VOS T, LIM S S, ABBAFATI C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990—2019: a systematic analysis for the Global Burden of Disease Study 2019[J] Lancet. . 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MOJTABAI R, OLFSON M, HAN B. National trends in the prevalence and treatment of depression in adolescents and young adults[J/OL] Pediatrics. . 2016;138(6):e20161878. doi: 10.1542/peds.2016-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MATHERS C D, LONCAR D. Projections of global mortality and burden of disease from 2002 to 2030[J/OL] PLoS Med. . 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ALAIE I, SSEGONJA R, PHILIPSON A, et al. Adolescent depression, early psychiatric comorbidities, and adulthood welfare burden: a 25-year longitudinal cohort study[J] Soc Psychiatry Psychiatr Epidemiol. . 2021;56(11):1993–2004. doi: 10.1007/s00127-021-02056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HAZELL P. Updates in treatment of depression in children and adolescents[J] Curr Opin Psychiatry. . 2021;34(6):593–599. doi: 10.1097/YCO.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 7.COUSINS L, GOODYER I M. Antidepressants and the adolescent brain[J] J Psychopharmacol. . 2015;29(5):545–555. doi: 10.1177/0269881115573542. [DOI] [PubMed] [Google Scholar]
- 8.MERUELO A D, BRUMBACK T, NAGEL B J, et al. Neuroimaging markers of adolescent depression in the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) study[J] J Affective Disord. . 2021;287:380–386. doi: 10.1016/j.jad.2021.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.高贵元, 黄 捷, 刘 丹, 等. 抑郁症的发病机制及抗抑郁药物的研究进展[J]. 中国医药导报, 2021, 18(1): 52-55, 70 ; GAO Guiyuan, HUANG Jie, LIU Dan, et al. The pathogenesis of depression and the research progress of antidepressants[J]. China Medical Herald, 2021, 18(1): 52-55, 70. (in Chinese)
- 10.FINDLING R L, PAGANO M E, MCNAMARA N K, et al. The short-term safety and efficacy of fluoxetine in depressed adolescents with alcohol and cannabis use disorders: a pilot randomized placebo-controlled trial[J] Child Adolesc Psychiatry Ment Health. . 2009;3(1):11. doi: 10.1186/1753-2000-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MARCH J, SILVA S, PETRYCKI S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression[J] JAMA. . 2004;292(7):807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 12.ATKINSON S D, PRAKASH A, ZHANG Q, et al. A double-blind efficacy and safety study of duloxetine flexible dosing in children and adolescents with major depressive disorder[J] J Child Adolesc Psychopharmacol. . 2014;24(4):180–189. doi: 10.1089/cap.2013.0146. [DOI] [PubMed] [Google Scholar]
- 13.ATTARI A, YADOLLAH M F, HASAN Z A, et al. Comparison of efficacy of fluoxetine with nortriptyline in treatment of major depression in children and adolescents: a double-blind study[J]. J Res Med Sci, 2006, 11: 24-30
- 14.DELBELLO M P, HOCHADEL T J, PORTLAND K B, et al. A double-blind, placebo-controlled study of selegiline transdermal system in depressed adolescents[J] J Child Adolesc Psychopharmacol. . 2014;24(6):311–317. doi: 10.1089/cap.2013.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KYE C H, WATERMAN G S, RYAN N D, et al. A randomized, controlled trial of amitriptyline in the acute treatment of adolescent major depression[J] J Am Acad Child Adolesc Psychiatry. . 1996;35(9):1139–1144. doi: 10.1097/00004583-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 16.WEIHS K L, MURPHY W, ABBAS R, et al. Desvenlafaxine versus placebo in a fluoxetine- referenced study of children and adolescents with major depressive disorder[J] J Child Adolesc Psychopharmacol. . 2018;28(1):36–46. doi: 10.1089/cap.2017.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.杜海霞, 郭 芳, 郭素芹, 等. 文拉法辛与氟西汀治疗儿童抑郁症对照研究[J]. 临床心身疾病杂志, 2008, 14(1): 7-9 ; DU Haixia, GUO Fang, GUO Suqin, et al. A control study of venlafaxine vs fluoxetine in the treatment of child depression[J]. Journal of Clinical Psychosomatic Diseases, 2008, 14(1): 7-9. (in Chinese)
- 18.BRESLAU N, DAVIS G C. Refining DSM-Ⅲ criteria in major depression. An assessment of the descriptive validity of criterion symptoms[J]. J Affect Disord, 1985, 9(3): 199-206 . [DOI] [PubMed]
- 19.KESSLER R C, WALTERS E E. Epidemiology of DSM-Ⅲ-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey[J]. Depress Anxiety, 1998, 7(1): 3-14 . [DOI] [PubMed]
- 20.SIMON G E, VON KORFF M. Medical co-morbidity and validity of DSM-Ⅳ depression criteria[J]. Psychol Med, 2006, 36(1): 27-36 . [DOI] [PubMed]
- 21.MASKE U E, BUTTERY A K, BEESDO-BAUM K, et al. Prevalence and correlates of DSM-Ⅳ-TR major depressive disorder, self-reported diagnosed depression and current depressive symptoms among adults in Germany[J]. J Affect Disord, 2016, 190: 167-177 . [DOI] [PubMed]
- 22.PEDERSEN S H, STAGE K B, BERTELSEN A, et al. ICD-10 criteria for depression in general practice[J]. J Affect Disord, 2001, 65(2): 191-194 . [DOI] [PubMed]
- 23.中华医学会精神科分会. 中国精神疾病分类方案与诊断标准[M]. 3版. 济南: 山东科学技术出版社, 2001: 87-88 ; Chinese Society of Psychiatry of Chinese Medical Association. Chinese classification of mental disorders (CCMD-3)[M]. 3rd ed. Jinan: Shandong Science and Technology Press, 2001: 87-88. (in Chinese)
- 24.RIEDEL M, MÖLLER H J, OBERMEIER M, et al. Response and remission criteria in major depression — a validation of current practice[J] J Psychiatric Res. . 2010;44(15):1063–1068. doi: 10.1016/j.jpsychires.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 25.张 渊, 杨智荣, 孙 凤, 等. 偏倚风险评估系列: (九) 如何应用偏倚风险评估的结果[J]. 中华流行病学杂志, 2018, 39(12): 1648-1654 . [DOI] [PubMed]; ZHANG Yuan, YANG Zhirong, SUN Feng, et al. Risk of bias assessment: (9) application of the risk of bias assessment results[J]. Chinese Journal of Epidemiology, 2018, 39(12): 1648-1654. (in Chinese) . [DOI] [PubMed]
- 26.张 超, 鄢金柱, 孙 凤, 等. 网状meta分析一致性的鉴别与处理方法[J]. 中国循证医学杂志, 2014, 14(7): 884-888 ; ZHANG Chao, YAN Jinzhu, SUN Feng, et al. Differentiation and handling of homogeneity in network meta- analysis[J]. Chinese Journal of Evidence-Based Medicine, 2014, 14(7): 884-888. (in Chinese)
- 27.SHIM S, YOON B H, SHIN I S, et al. Network meta-analysis: application and practice using Stata[J/OL] Epidemiol Health. . 2017;39:e2017047. doi: 10.4178/epih.e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WAGNER K D, JONAS J, FINDLING R L, et al. A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression[J] J Am Acad Child Adolesc Psychiatry. . 2006;45(3):280–288. doi: 10.1097/01.chi.0000192250.38400.9e. [DOI] [PubMed] [Google Scholar]
- 29.PUIG-ANTICH J, PEREL J M, LUPATKIN W, et al. Imipramine in prepubertal major depressive disorders[J] Arch Gen Psychiatry. . 1987;44(1):81–89. doi: 10.1001/archpsyc.1987.01800130093012. [DOI] [PubMed] [Google Scholar]
- 30.GELLER B, COOPER T B, GRAHAM D L, et al. Pharmacokinetically designed double-blind placebo-controlled study of nortriptyline in 6 to 12-year-olds with major depressive disorder[J] J Am Acad Child Adolesc Psychiatry. . 1992;31(1):34–44. doi: 10.1097/00004583-199201000-00007. [DOI] [PubMed] [Google Scholar]
- 31.FINDLING R L, ROBB A, BOSE A. Escitalopram in the treatment of adolescent depression: a randomized, double-blind, placebo-controlled extension trial[J] J Child Adolesc PsychoPharmacol. . 2013;23(7):468–480. doi: 10.1089/cap.2012.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.EMSLIE G J, RUSH A J, WEINBERG W A, et al. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression[J] Arch Gen Psychiatry. . 1997;54(11):1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- 33.WAGNER K D, AMBROSINI P, RYNN M, et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder two randomized controlled trials[J] JAMA. . 2003;290(8):1033–1041. doi: 10.1001/jama.290.8.1033. [DOI] [PubMed] [Google Scholar]
- 34.RYNN M, WAGNER K D, DONNELLY C, et al. Long-term sertraline treatment of children and adolescents with major depressive disorder[J] J Child Adolesc Psychopharmacol. . 2006;16(1-2):103–116. doi: 10.1089/cap.2006.16.103. [DOI] [PubMed] [Google Scholar]
- 35.LE NOURY J, NARDO J M, HEALY D, et al. Restoring Study 329: efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence[J] BMJ. . 2015;351:h4320. doi: 10.1136/bmj.h4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.KELLER M B, RYAN N D, STROBER M, et al. Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial[J] J Am Acad Child Adolesc Psychiatry. . 2001;40(7):762–772. doi: 10.1097/00004583-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 37.EMSLIE G J, PRAKASH A, ZHANG Q, et al. A double-blind efficacy and safety study of duloxetine fixed doses in children and adolescents with major depressive disorder[J] J Child Adolesc Psychopharmacol. . 2014;24(4):170–179. doi: 10.1089/cap.2013.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.BERARD R, FONG R, CARPENTER D J, et al. An international, multicenter, placebo-controlled trial of paroxetine in adolescents with major depressive disorder[J] J Child Adolesc Psychopharmacol. . 2006;16(1-2):59–75. doi: 10.1089/cap.2006.16.59. [DOI] [PubMed] [Google Scholar]
- 39.DURGAM S, CHEN C, MIGLIORE R, et al. A phase 3, double-blind, randomized, placebo-controlled study of vilazodone in adolescents with major depressive disorder[J] Pediatr Drugs. . 2018;20(4):353–363. doi: 10.1007/s40272-018-0290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EMSLIE G J, HEILIGENSTEIN J H, WAGNER K D, et al. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial[J] J Am Acad Child Adolesc Psychiatry. . 2002;41(10):1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 41.EMSLIE G J, VENTURA D, KOROTZER A, et al. Escitalopram in the treatment of adolescent depression: a randomized placebo-controlled multisite trial[J] J Am Acad Child Adolesc Psychiatry. . 2009;48(7):721–729. doi: 10.1097/CHI.0b013e3181a2b304. [DOI] [PubMed] [Google Scholar]
- 42.WAGNER K D, ROBB A S, FINDLING R L, et al. A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents[J] Am J Psychiatry. . 2004;161(6):1079–1083. doi: 10.1176/appi.ajp.161.6.1079. [DOI] [PubMed] [Google Scholar]
- 43.张锦绣. 艾司西酞普兰与舍曲林治疗青少年抑郁障碍的对照研究[J]. 中国民族民间医药, 2012, 21(16): 27-28 ; ZHANG Jinxiu. A control study of escitalopram vs sertraline in the treatment of juvenile depression[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy, 2012, 21(16): 27-28. (in Chinese)
- 44.季卫东, 郭田友, 杨 闯, 等. 盐酸文拉法辛缓释胶囊治疗青少年抑郁症对照研究[J]. 中国临床药理学与治疗学, 2008, 13(1): 117-120 ; JI Weidong, GUO Tianyou, YANG Chuang, et al. A randomized and double-blind clinical trail of venlafaxine hydrochloride sustained release capsules for treating juvenile depression[J]. Chinese Journal of Clinical Pharmacology and Therapeutics, 2008, 13(1): 117-120. (in Chinese)
- 45.EMSLIE G J, FINDLING R L, YEUNG P P, et al. Venlafaxine ER for the treatment of pediatric subjects with depression[J] J Am Acad Child Adolesc Psychiatry. . 2007;46(4):479–488. doi: 10.1097/chi.0b013e31802f5f03. [DOI] [PubMed] [Google Scholar]
- 46.EMSLIE G J, WAGNER K D, KUTCHER S, et al. Paroxetine treatment in children and adolescents with major depressive disorder: a randomized, multicenter, double-blind, placebo-controlled trial[J] J Am Acad Child Adolesc Psychiatry. . 2006;45(6):709–719. doi: 10.1097/01.chi.0000214189.73240.63. [DOI] [PubMed] [Google Scholar]
- 47.FINDLING R L, MCCUSKER E, STRAWN J R. A randomized, double-blind, placebo-controlled trial of vilazodone in children and adolescents with major depressive disorder with twenty-six-week open-label follow- up[J] J Child Adolesc Psychopharmacol. . 2020;30(6):355–365. doi: 10.1089/cap.2019.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.万 纯, 兰胜作, 王宇峰, 等. 西酞普兰与阿米替林治疗首发少年抑郁症的对照研究[J]. 中国行为医学科学, 2006, 15(12): 1137-1138 ; WAN Chun, LAN Shengzuo, WANG Yufeng, et al. A control study of citalopram vs amitriptyline in the treatment of juvenile depression[J]. Chinese Journal of Behavioral Medicine and Brain Science, 2006, 15(12): 1137-1138. (in Chinese)
- 49.闫凤娟, 刘雪峰. 艾司西酞普兰与氟西汀治疗儿童抑郁症对照研究[J]. 中国中西医结合儿科学, 2014, 6(2): 139-140 ; YAN Fengjuan, LIU Xuefeng. A control study of escitalopram vs fluoxetine in the treatment of children depression[J]. Chinese Pediatrics of Integrated Traditional and Western Medicine, 2014, 6(2): 139-140. (in Chinese)
- 50.KLEIN R G, MANNUZZA S, KOPLEWICZ H S, et al. Adolescent depression: controlled desipramine treatment and atypical features[J] Depress Anxiety. . 1998;7(1):15–31. doi: 10.1002/(SICI)1520-6394(1998)7:1<15::AID-DA3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.ATKINSON S, LUBACZEWSKI S, RAMAKER S, et al. Desvenlafaxine versus placebo in the treatment of children and adolescents with major depressive disorder[J] J Child Adolesc Psychopharmacol. . 2018;28(1):55–65. doi: 10.1089/cap.2017.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.IZAKI Y. Depression among adolescents: clinical features and interventions[J]. J Med Invest, 2021, 68(1.2): 22-28 . [DOI] [PubMed]
- 53.STAHL S M. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications[M]. 4th ed. Cambridge: Cambridge University Press, 2013: 433-468
- 54.ZHOU X, TENG T, ZHANG Y, et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta- analysis[J] Lancet Psychiatry. . 2020;7(7):581–601. doi: 10.1016/S2215-0366(20)30137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.BOADEN K, TOMLINSON A, CORTESE S, et al. Antidepressants in children and adolescents: meta-review of efficacy, tolerability and suicidality in acute treatment[J] Front Psychiatry. . 2020;11:717. doi: 10.3389/fpsyt.2020.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.李 玥, 贺 敏, 张磊阳, 等. 抗抑郁药物的研究进展[J]. 临床药物治疗杂志, 2017, 15(1): 8-13 ; LI Yue, HE Min, ZHANG Leiyang, et al. Progress on antidepressants[J]. Clinical Medication Journal, 2017, 15(1): 8-13. (in Chinese)
- 57.HETRICK S E, MCKENZIE J E, BAILEY A P, et al. New generation antidepressants for depression in children and adolescents: a network meta- analysis[J] Cochrane Database Syst Rev. . 2021;5(5):CD013674. doi: 10.1002/14651858.CD013674.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]