Abstract
Objectives
The distinction between bipolar I disorder (BD‐I) and bipolar II disorder (BD‐II) has been a topic of long‐lasting debate. This study examined differences between BD‐I and BD‐II in a large, global sample of OABD, focusing on general functioning, cognition and somatic burden as these domains are often affected in OABD.
Methods
Cross‐sectional analyses were conducted with data from the Global Aging and Geriatric Experiments in Bipolar Disorder (GAGE‐BD) database. The sample included 963 participants aged ≥50 years (714 BD‐I, 249 BD‐II). Sociodemographic and clinical factors were compared between BD subtypes including adjustment for study cohort. Multivariable analyses were conducted with generalized linear mixed models (GLMMs) and estimated associations between BD subtype and (1) general functioning (GAF), (2) cognitive performance (g‐score) and (3) somatic burden, with study cohort as random intercept.
Results
After adjustment for study cohort, BD‐II patients more often had a late onset ≥50 years (p = 0.008) and more current severe depression (p = 0.041). BD‐I patients were more likely to have a history of psychiatric hospitalization (p < 0.001) and current use of anti‐psychotics (p = 0.003). Multivariable analyses showed that BD subtype was not related to GAF, cognitive g‐score or somatic burden.
Conclusion
BD‐I and BD‐II patients did not differ in terms of general functioning, cognitive impairment or somatic burden. Some clinical differences were observed between the groups, which could be the consequence of diagnostic definitions. The distinction between BD‐I and BD‐II is not the best way to subtype OABD patients. Future research should investigate other disease specifiers in this population.
Keywords: bipolar disorder, cognition, comorbidities, diagnostic subtypes, elderly, functioning, geriatrics, impairment, older‐age bipolar disorder (OABD), psychiatry
1. INTRODUCTION
Bipolar disorder (BD) is clinically heterogeneous, and a distinction is often drawn between bipolar I disorder (BD‐I) and bipolar II disorder (BD‐II). 1 Although these subtypes have been included in the DSM since 1994, this separation has been a topic of a long‐lasting debate. 2 , 3 , 4 , 5 In particular, researchers question whether BD‐I and BD‐II differ etiologically and clinically. 6 , 7 , 8
One argument to keep the subtypes separated is that BD‐II is often considered as a “milder” form of BD‐I with different clinical features that require a different treatment strategy. 2 , 9 , 10 However, observed clinical differences in severity may be due to the DSM‐5 definition itself. 11 The DSM‐5 states that a manic episode with psychotic features, longer duration, hospitalization, or severe impairment can only exist within BD‐I, so by definition individuals with BD‐I experience more severe manic episodes than BD‐II patients. This DSM‐5 definition may also explain the higher hospitalization rates observed in BD‐I. 2 , 12 , 13
In contrast, studies in the general adult BD population have suggested that BD‐II could be “more severe” than BD‐I in other illness aspects, although available literature is conflicting. 13 , 14 In BD‐II, higher prevalences of depressive symptoms, 12 more episodes of major depression 2 , 13 , 15 and suicide attempts 13 are reported. A meta‐analysis could not find differences in comorbid anxiety disorders between subtypes, 16 but personality disorders seem to be more common in BD‐II. 12 , 17 A recent meta‐analysis observed subtle cognitive differences between BD subtypes, but these could be explained by differences in illness severity. 18 Maina et al. (2007) found that individuals with BD‐II experience lower quality of life than those with BD‐I. 19 While Vinberg et al. (2017) described lower overall functioning in BD‐II, 12 Karanti et al. (2020) reported that BD‐II patients were more likely to be financially self‐sustaining (i.e., not reliant on social benefits). 13
Examining BD subtypes is particularly interesting among individuals with older‐age bipolar disorder (OABD, defined as ≥50 years). 20 If any clinical differences between BD‐I and BD‐II exist, these may be more pronounced among older patients as illness duration is longer and the clinical phenotype has had more time to fully develop. Within a lifetime, patients can convert from BD‐II to BD‐I if they experience a full‐blown manic episode in later life, but cannot convert from BD‐I back to BD‐II. Thus, older patients who have a BD‐II diagnosis in later life may have a more “pure” version of that subtype compared to a younger sample of BD‐II in which some proportion may later convert to BD‐I.
It is of great clinical importance to identify determinants of (inter‐episodic) impairment and functioning in OABD, as investigation of clinically relevant subgroups might aid better, personalized care for OABD patients. 20 Up to date, only one study (N = 101) compared somatic comorbidities 21 and one study (N = 86) compared cognitive impairment 22 between BD subtypes in an older population specifically. The current study aimed to examine differences between BD‐I and BD‐II subtypes in OABD in a larger sample. Data were derived from the Global Aging & Geriatric Experiments in Bipolar Disorder database (GAGE‐BD), a harmonized dataset of archival studies from around the globe. 23 Differences in sociodemographic and clinical factors were explored between BD subtypes. We analyzed associations between BD subtype and (1) general functioning, (2) cognitive performance and (3) somatic burden in OABD in further detail, as these domains are often affected in the older BD population and have important implications for clinical outcomes. 20 , 24
2. MATERIALS AND METHODS
2.1. Study population
The current study analyzed cross‐sectional, multisite data from the GAGE‐BD project. The GAGE‐BD database includes pooled and harmonized international data from more than 1300 individuals with BD. The GAGE‐BD project aims to further characterize the group of individuals with OABD. 23 For the current analyses, data from Wave 1.75 (as of July 2021) were used. Detailed information on the GAGE‐BD project, sample characteristics, and meta‐data of contributing studies can be found elsewhere. 23 , 25 Participants were included in the present analysis if they were aged ≥50 years and if data on BD subtype were available. A total of 15 study cohorts from 10 sites contributed to the current analyses (Table S1). Approval to contribute data was obtained by each site's institutional review board or ethics committees and by the GAGE‐BD coordinating center (Case Western Reserve University School of Medicine, Cleveland, Ohio, USA). Table S2 shows the inclusion/exclusion criteria for the contributing studies.
2.2. Sociodemographic and clinical characteristics
Demographic variables (age, gender, education level, employment status, relationship status) and clinical variables (age of onset, illness duration, number of affective episodes, depression severity, rapid cycling, hospitalizations, lithium use, antipsychotic use, smoking) were harmonized across studies. 25 In all contributing studies, current mania severity was measured with the Young Mania Rating Scale (YMRS). 26 As current depressive symptoms were measured with the Hamilton Depression Rating Scale (HAM‐D), 27 Montgomery–Asberg Depression Rating Scale (MADRS), 28 or the Center for Epidemiologic Studies Depression Scale (CES‐D) 29 in different samples, these data were transformed into one categorical depression severity variable with three categories: 0 = No depression (HAM‐D ≤ 7; MADRS ≤6; CES‐D ≤ 15), 1 = Mild or moderate depression (HAM‐D 8–23; MADRS 7–34; CES‐D 16–27), and 2 = Severe depression (HAM‐D ≥ 24; MADRS ≥35; CES‐D ≥ 28).
2.3. Predictor of interest: BD subtype
The predictor was BD subtype (BD‐I vs. BD‐II). The participating studies gave this diagnosis based on the Structured Clinical Interview for DSM Disorders for DSM‐IV (SCID‐IV), 30 the Mini‐International Neuropsychiatric Interview (M.I.N.I.) for DSM‐IV, 31 or clinical evaluation (Table S2). Data on subtypes other than BD‐I and BD‐II, such as BD Not Otherwise Specified (BD‐NOS) or Cyclothymic Disorder, were not available in the GAGE‐BD database. Individuals with BD subtype “unknown” were removed from analysis.
2.4. Primary outcomes
2.4.1. General functioning
General functioning was measured with the continuous Global Assessment of Functioning (GAF) scale, which ranges from 0 to 100. 32
2.4.2. Cognitive performance
As neuropsychological assessments were heterogeneous across individual studies, the GAGE‐BD project harmonized the available cognitive data for each participant into a general cognitive ability “g” score. This method has been used before and has advantages for consortium analyses, as it allows all participants with cognitive data to be included regardless of the different batteries used across sites. 33 The method is based on findings that show that the g‐score derived from different studies with different batteries ranks patients almost identically (g factors approaching r = 1.0). 34
First, neuropsychological (NP) tests were assigned to a cognitive domain for each contributing study separately. Only NP tests in the domains of speed of processing, verbal learning, non‐verbal learning, working memory, and reasoning and problem solving were selected. A minimum of one and a maximum of two tests per domain and no more than one variable from each NP task were included. 33 Some NP tests were not selected due to a small study sample size (less than 10 participants per one NP variable). Table S3 shows the final selection of NP tests and the corresponding cognitive domains. For each participant a z‐score was calculated per NP test based on the group mean and standard deviation (SD) of the BD population within the respective study cohort. Extreme outlier scores were removed (z < −3.00 or > +3.00). To harmonize z‐scores across studies, a principal component analysis (PCA, unrotated, Eigenvalues >1.0) was performed with all selected measures. The first factor score of the PCA was used as individual cognitive g‐score (i.e., a continuous z‐score with mean = 0, SD = 1, range − 1.00 to +1.00). A negative g‐score represents cognitive performance worse than average, whereas a positive g‐score indicates cognitive performance above average.
2.4.3. Somatic burden
Across studies in the GAGE‐BD database, the presence of physical comorbidities was assessed in various ways, including standardized measures such as the Cumulative Illness Rating Scale (CIRS) 35 and the Charlson Comorbidity Index, 36 as well as using medical charts, self‐report, or physical examination. 25 The GAGE‐BD project harmonized these variables into eight binary variables, each representing a disease domain: cardiovascular, respiratory, gastrointestinal, liver, renal, genitourinary, musculoskeletal, and endocrine. 25 Each of the eight comorbidity variables represented 1 = presence and 0 = absence of an illness within that particular disease domain. The count variable “somatic burden” was created by summing the eight binary variables, but only for those participants with complete data on all eight disease domains. The procedure was similar to the development of the CIRS(G), which has been shown to have good interrater reliability and face validity in older‐aged psychiatric patients. 37
2.5. Statistical analysis
Analyses were carried out in IBM SPSS version 25. Since little is known about the potential differences between BD‐I and BD‐II in OABD, we employed an agnostic (hypothesis‐free) approach. A two‐sided alpha of 0.05 was considered statistically significant. Missing data were handled with pairwise deletion (available case analysis) and for the GLMMs with listwise deletion (complete case analysis).
2.5.1. Descriptive statistics
Non‐parametric tests (Mann–Whitney U test or Fisher exact test) were performed to compare sociodemographic and clinical factors between BD‐I and BD‐II. We also accounted for systematic differences between the contributing studies by performing generalized linear mixed models (GLMMs) with a binomial probability distribution with a logit link function. In these models a sociodemographic or clinical factor was entered as a predictor, BD subtype as the outcome variable, and study cohort as a random intercept.
2.5.2. Generalized linear mixed models (GLMMs)
We performed GLMMs with BD subtype as predictor and study cohort as random intercept. The residuals of both continuous outcomes (i.e., GAF and cognitive g‐score) were normally distributed within each of the 15 contributing studies. For these outcomes, we used a GLMM with a normal probability distribution with an identity link function. For the outcome somatic burden, a GLMM with a Poisson probability distribution with a log link function was employed, as is appropriate for count data. For each outcome, crude analyses (only controlling for study cohort) and analyses adjusted for age, education level, and gender were performed. For evaluation of missing data, binary missing data indicators (MDIs) were created for GAF, cognitive g‐score, and somatic burden, with 1 = missing and 0 = present. The association between BD subtype and missing data was evaluated with GLMMs with a binomial distribution and a logit link function, with BD subtype as predictor, MDI as outcome and study cohort as a random intercept.
2.5.3. Sensitivity analyses
We initially planned to adjust the GLMM analyses for eight potential confounders: age, gender, education level, number of affective episodes, late onset, depression severity, history of psychiatric hospitalizations, and anti‐psychotics use. However, many cohorts had substantial amounts of missing data on these variables. Therefore, we repeated the analyses in a subset of participants with complete data on these eight confounders to examine how likely it is that these confounders would substantially affect the estimated differences in outcomes between BD‐I and BD‐II.
3. RESULTS
3.1. Descriptive statistics
In total, 963 BD patients aged 50 or older were analyzed (Table 1). Overall, 714 participants were diagnosed with BD‐I (74.1%) and 249 with BD‐II (25.9%). The proportion of BD‐I patients in individual studies ranged from 47.8% to 100% (Figure 1). The selected sample of the “Inflammaging study” did not contain individuals with BD‐II, since all BD‐II patients in that study were aged <50 years. Most of the study participants were not highly symptomatic when assessed (low YMRS and on average 4.3% with severe depression).
TABLE 1.
Characteristics of the total sample and subsamples BD‐I disorder versus BD‐II disorder
| Total sample (max N = 963) | BD‐I (max N = 714, 74.1%) | BD‐II (max N = 249, 25.9%) | p‐value (Mann–Whitney U/Fisher exact) | Controlled for study cohort a t‐value, p‐value | |
|---|---|---|---|---|---|
| Sociodemographic variables | |||||
| Age (in years) | N = 963 | N = 714 | N = 249 | 0.048* | 1.069, 0.285 |
| M (SD) | 63.1 (8.8) | 62.8 (8.7) | 64.1 (8.9) | ||
| Gender | N = 963 | N = 714 | N = 249 | 1.000 | 0.148, 0.882 |
| Female | 56.1% (540) | 56.0% (400) | 56.2% (140) | ||
| Male | 43.9% (423) | 44.0% (314) | 43.8% (109) | ||
| Education level (in years of education) | N = 767 | N = 572 | N = 195 | 0.969 | 0.919, 0.359 |
| M (SD) | 13.1 (3.8) | 13.1 (3.8) | 13.1 (4.0) | ||
| Employment status | N = 634 | N = 460 | N = 174 | 0.214 | −0.707, 0.480 |
| Working | 24.3% (154) | 25.7% (118) | 20.7% (36) | ||
| Not working | 75.7% (480) | 74.3% (342) | 79.3% (138) | ||
| Relationship status | N = 799 | N = 595 | N = 204 | 0.359 | 0.082, 0.935 |
| Currently single | 58.4% (467) | 59.7% (355) | 54.9% (112) | ||
| In steady relationship | 41.6% (332) | 40.3% (240) | 45.1% (92) | ||
| Clinical variables | |||||
| Age of onset (in years) | N = 767 | N = 569 | N = 198 | 0.256 | 1.113, 0.266 |
| M (SD) | 31.7 (15.0) | 31.3 (14.8) | 33.2 (15.8) | ||
| Late onset (first episode ≥50 years) | N = 767 | N = 569 | N = 198 | 0.013* | 2.645, 0.008** |
| Yes | 14.2% (109) | 12.3% (70) | 19.7% (39) | ||
| No | 85.8% (658) | 87.7% (499) | 80.3% (159) | ||
| Duration since start illness or BD diagnosis (in years) | N = 874 | N = 656 | N = 218 | 0.491 | −1.190, 0.235 |
| M (SD) | 32.0 (13.7) | 32.2 (13.6) | 31.2 (14.0) | ||
| Number of affective episodes (lifetime) | N = 678 | N = 500 | N = 178 | 0.045* | 1.068, 0.286 |
| M (SD) | 20.0 (44.3) | 19.5 (47.7) | 21.3 (32.8) | ||
| Depression severity b | N = 841 | N = 634 | N = 207 | 0.007** |
Mild vs. no: −0.247, 0.805 Severe vs. no: 1.946, 0.052 Mild vs. severe: −2.045, 0.041* |
| No depression | 58.9% (495) | 59.0% (374) | 58.5% (121) | ||
| Mild to moderate depression | 36.9% (310) | 38.0% (241) | 33.3% (69) | ||
| Severe depression | 4.3% (36) | 3.0% (19) | 8.2% (17) | ||
| Manic symptoms (YMRS) | N = 881 | N = 665 | N = 216 | 0.773 | −0.582, 0.560 |
| M (SD) | 4.0 (5.4) | 4.0 (5.5) | 3.9 (5.0) | ||
| Rapid cycling (lifetime) | N = 426 | N = 309 | N = 117 | 0.093 | 1.526, 0.128 |
| No history of rapid cycling | 85.2% (363) | 87.1% (269) | 80.3% (94) | ||
| Possible/probable/definite history of rapid cycling | 14.8% (63) | 12.9% (40) | 19.7% (23) | ||
| Ever hospitalized for psychiatric condition | N = 613 | N = 448 | N = 165 | <0.001*** | −6.088, <0.001*** |
| Yes | 74.6% (457) | 80.8% (362) | 57.6% (95) | ||
| No | 25.4% (156) | 19.2% (86) | 42.4% (70) | ||
| Lithium use (current) | N = 909 | N = 675 | N = 234 | 0.488 | −0.775, 0.439 |
| Yes | 40.4% (367) | 39.7% (268) | 42.3% (99) | ||
| No | 59.6% (542) | 60.4% (407) | 57.7% (135) | ||
| Anti‐psychotic use (current) | N = 919 | N = 683 | N = 236 | 0.023* | −2.953, 0.003** |
| Yes | 45.5% (418) | 47.7% (326) | 39.0% (92) | ||
| No | 54.5% (501) | 52.3% (357) | 61.0% (144) | ||
| Smoking | N = 496 | N = 348 | N = 148 | 0.550 |
Lifetime vs. never: 0.201, 0.841 Current vs. never: 0.799, 0.424 Current vs. lifetime: 0.635, 0.526 |
| Never | 31.7% (157) | 33.0% (115) | 28.4% (42) | ||
| Lifetime smoker (past) | 30.6% (152) | 30.5% (106) | 31.1% (46) | ||
| Current smoker | 37.7% (187) | 36.5% (127) | 40.5% (60) | ||
| Outcome variables | |||||
| GAF score | N = 437 | N = 306 | N = 131 | 0.473 | −0.189, 0.851 |
| M (SD) | 61.0 (12.0) | 60.6 (12.1) | 62.0 (11.9) | ||
| Cognitive g‐score c | N = 324 | N = 230 | N = 94 | 0.191 | −0.398, 0.691 |
| M (SD) | 0.05 (0.94) | −0.002 (0.97) | 0.16 (0.85) | ||
| Somatic burden d | N = 513 | N = 413 | N = 100 | 0.831 | −0.083, 0.934 |
| M (SD) | 2.47 (2.1) | 2.47 (2.1) | 2.45 (2.1) | ||
Abbreviations: BD, Bipolar Disorder; BD‐I, bipolar I disorder; BD‐II, bipolar II disorder; CES‐D, Center for Epidemiologic Studies Depression Scale; GAF, Global Assessment of Functioning; HAMD, Hamilton Depression Rating Scale; M, mean; MDRS, Montgomery–Asberg Depression Rating Scale; SD, standard deviation; YMRS, Young Mania Rating Scale.
Note: *p < 0.05 **p < 0.01 ***p < 0.001.
Generalized linear mixed model (GLMM) using a binomial probability distribution with a logit link function and random effect of study cohort with outcome BD subtype.
The depression severity band was harmonized from MDRS, HAMD, and CES‐D, see text for cut‐offs.
Cognitive g‐score: a continuous z‐score scaling metric with mean = 0, SD = 1, range − 1.00 to +1.00, see text for procedure.
Somatic burden: total number of somatic diseases, out of eight disease categories. Only calculated for participants with available data for all of the eight disease categories (N = 513).
FIGURE 1.
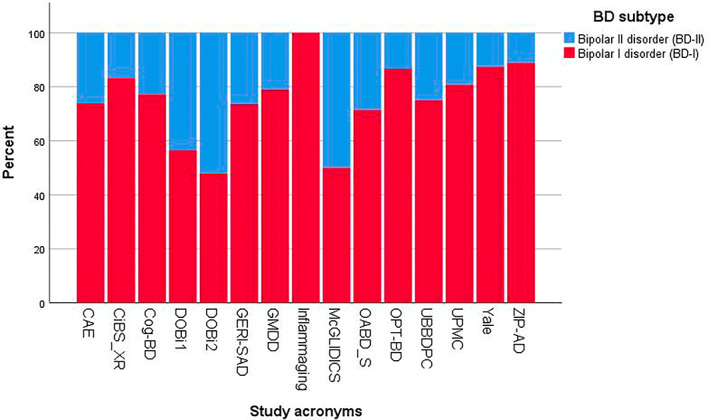
Percentage of BD subtypes per participating study. Notes: BD, bipolar disorder; CAE, “Treatment Adherence Enhancement in Bipolar Disorder” study; CiBS_XR, “Cognition in Bipolar Disorder XR” study; Cog‐BD, “Cognition in Euthymic Older Adults with Bipolar Disorder” study; DOBi 1, “Dutch Older Bipolar cohort study,” wave 1; DOBi 2, “Dutch Older Bipolar cohort study,” wave 2; GERI‐SAD, “Open‐label, Prospective Trial of Lamotrigine for Symptoms of Geriatric Bipolar Depression” study; GMDD, “Geriatric Psychiatry Mood Disorders Research Database”; Inflammaging, “Dynamic Inflammatory and Mood Predictors of Cognitive Aging in Bipolar Disorder” study; McGLIDICS, “The McGill Geriatric Lithium‐Induced Diabetes Insipidus Clinical Study”; OABD_S, “Cognitive Impairment and dementia in late life bipolar disorder” study; OPT‐BD, “Asenapine in the Treatment of Older Adults with Bipolar Disorder” study; UBBDPC, “University of Barcelona's Bipolar Disorder Program Cohort”; UPMC, “The Effect of Bipolar Disorder and its Comorbidities on Cognition in Older Adults” study; Yale, “Mood Disorders Research Program Database”; ZIP‐AD, “Ziprasidone switching in response to adherence in psychotropic‐related weight gain concerns among patients with bipolar disorder” study.
Overall, mean age was 63.1 years (SD 8.8), 56.1% was female, participants received on average 13.1 years of education (SD 3.8), 24.3% had a current paid job, and 41.4% was in a current steady relationship. Average GAF was 61.0 (SD 12.0) and mean somatic burden was 2.47 (SD 2.1). Controlled for study cohort, individuals with BD‐II more often had a late onset (≥50 years) of BD (19.7% vs. 12.3%, p = 0.008) and more often had a current severe rather than a mild/moderate depression (8.2%/33.3% vs. 3.0%/38.0%, p = 0.041). On the other hand, individuals with BD‐I more often had a history of psychiatric hospitalization (80.8% vs. 57.6%, p < 0.001) and more often were prescribed antipsychotic medications (47.7% vs. 39.0%, p = 0.003). For descriptive data on individual physical comorbidities, see Table 3.
TABLE 3.
Physical comorbidities for the total sample and subsamples of bipolar I disorder versus bipolar II disorder
| Total sample (max N = 963) | BD‐I (max N = 714, 74.1%) | BD‐II (max N = 249, 25.9%) | p‐value (Fisher exact) | Controlled for study cohort a t‐value, p‐value | |
|---|---|---|---|---|---|
| Cardiovascular comorbidity | N = 928 | N = 690 | N = 238 | 0.821 | 0.347, 0.729 |
| Yes | 45.6% (423) | 45.4% (313) | 46.2% (110) | ||
| No | 54.4% (505) | 54.6% (377) | 53.8% (128) | ||
| Respiratory comorbidity | N = 861 | N = 647 | N = 214 | 0.327 | 0.960, 0.337 |
| Yes | 36.8% (317) | 35.9% (232) | 39.7% (85) | ||
| No | 63.2% (544) | 64.1% (415) | 60.3% (129) | ||
| Gastrointestinal comorbidity | N = 749 | N = 554 | N = 195 | 0.777 | 0.613, 0.540 |
| Yes | 26.3% (197) | 26.0% (144) | 27.2% (53) | ||
| No | 73.7% (552) | 74.0% (410) | 72.8% (142) | ||
| Hepatic/Pancreatic comorbidity | N = 747 | N = 552 | N = 195 | 0.644 | 1.096, 0.274 |
| Yes | 7.9% (59) | 7.6% (42) | 8.7% (17) | ||
| No | 92.1% (688) | 92.4% (510) | 91.3% (178) | ||
| Renal comorbidity | N = 629 | N = 492 | N = 137 | 1.000 | 0.184, 0.854 |
| Yes | 7.9% (50) | 7.9% (39) | 8.0% (11) | ||
| No | 92.1% (579) | 92.1% (453) | 92.0% (126) | ||
| Genito‐urinary comorbidity | N = 541 | N = 427 | N = 114 | 0.448 | −0.944, 0.346 |
| Yes | 22.4% (121) | 23.2% (99) | 19.3% (22) | ||
| No | 77.6% (420) | 76.8% (328) | 80.7% (92) | ||
| Musculoskeletal comorbidity | N = 789 | N = 584 | N = 205 | 0.742 | 0.146, 0.884 |
| Yes | 41.7% (329) | 42.1% (246) | 40.5% (83) | ||
| No | 58.3% (460) | 57.9% (338) | 59.5% (122) | ||
| Endocrine comorbidity | N = 930 | N = 691 | N = 239 | 1.000 | 0.494, 0.622 |
| Yes | 36.1% (336) | 36.2% (250) | 36.0% (86) | ||
| No | 63.9% (594) | 63.8% (441) | 64.0% (153) |
Abbreviations: BD, bipolar disorder; BD‐I, bipolar I disorder; BD‐II, bipolar II disorder.
Note: *p < 0.05; **p < 0.01; ***p < 0.001.
Generalized linear mixed model (GLMM) using a binomial probability distribution with a logit link function and random effect of study cohort with outcome BD subtype.
3.2. Generalized linear mixed models (GLMMs)
3.2.1. General functioning
GAF was collected in 45.4% of the total sample (N = 437/963) (Table 2). Controlled for study cohort, BD subtype was not associated with missing data on GAF (p = 0.810). BD subtype was not significantly associated with GAF (crude analysis, B = 0.25, CI = ‐2.20–2.70, p = 0.839). Controlled for age, gender, and education level, the effect of BD subtype on GAF remained small and statistically not significant (adjusted analysis, B = 0.15, CI = ‐2.45–2.74, p = 0.911).
TABLE 2.
Association between BD subtype and GAF score, cognitive g‐score, and somatic burden
| GAF score | Cognitive g‐score a | Somatic burden b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N in model c | Coefficient | 95% CI | p‐value | N in model d | Coefficient | 95% CI | p‐value | N in model e | Coefficient | Risk ratio | 95% CI | p‐value | |
| BD‐I (vs. BD‐II) | 437 | 0.25 | −2.20 – 2.70 | 0.839 | 324 | 0.027 | −0.20 – 0.26 | 0.818 | 513 | 0.026 | 1.026 | 0.89–1.18 | 0.721 |
| Adjusted analysis 1 f | 402 | 0.15 | −2.45 – 2.74 | 0.911 | 318 | 0.052 | −0.18 – 0.28 | 0.658 | 375 | 0.059 | 1.061 | 0.88–1.28 | 0.545 |
Abbreviations: CI, confidence interval; BD, bipolar disorder; BD‐I, bipolar I disorder; BD‐II, bipolar II disorder; GAF, Global Assessment of Functioning. For every analysis, BD subtype was entered as a fixed predictor variable and study cohort was entered as a random effect.
Cognitive g‐score: a continuous z‐score scaling metric with mean = 0, SD = 1, range − 1.00 to +1.00, see text for procedure.
Somatic burden: the total number of present physical comorbidities, out of eight disease categories. This outcome variable was only calculated for participants with available data for all of the eight disease categories (N = 513).
Generalized Linear Mixed Model using a normal probability distribution with an identity link function.
Generalized Linear Mixed Model using a normal probability distribution with an identity link function.
Generalized Linear Mixed Model using a Poisson probability distribution with a log link function.
Corrected for age, gender, education level.
3.2.2. Cognitive performance
A cognitive g‐score was present in 33.6% of the total sample (N = 324/963). Controlled for study cohort, BD subtype was not associated with missing data on cognitive g‐score (p = 0.273). BD subtype was not significantly associated with cognitive g‐score (crude analysis, B = 0.027, CI = −0.20–0.26, p = 0.818). Controlled for age, gender, education level, the association between BD subtype on cognitive g‐score remained small and statistically not significant (adjusted analysis, B = 0.052, CI = ‐0.18–0.28, p = 0.658).
3.2.3. Somatic burden
Somatic burden was available for 53.3% of the total sample (N = 513/963). Controlled for study cohort, BD subtype was not associated with missing data on somatic burden (p = 0.832). BD subtype was not associated with higher relative risk of somatic burden (crude analysis, RR = 1.026, CI = 0.89–1.18, p = 0.721). Adjustment for age, gender, and education level did not change the association (adjusted analysis, RR = 1.061, CI = 0.88–1.28, p = 0.545).
3.3. Sensitivity analyses
A subset of N = 394 participants had complete data for eight potential confounders (age, gender, education level, number of affective episodes, late onset, depression severity, history of psychiatric hospitalizations, and antipsychotic use). The sample with complete data did not have a significantly different distribution of BD subtype than the sample without complete data (Table S4). However, controlled for study cohort, included participants were significantly older than those excluded. Controlled for eight variables, the association between BD subtype and GAF seemed stronger but remained statistically not significant (Table 4, coefficient −2.250 vs. 0.15 in Table 2). Further exploratory analyses showed that the possible increased strength was mainly driven by depression severity: in the subgroup of patients with current severe depression, GAF was significantly higher in BD‐II than BD‐I patients (p = 0.019, Figure S1). After adjustment for eight variables, the associations between BD subtype and cognitive g‐score (Table 4, coefficient 0.033 vs. 0.052 in Table 2) and between BD subtype and somatic burden (Table 4, coefficient 0.141 vs. 0.059 in Table 2) remained similar and statistically not significant.
TABLE 4.
Sensitivity analyses in subset with complete data for eight potential confounding variables
| GAF score | Cognitive g‐score a | Somatic burden b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N in model c | Coefficient | 95% CI | p‐value | N in model d | Coefficient | 95% CI | p‐value | N in model e | Coefficient | Risk ratio | 95% CI | p‐value | |
| BD‐I (vs. BD‐II) | 150 | −0.86 | −5.25 – 3.53 | 0.699 | 264 | −0.063 | −0.32 – 0.20 | 0.632 | 166 | 0.072 | 1.074 | 0.78–1.47 | 0.655 |
| Adjusted analysis 2 f | 150 | −2.250 | −6.49 – 1.99 | 0.296 | 264 | 0.033 | −0.24 – 0.31 | 0.812 | 166 | 0.141 | 1.151 | 0.80–1.65 | 0.441 |
Abbreviations: CI, confidence interval; BD, bipolar disorder; BD‐I, bipolar I disorder; BD‐II, bipolar II disorder; GAF, Global Assessment of Functioning. For every analysis, BD subtype was entered as a fixed predictor variable and study cohort was entered as a random effect.
Cognitive g‐score: a continuous z‐score scaling metric with mean = 0, SD = 1, range − 1.00 to +1.00, see text for procedure.
Somatic burden: the total number of present physical comorbidities, out of eight disease categories. This outcome variable was only calculated for participants with available data for all of the eight disease categories (N = 513).
Generalized Linear Mixed Model using a normal probability distribution with an identity link function.
Generalized Linear Mixed Model using a normal probability distribution with an identity link function.
Generalized Linear Mixed Model using a Poisson probability distribution with a log link function.
Corrected for age, gender, education level, number of affective episodes, late onset, depression severity, history of psychiatric hospitalizations, and anti‐psychotics use.
4. DISCUSSION
4.1. Main findings
This study investigated differences between bipolar I and bipolar II subtypes in 963 individuals with OABD. This is the first time a large international dataset has been used to study BD subtypes in this specific age group. BD‐I patients more often had a history of psychiatric hospitalization and current use of antipsychotics, BD‐II patients more often had a late onset ≥50 years. Also, there was some evidence that BD‐II patients had more severe current depression. Contrary to our predictions, BD‐I and BD‐II patients were not significantly different in terms of general functioning, cognitive performance, and somatic burden. These results suggest that despite possible clinical differences, BD subtype does not influence overall functioning or health in older age.
4.2. Clinical differences between BD‐I and BD‐II
In this OABD sample, individuals with BD‐II more often had a late onset (≥50 years). In younger patients, a review by Dell'Osso et al. (2016) reported that two studies found a higher age of onset in BD‐I, six studies a higher age of onset in BD‐II and nine studies could not find any differences. 38 Thus, this review concluded that age of onset could not reliably differentiate BD‐I from BD‐II.
We found tentative evidence that BD‐II patients more often suffered from severe depression. Also in the general BD population, researchers reported a higher prevalence of depressive symptoms, episodes of major depression and suicide attempts in BD‐II, 2 , 12 , 13 , 15 more depressive predominant polarity, 39 and higher rates of completed suicide. 40 However, it is important to note that these findings could also be the result of confounding by indication. As Guzman‐Parra et al. (2021) postulates, an increased severity and a higher number of depressive episodes in BD‐II patients is plausible when these have been recruited from an outpatient setting: It is likely that these patients sought clinical help for the (number of) depressive episodes, since by definition these patients are not severely impaired by manic episodes. 41
We also observed more antipsychotic use and past hospitalizations in BD‐I. These findings are in line with studies in younger BD patients. 2 , 9 , 12 , 13 We hypothesize that these differences are the consequence of the diagnostic criteria of these subtypes. Since the DSM‐5 states that a manic episode with psychotic features or hospitalization can only exist within BD‐I, 1 it is therefore definitional that higher rates of anti‐psychotic use and hospitalization are observed among BD‐I patients.
4.3. General functioning, cognitive performance, and somatic burden
BD subtype was not significantly related to general functioning in OABD. These findings are mostly in accordance with literature in younger BD samples. The large Swedish national registry study by Karanti et al. (2020) found that GAF did not differ between 4806 BD‐I and 3960 BD‐II patients. 13 Rosa et al. (2010) reported that BD‐I and BD‐II groups scored equally on the Functioning Assessment Short Test (FAST), but higher than healthy controls, meaning greater disability. 42 An exception is the study by Vinberg et al. (2017), who reported lower overall functioning in BD‐II on the FAST. 12 This discrepancy might be explained by differences in sampling: Part of the cohort used in the study by Vinberg et al. (2017) was referred to the study directly after discharge from inpatient units, while our study mostly included outpatients that were not highly symptomatic. Most of the BD‐II patients in Vinberg's study had recently been hospitalized due to depression, whereas this had been a manic episode for BD‐I patients. 12 Future studies could further examine if functioning differs between BD‐I and BD‐II patients in different phases of recovery.
This study did not identify differences in cognitive performance between BD‐I and BD‐II in OABD. This is in contrast with a small study (N = 38 with vs. N = 48 without cognitive impairment), which found that BD‐I (vs. BD‐II) was a predictor of cognitive impairment among OABD patients. 22 In the middle‐aged BD population on the other hand, a meta‐analysis of 48 reports could not find distinctive differences in cognitive performance between younger BD‐I and BD‐II patients. 18 Recent studies have identified cognitive subgroups within the OABD population that may explain the cognitive heterogeneity. 43 In particular, Martino et al. (2017) identified three subgroups based on the number of cognitive areas affected (intact, selective deficits, and globally impaired), but also these three groups also did not significantly differ in terms of BD subtype. 44
In our GAGE‐BD sample, somatic burden was similar in BD‐I and BD‐II older‐aged patients. This is in line with earlier studies by Dols et al. (2014) in OABD. 21 In younger adults, reports are conflicting: Amann et al. (2017) did not find differences in somatic comorbidity, 45 but Forty et al. (2014) found that individuals with BD‐II were more likely to have gastric ulcers, heart disease, Parkinson's disease, and rheumatoid arthritis, whereas kidney disease was more common in BD‐I. 46 However, these differences were not statistically significant following correction for multiple testing. 46 Karanti et al. reported that the presence of overall somatic comorbidity was similar between BD subtypes, but reported significantly higher rates of endocrine, nutritional, and metabolic diseases in BD‐I patients. 13
4.4. Clinical significance of subtyping and clinical implications
In the coming generation, the number of OABD patients will continue to increase. It is of great clinical importance to identify subtypes or disease specifiers that predict (future) disease severity and (inter‐episodic) functioning in OABD, as this could improve personalized care. 20 However, it is under debate if the current subtyping into BD‐I and BD‐II by the DSM has resulted in such clinically relevant subgroups.
This study found that BD‐I patients were more likely to have a history of psychiatric hospitalization and use of antipsychotics, while it provided some evidence that BD‐II patients more often had severe depression. This may suggest that BD‐I patients have higher rates of acute care utilization, whereas BD‐II patients are more likely to experience an illness course with chronic dysfunction in the outpatient setting. Importantly, the observation of these clinical differences could also be a consequence of the diagnostic definitions of BD‐I and BD‐II in the DSM. 47 Based on these variables, it is difficult to state which subtype is “more severe.”
Especially in the older population, BD is complicated by poor general functioning, high somatic burden, and poor cognitive performance, even during euthymia. 20 , 48 These outcomes reflect how BD affects daily life and thus represent disease severity. Our analyses show that BD subtype is not related to these outcomes in outpatients with OABD. BD‐II does not seem to be a “milder form” of BD‐I in OABD. Rather, poor to moderate general functioning and presence of somatic comorbidities was common in both subtypes. We therefore believe that the distinction between BD‐I and BD‐II is not the best way to subtype OABD patients. Clinicians should treat all OABD patients with an integrated care model that targets not only psychiatric functioning, but also general, physical, social, and cognitive functioning, irrespective of BD subtype. Future research should investigate other disease specifiers in BD, such as history of (mood‐congruent) psychotic features, illness duration, number of affective episodes, episode density, number of hospitalizations, and psychotropic medication use. Moreover, future research should be longitudinal and include patients across the entire lifespan to investigate the potential predictive power of new disease specifiers.
4.5. Strengths and limitations
One strength of this study is the large sample size of OABD patients, which enabled analyses that would not be possible using individual smaller and independent studies. The analyses controlled for sample effects as well as for potential confounders. The study investigated outcomes that are clinically very important in OABD.
A limitation is that the GAGE‐BD sample included mostly outpatients who were not in an acute affective episode and had limited manic symptoms. In addition, the proportion of BD‐I patients in individual studies ranged from 47.8% to 100%. In the total GAGE‐BD dataset, 74.1% of patients had BD‐I. A previous large nationwide study found a distribution of BD‐I/ BD‐II of all ages of around 55%/45%. 13 Although our study controlled for study cohort, this hints at a selection bias toward BD‐I patients and an underrepresentation of BD‐II in the GAGE‐BD dataset. Therefore, our findings may not necessarily generalize to the larger OABD population. Second, there is also a possibility that we did not observe differences between BD‐I and BD‐II patients due to a “healthy survivor” effect. This means that as patients with the most severe psychiatric illness or somatic comorbidities die sooner, potential differences become less visible in BD patients that survive into older age. Third, the cognitive g‐score measured relative, not absolute cognitive performance nor performance for individual cognitive domains. Other relevant aspects, such as social cognition, 49 were not assessed. Fourth, we were not able to draw definite conclusions on causality due to the cross‐sectional design. Using a prospective, longitudinal and homogeneous cohort design would have likely delivered stronger conclusions about causality. 50 Fifth, due to complete case analysis, the multivariable models were performed in smaller subsets of the total sample, which may have decreased statistical power and comparability. Given these smaller sample sizes and the relatively small proportion of BD‐II patients in the adjusted analyses, there is a possibility that the non‐significant differences in GAF, cognitive performance and somatic burden among BD subtypes are due to a power issue. Perhaps the current analyses can be replicated after more sites and OABD patients have joined the GAGE‐BD database in future. However, even if the very small differences observed are truly different, these do not reflect clinically meaningful changes. Last, although the sensitivity analyses controlled for eight potential confounders, it is possible that residual confounding was present.
4.6. Conclusions
In this GAGE‐BD study, some clinical differences in past treatment history and current presentation were observed between BD‐I and BD‐II patients, but these may in part be the result of diagnostic definitions (i.e., confounding by indication). BD‐I and BD‐II older‐aged patients appeared similar in general functioning, cognitive performance and somatic burden. BD‐I and BD‐II do not seem clinically relevant subtypes in OABD. More research is needed to identify other, potentially more meaningful psychiatric determinants of impairment in OABD. Hopefully, investigation of clinically relevant subgroups in OABD will result in the development of better, personalized, and targeted interventions that can enhance (inter‐episodic) functioning in this vulnerable group.
FUNDING INFORMATION
This project was supported by the International Society for Bipolar Disorders (ISBD) Bowden Massey Strategic Research Initiative; US National Institute of Mental Health (R01MH070902, R01MH113230, R01MH084921); Pfizer; Glaxo Smith Kline; Merck; National Health and Medical Research Council of Australia; National Institute for Biomarker Research in Neuropsychiatry, INBION (FAPESP 14/50873–3; 2016/01302–9 and CNPq 465412/2014–9); Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS); Canadian Institutes for Health Research, grant 200017; Ministry of Science and Technology, Taiwan; Spanish Ministry of Science and Innovation (PI15/00283, PI18/00805) integrated into the Plan Nacional de I + D + I and co‐financed by the ISCIII‐Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER); the Instituto de Salud Carlos III; the CIBER of Mental Health (CIBERSAM); the Secretaria d'Universitats i Recerca del Departament d'Economia i Coneixement (2017 SGR 1365); the CERCA Programme; and the Departament de Salut de la Generalitat de Catalunya for the PERIS grant SLT006/17/00357. This publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of ISBD. The ISBD is a 401c3 non‐profit organization whose mission is to foster international collaboration in education and research. For more information, visit www.isbd.org and www.gage‐bd.org.
CONFLICT OF INTEREST
Alexandra Beunders, Almar Kok, Sigfried Schouws, Ralph Kupka, Farren Briggs, Lisa Eyler, Orestes Forlenza, Ariel Gildengers, Kaylee Sarna, Ashley Sutherland, Joy Yala, Luca Villa, Nicole Korten and Annemiek Dols have no conflicts to report. Federica Klaus has no conflict of interest to report, but receives an Early. Postdoc Mobility Fellowship of the Swiss National Science Foundation (SNSF) (grant number P2ZHP3_181506) and Novartis Foundation for Medical‐Biological Research Fellowship. Hilary Blumberg received an honorarium from Aetna for a presentation. Brent Forester has received research grants from Biogen, Eisai, The Rogers Family Foundation, The Spier Family Foundation and the National Institutes of Health. He has previously served as a consultant for Acadia Pharmaceuticals and Biogen. He currently serves on the Advisory Board for Patina Health and the Pharmacy and Therapeutics Committee for CVS Health. Esther Jiménez declares no conflict of interest but has a research grant from the Instituto de Salud Carlos III (ISCIII) and the Spanish Ministry of Science and Innovation (PI20/00060)). Benoit Mulsant receives compensation from the Department of Psychiatry, University of Toronto, Toronto, Ontario; the Centre for Addiction and Mental Health (CAMH), Toronto, Ontario; and the University of Pittsburgh, Pennsylvania. Dr Mulsant is a member of the Board of Trustees, Centre for Addiction and Mental Health, Toronto, Ontario. Dr Mulsant currently receives research support from Brain Canada, the Canadian Institutes of Health Research, the CAMH Foundation, the Patient‐Centered Outcomes Research Institute (PCORI), the US National Institute of Health (NIH), Eli Lilly, Pfizer, Capital Solution Design LLC, and HAPPYneuron. He has also received research support from Bristol‐Myers Squibb, Eli Lilly and Pfizer. He directly owns stocks of General Electric. Regan Patrick receives partial salary support from Rogers Family Foundation, Biogen and NIH/NIA. Soham Rej receives salary support from a clinician‐investigator award from the Fonds de Recherche Québec Santé (FRQS) and owns shares in Aifred Health. Martha Sajatovic has received the following research grants within the past 3 years: Nuromate, Otsuka, International Society for Bipolar Disorders (ISBD), National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), and Patient‐Centered Outcomes Research Institute (PCORI). In the past year, she has been a consultant for Alkermes, Otsuka, Sunovion, Janssen, Lundbeck, Teva, Clinical Education Alliance, and Health Analytics. In the past year, she received royalties from Springer Press, Johns Hopkins University Press, Oxford Press, and UpToDate. She received compensation for preparation of/participation in CME activities in the past year from the American Physician's Institute (CMEtoGo, Psychopharmacology Institute, Novus, American Epilepsy Society, American Society of Clinical Psychopharmacology, and American Academy of Child and Adolescent Psychiatry. Eduard Vieta has received grants and served as consultant, advisor or CME speaker for the following entities: AB‐Biotics, Abbott, Allergan, Angelini, AstraZeneca, Biogen, Bristol‐Myers Squibb, Celon, Dainippon Sumitomo Pharma, Farmindustria, Ferrer, Forest Research Institute, Gedeon Richter, GH Research, Glaxo‐Smith‐Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sage, Sanofi‐Aventis, Servier, Shire, Sunovion, Takeda, the Brain and Behaviour Foundation, the Generalitat de Catalunya (PERIS), the Spanish Ministry of Science and Innovation (CIBERSAM), EU Horizon 2020 and the Stanley Medical Research Institute.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
The authors are deeply grateful to Dr. Charles L. Bowden, who passed away in March of 2022, and his wife Virginia Massey Bowden, for establishing the Bowden Massey Research Initiative, which has supported the GAGE‐BD project. Dr. Bowden's deep commitment to advancing care for individuals living with bipolar disorder is a continuously inspiring presence to our GAGE‐BD study team and the broader community of scientists focused on research in bipolar disorder. The authors thank Karin de Gooijer, Chien‐Lin Su, Julia Binnewies, Yuri Milaneschi, and Mariska Bot for their valuable input.
Beunders AJM, Klaus F, Kok AAL, et al. Bipolar I and bipolar II subtypes in older age: Results from the Global Aging and Geriatric Experiments in Bipolar Disorder (GAGE‐BD) project. Bipolar Disord. 2023;25:43‐55. doi: 10.1111/bdi.13271
DATA AVAILABILITY STATEMENT
Data are available as part of the GAGE‐BD project and subject to the completion of appropriate data use agreements. Qualified scientists who wish to access the data should contact the study lead author.
REFERENCES
- 1. American Psychiatric Publishing , ed. Diagnostic and Statistical Manual of Mental Disorders (5th Ed.). American Psychiatric Association; 2013. [Google Scholar]
- 2. Judd LL, Akiskal HS, Schettler PJ, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2003;73(1–2):19‐32. doi: 10.1016/S0165-0327(02)00324-5 [DOI] [PubMed] [Google Scholar]
- 3. Parker G, Spoelma MJ, Tavella G, et al. Categorical differentiation of the unipolar and bipolar disorders. Psychiatry Res. 2021;297:297. doi: 10.1016/J.PSYCHRES.2021.113719 [DOI] [PubMed] [Google Scholar]
- 4. Parker G. Polarised views about bipolar disorder(s): a critique of the 2020 college guidelines for mood disorders. Aust N Z J Psychiatry. 2021;55(6):548‐552. doi: 10.1177/00048674211020095 [DOI] [PubMed] [Google Scholar]
- 5. Malhi GS. Thing one and thing two 1: what “doctors use” to doctor you? Aust N Z J Psychiatry. 2021;55(6):536‐547. doi: 10.1177/00048674211022602 [DOI] [PubMed] [Google Scholar]
- 6. Vieta E, Gastó C, Otero A, Nieto E, Vallejo J. Differential features between bipolar I and bipolar II disorder. Compr Psychiatry. 1997;38(2):98‐101. doi: 10.1016/S0010-440X(97)90088-2 [DOI] [PubMed] [Google Scholar]
- 7. Merikangas KR, Lamers F. The “true” prevalence of bipolar II disorder. Curr Opin Psychiatry. 2012;25(1):19‐23. doi: 10.1097/YCO.0B013E32834DE3DE [DOI] [PubMed] [Google Scholar]
- 8. Song J, Kuja‐Halkola R, Sjölander A, et al. Specificity in etiology of subtypes of bipolar disorder: evidence from a Swedish population‐based family study. Biol Psychiatry. 2018;84(11):810‐816. doi: 10.1016/J.BIOPSYCH.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 9. Dell'Osso B, Cremaschi L, Arici C, et al. Differential core pharmacotherapy in bipolar I versus bipolar II disorder and European versus American patients not in a syndromal episode. Int Clin Psychopharmacol. 2020;35(1):8‐18. doi: 10.1097/YIC.0000000000000282 [DOI] [PubMed] [Google Scholar]
- 10. Parker G, Spoelma MJ, Tavella G, et al. The bipolar disorders: a case for their categorically distinct status based on symptom profiles. J Affect Disord. 2020;277:225‐231. doi: 10.1016/J.JAD.2020.08.014 [DOI] [PubMed] [Google Scholar]
- 11. Parker G, Fletcher K, McCraw S, Futeran S, Hong M. Identifying antecedent and illness course variables differentiating bipolar I, bipolar II and unipolar disorders. J Affect Disord. 2013;148(2–3):202‐209. doi: 10.1016/J.JAD.2012.11.061 [DOI] [PubMed] [Google Scholar]
- 12. Vinberg M, Mikkelsen RL, Kirkegaard T, Christensen EM, Kessing LV. Differences in clinical presentation between bipolar I and II disorders in the early stages of bipolar disorder: a naturalistic study. J Affect Disord. 2017;208:521‐527. doi: 10.1016/J.JAD.2016.10.031 [DOI] [PubMed] [Google Scholar]
- 13. Karanti A, Kardell M, Joas E, Runeson B, Pålsson E, Landén M. Characteristics of bipolar I and II disorder: a study of 8766 individuals. Bipolar Disord. 2020;22(4):392‐400. doi: 10.1111/BDI.12867 [DOI] [PubMed] [Google Scholar]
- 14. Dell'Osso B, Holtzman JN, Goffin KC, et al. American tertiary clinic‐referred bipolar II disorder compared to bipolar I disorder: more severe in multiple ways, but less severe in a few other ways. J Affect Disord. 2015;188:257‐262. doi: 10.1016/J.JAD.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 15. Baek JH, Park DY, Choi J, et al. Differences between bipolar I and bipolar II disorders in clinical features, comorbidity, and family history. J Affect Disord. 2011;131(1–3):59‐67. doi: 10.1016/J.JAD.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 16. Pavlova B, Perlis RH, Alda M, Uher R. Lifetime prevalence of anxiety disorders in people with bipolar disorder: a systematic review and meta‐analysis. Lancet Psychiatry. 2015;2(8):710‐717. doi: 10.1016/S2215-0366(15)00112-1 [DOI] [PubMed] [Google Scholar]
- 17. Fornaro M, Orsolini L, Marini S, et al. The prevalence and predictors of bipolar and borderline personality disorders comorbidity: systematic review and meta‐analysis. J Affect Disord. 2016;195:105‐118. doi: 10.1016/J.JAD.2016.01.040 [DOI] [PubMed] [Google Scholar]
- 18. Bora E. Neurocognitive features in clinical subgroups of bipolar disorder: a meta‐analysis. J Affect Disord. 2018;229:125‐134. doi: 10.1016/j.jad.2017.12.057 [DOI] [PubMed] [Google Scholar]
- 19. Maina G, Albert U, Bellodi L, et al. Health‐related quality of life in euthymic bipolar disorder patients: differences between bipolar I and II subtypes. J Clin Psychiatry. 2007;68(2):207‐212. doi: 10.4088/JCP.V68N0205 [DOI] [PubMed] [Google Scholar]
- 20. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older‐age bipolar disorder from the international society for bipolar disorders task force. Bipolar Disord. 2015;17(7):689‐704. doi: 10.1111/bdi.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dols A, Rhebergen D, Beekman A, Kupka R, Sajatovic M, Stek ML. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22:1066‐1074. doi: 10.1016/j.jagp.2013.12.176 [DOI] [PubMed] [Google Scholar]
- 22. Belvederi Murri M, Respino M, Proietti L, et al. Cognitive impairment in late life bipolar disorder: risk factors and clinical outcomes. J Affect Disord. 2019;257:166‐172. doi: 10.1016/j.jad.2019.07.052 [DOI] [PubMed] [Google Scholar]
- 23. Sajatovic M, Eyler LT, Rej S, et al. The global aging & geriatric experiments in bipolar disorder database (GAGE‐BD) project: understanding older‐age bipolar disorder by combining multiple datasets. Bipolar Disord. 2019;21(7):642‐649. doi: 10.1111/BDI.12795 [DOI] [PubMed] [Google Scholar]
- 24. Montejo L, Torrent C, Jiménez E, et al. Cognition in older adults with bipolar disorder: an ISBD task force systematic review and meta‐analysis based on a comprehensive neuropsychological assessment. Bipolar Disord. 2022;24:115‐136. doi: 10.1111/BDI.13175 [DOI] [PubMed] [Google Scholar]
- 25. Sajatovic M, Dols A, Rej S, et al. Bipolar symptoms, somatic burden, and functioning in older‐age bipolar disorder: analyses from the Global Aging & Geriatric Experiments in bipolar disorder database project. Bipolar Disord July 27. 2021;24:195‐206. doi: 10.1111/bdi.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429‐435. doi: 10.1192/bjp.133.5.429 [DOI] [PubMed] [Google Scholar]
- 27. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56‐62. doi: 10.1136/JNNP.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382‐389. doi: 10.1192/BJP.134.4.382 [DOI] [PubMed] [Google Scholar]
- 29. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385‐401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 30. First MB, Spitzer RL, Gibbon M, Williams J. In: Biometrics Research Department , ed. Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version. New York State Psychiatric Institute; 2002. Accessed March 29, 2022. https://ci.nii.ac.jp/naid/10027499505/ [Google Scholar]
- 31. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini‐international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry. 1998;59 Suppl 20:22‐33;quiz 34‐57. [PubMed] [Google Scholar]
- 32. Spitzer RL, Gibbon M, Williams JBW, Endicott J. Global assessment of functioning (GAF) scale. In: Sederer LI, Dickey B, eds. Outcomes Assessment in Clinical Practice. Baltimore, MD: Williams and Wilkins; 1996;76‐78. [Google Scholar]
- 33. Burdick KE, Millett CE, del M Bonnín C, et al. The international consortium investigating Neurocognition in bipolar disorder (ICONIC‐BD). Bipolar Disord. 2019;21(1):6‐10. doi: 10.1111/BDI.12748 [DOI] [PubMed] [Google Scholar]
- 34. Johnson W, Bouchard TJ, Krueger RF, McGue M, Gottesman II. Just one g: consistent results from three test batteries. Dermatol Int. 2004;32(1):95‐107. doi: 10.1016/S0160-2896(03)00062-X [DOI] [Google Scholar]
- 35. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622‐626. doi: 10.1111/J.1532-5415.1968.TB02103.X [DOI] [PubMed] [Google Scholar]
- 36. Chaudhry S, Jin L, Meltzer D. Use of a self‐report‐generated Charlson comorbidity index for predicting mortality. Med Care. 2005;43(6):607‐615. doi: 10.1097/01.MLR.0000163658.65008.EC [DOI] [PubMed] [Google Scholar]
- 37. Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the cumulative illness rating scale. Psychiatry Res. 1992;41(3):237‐248. doi: 10.1016/0165-1781(92)90005-N [DOI] [PubMed] [Google Scholar]
- 38. Dell'Osso B, Grancini B, Vismara M, et al. Age at onset in patients with bipolar I and II disorder: a comparison of large sample studies. J Affect Disord. 2016;201:57‐63. doi: 10.1016/J.JAD.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 39. Pallaskorpi S, Suominen K, Rosenström T, et al. Predominant polarity in bipolar I and II disorders: a five‐year follow‐up study. J Affect Disord. 2019;246:806‐813. doi: 10.1016/J.JAD.2018.12.093 [DOI] [PubMed] [Google Scholar]
- 40. Plans L, Nieto E, Benabarre A, Vieta E. Completed suicide in bipolar disorder patients: a cohort study after first hospitalization. J Affect Disord. 2019;257:340‐344. doi: 10.1016/J.JAD.2019.07.048 [DOI] [PubMed] [Google Scholar]
- 41. Guzman‐Parra J, Streit F, Forstner AJ, et al. Clinical and genetic differences between bipolar disorder type 1 and 2 in multiplex families. Transl Psychiatry. 2021;11(1):31. doi: 10.1038/S41398-020-01146-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosa AR, Bonnín CM, Vázquez GH, et al. Functional impairment in bipolar II disorder: is it as disabling as bipolar I? J Affect Disord. 2010;127(1–3):71‐76. doi: 10.1016/J.JAD.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 43. Montejo L, Jiménez E, Solé B, et al. Identifying neurocognitive heterogeneity in older adults with bipolar disorder: a cluster analysis. J Affect Disord. 2022;298(Pt A):522‐531. doi: 10.1016/J.JAD.2021.11.028 [DOI] [PubMed] [Google Scholar]
- 44. Martino DJ, Marengo E, Igoa A, Strejilevich SA. Neurocognitive heterogeneity in older adults with bipolar disorders. Psychiatry Res. 2018;262:510‐512. doi: 10.1016/J.PSYCHRES.2017.09.035 [DOI] [PubMed] [Google Scholar]
- 45. Amann BL, Radua J, Wunsch C, König B, Simhandl C. Psychiatric and physical comorbidities and their impact on the course of bipolar disorder: a prospective, naturalistic 4‐year follow‐up study. Bipolar Disord. 2017;19:225‐234. doi: 10.1111/bdi.12495 [DOI] [PubMed] [Google Scholar]
- 46. Forty L, Ulanova A, Jones L, et al. Comorbid medical illness in bipolar disorder. Br J Psychiatry. 2014;205(6):465‐472. doi: 10.1192/BJP.BP.114.152249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kessing LV, González‐Pinto A, Fagiolini A, et al. DSM‐5 and ICD‐11 criteria for bipolar disorder: implications for the prevalence of bipolar disorder and validity of the diagnosis—a narrative review from the ECNP bipolar disorders network. Eur Neuropsychopharmacol. 2021;47:54‐61. doi: 10.1016/J.EURONEURO.2021.01.097 [DOI] [PubMed] [Google Scholar]
- 48. Nivoli AMA, Murru A, Pacchiarotti I, et al. Bipolar disorder in the elderly: a cohort study comparing older and younger patients. Acta Psychiatr Scand. 2014;130(5):364‐373. doi: 10.1111/ACPS.12272 [DOI] [PubMed] [Google Scholar]
- 49. Miskowiak KW, Varo C. Social cognition in bipolar disorder: a proxy of psychosocial function and novel treatment target? Eur Neuropsychopharmacol. 2021;46:37‐38. doi: 10.1016/J.EURONEURO.2021.03.017 [DOI] [PubMed] [Google Scholar]
- 50. Vieta E, Angst J. Bipolar disorder cohort studies: crucial, but underfunded. Eur Neuropsychopharmacol. 2021;47:31‐33. doi: 10.1016/j.euroneuro.2021.03.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
Data are available as part of the GAGE‐BD project and subject to the completion of appropriate data use agreements. Qualified scientists who wish to access the data should contact the study lead author.


